Mind the Psychedelic Hype: Characterizing the Risks and Benefits of Psychedelics for Depression
Abstract
1. Introduction
2. Methods
Narrative Review Strategy
3. Results
3.1. Overview of the Benefits and Risks of Psychedelics
3.2. Overview of Individual Psychedelic Trials for Depression
| Study | Compound and Dose | Primary Outcome Measure | Follow-Up Time | Sample Size | Comparison Groups | Standardized Mean Difference a |
|---|---|---|---|---|---|---|
| von Rotz et al., 2022 [20] | Psilocybin, 0.215 mg/kg (1 session) | MADRS, BDI | 14 days | 52 (26 psilocybin, 26 placebo) | Randomized, double-blind, placebo-controlled clinical trial: psilocybin vs. placebo | MADRS: 0.92 (Day 14) |
| Goodwin et al., 2022 [40] | Psilocybin, 0.215 mg/kg (1 session) | MADRS | Week 3 | 233 (79 receive 25 mg, 75 received 10 mg, 79 received 1 mg) | Randomized double-blind, controlled trial: single dose for each group (1 mg vs. 10 mg vs. 25 mg) | MADRS: 0.61 (Week 3) |
| Davis et al., 2021 [18] | Psilocybin, 0.29, 0.43 mg/kg (2 sessions) | GRID-HAMD, QIDS-SR | Weeks 1 and 4 | 27 (15 immediate treatment, 12 waiting list control) | Randomized, waiting-list-controlled clinical trial: treatment condition group vs. delayed treatment condition group | GRID-HAMD: 2.21 (Week 4) |
| Griffiths et al., 2016 [22] | Psilocybin, High dose 22 or 30 mg/70 kg, low dose 1 or 3 mg/kg (2 sessions) | GRID-HAMD HAM-A | Week 5 and month 6 | 51 | Randomized double-blind, cross-over trial: comparison of low versus high psilocybin dose | GRID-HAMD: 1.25 (Week 5) |
| Ross et al., 2016 [23] | Psilocybin, 0.3 mg/kg (1 session) | BDI STAI-T | 7 weeks | 29 | Randomized, double-blind, placebo-controlled, crossover trial: psilocybin vs. placebo | BDI: 0.87 (Week 7) |
| Gasser et al., 2014 [21] b | LSD, 200 µg (2 sessions) | STAI-S STAI-T | 2 months and 12 months | 12 | Randomized, double-blind, active placebo-controlled pilot study | HADS-D: 2.7 (Week 8) |
| Palhano-Fontes et al., 2019 [41] b | Ayahuasca, 1.0 mL/kg (0.36 mg/mL of DMT, 1.86 mg/mL harmine) (1 session) | HAM-D | 1 week | 29 (15 placebo, 15 ayahuasca) | Randomized placebo-controlled trial: treatment vs. placebo | HAM-D: 1.46 (Day 7) |
