The Complexity in the Diagnosis and Treatment of Symptoms in Electronic Cigarette Users during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Material and Method
3. E-cigarette Components and Their Health Effects
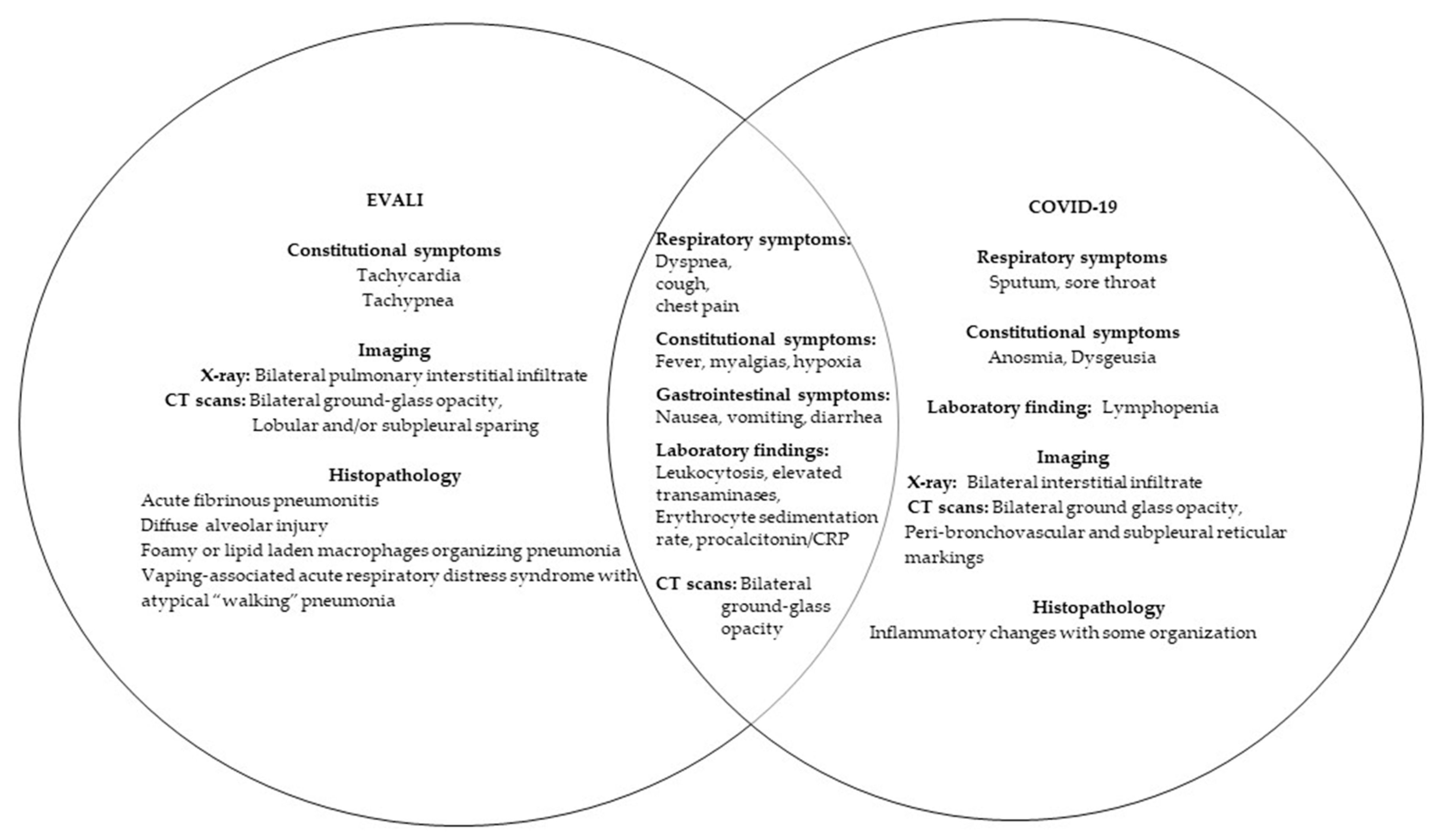
4. Electronic Cigarette Associated Lung Injury in the COVID-19 Pandemic
5. Disease Pathology in E-Cigarettes and COVID-19
6. E-Cigarettes, Microbiome, and COVID-19
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statements
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bals, R.; Boyd, J.; Esposito, S.; Foronjy, R.; Hiemstra, P.S.; Jimenez-Ruiz, C.A.; Katsaounou, P.; Lindberg, A.; Metz, C.; Schober, W.; et al. Electronic cigarettes: A task force report from the European Respiratory Society. Eur. Respir. J. 2019, 53, 1801151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann-Boyce, J.; McRobbie, H.; Bullen, C.; Begh, R.; Stead, L.F.; Hajek, P. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2016, 9, CD010216. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.; Brozek, G.; Lawson, J.; Skoczynski, S.; Zejda, J.E. E-smoking: Emerging public health problem? Int. J. Occup. Med. Env. Health 2017, 30, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoran, S.; Glantz, S.A. E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. Lancet Respir. Med. 2016, 4, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Regan, A.K.; Promoff, G.; Dube, S.R.; Arrazola, R. Electronic nicotine delivery systems: Adult use and awareness of the ’e-cigarette’ in the USA. Tob. Control 2013, 22, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Berry, K.M.; Fetterman, J.L.; Benjamin, E.J.; Bhatnagar, A.; Barrington-Trimis, J.L.; Leventhal, A.M.; Stokes, A. Association of Electronic Cigarette Use With Subsequent Initiation of Tobacco Cigarettes in US Youths. JAMA Netw. Open 2019, 2, e187794. [Google Scholar] [CrossRef] [Green Version]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The rise of e-cigarettes, pod mod devices, and JUUL among youth: Factors influencing use, health implications, and downstream effects. Drug Alcohol. Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef]
- Gomajee, R.; El-Khoury, F.; Goldberg, M.; Zins, M.; Lemogne, C.; Wiernik, E.; Lequy-Flahault, E.; Romanello, L.; Kousignian, I.; Melchior, M. Association Between Electronic Cigarette Use and Smoking Reduction in France. JAMA Intern. Med. 2019, 179, 1193–1200. [Google Scholar] [CrossRef]
- McMillen, R.; Klein, J.D.; Wilson, K.; Winickoff, J.P.; Tanski, S. E-Cigarette Use and Future Cigarette Initiation Among Never Smokers and Relapse Among Former Smokers in the PATH Study. Public Health Rep. 2019, 134, 528–536. [Google Scholar] [CrossRef]
- Kligerman, S.; Raptis, C.; Larsen, B.; Henry, T.S.; Caporale, A.; Tazelaar, H.; Schiebler, M.L.; Wehrli, F.W.; Klein, J.S.; Kanne, J. Radiologic, Pathologic, Clinical, and Physiologic Findings of Electronic Cigarette or Vaping Product Use-associated Lung Injury (EVALI): Evolving Knowledge and Remaining Questions. Radiology 2020, 294, 491–505. [Google Scholar] [CrossRef]
