Virtual Reality-Assisted, Single-Session Exposure for Public Speaking Anxiety: Improved Self-Reports and Heart Rate but No Significant Change in Heart Rate Variability
Abstract
1. Introduction
Present Study
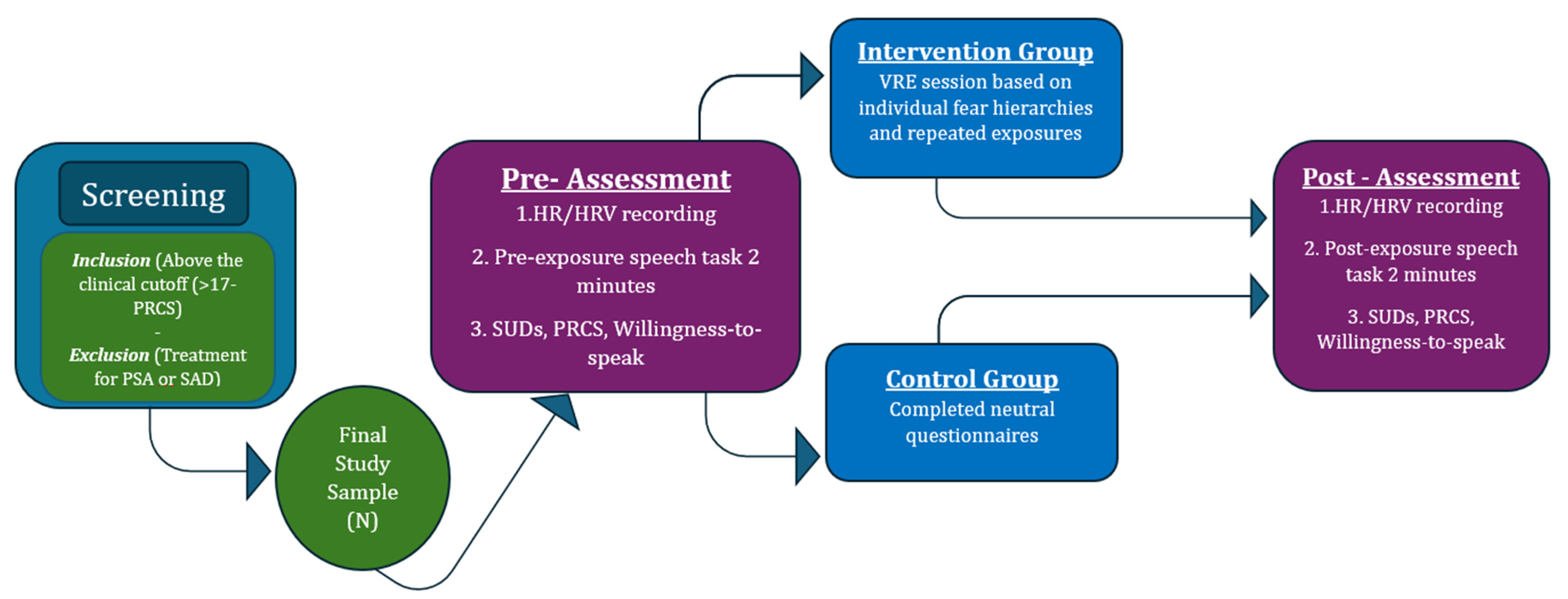
2. Materials and Methods
2.1. Participants
2.2. Measures
- Screening and baseline assessment: Online PRCS screening—all eligible participants completed baseline HR/HRV recordings and a pre-exposure speech task with SUD, PRCS, and willingness-to-speak ratings.
- Intervention or control condition: The VRE group underwent a personalized, graduated VRE session based on individual fear hierarchies and repeated exposures until SUD reduction; the CG completed neutral questionnaires.
- Post-intervention assessment: All participants completed the same 2 min speech task, post-exposure HR/HRV recordings, and repeated self-report measures (SUDs, PRCS, willingness to speak).