| Raison et al., 2023 [17] | Psilocybin, 25 mg (1 session) | MADRS | 43 days | 104 (53 placebo, 51 psilocybin) | Randomized Phase II double-blinded active placebo-controlled trial | MADRS: 0.92 (Day 43) |
| Carhart-Harris et al., 2021 [16] | Psilocybin, 25 mg (2 sessions) | QIDS-SR | 6 weeks | 59 (29 escitalopram and 1 mg psilocybin placebo, 30 psilocybin) | Randomized Phase II double-blinded placebo-controlled trial | QIDS-SR: 0.36 (Week 6) |
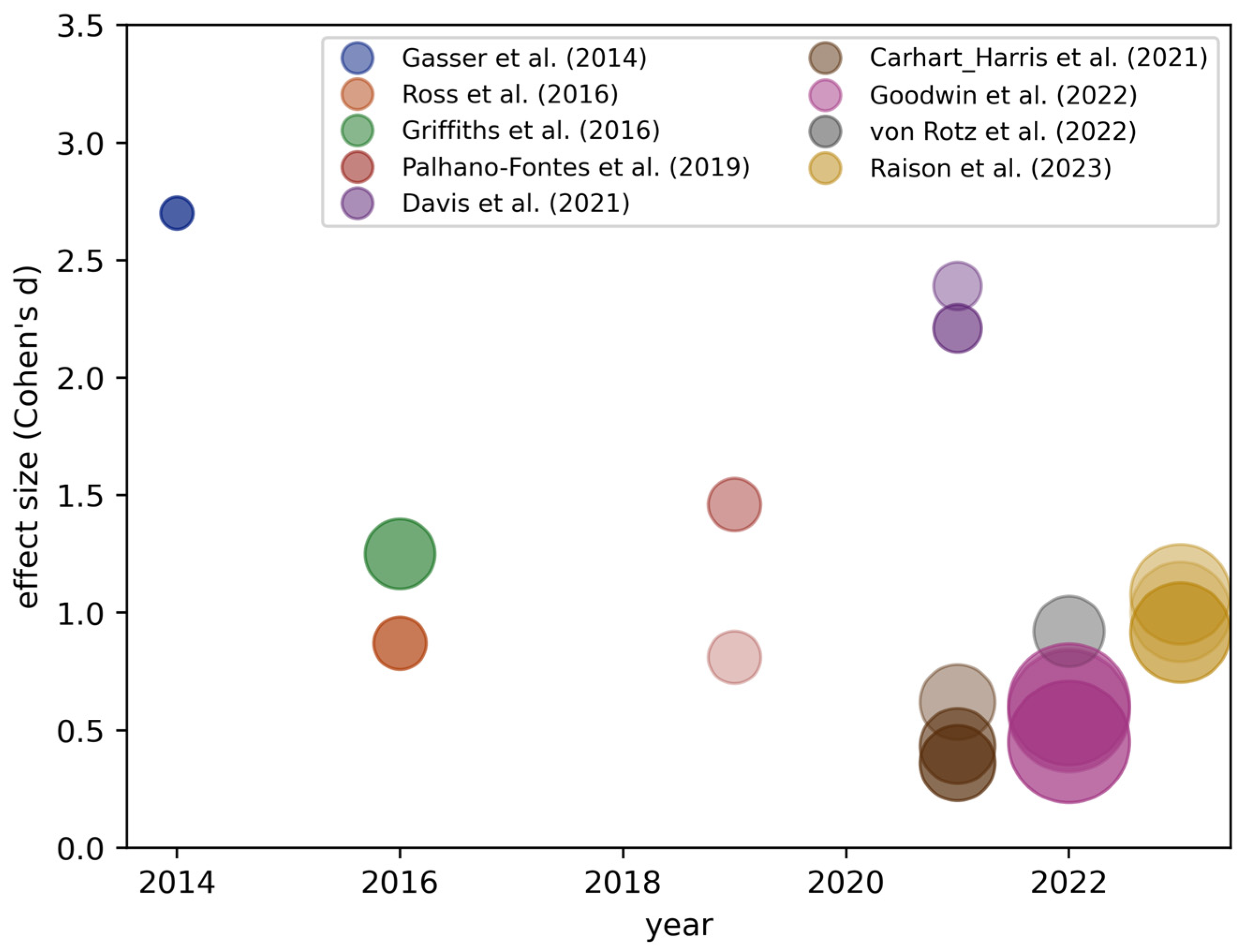
3.3. Overview of Meta-Analyses for Psychedelic Trials for Depression
3.4. Adverse Effects of Psychedelics
3.5. Comparing Psychedelics to Other Treatments and Interventions
3.6. Cognitive Behavioral Therapy
3.7. Mindfulness Interventions
3.8. SSRIs
3.9. Ketamine
3.10. Summary of the Comparisons between Treatment Modalities
4. Discussion
Why Might Effect Sizes Decrease over Time?
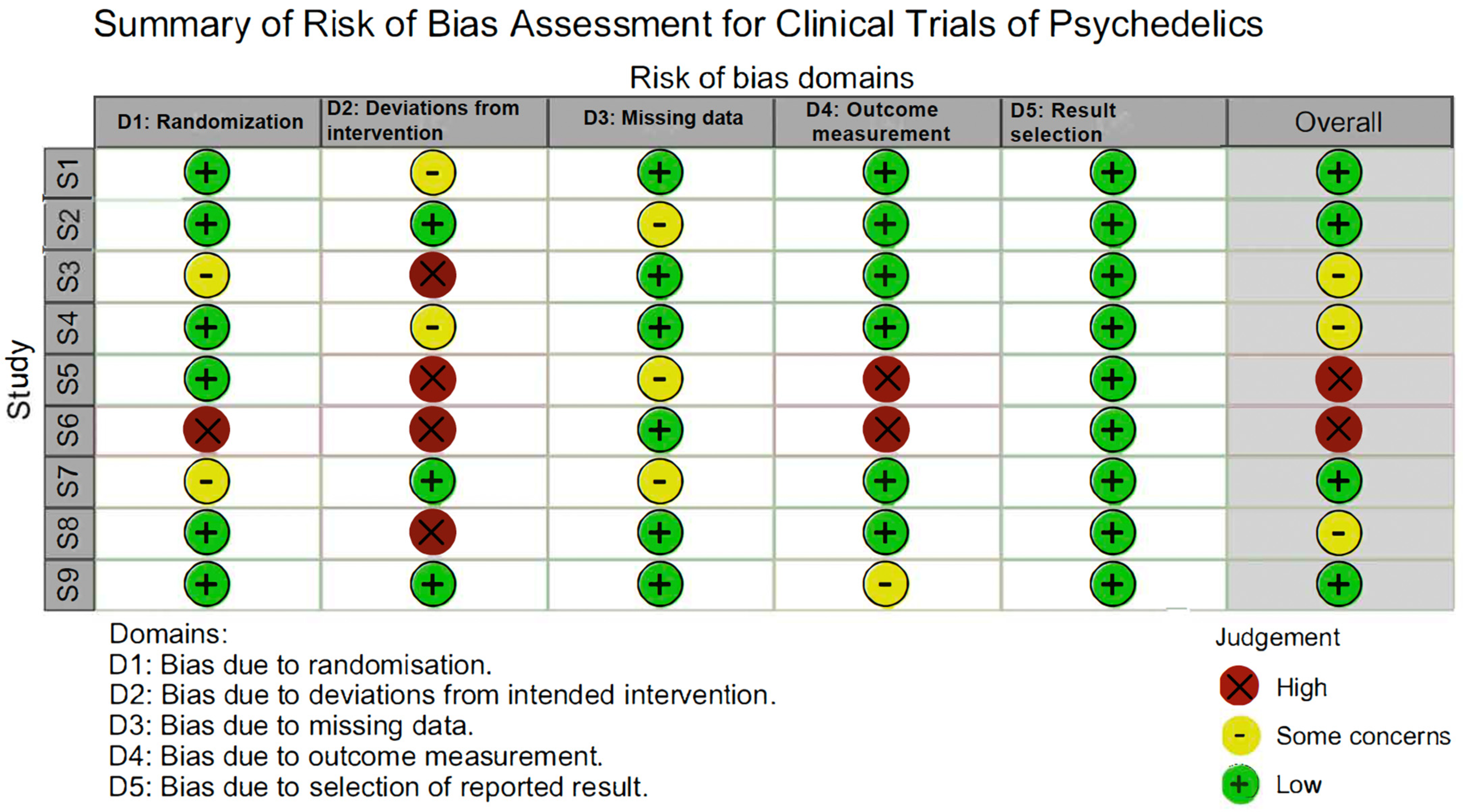
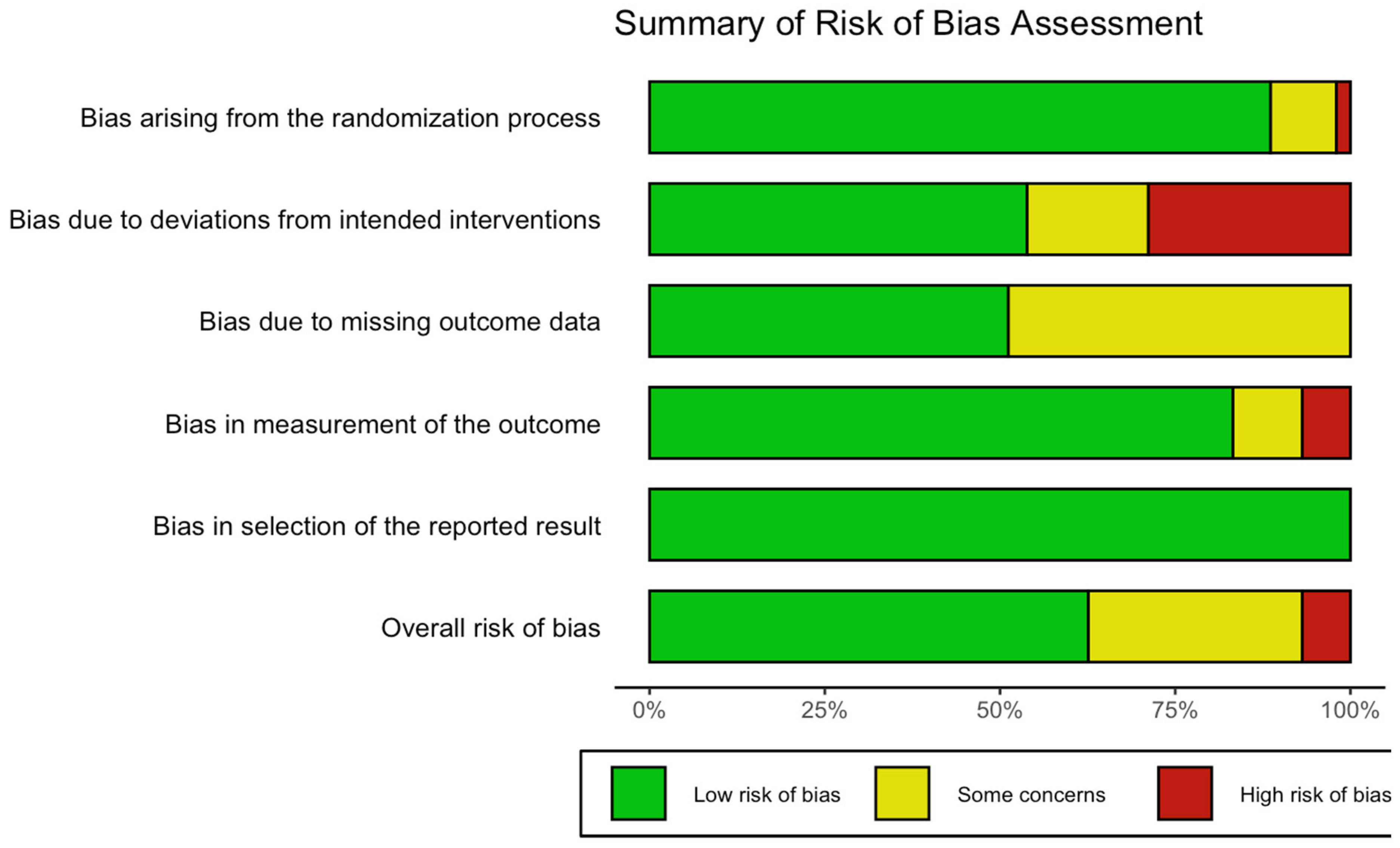
5. Study Design Considerations
5.1. Blinding and Expectancy Confounders in Psychedelic RCTs
5.2. Lack of Long-Term Follow-Up Measurements
5.3. Placebo Effect
6. Communicating Psychedelic Research Results
6.1. Overestimations in Published Effect Sizes Due to Publication Bias
6.2. Science Communication about Benefits and Risks
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Petranker, R.; Anderson, T.; Farb, N. Psychedelic Research and the Need for Transparency: Polishing Alice’s Looking Glass. Front. Psychol. 2020, 11, 1681. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Shechet, B.; Nicholas, C.R.; Ng, C.W.; Deole, G.; Chen, Z.; Raison, C.L. Post-acute psychological effects of classical serotonergic psychedelics: A systematic review and meta-analysis. Psychol. Med. 2020, 50, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Luoma, J.B.; Chwyl, C.; Bathje, G.J.; Davis, A.K.; Lancelotta, R. A Meta-Analysis of Placebo-Controlled Trials of Psychedelic-Assisted Therapy. J. Psychoact. Drugs 2020, 52, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, N.T.; van Vugt, M.K.; Vago, D.R.; Schmalzl, L.; Saron, C.D.; Olendzki, A.; Meissner, T.; Lazar, S.W.; Kerr, C.E.; Gorchov, J.; et al. Mind the Hype: A Critical Evaluation and Prescriptive Agenda for Research on Mindfulness and Meditation. Perspect. Psychol. Sci. 2017, 13, 36–61. