Virtual Reality Versus Conventional Exercise in Patients with Type 1 Diabetes: A Feasibility Randomized Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
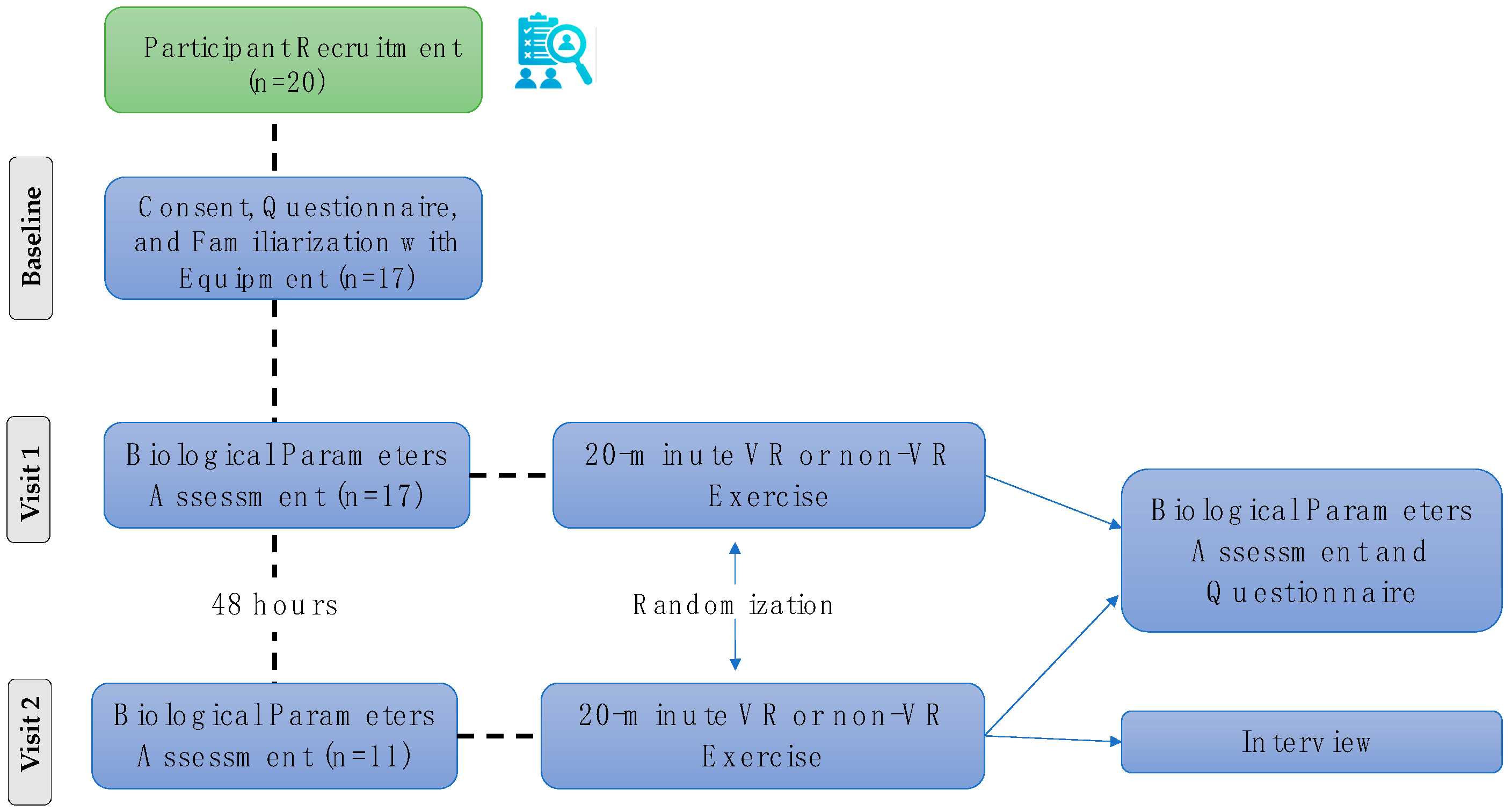
2.1. Trial Design and Ethics Approval
2.2. Participants
2.3. VR Cycling Application
2.4. Measures
2.5. Procedure and Experimental Protocol
2.6. Statistical Analysis
3. Results
3.1. Psychological Parameters and Feasibility of the VR System
3.2. Physiological and Biochemical Parameters and Mood
3.3. Semi-Structured Interview
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Physical Activity Fact Sheet; World Health Organization. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 22 September 2024).
