Abstract
Atherosclerosis is a pathological condition characterized by the accumulation of plaques in the arteries, leading to cardiovascular diseases. The deposition of cholesterol in peripheral cells increases the risk of atherosclerosis. Reverse cholesterol transport (RCT) is essential to reduce the risk of atherosclerosis because it removes excessive cholesterol from the peripheral tissues. ATP-binding cassette transporters such as ABCA1, ABCG1, ABCG5, and ABCG8 are involved in the efflux of cholesterol. The upregulation of these ABC transporters enhances RCT, thereby promoting the removal of excess cholesterol from the body. The expression and activity of ABC transporters are regulated by transcriptional and post-transcriptional mechanisms, as well as by post-translational modifications. In this review, the regulation of ABC transporters by nuclear receptors such as farnesoid X receptor, liver X receptor, retinoid X receptor, retinoic acid receptor, and peroxisome proliferator-activated receptors is discussed. Pharmacological and natural compounds serving as agonists for the nuclear receptors have been identified to elevate the mRNA levels of the transporters. Consequently, it is anticipated that these compounds will attenuate the development of atherosclerosis through stimulation of the ABC transporters, thereby enhancing RCT and fecal cholesterol excretion. Understanding these regulatory processes can aid in the development of therapeutic approaches to prevent atherosclerosis.
1. Atherosclerosis
Atherosclerosis is a pathological condition characterized by plaques within arterial walls, which are formed through the accumulation of lipids, inflammatory cells, and other substances [1,2]. Over time, plaques consisting of cholesterol, triglyceride, calcium, and other components grow, causing arteries to narrow and harden. This process can result in various cardiovascular diseases, including coronary heart disease and stroke.
Cholesterol is essential for the proper functioning of the body because it is a constituent of cellular membranes and precursors of steroid hormones and bile acids. Cholesterol is mainly synthesized in the liver and can also be obtained from foods. Lipoproteins, such as low-density lipoprotein (LDL) and high-density lipoprotein (HDL), transport cholesterol in the blood. Cholesterol, particularly LDL cholesterol, plays a key role in the development of atherosclerosis. Studies have shown that high levels of LDL cholesterol in the blood are associated with an increased risk of developing atherosclerosis and cardiovascular diseases [3] because it contributes to plaque formation in the arteries. Conversely, higher levels of HDL cholesterol are associated with a lower risk of cardiovascular disease [4,5] because HDL helps remove excess cholesterol from the peripheral cells and prevents plaque formation.
Lifestyle changes, such as a healthy diet, regular exercise, and smoking cessation, can effectively lower LDL cholesterol levels and increase HDL cholesterol levels, thereby reducing the risk of atherosclerosis and related diseases. A previous study showed that a Mediterranean-style diet, which is high in fruits, vegetables, whole grains, and healthy fats, can reduce the risk of cardiovascular disease by lowering LDL cholesterol levels [6]. In some cases, medications such as statins may be prescribed to lower cholesterol levels and reduce the risk of cardiovascular disease. Statins inhibit cholesterol production in the liver, thereby reducing LDL cholesterol levels and decreasing the risk of atherosclerosis and related diseases [3]. Regular physical activity has been shown to increase HDL cholesterol levels and reduce the risk of cardiovascular disease [7]. Additionally, the removal of accumulated cholesterol from cells is inversely associated with atherosclerotic events [8,9,10].
2. Reverse Cholesterol Transport
Reverse cholesterol transport (RCT) is a process by which excess cholesterol is removed from peripheral tissues, such as the arterial wall, and transported back to the liver for excretion to the bile and ultimately the feces [11]. The RCT pathway involves multiple steps and several different cell types, including macrophages, which play critical roles in this process (Figure 1). Excess cholesterol in cells is excreted by ATP-binding cassette (ABC) transporters, ABCA1 and ABCG1, and HDL is formed [12,13,14]. Subsequently, HDL is delivered to the liver, where it is taken up by hepatocytes through scavenger receptor class B type I (SR-BI), and cholesterol can either be excreted in the bile or used for bile acid synthesis. Cholesterol in the liver is excreted into the bile duct by ABC transporters, ABCG5 and ABCG8 [15,16].
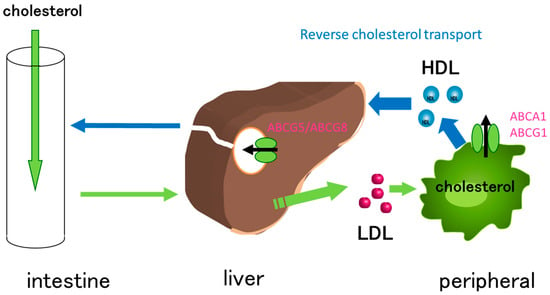
Figure 1.
Cholesterol transport facilitated by ABC transporters. Cholesterol is absorbed in the intestine and delivered to the liver via chylomicrons. Cholesterol, absorbed in the intestine and synthesized in the liver, is transferred to peripheral cells via LDL. The accumulation of cholesterol in peripheral cells, especially in macrophages, contributes to the development of atherosclerosis. The excess cholesterol is eliminated by ABCA1 and ABCG1, resulting in the formation of HDL. HDL is then delivered to the liver, where it is taken up by SR-BI. Finally, cholesterol is excreted to bile duct by ABCG5/ABCG8.
Impaired RCT has been implicated in the development of atherosclerosis, thereby increasing the risk of coronary heart disease and stroke [11]. Conversely, promoting RCT by exercise and dietary changes, as well as through pharmacological interventions, has been shown to prevent the progression of atherosclerosis [6,10,17,18]. Overall, RCT is an important physiological process for maintaining cholesterol homeostasis and preventing the development of cardiovascular diseases.
3. ABC Transporters Involved in Reverse Cholesterol Transport
ABC transporters are membrane proteins that consist of transmembrane domains (TMDs) and nucleotide-binding domains (NBDs) (Figure 2) [13]. ABC transporters transport substrates such as nutrients, metabolites, and xenobiotics using energy obtained from ATP hydrolysis. Multiple ABC transporters, including ABCA1, ABCA3, ABCA4, ABCA7, ABCA12, ABCB4, ABCB11, ABCD1, ABCG1, ABCG4, ABCG5, and ABCG8, are involved in lipid transport. ABCB4 transports phosphatidylcholine, whereas ABCB11 transports bile acids. Among them, ABCA1, ABCG1, ABCG4, ABCG5, and ABCG8 transport cholesterol. ABCA1 has two NBDs and two TMDs in a single molecule, whereas ABCG1, ABCG5, and ABCG8 are half-type ABC transporters that have one NBD and one TMD and function as a homodimer or a heterodimer (Figure 2). ABCG1 forms a homodimer [19,20,21,22], whereas ABCG5 and ABCG8 are highly homologous transporters and form a heterodimer [15,16]. ABCA1 and ABCG1 are ubiquitously expressed, but highly expressed in macrophages. ABCG5/ABCG8 heterodimer is expressed in the intestine and liver. ABCA1 mediates the efflux of cholesterol and phosphatidylcholine into apoA-I [23,24], whereas ABCG1 mediates the efflux of cholesterol and sphingomyelin to cholesterol-poor HDL (Figure 2A) [19,25,26,27]. Mutations in ABCA1 cause Tangier disease, a genetic disorder characterized by low HDL cholesterol levels and the accumulation of cholesterol in various tissues [28,29,30]. Studies have shown that ABCG1 also plays a critical role in macrophage cholesterol efflux and the prevention of atherosclerosis [31,32,33]. Mice lacking Abcg1 showed accumulation of cholesterol and triglyceride in macrophages of liver and lung [32]. Furthermore, mice lacking both Abca1 and Abcg1 showed greater accumulation of neutral lipids in tissues than mice lacking Abca1 or Abcg1 alone [34]. ABCA1 and ABCG1 are shown to transport cholesterol sequentially [26]. This suggests that ABCA1 and ABCG1 play important roles in the removal of excess cholesterol from peripheral cells, especially from macrophages, and that ABCA1 and ABCG1 function cooperatively. ABCG5 and ABCG8 mediate the efflux of cholesterol and plant sterols from the liver and intestines into the bile (Figure 2B) [15,16]. Mutations in either ABCG5 or ABCG8 cause sitosterolemia, a rare genetic disorder characterized by the accumulation of plant sterols and cholesterol in various tissues [35,36,37], indicating that ABCG5/ABCG8 suppresses the absorption of sterols in the intestine and enhances the excretion of sterols in the liver.
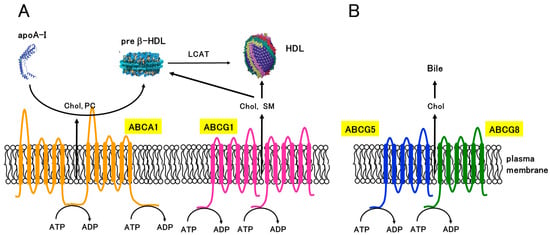
Figure 2.
Structures and functions of ABC transporters involved in cholesterol transport. (A) ABCA1 mediates the efflux of cholesterol (Chol) and phosphatidylcholine (PC) to apoA-I, which initiates the formation of nascent HDL (preβ-HDL). ABCG1 forms a homodimer and mediates the efflux of cholesterol and sphingomyelin (SM) to cholesterol-poor HDL. (B) ABCG5 and ABCG8 form a heterodimer and mediate the efflux of Chol to bile.