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Muthukumaraswamy, S.; Roseman, L.; Kaelen, M.; Droog, W.; Murphy, K.; Tagliazucchi, E.; Schenberg, E.E.; Nest, T.; Orban, C.; et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl. Acad. Sci. USA 2016, 113, 4853–4858. [Google Scholar] [CrossRef] [PubMed]
- Barrett, F.S.; Bradstreet, M.P.; Leoutsakos, J.-M.S.; Johnson, M.W.; Griffiths, R.R. The Challenging Experience Questionnaire: Characterization of challenging experiences with psilocybin mushrooms. J. Psychopharmacol. 2016, 30, 1279–1295. [Google Scholar] [CrossRef] [PubMed]
- Muttoni, S.; Ardissino, M.; John, C. Classical psychedelics for the treatment of depression and anxiety: A systematic review. J. Affect. Disord. 2019, 258, 11–24. [Google Scholar] [CrossRef] [PubMed]
- van Elk, M.; Yaden, D.B. Pharmacological, neural, and psychological mechanisms underlying psychedelics: A critical review. Neurosci. Biobehav. Rev. 2022, 140, 104793. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Yaden, D.B.; Yaden, M.E.; Griffiths, R.R. Psychedelics in Psychiatry—Keeping the Renaissance From Going Off the Rails. JAMA Psychiatry 2020, 78, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.T.; Danforth, A.; Daroff, R.; Stauffer, C.; Ekman, E.; Agin-Liebes, G.; Trope, A.; Boden, M.T.; Dilley, J.; Mitchell, J.; et al. Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study. EClinicalMedicine 2020, 27, 100538. [Google Scholar] [CrossRef] [PubMed]
- Hadar, A.; David, J.; Shalit, N.; Roseman, L.; Gross, R.; Sessa, B.; Lev-Ran, S. The Psychedelic Renaissance in Clinical Research: A Bibliometric Analysis of Three Decades of Human Studies with Psychedelics. J. Psychoact. Drugs 2022, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.W.; Sharma, B.; Griffiths, R.R.; Carhart-Harris, R. Trends in the Top-Cited Articles on Classic Psychedelics. J. Psychoact. Drugs 2021, 53, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Romeo, B.; Karila, L.; Martelli, C.; Benyamina, A. Efficacy of psychedelic treatments on depressive symptoms: A meta-analysis. J. Psychopharmacol. 