- Sharma, P.; Philpot, L.M.; Rosedahl, J.K.; Jose, T.T.; Ebbert, J.O. Electronic Vaping Product Use among Young Adults Who Receive Care at a Major Medical Institution. Subst. Use Misuse 2021, 56, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Grana, R.A.; Popova, L.; Ling, P.M. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern. Med. 2014, 174, 812–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goniewicz, M.L.; Kuma, T.; Gawron, M.; Knysak, J.; Kosmider, L. Nicotine levels in electronic cigarettes. Nicotine Tob. Res. 2013, 15, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.S.; LeBouf, R.F.; Son, Y.S.; Koutrakis, P.; Christiani, D.C. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Env. Health 2017, 16, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Toxicology of E-Cigarette Constituents. In Public Health Consequences of E-Cigarettes; Eaton, D.L., Kwan, L.Y., Stratton, K., Eds.; National Academies Press (US): Washington, DC, USA, 2018; p. 774. [Google Scholar]
- Calabrese, F.; Pezzuto, F.; Fortarezza, F.; Hofman, P.; Kern, I.; Panizo, A.; von der Thusen, J.; Timofeev, S.; Gorkiewicz, G.; Lunardi, F. Pulmonary pathology and COVID-19: Lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020, 477, 359–372. [Google Scholar] [CrossRef]
- Singal, C.M.S.; Jaiswal, P.; Seth, P. SARS-CoV-2, More than a Respiratory Virus: Its Potential Role in Neuropathogenesis. ACS Chem. Neurosci. 2020, 11, 1887–1899. [Google Scholar] [CrossRef]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med. Sci. Monit. 2020, 26, e928996. [Google Scholar] [CrossRef]
- Wang, Y.; Wong, L.Y.; Meng, L.; Pittman, E.N.; Trinidad, D.A.; Hubbard, K.L.; Etheredge, A.; Del Valle-Pinero, A.Y.; Zamoiski, R.; van Bemmel, D.M.; et al. Urinary concentrations of monohydroxylated polycyclic aromatic hydrocarbons in adults from the U.S. Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Environ. Int. 2019, 123, 201–208. [Google Scholar] [CrossRef]
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382, 697–705. [Google Scholar] [CrossRef]
- Crotty Alexander, L.E.; Ware, L.B.; Calfee, C.S.; Callahan, S.J.; Eissenberg, T.; Farver, C.; Goniewicz, M.L.; Jaspers, I.; Kheradmand, F.; King, T.E.; et al. E-Cigarette or Vaping Product Use-associated Lung Injury: Developing a Research Agenda. An NIH Workshop Report. Am. J. Respir Crit. Care Med. 2020, 202, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.R.; Etchey, B.; Ahmed, M. Explosions, Burn Injuries and Adverse Health Effects of Electronic Nicotine Delivery Systems: A Review of Current Regulations and Future Perspectives. J. Pharm. Pharm. Sci. 2021, 24, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Spyrou, A.; Tsimopoulou, K.; Stefopoulos, C.; Romagna, G.; Voudris, V. Nicotine absorption from electronic cigarette use: Comparison between first and new-generation devices. Sci. Rep. 2014, 4, 4133. [Google Scholar] [CrossRef] [Green Version]
- Lechasseur, A.; Jubinville, E.; Routhier, J.; Berube, J.C.; Hamel-Auger, M.; Talbot, M.; Lamothe, J.; Aubin, S.; Pare, M.E.; Beaulieu, M.J.; et al. Exposure to electronic cigarette vapors affects pulmonary and systemic expression of circadian molecular clock genes. Physiol. Rep. 2017, 5, e13440. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, D.N.; Glantz, S.A. Association of E-Cigarette Use With Respiratory Disease Among Adults: A Longitudinal Analysis. Am. J. Prev. Med. 2020, 58, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Tuder, R.M.; Cool, C.D.; Lynch, D.A.; Flores, S.C.; Voelkel, N.F. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am. J. Respir Crit. Care Med. 2001, 163, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Sheikh, T.; Williams, C. Electronic Vaping Product Use Among Adolescents in the Era of the COVID-19 Pandemic: An Updated Scientific Review for Clinicians. WMJ 2021, 120, 205–208. [Google Scholar]