- American Diabetes Association (ADA). Type 1 Diabetes; American Diabetes Association. Available online: https://diabetes.org/about-diabetes/type-1 (accessed on 22 September 2024).
- Lucier, J.; Mathias, P.M. Type 1 Diabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507713/ (accessed on 5 October 2024).
- Gallardo-Gómez, D.; Salazar-Martínez, E.; Alfonso-Rosa, R.M.; Ramos-Munell, J.; del Pozo-Cruz, J.; del Pozo Cruz, B.; Álvarez-Barbosa, F. Optimal dose and type of physical activity to improve glycemic control in people diagnosed with type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2024, 47, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Luo, B.; Xiang, D.; Ji, X.; Chen, X.; Li, R.; Zhang, S.; Meng, Y.; Nieman, D.C.; Chen, P. The anti-inflammatory effects of exercise on autoimmune diseases: A 20-year systematic review. J. Sport Health Sci. 2024, 13, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Wake, A.D. Protective effects of physical activity against health risks associated with type 1 diabetes: “Health benefits outweigh the risks”. World J. Diabetes 2022, 13, 161–184. [Google Scholar] [CrossRef]
- Basu, R.; Johnson, M.L.; Kudva, Y.C.; Basu, A. Exercise, hypoglycemia, and type 1 diabetes. Diabetes Technol. Ther. 2014, 16, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Cryer, P.E. Hypoglycemia in type 1 diabetes mellitus. Endocrinol. Metab. Clin. N. Am. 2010, 39, 641–654. [Google Scholar] [CrossRef]
- Egede, J.K.; Campbell, J.A.; Walker, R.J.; Egede, L.E. Perceived stress as a pathway for the relationship between neighborhood factors and glycemic control in adults with diabetes. Am. J. Health Promot. 2022, 36, 269–278. [Google Scholar] [CrossRef]
- Walker, R.J.; Garacci, E.; Campbell, J.A.; Egede, L.E. The influence of daily stress on glycemic control and mortality in adults with diabetes. J. Behav. Med. 2020, 43, 723–731. [Google Scholar] [CrossRef]
- Diabetes UK. Stress and Diabetes. Available online: https://www.diabetes.org.uk/living-with-diabetes/emotional-wellbeing/stress (accessed on 14 November 2024).
- Hermanns, N.; Scheff, C.; Kulzer, B.; Krichbaum, M.; Kubiak, T.; Haak, T. Association of glucose levels and glucose variability with mood in type 1 diabetic patients. Diabetologia 2007, 50, 930–933. [Google Scholar] [CrossRef]
- Van Tilburg, M.A.L.; McCaskill, C.C.; Lane, J.D.; Edwards, C.L.; Bethel, A.; Feinglos, M.N.; Surwit, R.S. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom. Med. 2001, 63, 551–555. [Google Scholar] [CrossRef]
- Sabag, A.; Chang, C.R.; Francois, M.E.; Keating, S.E.; Coombes, J.S.; Johnson, N.A.; Pastor-Valero, M.; Rey Lopez, J.P. The effect of exercise on quality of life in type 2 diabetes: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2023, 55, 1353–1365. [Google Scholar] [CrossRef]