3. Results
3.1. Preliminary Analyses
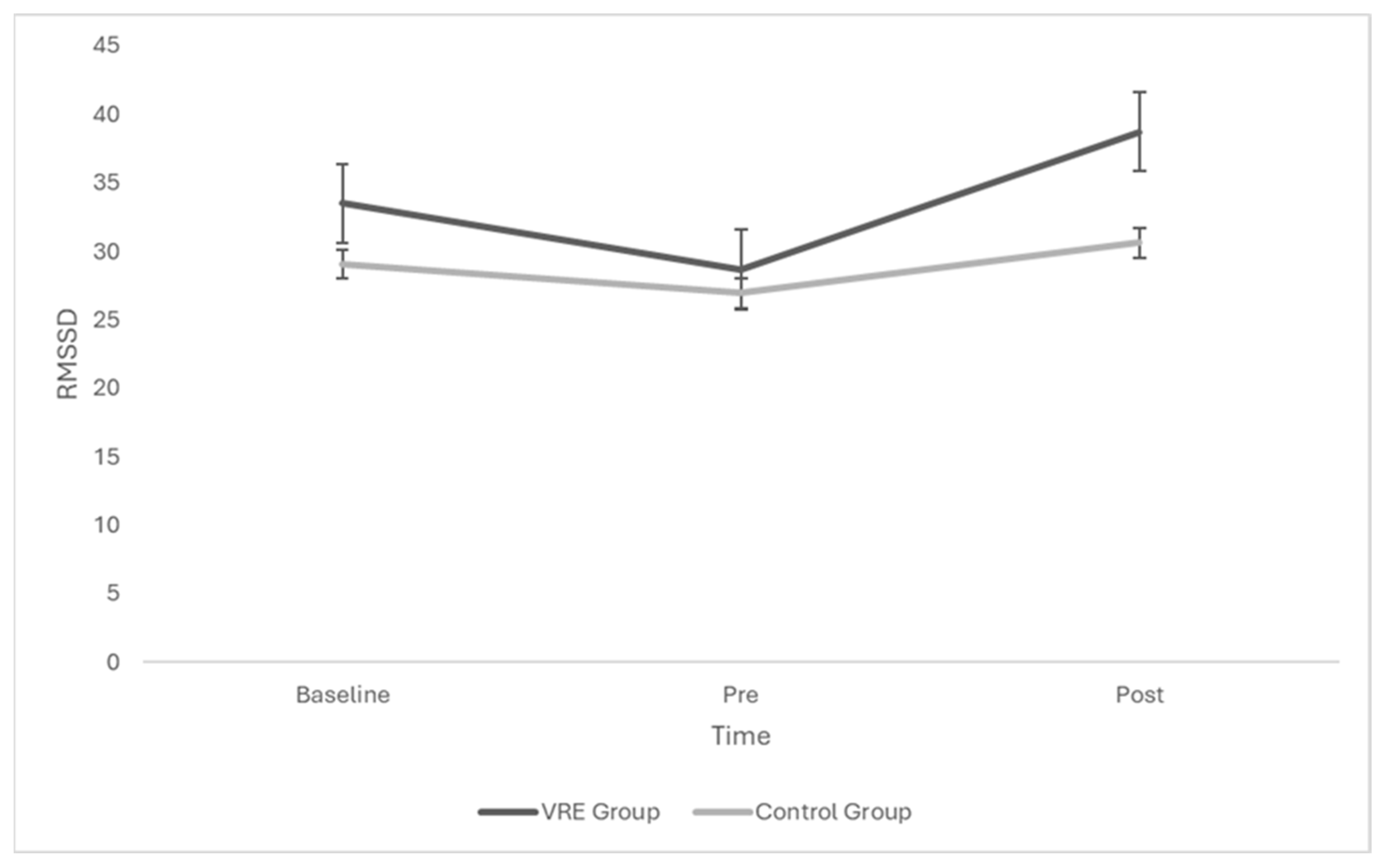
3.2. Effects of Group and Time
3.3. Within-Subject Effects of Audience Type
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, S.R.; Kemmerling, R.L.; North, M.M. Brief virtual reality therapy for public speaking anxiety. CyberPsychology Behav. 2002, 5, 543–550. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision (DSM-5-TR); American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Lintner, T.; Belovecová, B. Demographic predictors of public speaking anxiety among university students. Curr. Psychol. 2024, 43, 25215–25223. [Google Scholar] [CrossRef]
- Stein, M.B.; Walker, J.R.; Forde, D.R. Public-speaking fears in a community sample: Prevalence, impact on functioning, and diagnostic classification. Arch. Gen. Psychiatry 1996, 53, 169–174. [Google Scholar] [CrossRef]
- Furukawa, T.A.; Watanabe, N.; Kinoshita, Y.; Kinoshita, K.; Sasaki, T.; Nishida, A.; Okazaki, Y.; Shimodera, S. Public speaking fears and their correlates among 17,615 Japanese adolescents. Asia-Pac. Psychiatry 2014, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, S.; Bossé, J.; Loranger, C.; Klinger, É. Social anxiety disorder: Efficacy and virtual humans. In Series in Anxiety and Related Disorders. Advances in Virtual Reality and Anxiety Disorders; Wiederhold, B.K., Bouchard, S., Eds.; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2014; pp. 187–209. [Google Scholar] [CrossRef]
- Wong, K.P.; Lai, C.Y.Y.; Qin, J. Systematic review and meta-analysis of randomised controlled trials for evaluating the effectiveness of virtual reality therapy for social anxiety disorder. J. Affect. Disord. 2023, 333, 353–364. [Google Scholar] [CrossRef]
- Felnhofer, A.; Kothgassner, O.D.; Hetterle, T.; Beutl, L.; Hlavacs, H.; Kryspin-Exner, I. Afraid to be there? Evaluating the relation between presence, self-reported anxiety, and heart rate in a virtual public speaking task. Cyberpsychology Behav. Soc. Netw. 2014, 17, 310–316. [Google Scholar] [CrossRef]
- Slater, M.; Antley, A.; Davison, A.; Swapp, D.; Guger, C.; Barker, C.; Pistrang, N.; Sanchez-Vives, M.V.; Rustichini, A. A virtual reprise of the Stanley Milgram obedience experiments. PLoS ONE. 2006, 1, e39. [Google Scholar] [CrossRef]
- Owens, M.E.; Beidel, D.C. Can Virtual Reality Effectively Elicit Distress Associated with Social Anxiety Disorder? J. Psychopathol. Behav. Assess. 2015, 37, 296–305. [Google Scholar] [CrossRef]