- Arter, Z.L.; Wiggins, A.; Hudspath, C.; Kisling, A.; Hostler, D.C.; Hostler, J.M. Acute eosinophilic pneumonia following electronic cigarette use. Respir. Med. Case Rep. 2019, 27, 100825. [Google Scholar] [CrossRef]
- Sommerfeld, C.G.; Weiner, D.J.; Nowalk, A.; Larkin, A. Hypersensitivity Pneumonitis and Acute Respiratory Distress Syndrome From E-Cigarette Use. Pediatrics 2018, 141, e20163927. [Google Scholar] [CrossRef]
- Viswam, D.; Trotter, S.; Burge, P.S.; Walters, G.I. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef]
- Ganne, N.; Palraj, R.; Husted, E.; Shah, I. E-cigarette or vaping product use-associated lung injury (EVALI) masquerading as COVID-19. BMJ Case Rep. 2021, 14, e243885. [Google Scholar] [CrossRef] [PubMed]
- Pitlick, M.M.; Lang, D.K.; Meehan, A.M.; McCoy, C.P. EVALI: A Mimicker of COVID-19. Mayo. Clin. Proc. Innov. Qual. Outcomes 2021, 5, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell. 2020, 53, 514–529.e3. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Roa, A.A.; Lemos-Ramirez, J.C. E-Cigarette or Vaping Product Use-Associated Lung Injury (EVALI) Mimicking COVID-19 Disease. Case Rep. Pulmonol. 2020, 2020, 8821289. [Google Scholar] [CrossRef]
- Hoshina, Y. E-cigarette or vaping product use-associated lung injury: A great COVID-19 mimicker in young adult. Clin. Case Rep. 2021, 9, e05016. [Google Scholar] [CrossRef]
- Lilley, J.; Kravitz, S.; Haynes, Z.; Church, T.; McKay, S.; Mertz, A. E-cigarette, or vaping, product use associated lung injury and the risks and benefits of a thorough infectious work-up. Respir. Med. Case Rep. 2021, 33, 101465. [Google Scholar] [CrossRef]
- Adhikari, R.; Koritala, T.; Gotur, R.; Malayala, S.V.; Jain, N.K. EVALI - E-Cigarette or Vaping Product Use-Associated Lung Injury: A Case Report. Cureus 2021, 13, e13541. [Google Scholar] [CrossRef]
- Hassoun, A.; Brady, K.; Arefi, R.; Trifonova, I.; Tsirilakis, K. Vaping-Associated Lung Injury During COVID-19 Multisystem Inflammatory Syndrome Outbreak. J. Emerg. Med. 2021, 60, 524–530. [Google Scholar] [CrossRef]
- Kichloo, A.; Khan, A.; Siddiqui, N.; Ejaz, H.; Albosta, M.S.; Wani, F.; Lone, N. Habit Mimics the Illness: EVALI During the Era of the COVID-19 Pandemic. J. Investig Med. High Impact Case Rep. 2020, 8, 2324709620972243. [Google Scholar] [CrossRef]
- Patel, V.N.; Rouse, M.; Brown, C.; Pandya, S. Ground Glass Opacities Observed in a 26-Year-Old Coronavirus Disease 2019 (COVID-19) Rule-Out Patient With a History of Vape Use. Cureus 2020, 12, e10302. [Google Scholar] [CrossRef] [PubMed]
- Ansari-Gilani, K.; Petraszko, A.M.; Gilkeson, R.C. COVID-19 pneumonia versus EVALI, distinguishing the overlapping CT features in the COVID-19 era. Heart Lung 2020, 49, 885–886. [Google Scholar] [CrossRef] [PubMed]
- Galo, J.; Celli, D.; Gross, D.; Holt, G.; Campos, M. A presentation of E-Cigarette vaping associated lung injury (EVALI) caused by THC-Containing electronic smoking device. Respir Med. Case Rep. 2020, 31, 101154. [Google Scholar] [CrossRef] [PubMed]
- COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 25 January 2022).