2020, 34, 1079–1085. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. New Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Raison, C.L.; Sanacora, G.; Woolley, J.; Heinzerling, K.; Dunlop, B.W.; Brown, R.T.; Kakar, R.; Hassman, M.; Trivedi, R.P.; Robison, R.; et al. Single-Dose Psilocybin Treatment for Major Depressive Disorder: A Randomized Clinical Trial. JAMA 2023, 330, 843–853. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Atli, M.; Bennett, J.C.; Croal, M.; DeBattista, C.; Dunlop, B.W.; Feifel, D.; Hellerstein, D.J.; et al. Single-dose psilocybin for a treatment-resistant episode of major depression: Impact on patient-reported depression severity, anxiety, function, and quality of life. J. Affect. Disord. 2023, 327, 120–127. [Google Scholar] [CrossRef]
- Von Rotz, R.; Schindowski, E.M.; Jungwirth, J.; Schuldt, A.; Rieser, N.M.; Zahoranszky, K.; Seifritz, E.; Nowak, A.; Nowak, P.; Jancke, L.; et al. Single-Dose Psilocybin-Assisted Therapy in Major Depressive Disorder: A Placebo-Controlled, Double-Blind, Randomised Clinical Trial. EClinicalMedicine 2022, 56, 101809. [Google Scholar] [CrossRef]
- Gasser, P.; Holstein, D.; Michel, Y.; Doblin, R.; Yazar-Klosinski, B.; Passie, T.; Brenneisen, R. Safety and Efficacy of Lysergic Acid Diethylamide-Assisted Psychotherapy for Anxiety Associated With Life-threatening Diseases. J. Nerv. Ment. Dis. 2014, 202, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.C.; Mennenga, S.E.; Owens, L.T.; Podrebarac, S.K.; Baron, T.; Rotrosen, J.; Ross, S.; Forcehimes, A.A.; Bogenschutz, M.P. Psilocybin for alcohol use disorder: Rationale and design considerations for a randomized controlled trial. Contemp. Clin. Trials 2022, 123, 106976. [Google Scholar] [CrossRef] [PubMed]