4. Nuclear Receptors Involved in the Regulation of Cholesterol Transporters
Nuclear receptors translate hormonal, metabolic, and nutritional signals into alterations in gene expressions [38]. Most nuclear receptors consist of several domains; N-terminal activation function 1, DNA-binding, hinge, and ligand-binding domains (Figure 3). Multiple nuclear receptors are involved in lipid homeostasis by regulating the expression of genes related to lipid biosynthesis, absorption, and excretion [38,39]. Expression of ABCA1, ABCG1, ABCG5, and ABCG8 is regulated by nuclear receptors such as liver X receptor (LXR; NR1H2/NR1H3), retinoid X receptor (RXR; NR2B1/NR2B2/NR2B3), retinoic acid receptor (RAR: NR1B1/NR1B2/NR1B3), peroxisome proliferator-activated receptor (PPAR; NR1C1/NR1C2/NR1C3), and farnesoid X receptor (FXR; NR1H4) [13,40].
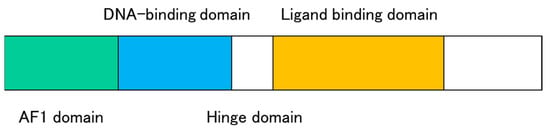
Figure 3.
Schematic structure of nuclear receptors. Nuclear receptors consist of an N-terminal ligand-independent activation function (AF1) domain, a DNA-binding domain, a hinge domain, and a ligand-binding domain with a ligand-dependent activation function (AF2).
4.1. LXR and RXR
LXR and RXR are members of the nuclear receptor superfamily that regulate various physiological processes including metabolism, inflammation, and immunity. LXR is activated by oxysterols, which are sterol metabolites, such as 22-(R)-hydroxycholesterol, 24-(S)-hydroxy-cholesterol, 25-hydroxycholesterol, 27-hydroxycholesterol, and 24-(S),25-epoxycholesterol [38,41,42,43], and it regulates the expression of genes involved in cholesterol transport and metabolism, as well as genes involved in inflammatory responses. The LXR is also involved in the regulation of the metabolism of other lipids such as fatty acids and triglycerides [44]. There are two types of LXR, LXRα (NR1H3) and LXRβ (NR1H2). LXRβ is ubiquitously expressed, whereas LXRα is expressed in the liver, adipose tissue, adrenal glands, intestine, lungs, kidneys, and myeloid cells. Human LXRα expression is autoregulated, whereas human LXRβ is stably expressed even in the absence of excess cholesterol.
RXR functions as a heterodimer with other nuclear receptors such as RAR, vitamin D receptors (VDRs), and PPAR [38,45,46]. There are three types of RXR, RXRα (NR2B1), RXRβ (NR2B2), and RXRγ (NR2B3). RXR itself can also bind to DNA as a homodimer, recognizing the RXR response element (RXRE), which is composed of two direct repeats of the core sequence, such as the DR1 motif, in which the two half-sites are separated by a single nucleotide. LXR regulates the expression of genes involved in cholesterol metabolism, whereas RXR regulates the expression of genes involved in cell differentiation and proliferation.
LXR and RXR form heterodimers that regulate gene expression by binding to the LXR response elements (LXREs) in the regulatory regions of target genes. The LXREs contain direct repeats of the core sequence (A/G)GGTCA separated by four nucleotides (DR4 motif). The binding of LXR to the LXREs leads to the recruitment of coactivator proteins, which in turn activates the transcription of target genes. The LXR/RXR heterodimer regulates the expression of genes involved in lipid metabolism, such as ABCA1, ABCG1, and sterol regulatory element-binding protein (SREBP)-1.
Activation of LXR and RXR has been shown to have beneficial effects on cholesterol metabolism and inflammation, and LXR agonists have been developed as potential therapeutic agents for the treatment of various diseases, including atherosclerosis, whereas they show adverse effects of increased fatty acid levels because of the activation of SREBP-1 [47,48].
4.2. RAR
RAR plays a crucial role in mediating the biological effects of retinoic acid, a derivative of vitamin A [45,49]. There are three types of RAR, RARα (NR1B1), RARβ (NR1B2), and RARγ (NR1B3). Retinoic acid serves as the ligand for RARs. Upon binding, retinoic acid induces conformational changes in the receptor, leading to the dissociation of corepressors and recruitment of coactivators. The activated RAR/RXR heterodimer then binds to retinoic acid response elements (RAREs) located in the regulatory regions of the target genes. RAR is involved in various physiological processes, including embryonic development, cell differentiation, and homeostasis [45,49].
4.3. PPAR
PPAR plays a key role in regulating metabolism, inflammation, and cell differentiation [39,46]. There are three types of PPARs, PPARα (NR1C1), PPARδ (NR1C2), and PPARγ (NR1C3). PPAR is activated by fatty acids and other lipid molecules and regulates the expression of genes involved in lipid metabolism and inflammation. PPARα is mainly expressed in the liver and is involved in the regulation of fatty acid oxidation and ketone body synthesis. PPARδ is expressed in various tissues and is involved in the regulation of fatty acid oxidation and glucose metabolism. PPARγ is mainly expressed in the adipose tissue and is involved in the regulation of adipogenesis and glucose metabolism.
Studies have shown that activation of PPAR can have beneficial effects on metabolism and inflammation, and PPAR agonists have been developed as drugs for the treatment of various metabolic disorders, including type 2 diabetes and dyslipidemia [50].
4.4. FXR
FXR plays a crucial role in regulating bile acid metabolism and homeostasis [38,43,51]. FXR is predominantly expressed in the liver and intestine, where it controls bile acid synthesis, transport, and enterohepatic circulation. FXR is activated by bile acids, which serve as endogenous ligands for FXR. Upon activation, the FXR or FXR/RXR heterodimer binds to the FXR response elements (FXREs) in the promoter regions of target genes. The activation of FXR has numerous physiological effects. One of its primary functions is to regulate bile acid synthesis by suppressing the expression of cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in bile acid biosynthesis, and upregulating the expression of small heterodimer partner (SHP), a repressor of CYP7A1. By reducing bile acid synthesis, FXR maintains bile acid homeostasis and prevents the accumulation of toxic levels of bile acids in the liver.
FXR also regulates the transport of bile acids by modulating the expression of various transporters, such as organic solute transporter α/β (OSTα/β), bile salt export pump (BSEP, ABCB4), and ileal bile acid transporter (IBAT). By controlling the expression of these transporters, FXR ensures efficient bile acid uptake in the intestine, promotes bile acid secretion into the bile, and limits its reabsorption from the intestine, thereby contributing to the enterohepatic circulation of bile acids. Furthermore, FXR has been implicated in various diseases, including cholestasis, non-alcoholic fatty liver disease (NAFLD), and inflammatory bowel disease (IBD) [51].
5. Transcriptional and Post-Transcriptional Regulation of ABCA1 and ABCG1
ABC transporters that mediate the efflux of cholesterol are regulated by multiple mechanisms. ABCA1, ABCG1, and ABCG5/ABCG8 are regulated at transcriptional, post-transcriptional, and post-translational levels.
The expression of ABCA1 and ABCG1 is tightly regulated transcriptionally and post-transcriptionally because ABCA1 and ABCG1 play important roles in lipid homeostasis in the body. Transcriptional regulation of ABCA1 and ABCG1 involves multiple transcription factors and co-regulators, including LXR, PPAR, and RXR (Figure 4A). When intracellular cholesterol levels rise, oxysterols, oxidized metabolites of cholesterol such as 25-hydroxy cholesterol, also increase. ABCA1 and ABCG1 expressions are physiologically induced by oxysterols via the LXR pathway [52,53,54]. When LXR is activated by oxysterols, the LXR/RXR heterodimers induce ABCA1 and ABCG1 by binding to the LXRE of promoters of the genes [55]. Agonists for PPARα and PPARγ also induce the expression of ABCA1 and ABCG1 via LXR [56,57,58]. In addition to nuclear receptors, other transcription factors are also involved in the regulation of ABCA1 expression, including SREBP, activator protein 2 (AP2), CCAAT/enhancer-binding protein (C/EBP), and zinc finger protein202 (ZNF202) [59,60,61,62,63]. These factors may bind to specific promoter regions and regulate ABCA1 and ABCG1 expression in response to various stimuli such as cholesterol and inflammatory signals.
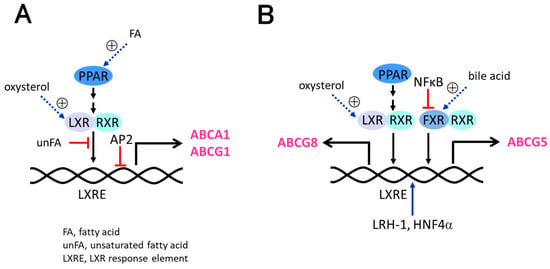
Figure 4.
Transcriptional regulation of ABC transporters. (A) ABCA1 and ABCG1 are induced by PPAR and LXR/RXR. AP2 suppresses the expression of ABCA1 and ABCG1. (B) ABCG5 and ABCG8 are induced by PPAR, LXR/RXR, and FXR. LRH-1 and HNF4α enhance the expression of ABCG5 and ABCG8, while NFκB suppresses it.