- Leung, J.M.; Sin, D.D. Smoking, ACE-2 and COVID-19: Ongoing controversies. Eur. Respir. J. 2020, 56, 2001759. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, K.D.; Eapen, M.S.; Lu, W.; Chia, C.; Haug, G.; Sohal, S.S. COVID-19 and vaping: Risk for increased susceptibility to SARS-CoV-2 infection? Eur. Respir. J. 2020, 56, 2001645. [Google Scholar] [CrossRef]
- McAlinden, K.D.; Eapen, M.S.; Lu, W.; Sharma, P.; Sohal, S.S. The rise of electronic nicotine delivery systems and the emergence of electronic-cigarette-driven disease. Am. J. Physiol Lung Cell Mol. Physiol. 2020, 319, L585–L595. [Google Scholar] [CrossRef]
- Jensen, K.; Nizamutdinov, D.; Guerrier, M.; Afroze, S.; Dostal, D.; Glaser, S. General mechanisms of nicotine-induced fibrogenesis. FASEB J. 2012, 26, 4778–4787. [Google Scholar] [CrossRef] [Green Version]
- Zia, S.; Ndoye, A.; Nguyen, V.T.; Grando, S.A. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res. Commun. Mol. Pathol. Pharm. 1997, 97, 243–262. [Google Scholar]
- Kuba, K.; Imai, Y.; Penninger, J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharm. 2006, 6, 271–276. [Google Scholar] [CrossRef]
- Jia, H.P.; Look, D.C.; Shi, L.; Hickey, M.; Pewe, L.; Netland, J.; Farzan, M.; Wohlford-Lenane, C.; Perlman, S.; McCray, P.B., Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005, 79, 14614–14621. [Google Scholar] [CrossRef] [Green Version]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, G.; Lungarella, G.; Rahman, I. SARS-CoV-2 COVID-19 susceptibility and lung inflammatory storm by smoking and vaping. J. Inflamm. (Lond.) 2020, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Naidu, V.; Zeki, A.A.; Sharma, P. Sex differences in the induction of angiotensin converting enzyme 2 (ACE-2) in mouse lungs after e-cigarette vapor exposure and its relevance to COVID-19. J. Investig. Med. 2021, 69, 954–961. [Google Scholar] [CrossRef]
- Cardinale, A.; Nastrucci, C.; Cesario, A.; Russo, P. Nicotine: Specific role in angiogenesis, proliferation and apoptosis. Crit. Rev. Toxicol. 2012, 42, 68–89. [Google Scholar] [CrossRef]
- Russo, P.; Bonassi, S.; Giacconi, R.; Malavolta, M.; Tomino, C.; Maggi, F. COVID-19 and smoking: Is nicotine the hidden link? Eur. Respir. J. 2020, 55, 2001116. [Google Scholar] [CrossRef]
- Olds, J.L.; Kabbani, N. Is nicotine exposure linked to cardiopulmonary vulnerability to COVID-19 in the general population? FEBS J. 2020, 287, 3651–3655. [Google Scholar] [CrossRef] [Green Version]
- Masso-Silva, J.A.; Moshensky, A.; Shin, J.; Olay, J.; Nilaad, S.; Advani, I.; Bojanowski, C.M.; Crotty, S.; Li, W.T.; Ongkeko, W.M.; et al. Chronic E-Cigarette Aerosol Inhalation Alters the Immune State of the Lungs and Increases ACE2 Expression, Raising Concern for Altered Response and Susceptibility to SARS-CoV-2. Front. Physiol. 2021, 12, 649604. [Google Scholar] [CrossRef]
- Sivaraman, V.; Parker, D.; Zhang, R.; Jones, M.M.; Onyenwoke, R.U. Vaping Exacerbates Coronavirus-Related Pulmonary Infection in a Murine Model. Front. Physiol. 2021, 12, 634839. [Google Scholar] [CrossRef]
- Gilpin, D.F.; McGown, K.A.; Gallagher, K.; Bengoechea, J.; Dumigan, A.; Einarsson, G.; Elborn, J.S.; Tunney, M.M. Electronic cigarette vapour increases virulence and inflammatory potential of respiratory pathogens. Respir. Res. 2019, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, L.; Suri, R.; Dearing, E.; Mudway, I.; Dove, R.E.; Neill, D.R.; Van Zyl-Smit, R.; Kadioglu, A.; Grigg, J. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur. Respir. J. 2018, 51, 1701592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atto, B.; Eapen, M.S.; Sharma, P.; Frey, U.; Ammit, A.J.; Markos, J.; Chia, C.; Larby, J.; Haug, G.; Weber, H.C.; et al. New therapeutic targets for the prevention of infectious acute exacerbations of COPD: Role of epithelial adhesion molecules and inflammatory pathways. Clin. Sci. (Lond.) 2019, 133, 1663–1703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Khan, N.A.; Muthumalage, T.; Lawyer, G.R.; McDonough, S.R.; Chuang, T.D.; Gong, M.; Sundar, I.K.; Rehan, V.K.; Rahman, I. Dysregulated repair and inflammatory responses by e-cigarette-derived inhaled nicotine and humectant propylene glycol in a sex-dependent manner in mouse lung. FASEB Bioadv. 2019, 1, 609–623. [Google Scholar] [CrossRef] [Green Version]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef]
- Merianos, A.L.; Russell, A.M.; Mahabee-Gittens, E.M.; Barry, A.E.; Yang, M.; Lin, H.C. Concurrent use of e-cigarettes and cannabis and associated COVID-19 symptoms, testing, and diagnosis among student e-cigarette users at four U.S. Universities. Addict. Behav. 2021, 107170, 126. [Google Scholar] [CrossRef]
- Callahan, S.J.; Harris, D.; Collingridge, D.S.; Guidry, D.W.; Dean, N.C.; Lanspa, M.J.; Blagev, D.P. Diagnosing EVALI in the Time of COVID-19. Chest 2020, 158, 2034–2037. [Google Scholar] [CrossRef]
- Rai, S.; Hormozdyaran, S.; Burns, J.; Amodio, J.B.; Quizon, A.I. Diagnosis of EVALI in Adolescents During the COVID-19 Pandemic: A Case Series. Hosp. Pediatr. 2022. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal. Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Diaz, C.; Garcia-Orozco, A.; Riera-Leal, A.; Padilla-Arellano, J.R.; Fafutis-Morris, M. Microbiota and Its Role on Viral Evasion: Is It With Us or Against Us? Front. Cell Infect. Microbiol. 2019, 9, 256. [Google Scholar] [CrossRef] [Green Version]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.H.; Fissel, J.A.; Fanelli, B.; Bergman, Y.; Gniazdowski, V.; Dadlani, M.; Carroll, K.C.; Colwell, R.R.; Simner, P.J. Metagenomic Next-Generation Sequencing of Nasopharyngeal Specimens Collected from Confirmed and Suspect COVID-19 Patients. mBio 2020, 11, e01969-20. [Google Scholar] [CrossRef]
- Zhou, H.; Li, C.; Hu, T.; Liu, T.; Ni, N.; Chen, W.; Zhao, H.; Ruan, S.; Li, J.; Wu, H.; et al. Total infectomes of 162 SARS-CoV-2 cases using meta-transcriptomic sequencing. J. Infect. 2021, 82, e44–e48. [Google Scholar] [CrossRef]
- Xu, R.; Lu, R.; Zhang, T.; Wu, Q.; Cai, W.; Han, X.; Wan, Z.; Jin, X.; Zhang, Z.; Zhang, C. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun. Biol. 2021, 4, 240. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S. Gastrointestinal symptoms associated with COVID-19: Impact on the gut microbiome. Transl. Res. 2020, 226, 57–69. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Voidarou, C.; Konstantinidis, T.; Bezirtzoglou, E. Unraveling the Interconnection Patterns Across Lung Microbiome, Respiratory Diseases, and COVID-19. Front. Cell. Infect. Microbiol. 2020, 10, 619075. [Google Scholar] [CrossRef] [PubMed]
- Haiminen, N.; Utro, F.; Seabolt, E.; Parida, L. Functional profiling of COVID-19 respiratory tract microbiomes. Sci. Rep. 2021, 11, 6433. [Google Scholar] [CrossRef]
- Amrock, S.M.; Zakhar, J.; Zhou, S.; Weitzman, M. Perception of e-cigarette harm and its correlation with use among U.S. adolescents. Nicotine Tob. Res. 2015, 17, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol Lung Cell Mol. Physiol. 2017, 313, L278–L292. [Google Scholar] [CrossRef]
- Sassano, M.F.; Davis, E.S.; Keating, J.E.; Zorn, B.T.; Kochar, T.K.; Wolfgang, M.C.; Glish, G.L.; Tarran, R. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018, 16, e2003904. [Google Scholar] [CrossRef]