- Schlag, A.K.; Aday, J.; Salam, I.; Neill, J.C.; Nutt, D.J. Adverse effects of psychedelics: From anecdotes and misinformation to systematic science. J. Psychopharmacol. 2022, 36, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Hartogsohn, I. Constructing drug effects: A history of set and setting. Drug Sci. Policy Law 2017, 3. [Google Scholar] [CrossRef]
- Cooper, H.L. War on Drugs Policing and Police Brutality. Subst. Use Misuse 2015, 50, 1188–1194. [Google Scholar] [CrossRef]
- Provine, D.M. Race and Inequality in the War on Drugs. Annu. Rev. Law Soc. Sci. 2011, 7, 41–60. [Google Scholar] [CrossRef]
- Aday, J.S.; Davoli, C.C.; Bloesch, E.K. 2018: A watershed year for psychedelic science. Drug Sci. Policy Law 2019, 5. [Google Scholar] [CrossRef]
- Yaden, D.B.; Potash, J.B.; Griffiths, R.R. Preparing for the Bursting of the Psychedelic Hype Bubble. JAMA Psychiatry 2022, 79, 943–944. [Google Scholar] [CrossRef]
- Haikazian, S.; Chen-Li, D.C.; Johnson, D.E.; Fancy, F.; Levinta, A.; Husain, M.I.; Mansur, R.B.; McIntyre, R.S.; Rosenblat, J.D. Psilocybin-assisted therapy for depression: A systematic review and meta-analysis. Psychiatry Res. 2023, 329, 115531. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Kopra, E.I.; Cleare, A.J.; Rucker, J.J. Psychedelic therapy for depressive symptoms: A systematic review and meta-analysis. J. Affect. Disord. 2023, 322, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, M.M.; Gukasyan, N.; Raison, C.L. Toward Risk-Benefit Assessments in Psychedelic- and MDMA-Assisted Therapies. JAMA Psychiatry 2022, 79, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Richards, W.; Griffiths, R. Human hallucinogen research: Guidelines for safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Breeksema, J.J.; Kuin, B.W.; Kamphuis, J.; Brink, W.v.D.; Vermetten, E.; Schoevers, R.A. Adverse events in clinical treatments with serotonergic psychedelics and MDMA: A mixed-methods systematic review. J. Psychopharmacol. 2022, 36, 1100–1117. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, J.T.; Graziosi, M.; Nayak, S.M.; Yaden, D.B. Adverse events in studies of classic psychedelics: A systematic review and meta-analysis. JAMA Psychiatry, accept.