The post-transcriptional regulation of ABCA1 and ABCG1 involves several mechanisms, including mRNA stability, alternative splicing, and microRNA (miRNA) regulation. The 3’-untranslated regions (UTRs) of ABCA1 and ABCG1 mRNA contain cis-acting elements that regulate mRNA stability and translation efficiency. For example, the RNA-binding protein human antigen R (HuR) binds to and stabilizes the 3’-UTR of ABCA1 mRNA. Conversely, the stability of ABCA1 and ABCG1 mRNA is negatively regulated by miRNAs (miR-33a and miR-33b), which exist in the introns of SREBP-2 [64]. Additionally, other miRNAs such as miR-17, miR-19b, miR-93, miR-101, and miR-144 also downregulate ABCA1 mRNA [65,66].
6. Post-Translational Regulation of ABCA1 by LXR
In addition to transcriptional regulation of ABCA1 by LXR, ABCA1 is also post-translationally regulated by LXR [67,68]. The LXRβ/RXR complex directly binds to the C-terminal region of ABCA1 on the plasma membrane of macrophages and influences cholesterol secretion [67,68].
Excessive elimination of cholesterol can be detrimental to cells since cholesterol is an essential component of cell membranes. Hence, ABCA1 protein is degraded by the calpain and proteasome pathways with a half-life of 1–2 h [12,69]. LXR suppresses the degradation of ABCA1, and the addition of exogenous LXR ligands, which mimic cholesterol accumulation, results in the dissociation of LXRβ from ABCA1, thereby reversing the effects of LXR on ABCA1 degradation [67].
Under conditions in which intracellular cholesterol does not accumulate, the ABCA1-LXRβ complex localizes to the plasma membrane, which makes ABCA1 inactive (Figure 5). However, when cholesterol accumulates and the intracellular concentration of oxysterols rises, LXRβ binds oxysterol and dissociates from ABCA1 [68]. This dissociation enables the restoration of ABCA1 activity and apoA-I-dependent cholesterol efflux, resulting in an immediate decrease in intracellular cholesterol levels. Therefore, LXR can elicit both a post-translational response by directly binding to ABCA1 and a transcriptional response to maintain cholesterol homeostasis (Figure 5).
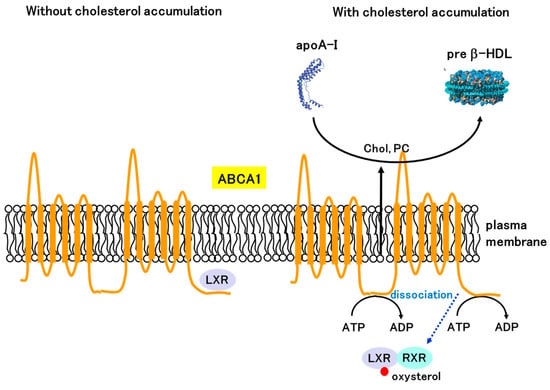
Figure 5.
Post-translational regulation of ABCA1 by LXR. LXRβ interacts with the C-terminal region of ABCA1 and inhibits ATP binding at the NBDs and apoA-I binding at the extracellular domain under cholesterol-depleted conditions. Under cholesterol-accumulated conditions, LXRβ binds oxysterol and dissociates from ABCA1, enabling ABCA1 to transport cholesterol using energy derived from ATP hydrolysis.
7. Transcriptional and Post-Transcriptional Regulation of ABCG5/ABCG8
The transcriptional regulation of ABCG5/ABCG8 is complex and involves a variety of mechanisms, including regulatory factors and genetic polymorphisms (Figure 4B). ABCG5 and ABCG8 possess a shared bidirectional promoter [35,70,71]. LXR and FXR have been shown to regulate the expression of ABCG5 and ABCG8 [72,73,74,75]. LXR binds to the LXREs within the promoters of ABCG5 and ABCG8 genes and enhances their transcription in combination with RXR [72,73]. The hepatic and intestinal expression of ABCG5/ABCG8 is modulated by bile acids via FXR [74,75]. FXR agonists have also been shown to regulate the mRNA expression of ABCG5 and ABCG8 in cultured hepatocytes [76]. In addition to nuclear receptors, other transcriptional regulators, including nuclear factor-kappa B (NF-kB), forkhead box protein O1 (FoxO1), PPARγ, liver receptor homolog-1 (LRH-1; NR5A2), hepatocyte nuclear factor 4α (HNF4α; NR2A1), and GATA4 have been shown to modulate ABCG5/ABCG8 expression [77,78,79,80,81]. Regulatory elements for HNF4α, LRH-1, NFκB, and FoxO1 were found within the intergenic regions of the initiation codons of ABCG5 and ABCG8 [78,79]. These factors can modulate the activity of LXR or directly interact with the promoter regions of ABCG5/ABCG8 to enhance or suppress their transcription.
Similar to ABCA1 and ABCG1, ABCG5 and ABCG8 are also post-transcriptionally regulated. Several miRNAs have been shown to regulate the expression of ABCG5/ABCG8. For instance, miR-33a downregulates the expression of both ABCG5 and ABCG8 [82]. Similarly, miR-223 has been shown to downregulate the expression of ABCG5/ABCG8 [83].
8. Transcriptional Activation of ABCA1, ABCG1, and ABCG5/ABCG8 by Pharmacological Compounds
Transcriptional activation of ABCA1, ABCG1, and ABCG5/ABCG8 can be achieved by various pharmacological compounds (Table 1). Synthetic LXR agonists, such as GW3965 and T0901317, have been shown to upregulate the expression of ABCA1 and ABCG1 in various cell types [84,85,86,87,88]. Moreover, these LXR ligands can prevent the development of atherosclerosis in vivo [84]. T0901317 highly induced the expression of ABCA1 and ABCG1, which increased cholesterol efflux and prevented the development of atherosclerosis [47,86,89,90]. However, LXR agonists exert adverse effects by enhancing lipogenesis, because they activate the SREBP-1c pathway [47]. Several PPAR agonists such as pioglitazone, rosiglitazone, WY14643, and GW501515 have been shown to induce the expression of ABCA1 and ABCG1 in various cell types [57,58,91,92,93]. Fibrates also induce the expression of ABCA1, ABCG1, and ABCG5/ABCG8 [91,94]. Statins, which are oral drugs used for the treatment of atherosclerosis by inhibiting HMG-CoA reductase, have not only demonstrated a reduction in cholesterol synthesis but also an upregulation of ABCA1 and ABCG1 expression [95,96]. Considering that both statins and fibrates are PPAR-activating compounds, it is plausible that they upregulate ABCA1 and ABCG1 via the PPAR-LXR pathway [93], although pitavastatin did not activate LXR but increased ABCA1 by PPARα-dependent protein stabilization [96] and the effects of statins depend on cellular conditions [97]. Agonists for RXR and RAR, including all-trans retinoic acid and 9-cis retinoic acid, increased ABCA1 and ABCG1 in a RAR/LXR/RXR pathway [52,98,99].
In the case of ABCG5/ABCG8 transcriptional activation, several pharmacological compounds have been demonstrated to be effective. Because ABCG5/ABCG8 is induced by LXR, LXR agonists, such as T0901317 and GW3936, increased ABCG5/ABCG8 expression [72,73]. Additionally, PPAR agonists, such as pioglitazone and rosiglitazone, have been reported to elevate ABCG5/ABCG8 expressions [80,81]. Treatment with metformin has also been shown to enhance ABCG5/ABCG8 expression in hepatocytes, possibly through the suppression of period2 and/or cryptochrome1, which are transcriptional repressors for ABCG5 and ABCG8 [100].

Table 1.
Transcriptional activation of ABCA1, ABCG1, and ABCG5/ABCG8 by pharmacological compounds.
Table 1.
Transcriptional activation of ABCA1, ABCG1, and ABCG5/ABCG8 by pharmacological compounds.
| Pharmacological Compound | Mechanism of Action | Target Transporters | References |
|---|---|---|---|
| LXR agonists (e.g., GW3965, T0901317) | Activation of LXR | ABCA1, ABCG1, ABCG5, ABCG8 | [72,73,84,85,86,87,88] |
| RXR agonists (e.g., 9-cis retinoic acid) | Activation of RXR | ABCA1, ABCG1 | [99] |
| RAR agonists (e.g., all-trans retinoic acid and 9-cis retinoic acid) | Activation of RAR | ABCA1, ABCG1 | [52,98,99] |
| PPAR agonists (e.g., fibrates, pioglitazone) | Activation of PPAR | ABCA1, ABCG1, ABCG5, ABCG8 | [57,58,80,81,91,92,93] |
| Statins (e.g., pitavastatin, atorvastatin) | Inhibition of HMG-CoA reductase | ABCA1, ABCG1 | [95,96] |
9. Transcriptional Activation of ABCA1, ABCG1, and ABCG5/ABCG8 by Natural Products
Multiple foods, dietary components, and natural compounds have been reported to activate transcription of ABCA1, ABCG1, ABCG5, and ABCG8 (Table 2). Cineole, a terpene oxide and a major constituent of eucalyptus and rosemary oils, increased ABCA1 mRNA levels [101]. 6-Gingerol, the pungent ingredient in ginger, increased ABCA1 mRNA, possibly by LXRα [102]. 8(R)-Hydroxyeicosapentaenoic acid (8R-HEPE) from North Pacific krill (Euphausia pacifica) induced ABCA1 and ABCG1 by activation of LXR [103]. Vitamin D upregulated ABCA1 and ABCG1 mRNA by increasing 27-hydroxycholesterol levels and activating LXR [104]. Riccardin C, a non-sterol natural product isolated from liverworts that functions as an LXRα agonist and an LXRβ antagonist, induced ABCA1 and ABCG1 expression [105]. Quercetin, a flavonoid, enhanced ABCA1 expression and cholesterol efflux through p38- and LXRα-dependent pathways in macrophages [106].