- Harber, P.; Saechao, K.; Boomus, C. Diacetyl-induced lung disease. Toxicol. Rev. 2006, 25, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Holden, V.K.; Hines, S.E. Update on flavoring-induced lung disease. Curr. Opin Pulm. Med. 2016, 22, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Paul, B.; Li, Q.; Yang, J.; Vasconcelos, R.; Makwana, S.; Gonzalez, J.M.; Shah, S.; Xie, C.; Janal, M.N.; et al. Electronic Cigarette Aerosol Modulates the Oral Microbiome and Increases Risk of Infection. iScience 2020, 23, 100884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haran, J.P.; Bradley, E.; Zeamer, A.L.; Cincotta, L.; Salive, M.C.; Dutta, P.; Mutaawe, S.; Anya, O.; Meza-Segura, M.; Moormann, A.M.; et al. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight 2021, 6, e152346. [Google Scholar] [CrossRef] [PubMed]
- Wolff, L.; Martiny, D.; Deyi, V.Y.M.; Maillart, E.; Clevenbergh, P.; Dauby, N. COVID-19-Associated Fusobacterium nucleatum Bacteremia, Belgium. Emerg. Infect. Dis. 2021, 27, 975–977. [Google Scholar] [CrossRef]
- Holliday, R.; Chaffee, B.W.; Jakubovics, N.S.; Kist, R.; Preshaw, P.M. Electronic Cigarettes and Oral Health. J. Dent. Res. 2021, 100, 906–913. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Renu, K.; Prasanna, P.L.; Valsala Gopalakrishnan, A. Coronaviruses pathogenesis, comorbidities and multi-organ damage—A review. Life Sci. 2020, 255, 117839. [Google Scholar] [CrossRef]
- Dickson, R.P.; Singer, B.H.; Newstead, M.W.; Falkowski, N.R.; Erb-Downward, J.R.; Standiford, T.J.; Huffnagle, G.B. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016, 1, 16113. [Google Scholar] [CrossRef]
- Ganesan, S.M.; Dabdoub, S.M.; Nagaraja, H.N.; Scott, M.L.; Pamulapati, S.; Berman, M.L.; Shields, P.G.; Wewers, M.E.; Kumar, P.S. Adverse effects of electronic cigarettes on the disease-naive oral microbiome. Sci. Adv. 2020, 6, eaaz0108. [Google Scholar] [CrossRef]
- Kazachkov, M.; Pirzada, M. Diagnosis of EVALI in the COVID-19 era. Lancet Respir Med. 2020, 8, 1169–1170. [Google Scholar] [CrossRef]
- Arrazola, R.A.; Singh, T.; Corey, C.G.; Husten, C.G.; Neff, L.J.; Apelberg, B.J.; Bunnell, R.E.; Choiniere, C.J.; King, B.A.; Cox, S.; et al. Tobacco use among middle and high school students - United States, 2011-2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 381–385. [Google Scholar] [PubMed]
- Pokhrel, P.; Fagan, P.; Kehl, L.; Herzog, T.A. Receptivity to e-cigarette marketing, harm perceptions, and e-cigarette use. Am. J. Health Behav. 2015, 39, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Reference | Age/Gender/Medical History | Clinical Presentation | Lab Examination | Chest Radiography | COVID Test- Negative Result |
|---|---|---|---|---|---|
| Rodriguez et al. [36] 10.1155/2020/8821289 | 23 years Male Past history of smoking marijuana in the e-cigarette three times a week. Past history of childhood asthma | Blood pressure: 135/65 mmHg Temperature: 39 °C Pulse Rate: 134 Respiratory rate: 22 Oxygen saturation at room temperature SPO2 (RT): 96% | White blood cells: 15.3 × 103/μL Alanine transaminase (ALT): 69 U/L Aspartate transaminase (AST): 66 U/L Ferritin: 375.6 ng/mL C-reactive protein (CRP): 27.70 mg/dL Procalcitonin: 1.43 ng/mL. | X-ray: Bilateral interstitial infiltrates | 1. Respiratory pathogen panel. 2. SARS-CoV-2 nasopharyngeal swab polymerase chain reaction (PCR) test |
| Hoshina. [37] 10.1002/ccr3.5016 | 20 years Male Past history of occasionally vaping Tetrahydrocannabinol (THC) | Dyspnea Temperature: 37.9 °C Tachycardia Tachypnea SPO2 (RT): 93% Bibasilar crackles | Leukocytosis: 19.0 × 103/μL | Computed tomography (CT): Diffuse, bilateral subsegmental ground-glass opacities | SARS-CoV-2 nasopharyngeal swab PCR test |