- Carbonaro, T.M.; Bradstreet, M.P.; Barrett, F.S.; MacLean, K.A.; Jesse, R.; Johnson, M.W.; Griffiths, R.R. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J. Psychopharmacol. 2016, 30, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Koslowski, M.; Johnson, M.W.; Gründer, G.; Betzler, F. Novel Treatment Approaches for Substance Use Disorders: Therapeutic Use of Psychedelics and the Role of Psychotherapy. Curr. Addict. Rep. 2021, 9, 48–58. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. New Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; dos Santos, R.G.; et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol. Med. 2019, 49, 655–663. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Osório, F.d.L.; Sanches, R.F.; Macedo, L.R.; dos Santos, R.G.; Maia-De-Oliveira, J.P.; Wichert-Ana, L.; de Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A preliminary report. Rev. Bras. Psiquiatr. 2015, 37, 13–20. [Google Scholar] [CrossRef]
- Cuijpers, P.; Berking, M.; Andersson, G.; Quigley, L.; Kleiboer, A.; Dobson, K.S. A Meta-Analysis of Cognitive-Behavioural Therapy for Adult Depression, Alone and in Comparison with other Treatments. Can. J. Psychiatry 2013, 58, 376–385. [Google Scholar] [CrossRef]
- David, D.; Cristea, I.; Hofmann, S.G. Why Cognitive Behavioral Therapy Is the Current Gold Standard of Psychotherapy. Front. Psychiatry 2018, 9, 4. [Google Scholar] [CrossRef]
- Kaczkurkin, A.N.; Foa, E.B. Cognitive-behavioral therapy for anxiety disorders: An update on the empirical evidence. Dialog-Clin. Neurosci. 2015, 17, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Lepping, P.; Whittington, R.; Sambhi, R.; Lane, S.; Poole, R.; Leucht, S.; Cuijpers, P.; McCabe, R.; Waheed, W. Clinical relevance of findings in trials of CBT for depression. Eur. Psychiatry 2017, 45, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, T.J.; Friborg, O. The effects of cognitive behavioral therapy as an anti-depressive treatment is falling: A meta-analysis. Psychol. Bull. 2015, 141, 747–768. [Google Scholar] [CrossRef]
- Button, K.S.; Kounali, D.; Thomas, L.; Wiles, N.J.; Peters, T.J.; Welton, N.J.; Ades, A.E.; Lewis, G. Minimal clinically important difference on the Beck Depression Inventory—II according to the patient’s perspective. Psychol. Med. 2015, 45, 3269–3279. [Google Scholar] [CrossRef] [PubMed]
- Ljótsson, B.; Hedman, E.; Mattsson, S.; Andersson, E. The effects of cognitive–behavioral therapy for depression are not falling: A re-analysis of Johnsen and Friborg (2015). Psychol. Bull. 2017, 143, 321–325. [Google Scholar] [CrossRef]
- Cuijpers, P.; Reijnders, M.; Karyotaki, E.; de Wit, L.; Ebert, D.D. Negative effects of psychotherapies for adult depression: A meta-analysis of deterioration rates. J. Affect. Disord. 2018, 239, 138–145. [Google Scholar] [CrossRef]
- Barlow, D.H. Negative Effects from Psychological Treatments: A Perspective. Am. Psychol. 2010, 65, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Parker, G. The elephant on the couch: Side-effects of psychotherapy. Aust. New Zealand J. Psychiatry 2009, 43, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.; Schermuly-Haupt, M.-L. Definition, assessment and rate of psychotherapy side effects. World Psychiatry 2014, 13, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Moritz, S.; Nestoriuc, Y.; Rief, W.; Klein, J.P.; Jelinek, L.; Peth, J. It can’t hurt, right? Adverse effects of psychotherapy in patients with depression. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 269, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.G.; Kabat-Zinn, J. Mindfulness: Diverse perspectives on its meaning, origins, and multiple applications at the intersection of science and dharma. Contemp. Buddhism 2011, 12, 1–18. [Google Scholar] [CrossRef]
- Wielgosz, J.; Goldberg, S.B.; Kral, T.R.; Dunne, J.D.; Davidson, R.J. Mindfulness Meditation and Psychopathology. Annu. Rev. Clin. Psychol. 2019, 15, 285–316. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B. A common factors perspective on mindfulness-based interventions. Nat. Rev. Psychol. 2022, 1, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Dunning, D.L.; Griffiths, K.; Kuyken, W.; Crane, C.; Foulkes, L.; Parker, J.; Dalgleish, T. Research Review: The effects of mindfulness-based interventions on cognition and mental health in children and adolescents—A meta-analysis of randomized controlled trials. J. Child. Psychol. Psychiatry 2018, 60, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Khoury, B.; Lecomte, T.; Fortin, G.; Masse, M.; Therien, P.; Bouchard, V.; Chapleau, M.-A.; Paquin, K.; Hofmann, S.G. Mindfulness-based therapy: A comprehensive meta-analysis. Clin. Psychol. Rev. 2013, 33, 763–771. [Google Scholar] [CrossRef]
- Goyal, M.; Singh, S.; Sibinga, E.M.S.; Gould, N.F.; Rowland-Seymour, A.; Sharma, R.; Berger, Z.; Sleicher, D.; Maron, D.D.; Shihab, H.M.; et al. Meditation Programs for Psychological Stress and Well-being. JAMA Intern. Med. 2014, 174, 357–368. [Google Scholar] [CrossRef]
- Van Gordon, W.; Shonin, E.; Garcia-Campayo, J. Are there adverse effects associated with mindfulness? Aust. New Zealand J. Psychiatry 2017, 51, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Britton, W.B.; Lindahl, J.R.; Cooper, D.J.; Canby, N.K.; Palitsky, R. Defining and Measuring Meditation-Related Adverse Effects in Mindfulness-Based Programs. Clin. Psychol. Sci. 2021, 9, 1185–1204. [Google Scholar] [CrossRef] [PubMed]
- Selective Serotonin Reuptake Inhibitors—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554406/ (accessed on 27 March 2024).