The activation of PPAR by natural products has been reported to upregulate ABCA1 and ABCG1 through the PPAR-LXRα pathway. Leonurine, an alkaloid compound of Herba leonuri, can prevent the development of atherosclerosis in the PPARγ-LXRα signaling pathway [107]. Allicin increased ABCA1 mRNA levels in THP-1 cells by acting through the PPARγ-LXRα pathway [108]. Anthocyanins induced ABCA1 in the PPARγ-LXRα pathway, which was blocked by the PPAR antagonist GW9662 [109]. Hydroxytyrosol, a phenolic compound, also enhances ABCA1 expression in a PPARγ-LXRα pathway, resulting in reduced foam cell formation [110]. Lycopene induced ABCA1 mRNA by the PPARγ-LXRα pathway in prostate cancer cells, although the induction was slow [111]. Evodiamine, one of the main alkaloids obtained from the medicinal evodia fruit of the plant Evodia rutaecarpa Benth, induced ABCG1 mRNA via the PPARγ-LXRα pathway [112]. Baicalin, a major flavonoid in Scutellaria baicalensis, induced ABCA1 and ABCG1 expression in the PPARγ-LXRα pathway [113]. Similarly, curcumin, a polyphenolic compound found in turmeric, has been shown to induce the expression of ABCA1 and ABCG1 in macrophages and adipocytes, possibly mediated by the activation of PPARγ and LXR [114,115]. In macrophages, the binding of 13-hydroxy linoleic acid to PPAR has been shown to induce the expression of ABCA1 and ABCG1 [116]. Mangiferin, a xanthonoid from Salacia oblonga, increased ABCA1 and ABCG1 via the PPARα-LXR pathway in macrophage Raw264.7 cells and decreased atherosclerotic plaque size in apoE knockout mice [117]. Resveratrol, a polyphenolic compound found in grapes and red wine, has been reported to induce the expression of ABCA1 and ABCG1 in macrophages [118,119]. These effects are thought to be mediated by activation of PPARγ and LXRα. While the natural products described above increased the expression of ABCA1 and ABCG1, unsaturated fatty acids such as eicosapentaenoic acid and linoleic acid inhibited the LXR/RXR pathway via DR4 and suppressed the transcription of ABCA1 and ABCG1 [120].
Dietary soy protein induced hepatic ABCG5/ABCG8 mRNA expression and promoted cholesterol efflux [121]. The cell wall of lactobacillus promoted the expression of ABCG5/ABCG8 protein and mRNA [72]. Marine-derived furanone, 5-hydroxy-3-methoxy-5-methyl-4-butylfuran-2(5H)-one, isolated from the fungus Setosphaeria increased ABCA1, ABCG1, and ABCG5/ABCG8 in a manner dependent on LXRα and PPARα [122]. Diosgenin, the aglycone form of bioactive saponin found in wild yam (Dioscorea villosa Linn) increased ABCG5/ABCG8 mRNA possibly through indirect activation of LXRα [123]. In addition to ABCA1 and ABCG1, intestinal ABCG5/ABCG8 was also induced by resveratrol dependent on the LXRα pathway, but liver ABCG5/ABCG8 was not upregulated [124]. Plant sterols and stanols, which are commonly found in foods such as nuts, seeds, and vegetable oils, have been reported to activate the transcription of ABCA1, ABCG1, and ABCG5/ABCG8 in hepatocytes and enterocytes by the activation of LXR [125,126]. Taurine (2-aminoethanesulfonic acid), which is abundant in seafood and traditionally used to treat heart and liver disorders, increased ABCA1 and ABCG1 mRNAs levels in THP-1 cells and ABCA1, ABCG5, and ABCG8 mRNAs in HepG2 and Caco2 cells by binding to LXRα [127]. The effects of these natural products on ABCA1, ABCG1, ABCG5, and ABCG8 expression may vary depending on the specific cell type and experimental conditions used in the studies, and the mechanisms underlying these effects are likely multifactorial. Since many natural products targeting LXR, RXR, PPAR, or FXR have been reported [38,128,129], we expect that increasing numbers of natural products will activate transcription of ABCA1, ABCG1, ABCG5, and ABCG8 in the future.

Table 2.
Transcriptional activation of ABCA1, ABCG1, and ABCG5/ABCG8 by natural compounds.
Table 2.
Transcriptional activation of ABCA1, ABCG1, and ABCG5/ABCG8 by natural compounds.
| Natural Compound | Mechanism of Action | Target Transporters | References |
|---|---|---|---|
| Allicin | Activation of PPAR and LXR | ABCA1 | [108] |
| Anthocyanins | Activation of PPAR and LXR | ABCA1 | [109] |
| Baicalin | Activation of PPAR and LXR | ABCA1, ABCG1 | [113] |
| Cineole | ? | ABCA1 | [101] |
| Curcumin | Activation of LXR | ABCA1, ABCG1 | [114,115] |
| Diosgenin | Activation of LXR | ABCG5, ABCG8 | [123] |
| Evodiamine | Activation of PPAR and LXR | ABCG1 | [112] |
| 6-Gingerol | Activation of LXR | ABCA1 | [102] |
| 8(R)-hydroxyeicosapentaenoic acid | Activation of LXR | ABCA1, ABCG1 | [103] |
| 13-hydroxy linoleic acid | Activation of PPAR | ABCA1, ABCG1 | [116] |
| 5-hydroxy-3-methoxy-5-methyl-4-butylfuran-2(5H)-one | Activation of LXR | ABCA1, ABCG1, ABCG5, ABCG8 | [122]. |
| Lycopene | Activation of PPAR and LXR | ABCA1 | [111] |
| Mangiferin | Activation of PPAR and LXR | ABCA1, ABCG1 | [117] |
| Quercetin | Activation of LXR | ABCA1 | [106] |
| Resveratrol | Activation of PPAR and LXR | ABCA1, ABCG1, ABCG5, ABCG8 | [118,119,124] |
| Riccardin C | Activation of LXR | ABCA1, ABCG1 | [105] |
| Soy protein | ? | ABCG5, ABCG8 | [121] |
| Taurine | Activation of LXR | ABCA1, ABCG1, ABCG5, ABCG8 | [127] |
| Vitamin D | Activation of LXR | ABCA1, ABCG1 | [104] |
10. Conclusions and Perspectives
ABCA1, ABCG1, and ABCG5/ABCG8, the key players in cholesterol removal from the body, are good candidates for the prevention of atherosclerosis. The development of compounds that induce the expression of ABCA1, ABCG1, ABCG5, and ABCG8 by transcriptional regulation is desired. A synthetic ligand for LXR is one of the candidates that can promote transcription and activate ABCA1 and ABCG1 by increasing their expression levels; however, LXR agonists show an adverse effect due to the induction of SREBP-1c, which regulates fatty acid synthesis, resulting in elevated serum triglyceride levels [47,101,127]. Consequently, possible candidates are LXR agonists capable of inducing ABCA1, ABCG1, and ABCG5/ABCG8 without upregulating SREBP-1c [130]. Another possible target is PPAR, which induces the expression of ABCA1, ABCG1, ABCG5, and ABCG8 without increasing fatty acid synthesis. Combining PPAR agonists with LXR agonists may serve as a promising strategy to overcome the aforementioned adverse effects because ABCA1 and ABCG1 are induced by LXR agonists and PPAR stimulation suppresses triglyceride levels by increasing β-oxidation and liver lipoprotein lipase [47]. Furthermore, transcription factors could be therapeutic targets. Compounds that suppress AP-2 or activate LRH-4 and HNF4α are expected to elevate ABCA1, ABCG1, and ABCG5/ABCG8 expression. Further research is needed to fully understand the molecular mechanisms underlying this regulation and its potential therapeutic implications. In the future, it is considered that new strategies to prevent and cure atherosclerosis and cardiovascular diseases using pharmacological and natural compounds will be developed.
Funding
This research was funded by JSPS KAKENHI grant number JP20K11592 from the Japan Society for the Promotion of Science.
Data Availability Statement
The data presented in this study are available on request from the author.
Conflicts of Interest
The author declares no conflict of interest.