- Lim, M.H.; Aryadoust, V.; Esposito, G. A meta-analysis of the effect of virtual reality on reducing public speaking anxiety. Curr. Psychol. 2022, 42, 12912–12928. [Google Scholar] [CrossRef]
- Morina, N.; Kampmann, I.; Emmelkamp, P.; Barbui, C.; Hoppen, T.H. Meta-analysis of virtual reality exposure therapy for social anxiety disorder. Psychol. Med. 2023, 53, 2176–2178. [Google Scholar] [CrossRef]
- Wechsler, T.F.; Kümpers, F.; Mühlberger, A. Inferiority or even superiority of virtual reality exposure therapy in phobias?—A systematic review and quantitative meta-analysis on randomized controlled trials specifically comparing the efficacy of virtual reality exposure to gold standard in vivo exposure in agoraphobia, specific phobia, and social phobia. Front. Psychol. 2019, 10, 1758. [Google Scholar] [CrossRef]
- Chesham, R.K.; Malouff, J.M.; Schutte, N.S. Meta-analysis of the efficacy of virtual reality exposure therapy for social anxiety. Behav. Chang. 2018, 35, 152–166. [Google Scholar] [CrossRef]
- Tan, Y.L.; Chang, V.Y.X.; Ang, W.H.D.; Ang, W.W.; Lau, Y. Virtual reality exposure therapy for social anxiety disorders: A meta-analysis and meta-regression of randomized controlled trials. Anxiety Stress Coping 2024, 38, 141–160. [Google Scholar] [CrossRef]
- Seuling, P.D.; Czernin, N.S.; Schiele, M.A. Virtual Reality exposure therapy in the treatment of public speaking anxiety and social anxiety disorder. Neurosci. Appl. 2024, 3, 104074. [Google Scholar] [CrossRef]
- Premkumar, P.; Heym, N.; Brown, D.J.; Battersby, S.; Sumich, A.; Huntington, B.; Daly, R.; Zysk, E. The effectiveness of self-guided virtual-reality exposure therapy for public-speaking anxiety. Front. Psychiatry 2021, 12, 694610. [Google Scholar] [CrossRef] [PubMed]
- Stupar-Rutenfrans, S.; Ketelaars, L.E.H.; Van Gisbergen, M.S. Beat the Fear of Public Speaking: Mobile 360° Video Virtual Reality Exposure Training in Home Environment Reduces Public Speaking Anxiety. Cyberpsychology Behav. Soc. Netw. 2017, 20, 624–633. [Google Scholar] [CrossRef]
- Reeves, R.; Curran, D.; Gleeson, A.; Hanna, D. A meta-analysis of the efficacy of virtual reality and in vivo exposure therapy as psychological interventions for public speaking anxiety. Behav. Modif. 2022, 46, 937–965. [Google Scholar] [CrossRef]