- Leucht, S.; Hierl, S.; Kissling, W.; Dold, M.; Davis, J.M. Putting the efficacy of psychiatric and general medicine medication into perspective: Review of meta-analyses. Br. J. Psychiatry 2012, 200, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Arroll, B. Efficacy and tolerability of tricyclic antidepressants and ssris compared with placebo for treatment of depression in primary care: A meta-analysis. Ann. Fam. Med. 2005, 3, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, K.; Paludan-Müller, A.S.; Boesen, K. Considering the methodological limitations in the evidence base of antidepressants for depression: A reanalysis of a network meta-analysis. BMJ Open 2019, 9, e024886. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Perlis, C.S.; Wu, Y.; Hwang, C.; Joseph, M.; Nierenberg, A.A. Industry Sponsorship and Financial Conflict of Interest in the Reporting of Clinical Trials in Psychiatry. Am. J. Psychiatry 2005, 162, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, I. Challenging Received Wisdom: Antidepressants and the Placebo Effect. McGill J. Med. 2008, 11, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.C.; Katakam, K.K.; Schou, A.; Hellmuth, S.G.; Stallknecht, S.E.; Leth-Møller, K.; Iversen, M.; Banke, M.B.; Petersen, I.J.; Klingenberg, S.L.; et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry 2017, 17, 58. [Google Scholar] [CrossRef]
- Ferguson, J.M. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim Care Companion J Clin Psychiatry. 2001, 3, 22–27. [Google Scholar] [CrossRef]
- Selective Serotonin Re-Uptake Inhibitors: An Overview—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30439857/ (accessed on 27 March 2024).
- Scheidegger, M.; Henning, A.; Walter, M.; Boeker, H.; Weigand, A.; Seifritz, E.; Grimm, S. Effects of ketamine on cognition–emotion interaction in the brain. NeuroImage 2016, 124, 8–15. [Google Scholar] [CrossRef]
- Fond, G.; Loundou, A.; Rabu, C.; Macgregor, A.; Lançon, C.; Brittner, M.; Micoulaud-Franchi, J.-A.; Richieri, R.; Courtet, P.; Abbar, M.; et al. Ketamine administration in depressive disorders: A systematic review and meta-analysis. Psychopharmacol. 2014, 231, 3663–3676. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.M.; Laws, K.R. The use of ketamine as an antidepressant: A systematic review and meta-analysis. Hum. Psychopharmacol. Clin. Exp. 2015, 30, 152–163. [Google Scholar] [CrossRef]
- Nikolin, S.; Rodgers, A.; Schwaab, A.; Bahji, A.; Zarate, C.; Vazquez, G.; Loo, C. Ketamine for the treatment of major depression: A systematic review and meta-analysis. EClinicalMedicine 2023, 62, 102127. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, N.; Lai, R.; Somogyi, A.; Mitchell, P.B.; Glue, P.; Loo, C.K. Ketamine as a new treatment for depression: A review of its efficacy and adverse effects. Aust. New Zealand J. Psychiatry 2013, 47, 710–727. [Google Scholar] [CrossRef] [PubMed]
- Strong, C.; Kabbaj, M. On the safety of repeated ketamine infusions for the treatment of depression: Effects of sex and developmental periods. Neurobiol. Stress 2018, 9, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, K.A.; Heller, C.Y. Ketamine sensitization: Influence of dose, environment, social isolation and treatment interval. Behav. Brain Res. 2019, 378, 112271. [Google Scholar] [CrossRef] [PubMed]
- van Elk, M.; Fried, E.I. History repeating: Guidelines to address common problems in psychedelic science. Ther. Adv. Psychopharmacol. 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Contradicted and Initially Stronger Effects in Highly Cited Clinical Research. JAMA 2005, 294, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.V.; Horwitz, R.I.; Ioannidis, J.P.A. Empirical Evaluation of Very Large Treatment Effects of Medical Interventions. JAMA 2012, 308, 1676–1684. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Forsyth, A.; Lumley, T. Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev. Clin. Pharmacol. 2021, 14, 1133–1152. [Google Scholar] [CrossRef]