References
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Groenen, A.G.; Halmos, B.; Tall, A.R.; Westerterp, M. Cholesterol efflux pathways, inflammation, and atherosclerosis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. LDL cholesterol: Controversies and future therapeutic directions. Lancet 2014, 384, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Sacks, F.M.; Salvini, S.; Willett, W.C.; Hennekens, C.H. A Prospective Study of Cholesterol, Apolipoproteins, and the Risk of Myocardial Infarction. N. Engl. J. Med. 1991, 325, 373–381. [Google Scholar] [CrossRef]
- Miller, N.E.; Thelle, D.S.; Forde, O.H.; Mjos, O.D. The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: A prospective case-control study. Lancet 1977, 1, 965–968. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Leon, A.S.; Sanchez, O.A. Response of blood lipids to exercise training alone or combined with dietary intervention. Med. Sci. Sports Exerc. 2001, 33, S502–S515. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef]
- Shea, S.; Stein, J.H.; Jorgensen, N.W.; McClelland, R.L.; Tascau, L.; Shrager, S.; Heinecke, J.W.; Yvan-Charvet, L.; Tall, A.R. Cholesterol Mass Efflux Capacity, Incident Cardiovascular Disease, and Progression of Carotid Plaque. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 89–96. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Rader, D.J.; Alexander, E.T.; Weibel, G.L.; Billheimer, J.; Rothblat, G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009, 50, S189–S194. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Vaughan, A.M. ATP-Binding Cassette Cholesterol Transporters and Cardiovascular Disease. Circ. Res. 2006, 99, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. ATP-binding cassette proteins involved in glucose and lipid homeostasis. Biosci. Biotechnol. Biochem. 2010, 74, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Collins, H.L.; Ranalletta, M.; Fuki, I.V.; Billheimer, J.T.; Rothblat, G.H.; Tall, A.R.; Rader, D.J. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2007, 117, 2216–2224. [Google Scholar] [CrossRef]
- Tachibana, S.; Hirano, M.; Hirata, T.; Matsuo, M.; Ikeda, I.; Ueda, K.; Sato, R. Cholesterol and plant sterol efflux from cultured intestinal epithelial cells is mediated by ATP-binding cassette transporters. Biosci. Biotechnol. Biochem. 2007, 71, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Graf, G.A.; Yu, L.; Li, W.-P.; Gerard, R.; Tuma, P.L.; Cohen, J.C.; Hobbs, H.H. ABCG5 and ABCG8 Are Obligate Heterodimers for Protein Trafficking and Biliary Cholesterol Excretion. J. Biol. Chem. 2003, 278, 48275–48282. [Google Scholar] [CrossRef]
- Leaf, D.A. The effect of physical exercise on reverse cholesterol transport. Metabolism 2003, 52, 950–957. [Google Scholar] [CrossRef]
- Rahmati-Ahmadabad, S.; Broom, D.R.; Ghanbari-Niaki, A.; Shirvani, H. Effects of exercise on reverse cholesterol transport: A systemized narrative review of animal studies. Life Sci. 2019, 224, 139–148. [Google Scholar] [CrossRef]
- Kobayashi, A.; Takanezawa, Y.; Hirata, T.; Shimizu, Y.; Misasa, K.; Kioka, N.; Arai, H.; Ueda, K.; Matsuo, M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 2006, 47, 1791–1802. [Google Scholar] [CrossRef]
- Kage, K.; Tsukahara, S.; Sugiyama, T.; Asada, S.; Ishikawa, E.; Tsuruo, T.; Sugimoto, Y. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int. J. Cancer 2002, 97, 626–630. [Google Scholar] [CrossRef]
- Rocchi, E.; Khodjakov, A.; Volk, E.L.; Yang, C.-H.; Litman, T.; Bates, S.E.; Schneider, E. The Product of the ABC Half-Transporter Gene ABCG2 (BCRP/MXR/ABCP) Is Expressed in the Plasma Membrane. Biochem. Biophys. Res. Commun. 2000, 271, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Cserepes, J.; Szentpetery, Z.; Seres, L.; Ozvegy-Laczka, C.; Langmann, T.; Schmitz, G.; Glavinas, H.; Klein, I.; Homolya, L.; Varadi, A.; et al. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: Indications for heterodimerization. Biochem. Biophys. Res. Commun. 2004, 320, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Parks, J.S. ATP-binding cassette transporter AI and its role in HDL formation. Curr. Opin. Lipidol. 2005, 16, 19–25. [Google Scholar] [CrossRef]
- Tanaka, A.R.; Abe-Dohmae, S.; Ohnishi, T.; Aoki, R.; Morinaga, G.; Okuhira, K.-i.; Ikeda, Y.; Kano, F.; Matsuo, M.; Kioka, N.; et al. Effects of Mutations of ABCA1 in the First Extracellular Domain on Subcellular Trafficking and ATP Binding/Hydrolysis. J. Biol. Chem. 2003, 278, 8815–8819. [Google Scholar] [CrossRef]
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Oram, J.F. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 2006, 47, 2433–2443. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, N.; Wang, N.; Yvan-Charvet, L.; Tall, A.R. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA 2007, 104, 15093–15098. [Google Scholar] [CrossRef]
- Brooks-Wilson, A.; Marcil, M.; Clee, S.M.; Zhang, L.H.; Roomp, K.; van Dam, M.; Yu, L.; Brewer, C.; Collins, J.A.; Molhuizen, H.O.; et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 1999, 22, 336–345. [Google Scholar] [CrossRef]
- Bodzioch, M.; Orso, E.; Klucken, J.; Langmann, T.; Bottcher, A.; Diederich, W.; Drobnik, W.; Barlage, S.; Buchler, C.; Porsch-Ozcurumez, M.; et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 1999, 22, 347–351. [Google Scholar] [CrossRef]
- Rust, S.; Rosier, M.; Funke, H.; Real, J.; Amoura, Z.; Piette, J.C.; Deleuze, J.F.; Brewer, H.B.; Duverger, N.; Denefle, P.; et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999, 22, 352–355. [Google Scholar] [CrossRef]
- Singaraja, R.R.; Fievet, C.; Castro, G.; James, E.R.; Hennuyer, N.; Clee, S.M.; Bissada, N.; Choy, J.C.; Fruchart, J.C.; McManus, B.M.; et al. Increased ABCA1 activity protects against atherosclerosis. J. Clin. Investig. 2002, 110, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldan, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Münch, G.; Bültmann, A.; Li, Z.; Holthoff, H.-P.; Ullrich, J.; Wagner, S.; Ungerer, M. Overexpression of ABCG1 protein attenuates arteriosclerosis and endothelial dysfunction in atherosclerotic rabbits. Heart Int. 2012, 7, e12. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Ranalletta, M.; Wang, N.; Han, S.; Terasaka, N.; Li, R.; Welch, C.; Tall, A.R. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Investig. 2007, 117, 3900–3908. [Google Scholar] [CrossRef] [PubMed]
- Berge, K.E.; Tian, H.; Graf, G.A.; Yu, L.; Grishin, N.V.; Schultz, J.; Kwiterovich, P.; Shan, B.; Barnes, R.; Hobbs, H.H. Accumulation of Dietary Cholesterol in Sitosterolemia Caused by Mutations in Adjacent ABC Transporters. Science 2000, 290, 1771–1775. [Google Scholar] [CrossRef]
- Heimerl, S.; Langmann, T.; Moehle, C.; Mauerer, R.; Dean, M.; Beil, F.U.; von Bergmann, K.; Schmitz, G. Mutations in the human ATP-binding cassette transporters ABCG5 and ABCG8 in sitosterolemia. Hum. Mutat. 2002, 20, 151–155. [Google Scholar] [CrossRef]
- Hubacek, J.A.; Berge, K.E.; Cohen, J.C.; Hobbs, H.H. Mutations in ATP-cassette binding proteins G5 (ABCG5) and G8 (ABCG8) causing sitosterolemia. Hum. Mutat. 2001, 18, 359–360. [Google Scholar] [CrossRef]
- Hiebl, V.; Ladurner, A.; Latkolik, S.; Dirsch, V.M. Natural products as modulators of the nuclear receptors and metabolic sensors LXR, FXR and RXR. Biotechnol. Adv. 2018, 36, 1657–1698. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef]
- Janowski, B.A.; Grogan, M.J.; Jones, S.A.; Wisely, G.B.; Kliewer, S.A.; Corey, E.J.; Mangelsdorf, D.J. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl. Acad. Sci. USA 1999, 96, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Kliewer, S.A.; Moore, L.B.; Smith-Oliver, T.A.; Oliver, B.B.; Su, J.-L.; Sundseth, S.S.; Winegar, D.A.; Blanchard, D.E.; Spencer, T.A.; et al. Activation of the Nuclear Receptor LXR by Oxysterols Defines a New Hormone Response Pathway. J. Biol. Chem. 1997, 272, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Calkin, A.C.; Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012, 13, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Collins, J.L.; Osborne, T.F.; Tontonoz, P. Direct and Indirect Mechanisms for Regulation of Fatty Acid Synthase Gene Expression by Liver X Receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef]