- Wilkie, L.; Mitchell, F.; Robertson, K.; Kirk, A. Motivations for physical activity in youth with type 1 diabetes participating in the ActivPals project: A qualitative study. Pract. Diabetes 2017, 34, 151–155. [Google Scholar] [CrossRef][Green Version]
- Alarcón-Gómez, J.; Chulvi-Medrano, I.; Martin-Rivera, F.; Calatayud, J. Effect of High-Intensity Interval Training on Quality of Life, Sleep Quality, Exercise Motivation and Enjoyment in Sedentary People with Type 1 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2021, 18, 12612. [Google Scholar] [CrossRef]
- Buckworth, J.; Lee, R.E.; Regan, G.; Schneider, L.K.; DiClemente, C.C. Decomposing intrinsic and extrinsic motivation for exercise: Application to stages of motivational readiness. Psychol. Sport Exerc. 2007, 8, 441–461. [Google Scholar] [CrossRef]
- Klompstra, L.; Deka, P.; Almenar, L.; Jaarsma, T.; Strömberg, A. Physical activity enjoyment, exercise motivation, and physical activity in patients with heart failure: A mediation analysis. Clin. Rehabil. 2022, 36, 1324–1331. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef]
- Rajati, F.; Sadeghi, M.; Feizi, A.; Sharifirad, G.; Hasandokht, T.; Mostafavi, F. Self-efficacy strategies to improve exercise in patients with heart failure: A systematic review. ARYA Atheroscler. 2014, 10, 319–333. [Google Scholar]
- Iannotti, R.J.; Schneider, S.; Nansel, T.R.; Haynie, D.L.; Plotnick, L.P.; Clark, L.M.; Sobel, D.O.; Simons-Morton, B. Self-efficacy, outcome expectations, and diabetes self-management in adolescents with Type 1 diabetes. J. Dev. Behav. Pediatr. 2006, 27, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, N. Virtual reality meets diabetes. J. Diabetes Sci. Technol. 2025, 19, 810–819. [Google Scholar] [CrossRef]
- Reagan, L.; Pereira, K.; Jefferson, V.; Evans Kreider, K.; Totten, S.; D’Eramo Melkus, G.; Johnson, C.; Vorderstrasse, A. Diabetes self-management training in a virtual environment. Diabetes Educ. 2017, 43, 413–421. [Google Scholar] [CrossRef]
- Gruber, N.; Shemesh-Iron, M.; Kraft, E.; Mitelberg, K.; Mauda, E.; Ben-Ami, M.; Mazor-Aronovitch, K.; Levy-Shraga, Y.; Levran, N.; Levek, N.; et al. Virtual reality’s impact on children with type 1 diabetes: A proof-of-concept randomized cross-over trial on anxiety, pain, adherence, and glycemic control. Acta Diabetol. 2024, 61, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yim, Y.R.; Hur, M.H. Effects of virtual reality program on glycated hemoglobin, static and dynamic balancing ability, and falls efficacy for diabetic patients: A systematic review and meta-analysis. J. Korean Acad. Fundam. Nurs. 2023, 30, 155–167. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Vavougios, G.D.; Kalogiannis, P.; Tachoulas, K.; Touloudi, E.; Astara, K.; Mysiris, D.S.; Tsirimona, G.; Papayianni, E.; Boutlas, S.; et al. Breathlessness and exercise with virtual reality system in long-post-coronavirus disease 2019 patients. Front. Public Health 2023, 11, 1115393. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Fang, C.; Che, Y.; Peng, X.; Zhang, X.; Lin, D. Reward feedback mechanism in virtual reality serious games in interventions for children with attention deficits: Pre- and posttest experimental control group study. JMIR Serious Games 2025, 13, 67338. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Barnes-Horowitz, N.; Treanor, M.; Sun, M.; Young, K.S.; Craske, M.G. Virtual reality reward training for anhedonia: A pilot study. Front. Psychol. 2021, 11, 613617. [Google Scholar] [CrossRef]
- Hajder, Đ.; Bjelica, B.; Bubanj, S.; Aksović, N.; Marković, M.; Arsenijević, R.; Lupu, G.-S.; Gašić, T.; Sufaru, C.; Toskić, L.; et al. A systematic review and meta-analysis of virtual and traditional physical activity programs: Effects on physical, health, and cognitive outcomes. Healthcare 2025, 13, 711. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S. Effectiveness of virtual reality using video gaming technology in elderly adults with diabetes mellitus. Diabetes Technol. Ther. 2013, 15, 489–496. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hong, J.H.; Hur, M.H.; Seo, E.Y. Effects of virtual reality exercise program on blood glucose, body composition, and exercise immersion in patients with type 2 diabetes. Int. J. Environ. Res. Public Health 2023, 20, 4178. [Google Scholar] [CrossRef]
- Senior, H.; Henwood, T.; de Souza, D.; Mitchell, G. Investigating innovative means of prompting activity uptake in older adults with type 2 diabetes: A feasibility study of exergaming. J. Sports Med. Phys. Fit. 2016, 56, 1221–1225. [Google Scholar]
- Mallik, R.; Patel, M.; Atkinson, B.; Kar, P. Exploring the role of virtual reality to support clinical diabetes training—A pilot study. J. Diabetes Sci. Technol. 2022, 16, 844–851. [Google Scholar] [CrossRef]
- Theng, Y.L.; Lee, J.W.Y.; Patinadan, P.V.; Foo, S.S.B. The use of videogames, gamification, and virtual environments in the self-management of diabetes: A systematic review of evidence. Games Health J. 2015, 4, 352–361. [Google Scholar] [CrossRef]
- Brito Gomes, J.L.; Vancea, D.M.M.; Farinha, J.B.; Barros, C.B.A.; Costa, M.C. 24-Hour blood glucose responses after exergame and running in type-1 diabetes: An intensity- and duration-matched randomized trial. Sci. Sports 2023, 38, 726–733. [Google Scholar] [CrossRef]