- Whiteford, H.A.; Harris, M.G.; McKeon, G.; Baxter, A.; Pennell, C.; Barendregt, J.J.; Wang, J. Estimating remission from untreated major depression: A systematic review and meta-analysis. Psychol. Med. 2012, 43, 1569–1585. [Google Scholar] [CrossRef] [PubMed]
- Rief, W.; Nestoriuc, Y.; Weiss, S.; Welzel, E.; Barsky, A.J.; Hofmann, S.G. Meta-analysis of the placebo response in antidepressant trials. J. Affect. Disord. 2009, 118, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Button, K.S.; Ioannidis, J.P.A.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.J.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V. Estimation of Effect Size under Nonrandom Sampling: The Effects of Censoring Studies Yielding Statistically Insignificant Mean Differences. J. Educ. Stat. 1984, 9, 61–85. [Google Scholar] [CrossRef]
- Lane, D.M.; Dunlap, W.P. Estimating effect size: Bias resulting from the significance criterion in editorial decisions. Br. J. Math. Stat. Psychol. 1978, 31, 107–112. [Google Scholar] [CrossRef]
- Gelman, A.; Carlin, J. Beyond Power Calculations. Perspect. Psychol. Sci. 2014, 9, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Ciubotariu, I.I.; Bosch, G. Improving research integrity: A framework for responsible science communication. BMC Res. Notes 2022, 15, 177. [Google Scholar] [CrossRef]
- Fage-Butler, A. A values-based approach to knowledge in the public’s representations of climate change on social media. Front. Commun. 2022, 7, 978670. [Google Scholar] [CrossRef]

| Psychedelic Treatments | Cognitive-Behavioral Therapy | Mindfulness Interventions | SSRIs | Ketamine |
|---|---|---|---|---|
| Cohen’s d = 1.46 at day 7 [31] | Hedges’ g = 0.53 at the end of the treatment [44] | Cohen’s d = 0.59 [58] | Cohen’s d = 0.32 at the end of the treatment [65] | Hedges’ g = 1.29 (after 4 h) [75] |
| Cohen’s d = 0.92 at day 14 [31] | Hedges’ g = 1.37 at the end of the treatment [48] | Hedges’ g = 0.53 at the end of the intervention [60] | Cohen’s d = 0.29 [67] a | Hedges’ g = 1.24 (after 24 h) [75] |
| Cohen’s d = 2.21 at week 4 [31] | Cohen’s d = 0.30 at 8 weeks [61] | Hedges’ g = 1.06 (after 7 days) [75] | ||
| Cohen’s d = 2.7 at week 8 [31] | Cohen’s d = 0.23 at 3–6 months [61] | Hedges’ g = 1.67 (after 12–14 days) [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meling, D.; Ehrenkranz, R.; Nayak, S.M.; Aicher, H.D.; Funk, X.; van Elk, M.; Graziosi, M.; Bauer, P.R.; Scheidegger, M.; Yaden, D.B. Mind the Psychedelic Hype: Characterizing the Risks and Benefits of Psychedelics for Depression. Psychoactives 2024, 3, 215-234. https://doi.org/10.3390/psychoactives3020014
Meling D, Ehrenkranz R, Nayak SM, Aicher HD, Funk X, van Elk M, Graziosi M, Bauer PR, Scheidegger M, Yaden DB. Mind the Psychedelic Hype: Characterizing the Risks and Benefits of Psychedelics for Depression. Psychoactives. 2024; 3(2):215-234. https://doi.org/10.3390/psychoactives3020014
Chicago/Turabian StyleMeling, Daniel, Rebecca Ehrenkranz, Sandeep M. Nayak, Helena D. Aicher, Xaver Funk, Michiel van Elk, Marianna Graziosi, Prisca R. Bauer, Milan Scheidegger, and David B. Yaden. 2024. "Mind the Psychedelic Hype: Characterizing the Risks and Benefits of Psychedelics for Depression" Psychoactives 3, no. 2: 215-234. https://doi.org/10.3390/psychoactives3020014
APA StyleMeling, D., Ehrenkranz, R., Nayak, S. M., Aicher, H. D., Funk, X., van Elk, M., Graziosi, M., Bauer, P. R., Scheidegger, M., & Yaden, D. B. (2024). Mind the Psychedelic Hype: Characterizing the Risks and Benefits of Psychedelics for Depression. Psychoactives, 3(2), 215-234. https://doi.org/10.3390/psychoactives3020014