- Daniels, M.M.; Palaoag, T.; Daniels, M. Efficacy of virtual reality in reducing fear of public speaking: A systematic review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 803, 012003. [Google Scholar] [CrossRef]
- Boetje, J.; van Ginkel, S. The added benefit of an extra practice session in virtual reality on the development of presentation skills: A randomized control trial. Comput. Educ. 2020, 37, 253–264. [Google Scholar] [CrossRef]
- Siegel, P.; Warren, R.; Wang, Z.; Yang, J.; Cohen, D.; Anderson, J.F.; Murray, L.; Peterson, B.S. Less is more: Neural activity during very brief and clearly visible exposure to phobic stimuli. Hum. Brain Mapp. 2017, 38, 2466–2481. [Google Scholar] [CrossRef]
- Miloff, A.; Lindner, P.; Dafgård, P.; Deak, S.; Garke, M.; Hamilton, W.; Heinsoo, J.; Kristoffersson, G.; Rafi, J.; Sindemark, K.; et al. Automated virtual reality exposure therapy for spider phobia vs. in-vivo one-session treatment: A randomized non-inferiority trial. Behav. Res. Ther. 2019, 118, 130–140. [Google Scholar] [CrossRef]
- Takac, M.; Collett, J.; Blom, K.J.; Conduit, R.; Rehm, I.; De Foe, A. Public speaking anxiety decreases within repeated virtual reality training sessions. PLoS ONE 2019, 14, e0216288. [Google Scholar] [CrossRef] [PubMed]
- Lindner, P.; Miloff, A.; Fagernäs, S.; Andersen, J.; Sigeman, M.; Andersson, G.; Furmark, T.; Carlbring, P. Therapist-led and self-led one-session virtual reality exposure therapy for public speaking anxiety with consumer hardware and software: A randomized controlled trial. J. Anxiety Disord. 2019, 61, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Banakou, D.; Johnston, T.; Beacco, A.; Senel, G.; Slater, M. Desensitizing anxiety through imperceptible change: Feasibility study on a paradigm for single-session exposure therapy for fear of public speaking. JMIR Form. Res. 2024, 8, e52212. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Carpenter, W.T.; Carretta, C.; Papanastasiou, E.; Vaidyanathan, U. Precision psychiatry and Research Domain Criteria: Implications for clinical trials and future practice. CNS Spectr. 2024, 29, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Lindner, P.; Miloff, A.; Fagernäs, S.; Andersen, J.; Sigeman, M.; Andersson, G.; Wilsdon, L.; Fullwood, C. The effect of immersion and presence in a virtual reality public speaking task. Annu. Rev. CyberTherapy Telemed. 2017, 17, 211–213. Available online: http://hdl.handle.net/2436/622249 (accessed on 31 July 2017).