- Gomes, J.L.d.B.; Vancea, D.M.M.; Araújo, R.C.d.; Soltani, P.; Guimarães, F.J.d.S.P.; Costa, M.d.C. Cardiovascular and enjoyment comparisons after active videogame and running in type 1 diabetes patients: A randomized crossover trial. Games Health J. 2021, 10, 339–346. [Google Scholar] [CrossRef]
- World Medical Association. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 11 January 2025).
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Touloudi, E.; Hassandra, M.; Stavrou, V.T.; Panagiotounis, F.; Galanis, E.; Goudas, M.; Theodorakis, Y. Exploring the acute effects of immersive virtual reality biking on self-efficacy and attention of individuals in the treatment of substance use disorders: A feasibility study. Brain Sci. 2024, 14, 724. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Petropoulakos, K.; Papakonstantinou, V.; Pentsi, S.; Souzou, E.; Dimitriadis, Z.; Billis, E.; Koumantakis, G.; Poulis, I.; Spanos, S. Validity and reliability of the Greek Version of Pittsburgh Sleep Quality Index in chronic non-specific low back pain patients. Healthcare 2024, 12, 557. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Michopoulos, I.; Douzenis, A.; Kalkavoura, C.; Christodoulou, C.; Michalopoulou, P.; Kalemi, G.; Fineti, K.; Patapis, P.; Protopapas, K.; Lykouras, L. Hospital Anxiety and Depression Scale (HADS): Validation in a Greek general hospital sample. Ann. Gen. Psychiatry 2008, 7, 4. [Google Scholar] [CrossRef]
- Touloudi, E.; Hassandra, M.; Galanis, E.; Goudas, M.; Theodorakis, Y. Applicability of an immersive virtual reality exercise training system for office workers during working hours. Sports 2022, 10, 104. [Google Scholar] [CrossRef]
- Hassandra, M.; Galanis, E.; Hatzigeorgiadis, A.; Goudas, M.; Mouzakidis, C.; Karathanasi, E.M.; Petridou, N.; Tsolaki, M.; Zikas, P.; Evangelou, G.; et al. Exercise program effects on alzheimer’s disease risk factors: A study on older adults. JMIR Serious Games 2021, 9, e24170. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.; Lorr, M.; Doppleman, L. POMS Manual for the Profile of Mood States, 27th ed.; Educational and Industrial Testing Service: San Diego, CA, USA, 1971. [Google Scholar]
- Maggouritsa, M.; Kokaridas, D.; Theodorakis, Y.; Patsiaouras, A. The effect of a physical activity programme on improving mood profile of patients with schizophrenia. Int. J. Sport Exerc. Psychol. 2014, 12, 253–268. [Google Scholar] [CrossRef]
- Goudas, M.; Biddle, S.; Fox, K. Perceived locus of causality, goal orientations, and perceived competence in school physical education classes. Br. J. Educ. Psychol. 1994, 64, 453–463. [Google Scholar] [CrossRef]
- Goudas, M.; Dermitzaki, I.; Bagiatis, K. Predictors of student’s intrinsic motivation in school physical education. Eur. J. Psychol. Educ. 2000, 15, 271–280. [Google Scholar] [CrossRef]