| Lilley et al. [38] 10.1016/j.rmcr.2021.101465 | 29 years Female Past history of vaping THC daily for 5 years | Temperature: 38.9 °C Three-day history of watery, non-bloody bowel movements and non-bloody, non-bilious emesis. Tachycardia Sepsis | WBC count: 12,700 cells/mm | X-ray: Mild patchy alveolar opacities bilaterally in the basilar lung fields. Atypical pneumonitis | 1. Autoimmune serologies test. 2. SARS-CoV-2 nasopharyngeal swab PCR test |
| Ganne et al. [32] 10.1136/bcr-2021-243885 | 32 years Female E-cigarette user (Five times per week) History of opioid use, generalized anxiety disorder, and latent tuberculosis | Relapsing fevers Shortness of breath Tachycardia Tachypnea Diaphoretia Anxiety Cough Myalgias Fatigue Pain with inspiration Lower abdominal pain Nausea Vomiting Headache | Decreased air movement in all lobes with scant rhonchi and wheezing Elevated CRP Elevated D-dimer Ferritin Lactate dehydrogenase | X-ray: Diffuse bilateral pulmonary infiltrates in upper and lower lungs. CT scan: Diffuse bilateral pulmonary interstitial and ground-glass opacities | 1. SARS-CoV-2 nasopharyngeal swab PCR test. 2. Total antibody test |
| Pitlick et al. [33] 10.1016/j.mayocpiqo.2021.03.002/ | 34 years Male E-cigarette user with cannabis oil, polysubstance abuse. Past history of depression, anxiety, attention-deficit/hyperactivity disorder, gastroesophageal reflux disease, and hypertension | Shortness of breath Nonproductive cough Pleuritic chest pain Fevers Myalgias Abdominal pain Nausea Headaches | Leukocytosis Neutrophilia Lymphopenia Elevated CRP Elevated D-dimer | X-ray: Asymmetric bilateral patchy basilar opacities. CT scan: Diffuse, midlung-predominant, ill-defined ground-glass opacities with interlobular septal thickening | SARS-CoV-2 nasopharyngeal swab PCR test |
| Pitlick et al. [33] 10.1016/j.mayocpiqo.2021.03.002/ | 47 years Male History of vaping with cannabis oil several times daily in the weeks before admission | Fever Nonproductive cough Myalgias Malaise Nausea Diarrhea | Leukocytosis Lymphopenia Elevated CRP Elevated platelets Elevated D-dimer | X-ray: Bilateral patchy airspace opacities. CT scan: Extensive bilateral ground-glass opacities in a predominantly central distribution with associated interlobular septal thickening | 1. SARS-CoV-2 nasopharyngeal swab PCR test. 2. Respiratory pathogen panel |
| Pitlick et al. [33] 10.1016/j.mayocpiqo.2021.03.002/ | 20 years Male History of vaping tetrahydrocannabinol products | Shortness of breath Nonproductive cough Nausea Temperature: 38.4 °C Tachycardia (heart rate:110 beats/min) | Mild leukocytosis Lymphopenia Elevated CRP Elevated D-dimer | CT scan: Diffuse bilateral ground-glass opacities with a peripheral predominance | SARS-CoV-2 nasopharyngeal swab PCR test |
| Adhikari et al. [39] 10.7759/cureus.13541 | 23 years Male Vaping history of 8 years, immunocompetent | Fever Shortness of breath Tachypnea Nausea Diarrhea | Leukocytosis Lymphopenia Elevated procalcitonin Elevated erythrocyte sedimentation rate (ESR) Elevated CRP | X-ray: Bilateral pneumonia CT scan: Bilateral lung infiltrates | |
| Hassoun et al. [40] 10.1016/j.jemermed.2020.12.005 | 19 years Female Vaping tetrahydrocannabinol (THC) | Abdominal pain Vomiting Diarrhea Fevers Shortness of breath Vomiting Transient oxygen desaturation: 92% Tachycardia Tachypnea Diffuse abdominal tenderness | Ketonuria Elevated WBC count Neutrophilia Elevated CRP Elevated ESR Elevated D-dimer Elevated fibrinogen | X-ray: Mild left perihilar opacity. CT scan: Bilateral ground-glass opacities, with consolidation in the left lower lobe | 1. Autoimmune serologies test. 2. SARS-CoV-2 nasopharyngeal swab PCR test |