- Cacciopo, J.T.; Tassinary, L.G.; Berntson, G.G. Handbook of Psychophysiology, 4th ed.; Cambridge University Press: Cambridge, UK, 2016; pp. I–Ii. [Google Scholar]
- Benito, K.G.; Walther, M. Therapeutic process during exposure: Habituation model. J. Obs.-Compuls. Relat. Disord. 2015, 6, 147–157. [Google Scholar] [CrossRef]
- Sakib, M.N.; Yadav, M.; Chaspari, T.; Behzadan, A.H. Coupling virtual reality and physiological markers to improve public speaking performance. In Proceedings of the 19th International Conference on Construction Applications of Virtual Reality (CONVR2019), Bangkok, Thailand, 13–15 November 2019. [Google Scholar]
- Yadav, M.; Sakib, M.N.; Nirjhar, E.H.; Feng, K.; Behzadan, A.; Chaspari, T. Exploring individual differences of public speaking anxiety in real-life and virtual presentations. IEEE Trans. Affect. Comput. 2020, 13, 1168–1182. [Google Scholar] [CrossRef]
- Craske, M.G.; Treanor, M.; Conway, C.C.; Zbozinek, T.; Vervliet, B. Maximizing exposure therapy: An inhibitory learning approach. Behav. Res. Ther. 2014, 58, 10–23. [Google Scholar] [CrossRef]
- Mather, M.; Thayer, J.F. How heart rate variability affects emotion regulation brain networks. Curr. Opin. Behav. Sci. 2018, 19, 98–104. [Google Scholar] [CrossRef]
- McCraty, R.; Shaffer, F. Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Bigger, J.T.; Kleiger, R.E.; Fleiss, J.L.; Rolnitzky, L.M.; Steinman, R.C.; Miller, J.P. Components of heart rate variability measured during healing of acute myocardial infarction. Am. J. Cardiol. 1988, 61, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Lischke, A.; Jacksteit, R.; Mau-Moeller, A.; Pahnke, R.; Hamm, A.O.; Weippert, M. Heart rate variability is associated with psychosocial stress in distinct social domains. J. Psychosom. Res. 2018, 106, 56–61. [Google Scholar] [CrossRef]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Kothgassner, O.D.; Felnhofer, A.; Hlavacs, H.; Beutl, L.; Palme, R.; Kryspin-Exner, I.; Glenk, L.M. Salivary cortisol and cardiovascular reactivity to a public speaking task in a virtual and real-life environment. Comput. Hum. Behav. 2016, 62, 124–135. [Google Scholar] [CrossRef]
- Biesmans, L.E.J.; Van Hees, P.J.M.; Rombout, L.E.; Alimardani, M.; Fukuda, E. The effects of ingroup bias on public speaking anxiety in virtual reality. In Proceedings of the 15th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2020), Valletta, Malta, 27–29 February 2020; Volume 2. [Google Scholar] [CrossRef]
- van Dis, E.A.M.; Landkroon, E.; Hagenaars, M.A.; van der Does, F.H.S.; Engelhard, I.M. Old Fears Die Hard: Return of Public Speaking Fear in a Virtual Reality Procedure. Behav. Ther. 2021, 52, 1188–1197. [Google Scholar] [CrossRef]
- Russell, G.; Topham, P. The Impact of Social Anxiety on Student Learning and Well-being in Higher Education. J. Ment. Health 2012, 21, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Grieve, R.; Woodley, J.; Hunt, S.E.; McKay, A. Student fears of oral presentations and public speaking in higher education: A qualitative survey. J. Furth. High. Educ. 2021, 45, 1281–1293. [Google Scholar] [CrossRef]