- Bandura, A. Guide for Constructing Self-Efficacy Scales. In Self-Efficacy Beliefs of Adolescents; Pajares, T., Urdan, T., Eds.; Information Age Publishing: Greenwich, CT, USA, 2006; pp. 307–337. [Google Scholar]
- Megakli, T.; Vlachopoulos, S.; Thøgersen-Ntoumani, C.; Theodorakis, Y. Impact of aerobic and resistance exercise combination on physical self-perceptions and self-esteem in women with obesity with one-year follow-up. Int. J. Sport Exerc. Psychol. 2017, 15, 236–257. [Google Scholar] [CrossRef]
- Agarwal, R.; Prasad, J. A Conceptual and operational definition of personal innovativeness in the domain of information technology. Inf. Syst. Res. 1998, 9, 204–215. [Google Scholar] [CrossRef]
- Brooke, J. SUS—A Quick and Dirty Usability Scale. In Usability Evaluation in Industry; Jordan, P.W., Thomas, B., McClelland, I.L., Weerdmeester, B., Eds.; Taylor & Francis Ltd.: Bristol, UK, 1996; pp. 189–194. [Google Scholar]
- Mrakic-Sposta, S.; Di Santo, S.G.; Franchini, F.; Arlati, S.; Zangiacomi, A.; Greci, L.; Moretti, S.; Jesuthasan, N.; Marzorati, M.; Rizzo, G.; et al. Effects of combined physical and cognitive virtual reality-based training on cognitive impairment and oxidative stress in MCI patients: A pilot study. Front. Aging Neurosci. 2018, 10, 282. [Google Scholar] [CrossRef]
- Rasimah, C.M.Y.; Ahmad, A.; Zaman, H.B. Evaluation of user acceptance of mixed reality technology. Australas. J. Educ. Technol. 2011, 27, 1369–1387. [Google Scholar] [CrossRef]
- Ajzen, I. Constructing a Theory of Planned Behavior Questionnaire. 2006. Available online: https://people.umass.edu/aizen/ (accessed on 25 January 2023).
- Lim, C.Y.; In, J. Randomization in clinical studies. Korean J. Anesthesiol. 2019, 72, 221–232. [Google Scholar] [CrossRef]
- Turpin, N.A.; Watier, B. Cycling biomechanics and its relationship to performance. Appl. Sci. 2020, 10, 4112. [Google Scholar] [CrossRef]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538226/# (accessed on 25 January 2025).
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Whipp, B.J. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Elsholz, S.; Pham, K.; Zarnekow, R. A taxonomy of virtual reality sports applications. Virtual Real. 2025, 29, 16. [Google Scholar] [CrossRef]
- Surwit, R.S.; Schneider, M.S.; Feinglos, M.N. Stress and diabetes mellitus. Diabetes Care 1992, 15, 1413–1422. [Google Scholar] [CrossRef]
- Shaw, A.J.; Lubetzky, A.V. A short bout of exercise with and without an immersive virtual reality game can reduce stress and anxiety in adolescents: A pilot randomized controlled trial. Front. Virtual Real. 2020, 1, 598506. [Google Scholar] [CrossRef]