- Altucci, L.; Leibowitz, M.D.; Ogilvie, K.M.; de Lera, A.R.; Gronemeyer, H. RAR and RXR modulation in cancer and metabolic disease. Nat. Rev. Drug Discov. 2007, 6, 793–810. [Google Scholar] [CrossRef]
- Gronemeyer, H.; Gustafsson, J.-Å.; Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004, 3, 950–964. [Google Scholar] [CrossRef]
- Beyer, T.P.; Schmidt, R.J.; Foxworthy, P.; Zhang, Y.; Dai, J.; Bensch, W.R.; Kauffman, R.F.; Gao, H.; Ryan, T.P.; Jiang, X.-C.; et al. Coadministration of a Liver X Receptor Agonist and a Peroxisome Proliferator Activator Receptor-α Agonist in Mice: Effects of Nuclear Receptor Interplay on High-Density Lipoprotein and Triglyceride Metabolism in Vivo. J. Pharmacol. Exp. Ther. 2004, 309, 861–868. [Google Scholar] [CrossRef]
- Quinet, E.M.; Savio, D.A.; Halpern, A.R.; Chen, L.; Miller, C.P.; Nambi, P. Gene-selective modulation by a synthetic oxysterol ligand of the liver X receptor. J. Lipid Res. 2004, 45, 1929–1942. [Google Scholar] [CrossRef]
- Brown, G. Retinoic acid receptor regulation of decision-making for cell differentiation. Front. Cell Dev. Biol. 2023, 11, 1182204. [Google Scholar] [CrossRef]
- Staels, B.; Fruchart, J.-C. Therapeutic Roles of Peroxisome Proliferator–Activated Receptor Agonists. Diabetes 2005, 54, 2460–2470. [Google Scholar] [CrossRef]
- Sun, L.; Cai, J.; Gonzalez, F.J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Costet, P.; Lalanne, F.; Gerbod-Giannone, M.C.; Molina, J.R.; Fu, X.; Lund, E.G.; Gudas, L.J.; Tall, A.R. Retinoic Acid Receptor-Mediated Induction of ABCA1 in Macrophages. Mol. Cell. Biol. 2003, 23, 7756–7766. [Google Scholar] [CrossRef]
- Repa, J.J.; Turley, S.D.; Lobaccaro, J.A.; Medina, J.; Li, L.; Lustig, K.; Shan, B.; Heyman, R.A.; Dietschy, J.M.; Mangelsdorf, D.J. Regulation of Absorption and ABC1-Mediated Efflux of Cholesterol by RXR Heterodimers. Science 2000, 289, 1524–1529. [Google Scholar] [CrossRef]
- Venkateswaran, A.; Laffitte, B.A.; Joseph, S.B.; Mak, P.A.; Wilpitz, D.C.; Edwards, P.A.; Tontonoz, P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRalpha. Proc. Natl. Acad. Sci. USA 2000, 97, 12097–12102. [Google Scholar] [CrossRef]
- Sabol, S.L.; Brewer, H.B., Jr.; Santamarina-Fojo, S. The human ABCG1 gene: Identification of LXR response elements that modulate expression in macrophages and liver. J. Lipid Res. 2005, 46, 2151–2167. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.E.; Sakai, S.; Lambert, G.; Nicol, C.J.; Matsusue, K.; Pimprale, S.; Lee, Y.-H.; Ricote, M.; Glass, C.K.; Brewer, H.B., Jr.; et al. Conditional Disruption of the Peroxisome Proliferator-Activated Receptor {gamma} Gene in Mice Results in Lowered Expression of ABCA1, ABCG1, and apoE in Macrophages and Reduced Cholesterol Efflux. Mol. Cell. Biol. 2002, 22, 2607–2619. [Google Scholar] [CrossRef]
- Ozasa, H.; Ayaori, M.; Iizuka, M.; Terao, Y.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Uehara, Y.; et al. Pioglitazone enhances cholesterol efflux from macrophages by increasing ABCA1/ABCG1 expressions via PPARγ/LXRα pathway: Findings from in vitro and ex vivo studies. Atherosclerosis 2011, 219, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chinetti, G.; Lestavel, S.; Bocher, V.; Remaley, A.T.; Neve, B.; Torra, I.P.; Teissier, E.; Minnich, A.; Jaye, M.; Duverger, N.; et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001, 7, 53–58. [Google Scholar] [CrossRef]
- Iwamoto, N.; Abe-Dohmae, S.; Ayaori, M.; Tanaka, N.; Kusuhara, M.; Ohsuzu, F.; Yokoyama, S. ATP-Binding Cassette Transporter A1 Gene Transcription Is Downregulated by Activator Protein 2α: Doxazosin Inhibits Activator Protein 2α and Increases High-Density Lipoprotein Biogenesis Independent of α1-Adrenoceptor Blockade. Circ. Res. 2007, 101, 156–165. [Google Scholar] [CrossRef]
- Wang, S.M.; Lin, W.C.; Lin, H.Y.; Chen, Y.L.; Ko, C.Y.; Wang, J.M. CCAAT/Enhancer-binding protein delta mediates glioma stem-like cell enrichment and ATP-binding cassette transporter ABCA1 activation for temozolomide resistance in glioblastoma. Cell Death Discov. 2021, 7, 8. [Google Scholar] [CrossRef]
- Zeng, L.; Liao, H.; Liu, Y.; Lee, T.-S.; Zhu, M.; Wang, X.; Stemerman, M.B.; Zhu, Y.; Shyy, J.Y.J. Sterol-responsive Element-binding Protein (SREBP) 2 Down-regulates ATP-binding Cassette Transporter A1 in Vascular Endothelial Cells: A NOVEL ROLE OF SREBP IN REGULATING CHOLESTEROL METABOLISM. J. Biol. Chem. 2004, 279, 48801–48807. [Google Scholar] [CrossRef] [PubMed]
- Porsch-Ozcurumez, M.; Langmann, T.; Heimerl, S.; Borsukova, H.; Kaminski, W.E.; Drobnik, W.; Honer, C.; Schumacher, C.; Schmitz, G. The zinc finger protein 202 (ZNF202) is a transcriptional repressor of ATP binding cassette transporter A1 (ABCA1) and ABCG1 gene expression and a modulator of cellular lipid efflux. J. Biol. Chem. 2001, 276, 12427–12433. [Google Scholar] [CrossRef] [PubMed]
- Tamehiro, N.; Shigemoto-Mogami, Y.; Kakeya, T.; Okuhira, K.-i.; Suzuki, K.; Sato, R.; Nagao, T.; Nishimaki-Mogami, T. Sterol Regulatory Element-binding Protein-2- and Liver X Receptor-driven Dual Promoter Regulation of Hepatic ABC Transporter A1 Gene Expression: Mechanism Underlying the Unique Response to Cellular Cholesterol Status. J. Biol. Chem. 2007, 282, 21090–21099. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Suárez, Y.; Dávalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernández-Hernando, C. MiR-33 Contributes to the Regulation of Cholesterol Homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef]
- He, Y.; Lin, L.; Cao, J.; Mao, X.; Qu, Y.; Xi, B. Up-regulated miR-93 contributes to coronary atherosclerosis pathogenesis through targeting ABCA1. Int. J. Clin. Exp. Med. 2015, 8, 674–681. [Google Scholar]
- Aryal, B.; Singh, A.K.; Rotllan, N.; Price, N.; Fernández-Hernando, C. MicroRNAs and lipid metabolism. Curr. Opin. Lipidol. 2017, 28, 273–280. [Google Scholar] [CrossRef]
- Hozoji, M.; Munehira, Y.; Ikeda, Y.; Makishima, M.; Matsuo, M.; Kioka, N.; Ueda, K. Direct Interaction of Nuclear Liver X Receptor-{beta} with ABCA1 Modulates Cholesterol Efflux. J. Biol. Chem. 2008, 283, 30057–30063. [Google Scholar] [CrossRef]
- Hozoji-Inada, M.; Munehira, Y.; Nagao, K.; Kioka, N.; Ueda, K. Liver X Receptor β (LXRβ) Interacts Directly with ATP-binding Cassette A1 (ABCA1) to Promote High Density Lipoprotein Formation during Acute Cholesterol Accumulation. J. Biol. Chem. 2011, 286, 20117–20124. [Google Scholar] [CrossRef]
- Ogura, M.; Ayaori, M.; Terao, Y.; Hisada, T.; Iizuka, M.; Takiguchi, S.; Uto-Kondo, H.; Yakushiji, E.; Nakaya, K.; Sasaki, M.; et al. Proteasomal Inhibition Promotes ATP-Binding Cassette Transporter A1 (ABCA1) and ABCG1 Expression and Cholesterol Efflux From Macrophages In Vitro and In Vivo. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1980–1987. [Google Scholar] [CrossRef]
- Lee, M.H.; Lu, K.; Hazard, S.; Yu, H.; Shulenin, S.; Hidaka, H.; Kojima, H.; Allikmets, R.; Sakuma, N.; Pegoraro, R.; et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 2001, 27, 79–83. [Google Scholar] [CrossRef]
- Lu, K.; Lee, M.H.; Hazard, S.; Brooks-Wilson, A.; Hidaka, H.; Kojima, H.; Ose, L.; Stalenhoef, A.F.; Mietinnen, T.; Bjorkhem, I.; et al. Two genes that map to the STSL locus cause sitosterolemia: Genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am. J. Hum. Genet. 2001, 69, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-s.; Ju, J.-h.; Kim, H.; Lee, J.; Park, H.-j.; Ji, Y.; Shin, H.-k.; Do, M.-S.; Lee, J.-m.; Holzapfel, W. Lactobacillus rhamnosus BFE 5264 and Lactobacillus plantarum NR74 Promote Cholesterol Excretion Through the Up-Regulation of ABCG5/8 in Caco-2 Cells. Probiotics Antimicrob Proteins 2011, 3, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Berge, K.E.; Pomajzl, C.; Richardson, J.A.; Hobbs, H.; Mangelsdorf, D.J. Regulation of ATP-binding Cassette Sterol Transporters ABCG5 and ABCG8 by the Liver X Receptors alpha and beta. J. Biol. Chem. 2002, 277, 18793–18800. [Google Scholar] [CrossRef]