- Paul, G.L. Insight vs. Desensitization in Psychotherapy: An Experiment in Anxiety Reduction; Stanford University Press: Redwood City, CA, USA, 1996. [Google Scholar]
- Culver, N.C.; Stoyanova, M.; Craske, M.G. Emotional variability and sustained arousal during exposure. J. Behav. Ther. Exp. Psychiatry 2012, 43, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.L.; Price, M.; Edwards, S.M.; Obasaju, M.A.; Schmertz, S.K.; Zimand, E.; Calamaras, M.R. Virtual reality exposure therapy for social anxiety disorder: A randomized controlled trial. J. Consult. Clin. Psychol. 2013, 81, 751. [Google Scholar] [CrossRef]
- Hook, J.N.; Smith, C.A.; Valentiner, D.P. A short-form of the Personal Report of Confidence as a Speaker. Personal. Individ. Differ. 2008, 44, 1306–1313. [Google Scholar] [CrossRef]
- Wolpe, J. The Practice of Behavior Therapy, 4th ed.; Pergamon Press: Oxford, UK, 1990. [Google Scholar]
- Benjamin, C.L.; O’Neil, K.A.; Crawley, S.A.; Beidas, R.S.; Coles, M.; Kendall, P.C. Patterns and predictors of subjective units of distress in anxious youth. Behav. Cogn. Psychother. 2010, 38, 497. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.; Sütterlin, S.; Schulz, S.M.; Vögele, C. ARTiiFACT: A tool for heart rate artifact processing and heart rate variability analysis. Behav. Res. Methods 2011, 43, 1161–1170. [Google Scholar] [CrossRef]
- Panayiotou, G.; Karekla, M.; Georgiou, D.; Constantinou, E.; Paraskeva-Siamata, M. Psychophysiological and self-reported reactivity associated with social anxiety and public speaking fear symptoms: Effects of fear versus distress. Psychiatry Res. 2017, 255, 278–286. [Google Scholar] [CrossRef]
- Hindo, C.S.; González-Prendes, A.A. One-session exposure treatment for social anxiety with specific fear of public speaking. Res. Soc. Work Pract. 2011, 21, 528–538. [Google Scholar] [CrossRef]
- Foa, E.B.; Kozak, M.J. Emotional processing of fear: Exposure to corrective information. Psychol. Bull. 1986, 99, 20. [Google Scholar] [CrossRef]
- Scheveneels, S.; Boddez, Y.; Van Daele, T.; Hermans, D. Virtually unexpected: No role for expectancy violation in virtual reality exposure for public speaking anxiety. Front. Psychol. 2019, 10, 2849. [Google Scholar] [CrossRef]
- Hill, L.K.; Siebenbrock, A. Are all measures created equal? Heart rate variability and respiration. Biomed. Sci. Instrum. 2009, 45, 71–76. [Google Scholar] [PubMed]
- McEwen, B.S.; Akil, H. Revisiting the stress concept: Implications for affective disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Rothbaum, B.O.; Hodges, L.F. Virtual reality exposure in the treatment of social anxiety. Cogn. Behav. Pract. 2003, 10, 240–247. [Google Scholar] [CrossRef]
- Anderson, P.L.; Zimand, E.; Hodges, L.F.; Rothbaum, B.O. Cognitive behavioral therapy for public-speaking anxiety using virtual reality for exposure. Depress. Anxiety 2005, 22, 156–158. [Google Scholar] [CrossRef]
- Carl, E.; Stein, A.T.; Levihn-Coon, A.; Pogue, J.R.; Rothbaum, B.; Emmelkamp, P.; Asmundson, G.J.; Carlbring, P.; Powers, M.B. Virtual reality exposure therapy for anxiety and related disorders: A meta-analysis of randomized controlled trials. J. Anxiety Disord. 2019, 61, 27–36. [Google Scholar] [CrossRef]