| Hassoun et al. [40] 10.1016/j.jemermed.2020.12.005 | 19 years Male History of vaping nicotine and THC oils | Nausea Lightheadedness Anorexia Mild sore throat Cough Fever | Elevated WBC Elevated ESR Elevated CRP | CT scan: Bilateral ground-glass opacities with reticulation and interlobular septal thickening, most prominently at the right lower lobe and left lingula | 1. SARS-CoV-2 nasopharyngeal swab PCR test 2. Group A streptococcus test was positive |
| Hassoun et al. [40] 10.1016/j.jemermed.2020.12.005 | 21 years Male | Febrile Tachycardia Tachypnea Hypoxic | Slightly elevated WBC Markedly elevated CRP Elevated ESR Elevated fibrinogen | CT scan: Bilateral subtle scattered ground-glass opacities. CT scan: Possible airway inflammation | 1. Autoimmune serologies test. 2. SARS-CoV-2 nasopharyngeal swab PCR test |
| Kichloo et al. [41] 10.1177/2324709620972243 | 31 years Male Binge smoking disposable E-cigarette pods Vaping nicotine, tetrahydrocannabinol and cannabidiol Medical history of Crohn’s disease | Fever Fatigue Dry cough Dyspnea Tachycardia SpO2 (RT): 86% | Elevated serum ACE Elevated lactate dehydrogenase (LDH) | X-ray: Faint bilateral pulmonary interstitial opacities. CT scan: Diffuse ground-glass opacities | SARS-CoV-2 nasopharyngeal swab PCR test |
| Patel et al. [42]10.7759/cureus.10302 | 26 years Female History of daily marijuana vaping use | Idiopathic chronic abdominal pain Diarrhea Cough Acute onset nausea Vomiting Abdominal pain Afebrile SPO2 (RT): 99% Tachycardia | Anion gap metabolic acidosis Elevated lactate Mild hyponatremia Leukocytosis Lymphopenia | CT scan: Ground glass opacities in bilateral lung bases | SARS-CoV-2 nasopharyngeal swab PCR test |
| Ansari-Gilani et al. [43] 10.1016/j.hrtlng.2020.06.008 | 37 years Male History of marijuana vape use | Fever Cough Shortness of breath SPO2 (RT): 92% | CT scan: Extensive ground-glass opacities in both lungs with slight lower lung predominance | SARS-CoV-2 nasopharyngeal swab PCR test | |
| Galo et al. [44] 10.1016/j.rmcr.2020.101154 | 36 years Male History of vaping THC-containing e-cigarette, irritable bowel syndrome and major depressive disorder | Worsening dyspnea on exertion Shortness of breath Cough Myalgias Fever Desaturation Hypoxemic SPO2 (RT): 86% | Elevated CRP: 269 mg/L. Bronchoscopy lavage indicated clusters of small foamy macrophage with intracellular lipid droplets | X-ray: Indeterminate infiltrate lungs, X-ray: Bilateral peripheral and basilar ground-glass opacities with mediastinal adenopathy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.R.; Ahmed, M. The Complexity in the Diagnosis and Treatment of Symptoms in Electronic Cigarette Users during the COVID-19 Pandemic. Pharmacoepidemiology 2022, 1, 49-63. https://doi.org/10.3390/pharma1020006
Ahmed AR, Ahmed M. The Complexity in the Diagnosis and Treatment of Symptoms in Electronic Cigarette Users during the COVID-19 Pandemic. Pharmacoepidemiology. 2022; 1(2):49-63. https://doi.org/10.3390/pharma1020006
Chicago/Turabian StyleAhmed, Ayesha Rahman, and Mahiba Ahmed. 2022. "The Complexity in the Diagnosis and Treatment of Symptoms in Electronic Cigarette Users during the COVID-19 Pandemic" Pharmacoepidemiology 1, no. 2: 49-63. https://doi.org/10.3390/pharma1020006
APA StyleAhmed, A. R., & Ahmed, M. (2022). The Complexity in the Diagnosis and Treatment of Symptoms in Electronic Cigarette Users during the COVID-19 Pandemic. Pharmacoepidemiology, 1(2), 49-63. https://doi.org/10.3390/pharma1020006