- Plante, T.G.; Aldridge, A.; Bogden, R.; Hanelin, C. Might virtual reality promote the mood benefits of exercise? Comput. Hum. Behav. 2003, 19, 495–509. [Google Scholar] [CrossRef]
- Maturo, C.C.; Cunningham, S.A. Influence of friends on children’s physical activity: A review. Am. J. Public Health 2013, 103, 23–38. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, N.; Pope, Z.C.; McDonough, D.J.; Gao, Z. Acute effects of immersive virtual reality exercise on young adults’ situational motivation. J. Clin. Med. 2019, 8, 1947. [Google Scholar] [CrossRef]
- Lemmens, J.S. Persistence and pleasure in VR: Enhancing exercise endurance and enjoyment through virtual environments. Psychol. Sport. Exerc. 2023, 69, 102494. [Google Scholar] [CrossRef]
- Evans, E.; Naugle, K.E.; Kaleth, A.S.; Arnold, B.; Naugle, K.M. Physical activity intensity, perceived exertion, and enjoyment during head-mounted display virtual reality games. Games Health J. 2021, 10, 314–320. [Google Scholar] [CrossRef]
- Ochi, G.; Ohno, K.; Kuwamizu, R.; Yamashiro, K.; Fujimoto, T.; Ikarashi, K.; Kodama, N.; Onishi, H.; Sato, D. Exercising with virtual reality is potentially better for the working memory and positive mood than cycling alone. Ment. Health Phys. Act. 2024, 27, 100641. [Google Scholar] [CrossRef]
- Poli, L.; Greco, G.; Gabriele, M.; Pepe, I.; Centrone, C.; Cataldi, S.; Fischetti, F. Effect of outdoor cycling, virtual and enhanced reality indoor cycling on heart rate, motivation, enjoyment and intention to perform green exercise in healthy adults. J. Funct. Morphol. Kinesiol. 2024, 9, 183. [Google Scholar] [CrossRef]
- Neumann, D.L.; Moffitt, R.L.; Thomas, P.R.; Loveday, K.; Watling, D.P.; Lombard, C.L.; Antonova, S.; Tremeer, M.A. A systematic review of the application of interactive virtual reality to sport. Virtual Real. 2018, 22, 183–198. [Google Scholar] [CrossRef]
- Schofield, J.; Ho, J.; Soran, H. Cardiovascular risk in type 1 diabetes mellitus. Diabetes Ther. 2019, 10, 773–789. [Google Scholar] [CrossRef]
- Porges, S.W. Cardiac Vagal Tone: A physiological index of stress. Neurosci. Biobehav. Rev. 1995, 19, 225–233. [Google Scholar] [CrossRef]
- Julián, M.T.; Pérez-Montes de Oca, A.; Julve, J.; Alonso, N. The double burden: Type 1 diabetes and heart failure—A comprehensive review. Cardiovasc. Diabetol. 2024, 23, 65. [Google Scholar] [CrossRef]
- Fang, Y.-M. Exploring usability, emotional responses, flow experience, and technology acceptance in VR: A comparative analysis of freeform creativity and goal-directed training. Appl. Sci. 2024, 14, 6737. [Google Scholar] [CrossRef]
- Greene, D.R.; Rougeau, K.M. Punching up the fun: A Comparison of enjoyment and in-task valence in virtual reality boxing and treadmill running. Psychol. Int. 2024, 6, 842–854. [Google Scholar] [CrossRef]