- Pallant, J. SPSS Survival Manual: A Step-by-Step Guide to Data Analysis Using IBM SPSS, 6th ed.; Open University Press, McGraw-Hill: Maidenhead, UK, 2016. [Google Scholar]
- Mohr, D.C.; Spring, B.; Freedland, K.E.; Beckner, V.; Arean, P.; Hollon, S.D.; Ockene, J.; Kaplan, R. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother. Psychosom. 2009, 78, 275–284. [Google Scholar] [CrossRef]
- Emmelkamp, P.M.; Meyerbröker, K.; Morina, N. Virtual reality therapy in social anxiety disorder. Curr. Psychiatry Rep. 2020, 22, 32. [Google Scholar] [CrossRef]
- Pelissolo, A.; Zaoui, M.; Aguayo, G.; Yao, S.N.; Roche, S.; Ecochard, R.; Gueyffier, F.; Pull, C.; Berthoz, A.; Jouvent, R. Virtual reality exposure therapy versus cognitive behavior therapy for panic disorder with agoraphobia: A randomized comparison study. J. Cybertherapy Rehabil. 2012, 5, 35–43. [Google Scholar]


| Audience Level | |||||
|---|---|---|---|---|---|
| Exp1 | Exp2 | Exp3 | Exp4 | Exp5 | |
| 1 | M = 55 (SD = 22.47) | M = 48 (SD = 22.14) | M = 37.7 (SD = 21.48) | M = 43.75 (SD = 22.87) N = 12 | M = 41.11 (SD = 17.81) N = 9 |
| 2 | M = 52.75 (SD = 18.60) | M = 45 (SD = 16.85) | M = 36.5 (SD = 17.99) | M = 33.21 (SD = 15.14) N = 14 | M = 30 (SD = 12.90) N = 10 |
| 3 | M = 61.35 (SD = 18.62) | M = 54.15 (SD = 20.12) | M = 45.75 (SD = 19.07) | M = 43.12 (SD = 16.62) N = 16 | M = 38.46 (SD = 16.88) N = 13 |
| 4 | M = 63.75 (SD = 16.13) | M = 59.50 (SD = 19.86) | M = 52.95 (SD = 16.91) | M = 45.79 (SD = 17.50), N = 19 | M = 43.52 (SD = 12.59) N = 17 |
| 5 | M = 84.90 (SD = 17.29) | M = 80.95 (SD = 17.46) | M = 79.60 (SD = 19.60) | M = 72.26 (SD = 20.25), N = 19 | M = 70.15 (SD = 21.62), N = 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artemi, T.-F.; Konstantinou, T.; Naziri, S.; Panayiotou, G. Virtual Reality-Assisted, Single-Session Exposure for Public Speaking Anxiety: Improved Self-Reports and Heart Rate but No Significant Change in Heart Rate Variability. Virtual Worlds 2025, 4, 27. https://doi.org/10.3390/virtualworlds4020027
Artemi T-F, Konstantinou T, Naziri S, Panayiotou G. Virtual Reality-Assisted, Single-Session Exposure for Public Speaking Anxiety: Improved Self-Reports and Heart Rate but No Significant Change in Heart Rate Variability. Virtual Worlds. 2025; 4(2):27. https://doi.org/10.3390/virtualworlds4020027
Chicago/Turabian StyleArtemi, Tonia-Flery, Thekla Konstantinou, Stephany Naziri, and Georgia Panayiotou. 2025. "Virtual Reality-Assisted, Single-Session Exposure for Public Speaking Anxiety: Improved Self-Reports and Heart Rate but No Significant Change in Heart Rate Variability" Virtual Worlds 4, no. 2: 27. https://doi.org/10.3390/virtualworlds4020027
APA StyleArtemi, T.-F., Konstantinou, T., Naziri, S., & Panayiotou, G. (2025). Virtual Reality-Assisted, Single-Session Exposure for Public Speaking Anxiety: Improved Self-Reports and Heart Rate but No Significant Change in Heart Rate Variability. Virtual Worlds, 4(2), 27. https://doi.org/10.3390/virtualworlds4020027