- Kamisako, T.; Ogawa, H. Alteration of the expression of adenosine triphosphate-binding cassette transporters associated with bile acid and cholesterol transport in the rat liver and intestine during cholestasis. J. Gastroenterol. Hepatol. 2005, 20, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gupta, S.; Xu, F.; Liverman, A.D.B.; Moschetta, A.; Mangelsdorf, D.J.; Repa, J.J.; Hobbs, H.H.; Cohen, J.C. Expression of ABCG5 and ABCG8 Is Required for Regulation of Biliary Cholesterol Secretion. J. Biol. Chem. 2005, 280, 8742–8747. [Google Scholar] [CrossRef]
- Li, T.; Matozel, M.; Boehme, S.; Kong, B.; Nilsson, L.M.; Guo, G.; Ellis, E.; Chiang, J.Y. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 2011, 53, 996–1006. [Google Scholar] [CrossRef]
- Balasubramaniyan, N.; Ananthanarayanan, M.; Suchy, F.J. Nuclear factor-κB regulates the expression of multiple genes encoding liver transport proteins. Am. J. Physiol. Gasterointest. Liver Physiol. 2016, 310, G618–G628. [Google Scholar] [CrossRef]
- Freeman, L.A.; Kennedy, A.; Wu, J.; Bark, S.; Remaley, A.T.; Santamarina-Fojo, S.; Brewer, H.B., Jr. The orphan nuclear receptor LRH-1 activates the ABCG5/ABCG8 intergenic promoter. J. Lipid Res. 2004, 45, 1197–1206. [Google Scholar] [CrossRef]
- Sumi, K.; Tanaka, T.; Uchida, A.; Magoori, K.; Urashima, Y.; Ohashi, R.; Ohguchi, H.; Okamura, M.; Kudo, H.; Daigo, K.; et al. Cooperative Interaction between Hepatocyte Nuclear Factor 4{alpha} and GATA Transcription Factors Regulates ATP-Binding Cassette Sterol Transporters ABCG5 and ABCG8. Mol. Cell. Biol. 2007, 27, 4248–4260. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, M.; Guo, F.; Yang, X.; Chen, Y.; Ma, C.; Li, Q.; Wei, Z.; Li, X.; Wang, H.; et al. Rosiglitazone alleviates intrahepatic cholestasis induced by α-naphthylisothiocyanate in mice: The role of circulating 15-deoxy-Δ(12,14) -PGJ(2) and Nogo. Br. J. Pharmacol. 2020, 177, 1041–1060. [Google Scholar] [CrossRef]
- Han, T.; Lv, Y.; Wang, S.; Hu, T.; Hong, H.; Fu, Z. Pioglitazone prevents cholesterol gallstone formation through the regulation of cholesterol homeostasis in guinea pigs with a lithogenic diet. Lipids Health Dis. 2019, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Francl, J.M.; Boehme, S.; Chiang, J.Y. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7α-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology 2013, 58, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ma, S.; Zhou, Y.; Wei, B.; Hao, Z.; Cui, X.; Xing, L.; Liu, G.; Jin, L.; Ma, T.; et al. miRNA-223 Suppresses Mouse Gallstone Formation by Targeting Key Transporters in Hepatobiliary Cholesterol Secretion Pathway. Int. J. Biol. Sci. 2021, 17, 4459–4473. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; McKilligin, E.; Pei, L.; Watson, M.A.; Collins, A.R.; Laffitte, B.A.; Chen, M.; Noh, G.; Goodman, J.; Hagger, G.N.; et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 7604–7609. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Pape, T.D.; Parks, J.S.; Kuipers, F.; Hayden, M.R. Tissue-Specific Induction of Intestinal ABCA1 Expression With a Liver X Receptor Agonist Raises Plasma HDL Cholesterol Levels. Circ. Res. 2006, 99, 672–674. [Google Scholar] [CrossRef]
- Fukumoto, H.; Deng, A.; Irizarry, M.C.; Fitzgerald, M.L.; Rebeck, G.W. Induction of the Cholesterol Transporter ABCA1 in Central Nervous System Cells by Liver X Receptor Agonists Increases Secreted Abeta Levels. J. Biol. Chem. 2002, 277, 48508–48513. [Google Scholar] [CrossRef]
- Murthy, S.; Born, E.; Mathur, S.N.; Field, F.J. Liver-X-receptor-mediated increase in ATP-binding cassette transporter A1 expression is attenuated by fatty acids in CaCo-2 cells: Effect on cholesterol efflux to high-density lipoprotein. Biochem. J. 2004, 377, 545–552. [Google Scholar] [CrossRef]
- Fujiyoshi, M.; Ohtsuki, S.; Hori, S.; Tachikawa, M.; Terasaki, T. 24S-hydroxycholesterol induces cholesterol release from choroid plexus epithelial cells in an apical- and apoE isoform-dependent manner concomitantly with the induction of ABCA1 and ABCG1 expression. J. Neurochem. 2007, 100, 968–978. [Google Scholar] [CrossRef]
- Murthy, S.; Born, E.; Mathur, S.N.; Field, F.J. LXR/RXR activation enhances basolateral efflux of cholesterol in CaCo-2 cells. J. Lipid Res. 2002, 43, 1054–1064. [Google Scholar] [CrossRef]
- Wang, N.; Ranalletta, M.; Matsuura, F.; Peng, F.; Tall, A.R. LXR-Induced Redistribution of ABCG1 to Plasma Membrane in Macrophages Enhances Cholesterol Mass Efflux to HDL. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1310–1316. [Google Scholar] [CrossRef]
- Arakawa, R.; Tamehiro, N.; Nishimaki-Mogami, T.; Ueda, K.; Yokoyama, S. Fenofibric Acid, an Active Form of Fenofibrate, Increases Apolipoprotein A-I–Mediated High-Density Lipoprotein Biogenesis by Enhancing Transcription of ATP-Binding Cassette Transporter A1 Gene in a Liver X Receptor–Dependent Manner. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.R.; Shenk, J.L.; Snaith, M.R.; Russell, C.S.; Plunket, K.D.; Bodkin, N.L.; Lewis, M.C.; Winegar, D.A.; Sznaidman, M.L.; Lambert, M.H.; et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc. Natl. Acad. Sci. USA 2001, 98, 5306–5311. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.L.; Patel, D.D.; Humphreys, S.M.; Wiggins, D.; Gibbons, G.F. Inhibition of cholesterol absorption associated with a PPARα-dependent increase in ABC binding cassette transporter A1 in mice. J. Lipid Res. 2003, 44, 2049–2058. [Google Scholar] [CrossRef]
- Kamisako, T.; Ogawa, H. Effects of pravastatin and bezafibrate on biliary lipid excretion and hepatic expression of Abcg5 and Abcg8 in the rat. J. Gastroenterol. Hepatol. 2004, 19, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Argmann, C.A.; Edwards, J.Y.; Sawyez, C.G.; O’Neil, C.H.; Hegele, R.A.; Pickering, J.G.; Huff, M.W. Regulation of Macrophage Cholesterol Efflux through Hydroxymethylglutaryl-CoA Reductase Inhibition: A role for RhoA IN ABCA1-mediated cholesterol efflux. J. Biol. Chem. 2005, 280, 22212–22221. [Google Scholar] [CrossRef] [PubMed]
- Maejima, T.; Sugano, T.; Yamazaki, H.; Yoshinaka, Y.; Doi, T.; Tanabe, S.; Nishimaki-Mogami, T. Pitavastatin Increases ABCA1 Expression by Dual Mechanisms: SREBP2-Driven Transcriptional Activation and PPARα-Dependent Protein Stabilization but Without Activating LXR in Rat Hepatoma McARH7777 Cells. J. Pharmacol. Sci. 2011, 116, 107–115. [Google Scholar] [CrossRef]
- Wong, J.; Quinn, C.M.; Gelissen, I.C.; Jessup, W.; Brown, A.J. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 2008, 196, 180–189. [Google Scholar] [CrossRef]
- Ayaori, M.; Yakushiji, E.; Ogura, M.; Nakaya, K.; Hisada, T.; Uto-Kondo, H.; Takiguchi, S.; Terao, Y.; Sasaki, M.; Komatsu, T.; et al. Retinoic acid receptor agonists regulate expression of ATP-binding cassette transporter G1 in macrophages. Biochim. Biophys. Acta 2012, 1821, 561–572. [Google Scholar] [CrossRef]
- Bechor, S.; Zolberg Relevy, N.; Harari, A.; Almog, T.; Kamari, Y.; Ben-Amotz, A.; Harats, D.; Shaish, A. 9-cis beta-Carotene Increased Cholesterol Efflux to HDL in Macrophages. Nutrients 2016, 8, 435. [Google Scholar] [CrossRef]
- Molusky, M.M.; Hsieh, J.; Lee, S.X.; Ramakrishnan, R.; Tascau, L.; Haeusler, R.A.; Accili, D.; Tall, A.R. Metformin and AMP Kinase Activation Increase Expression of the Sterol Transporters ABCG5/8 (ATP-Binding Cassette Transporter G5/G8) With Potential Antiatherogenic Consequences. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1493–1503. [Google Scholar] [CrossRef]