- Tussyadiah, I.P.; Wang, D.; Jung, T.H.; Tom Dieck, M.C. Virtual reality, presence, and attitude change: Empirical evidence from tourism. Tour. Manag. 2018, 66, 140–154. [Google Scholar] [CrossRef]
| VR Condition | Non-VR Condition | ||
|---|---|---|---|
| Post VR—Non-VR Comparison | Mean ± SD | Mean ± SD | |
| Self-Efficacy | 41.4 ± 6.4 | 40.2 ± 10.9 | |
| Self-Efficacy Expectations | 54.5 ± 10.2 | 49.5 ± 13.5 | |
| Interest/Enjoyment | 4.5 ± 0.5 * | 3.4 ± 0.9 | |
| Attitudes | 6.2 ± 0.7 | 5.7 ± 1.0 | |
| Post VR Trial | Personal Innovativeness | 3.9 ± 0.7 | - |
| Perceived Enjoyment | 4.6 ± 0.3 | - | |
| IFU | 4.4 ± 0.6 | - | |
| Usability | 83.4 ± 12.1 | - | |
| VR Equipment | 4.1 ± 0.5 | - | |
| VR Condition | Non-VR Condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Unit | Baseline | After Trial | p-Value | Cohen’s d | Baseline | After Trial | p-Value | Cohen’s d |
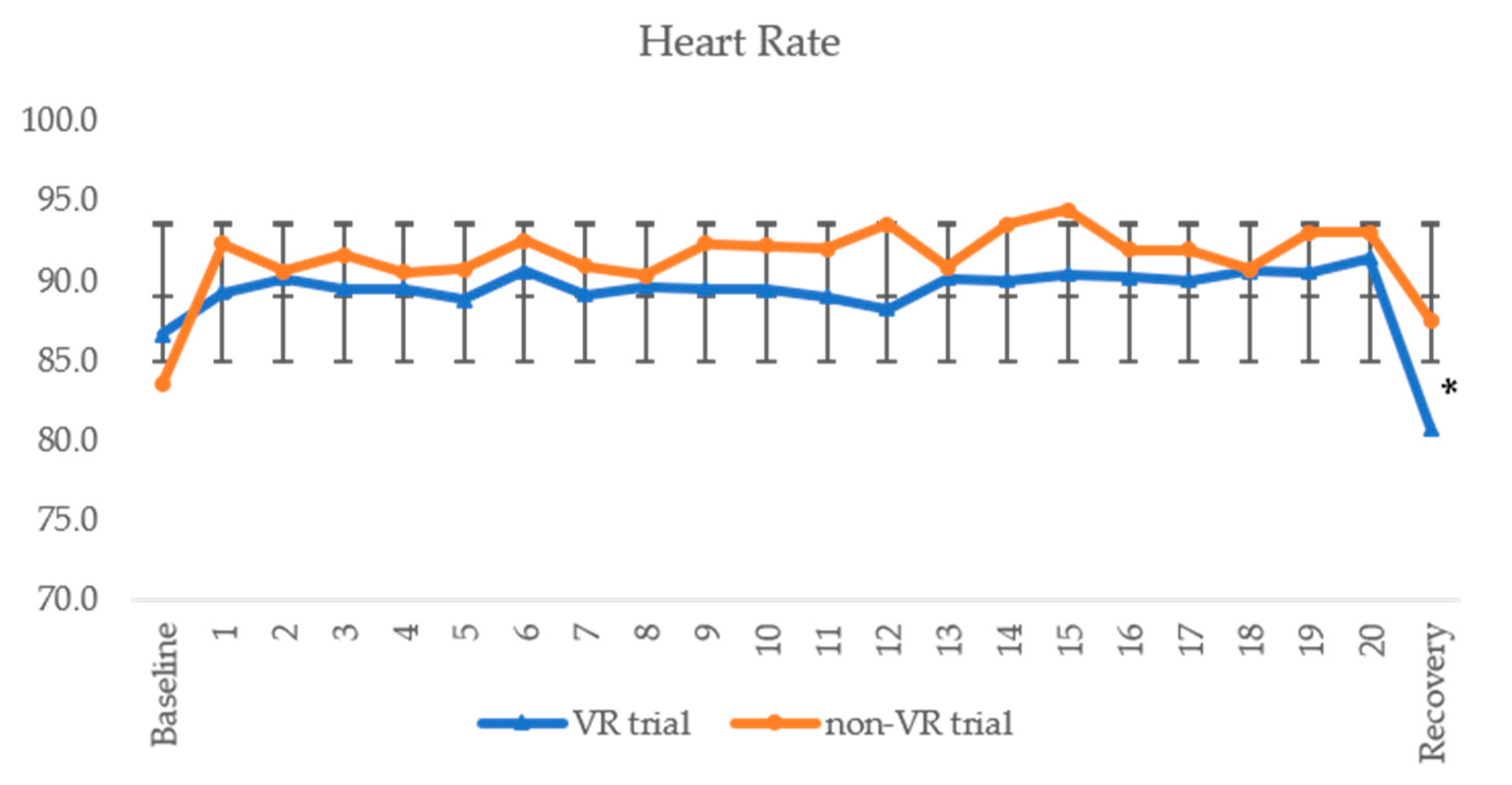
| HR | bpm | 83.0 ± 10.6 | 80.8 ± 14.3 | 0.594 | 0.17 | 83.5 ± 9.2 | 87.3 ± 8.8 | 0.202 | 0.42 |
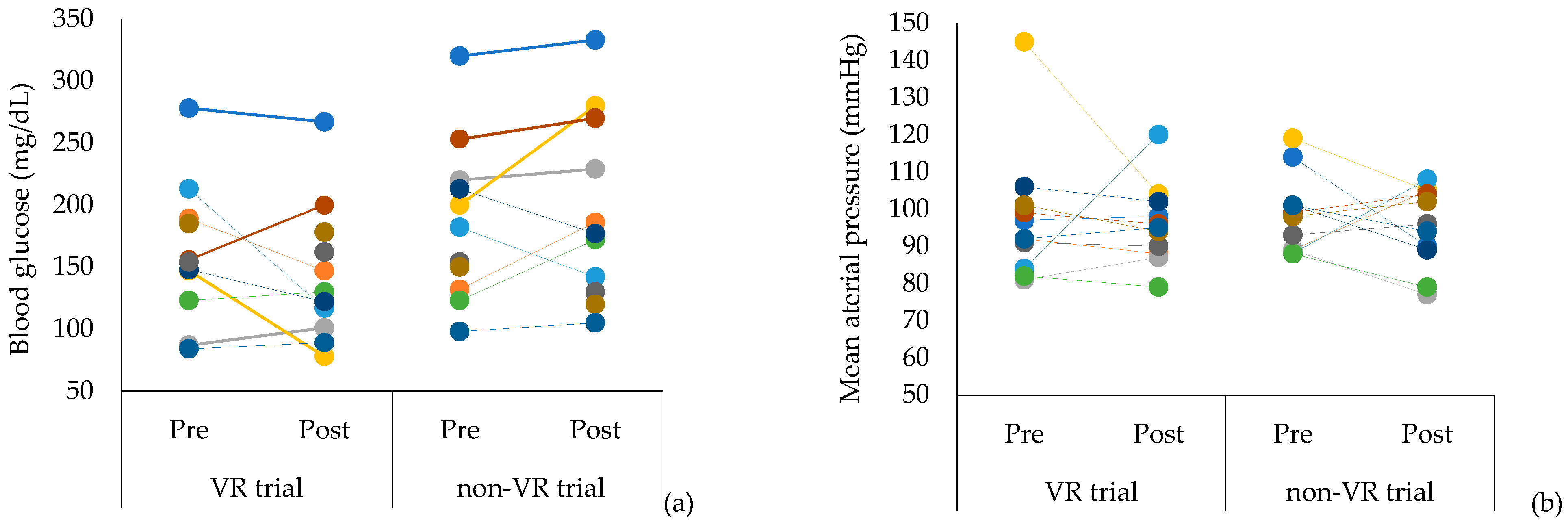
| BG | mg/dL | 173.2 ± 61.6 | 144.6 ± 55.1 | 0.091 | 0.49 | 185.9 ± 64.4 | 181.6 ± 58.2 | 0.990 | 0.07 |
| MAP | mmHg | 92.1 ± 24.3 | 95.7 ± 10.7 | 0.858 | 0.19 | 88.2 ± 25.8 | 96.7 ± 10.9 | 0.328 | 0.43 |
| Mood | score | 59.1 ± 5.9 | 64.0 ± 3.9 | 0.05 * | 0.98 | 59.7 ± 6.6 | 61.5 ± 5.0 | 0.439 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touloudi, E.; Stavrou, V.T.; Galanis, E.; Bargiota, A.; Goudas, M.; Dafoulas, G.; Hassandra, M.; Theodorakis, Y. Virtual Reality Versus Conventional Exercise in Patients with Type 1 Diabetes: A Feasibility Randomized Crossover Trial. Virtual Worlds 2025, 4, 32. https://doi.org/10.3390/virtualworlds4030032
Touloudi E, Stavrou VT, Galanis E, Bargiota A, Goudas M, Dafoulas G, Hassandra M, Theodorakis Y. Virtual Reality Versus Conventional Exercise in Patients with Type 1 Diabetes: A Feasibility Randomized Crossover Trial. Virtual Worlds. 2025; 4(3):32. https://doi.org/10.3390/virtualworlds4030032
Chicago/Turabian StyleTouloudi, Evlalia, Vasileios T. Stavrou, Evangelos Galanis, Alexandra Bargiota, Marios Goudas, George Dafoulas, Mary Hassandra, and Yannis Theodorakis. 2025. "Virtual Reality Versus Conventional Exercise in Patients with Type 1 Diabetes: A Feasibility Randomized Crossover Trial" Virtual Worlds 4, no. 3: 32. https://doi.org/10.3390/virtualworlds4030032
APA StyleTouloudi, E., Stavrou, V. T., Galanis, E., Bargiota, A., Goudas, M., Dafoulas, G., Hassandra, M., & Theodorakis, Y. (2025). Virtual Reality Versus Conventional Exercise in Patients with Type 1 Diabetes: A Feasibility Randomized Crossover Trial. Virtual Worlds, 4(3), 32. https://doi.org/10.3390/virtualworlds4030032