- Jun, H.-J.; Hoang, M.-H.; Yeo, S.-K.; Jia, Y.; Lee, S.-J. Induction of ABCA1 and ABCG1 expression by the liver X receptor modulator cineole in macrophages. Bioorg. Med. Chem. Lett. 2013, 23, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.; Liang, N.; Jiang, X.; Song, Y.; Ou, S.; Hu, Y.; Jiao, R.; Bai, W. 6-Gingerol Regulates Hepatic Cholesterol Metabolism by Up-regulation of LDLR and Cholesterol Efflux-Related Genes in HepG2 Cells. Front. Pharmacol. 2018, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Uemura, A.; Miyasaka, R. 8(R)-Hydroxyeicosapentaenoic acid (8R-HEPE) induces transcription of cholesterol efflux receptors via activation of liver X receptor in macrophages. Biosci. Biotechnol. Biochem. 2023, 87, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; You, Y.; Swier, V.; Tang, L.; Radwan, M.M.; Pandya, A.N.; Agrawal, D.K. Vitamin D Protects Against Atherosclerosis via Regulation of Cholesterol Efflux and Macrophage Polarization in Hypercholesterolemic Swine. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2432–2442. [Google Scholar] [CrossRef]
- Tamehiro, N.; Sato, Y.; Suzuki, T.; Hashimoto, T.; Asakawa, Y.; Yokoyama, S.; Kawanishi, T.; Ohno, Y.; Inoue, K.; Nagao, T.; et al. Riccardin C: A natural product that functions as a liver X receptor (LXR)[alpha] agonist and an LXR[beta] antagonist. FEBS Lett. 2005, 579, 5299–5304. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Lee, T.-S.; Chiang, A.-N. Quercetin enhances ABCA1 expression and cholesterol efflux through a p38-dependent pathway in macrophages. J. Lipid Res. 2012, 53, 1840–1850. [Google Scholar] [CrossRef]
- Jiang, T.; Ren, K.; Chen, Q.; Li, H.; Yao, R.; Hu, H.; Lv, Y.C.; Zhao, G.J. Leonurine Prevents Atherosclerosis Via Promoting the Expression of ABCA1 and ABCG1 in a Pparγ/Lxrα Signaling Pathway-Dependent Manner. Cell. Physiol. Biochem. 2017, 43, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-L.; Hu, H.-J.; Liu, Y.-B.; Hu, X.-M.; Fan, X.-J.; Zou, W.-W.; Pan, Y.-Q.; Zhou, W.-Q.; Peng, M.-W.; Gu, C.-H. Allicin induces the upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. Int. J. Mol. Med. 2017, 39, 1452–1460. [Google Scholar] [CrossRef]
- Xia, M.; Hou, M.; Zhu, H.; Ma, J.; Tang, Z.; Wang, Q.; Li, Y.; Chi, D.; Yu, X.; Zhao, T.; et al. Anthocyanins Induce Cholesterol Efflux from Mouse Peritoneal Macrophages: THE ROLE OF THE PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR γ-LIVER X RECEPTOR α-ABCA1 PATHWAY. J. Biol. Chem. 2005, 280, 36792–36801. [Google Scholar] [CrossRef]
- Franceschelli, S.; De Cecco, F.; Pesce, M.; Ripari, P.; Guagnano, M.T.; Nuevo, A.B.; Grilli, A.; Sancilio, S.; Speranza, L. Hydroxytyrosol Reduces Foam Cell Formation and Endothelial Inflammation Regulating the PPARγ /LXRα/ABCA1 Pathway. Int. J. Mol. Sci. 2023, 24, 2057. [Google Scholar] [CrossRef]
- Yang, C.-M.; Lu, I.H.; Chen, H.-Y.; Hu, M.-L. Lycopene inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARγ-LXRα-ABCA1 pathway. J. Nutri. Biochem. 2012, 23, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Malainer, C.; Atanasov, A.G.; Heiß, E.H.; Dirsch, V.M.; Wang, L.; Wang, K. Evodiamine Lowers Blood Lipids by Up-Regulating the PPARγ/ABCG1 Pathway in High-Fat-Diet-Fed Mice. J. Nat. Prod. 2021, 84, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- He, X.-W.; Yu, D.; Li, W.-L.; Zheng, Z.; Lv, C.-L.; Li, C.; Liu, P.; Xu, C.-Q.; Hu, X.-F.; Jin, X.-P. Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ-LXRα-ABCA1/ABCG1 pathway. Biomed. Pharmacother. 2016, 83, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-F.; Ching, L.-C.; Huang, Y.-C.; Chen, C.-Y.; Chiang, A.-N.; Kou, Y.R.; Shyue, S.-K.; Lee, T.-S. Molecular mechanism of curcumin on the suppression of cholesterol accumulation in macrophage foam cells and atherosclerosis. Mol. Nutr. Food Res. 2012, 56, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.-z.; Zhao, S.-p.; Wu, Z.-h.; Yang, J.; Xie, X.-z.; Yu, B.-l.; Nie, S. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma–LXRalpha–ABCA1 passway. Mol. Cell. Biochem. 2011, 358, 281–285. [Google Scholar] [CrossRef]
- Kammerer, I.; Ringseis, R.; Biemann, R.; Wen, G.; Eder, K. 13-hydroxy linoleic acid increases expression of the cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates apoA-I-dependent cholesterol efflux in RAW264.7 macrophages. Lipids Health Dis. 2011, 10, 222. [Google Scholar] [CrossRef]
- Ren, K.; Li, H.; Zhou, H.-F.; Liang, Y.; Tong, M.; Chen, L.; Zheng, X.-L.; Zhao, G.-J. Mangiferin promotes macrophage cholesterol efflux and protects against atherosclerosis by augmenting the expression of ABCA1 and ABCG1. Aging 2019, 11, 10992–11009. [Google Scholar] [CrossRef]
- Voloshyna, I.; Hai, O.; Littlefield, M.J.; Carsons, S.; Reiss, A.B. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARγ and adenosine. Eur. J. Pharmacol. 2013, 698, 299–309. [Google Scholar] [CrossRef]
- Sevov, M.; Elfineh, L.; Cavelier, L.B. Resveratrol regulates the expression of LXR-α in human macrophages. Biochem. Biophys. Res. Commun. 2006, 348, 1047–1054. [Google Scholar] [CrossRef]
- Uehara, Y.; Miura, S.-i.; von Eckardstein, A.; Abe, S.; Fujii, A.; Matsuo, Y.; Rust, S.; Lorkowski, S.; Assmann, G.; Yamada, T.; et al. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis 2007, 191, 11–21. [Google Scholar] [CrossRef]
- Ikeda, I.; Kudo, M.; Hamada, T.; Nagao, K.; Oshiro, Y.; Kato, M.; Sugawara, T.; Yamahira, T.; Ito, H.; Tamaru, S.; et al. Dietary Soy Protein Isolate and Its Undigested High Molecular Fraction Upregulate Hepatic ATP-Binding Cassette Transporter G5 and ATP-Binding Cassette Transporter G8 mRNA and Increase Biliary Secretion of Cholesterol in Rats. J. Nutri. Sci. Vitaminol. 2009, 55, 252–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, T.; Hu, S.M.; Pang, X.Y.; Wang, J.F.; Yin, J.Y.; Li, F.H.; Wang, J.; Yang, X.Q.; Xia, B.; Liu, Y.H.; et al. The marine-derived furanone reduces intracellular lipid accumulation in vitro by targeting LXRα and PPARα. J. Cell. Mol. Med. 2020, 24, 3384–3398. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Shi, J.; Yu, Y.; Lu, H.; Yu, L.; Liu, Y.; Zhang, F. Diosgenin regulates cholesterol metabolism in hypercholesterolemic rats by inhibiting NPC1L1 and enhancing ABCG5 and ABCG8. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xu, H.; Wang, X.; Chen, X.; Li, Q.; Liu, Q.; You, Y.; Zhang, H.; Xu, Z.; Zhao, Y.; et al. Resveratrol enhances trans-intestinal cholesterol excretion through selective activation of intestinal liver X receptor alpha. Biochem. Pharmacol. 2021, 186, 114481. [Google Scholar] [CrossRef]
- Sabeva, N.S.; McPhaul, C.M.; Li, X.; Cory, T.J.; Feola, D.J.; Graf, G.A. Phytosterols differentially influence ABC transporter expression, cholesterol efflux and inflammatory cytokine secretion in macrophage foam cells. J. Nutri. Biochem. 2011, 22, 777–783. [Google Scholar] [CrossRef]
- Plat, J.; Nichols, J.A.; Mensink, R.P. Plant sterols and stanols: Effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 2005, 46, 2468–2476. [Google Scholar] [CrossRef]
- Hoang, M.-H.; Jia, Y.; Jun, H.-j.; Lee, J.H.; Hwang, K.-Y.; Choi, D.-W.; Um, S.-J.; Lee, B.-Y.; You, S.-G.; Lee, S.-J. Taurine is a liver X receptor-α ligand and activates transcription of key genes in the reverse cholesterol transport without inducing hepatic lipogenesis. Mol. Nutr. Food Res. 2012, 56, 900–911. [Google Scholar] [CrossRef]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef]
- She, J.; Gu, T.; Pang, X.; Liu, Y.; Tang, L.; Zhou, X. Natural Products Targeting Liver X Receptors or Farnesoid X Receptor. Front. Pharmacol. 2021, 12, 772435. [Google Scholar] [CrossRef]
- Ben Aissa, M.; Lewandowski, C.T.; Ratia, K.M.; Lee, S.H.; Layden, B.T.; LaDu, M.J.; Thatcher, G.R.J. Discovery of Nonlipogenic ABCA1 Inducing Compounds with Potential in Alzheimer’s Disease and Type 2 Diabetes. ACS Pharmacol. Transl. Sci. 2021, 4, 143–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).