Advancements in Peripheral Nerve Injury Research Using Lab Animals
Abstract
1. Introduction
2. Selection of Peripheral Nerve Model in Animal Studies
2.1. Sciatic Nerve
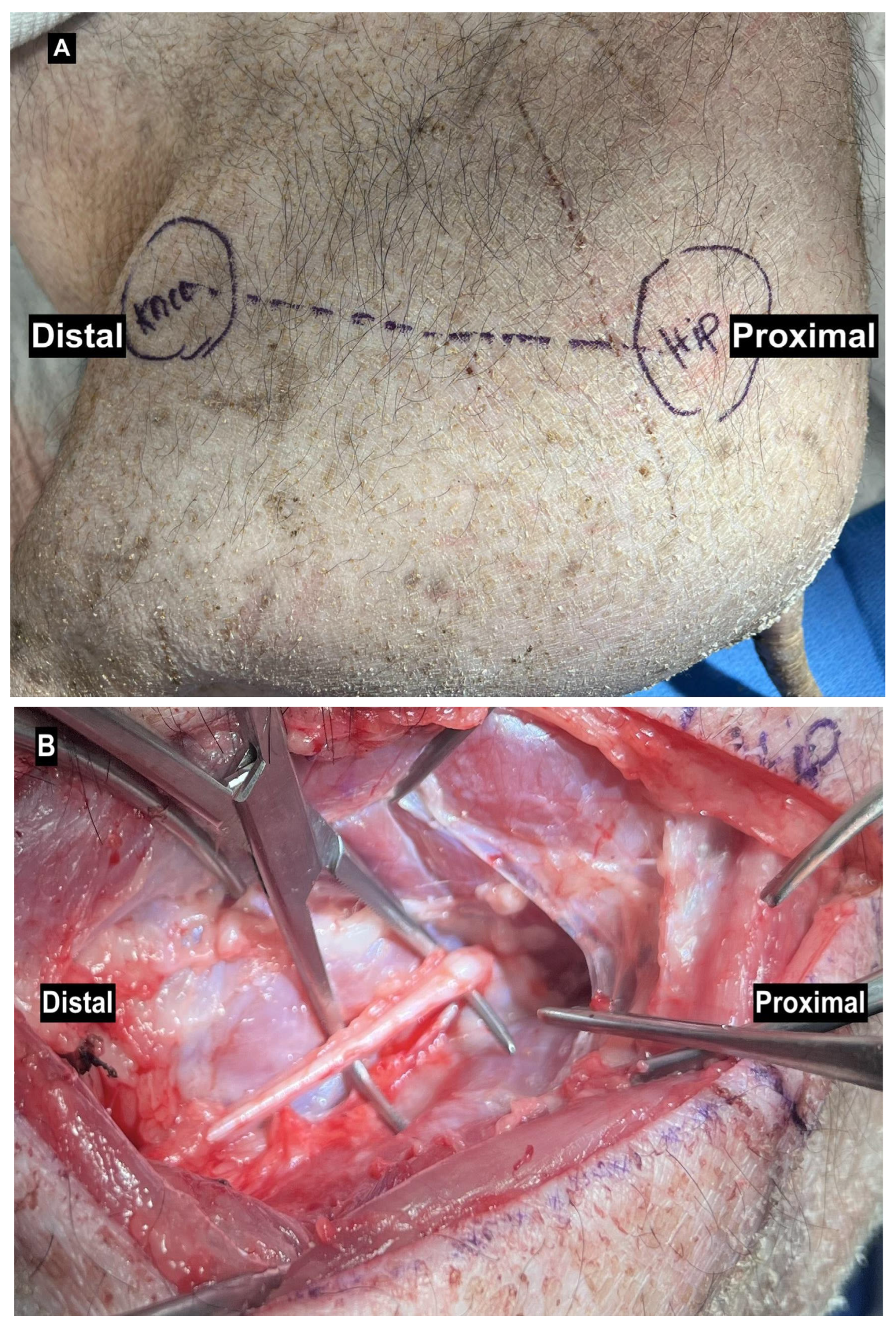
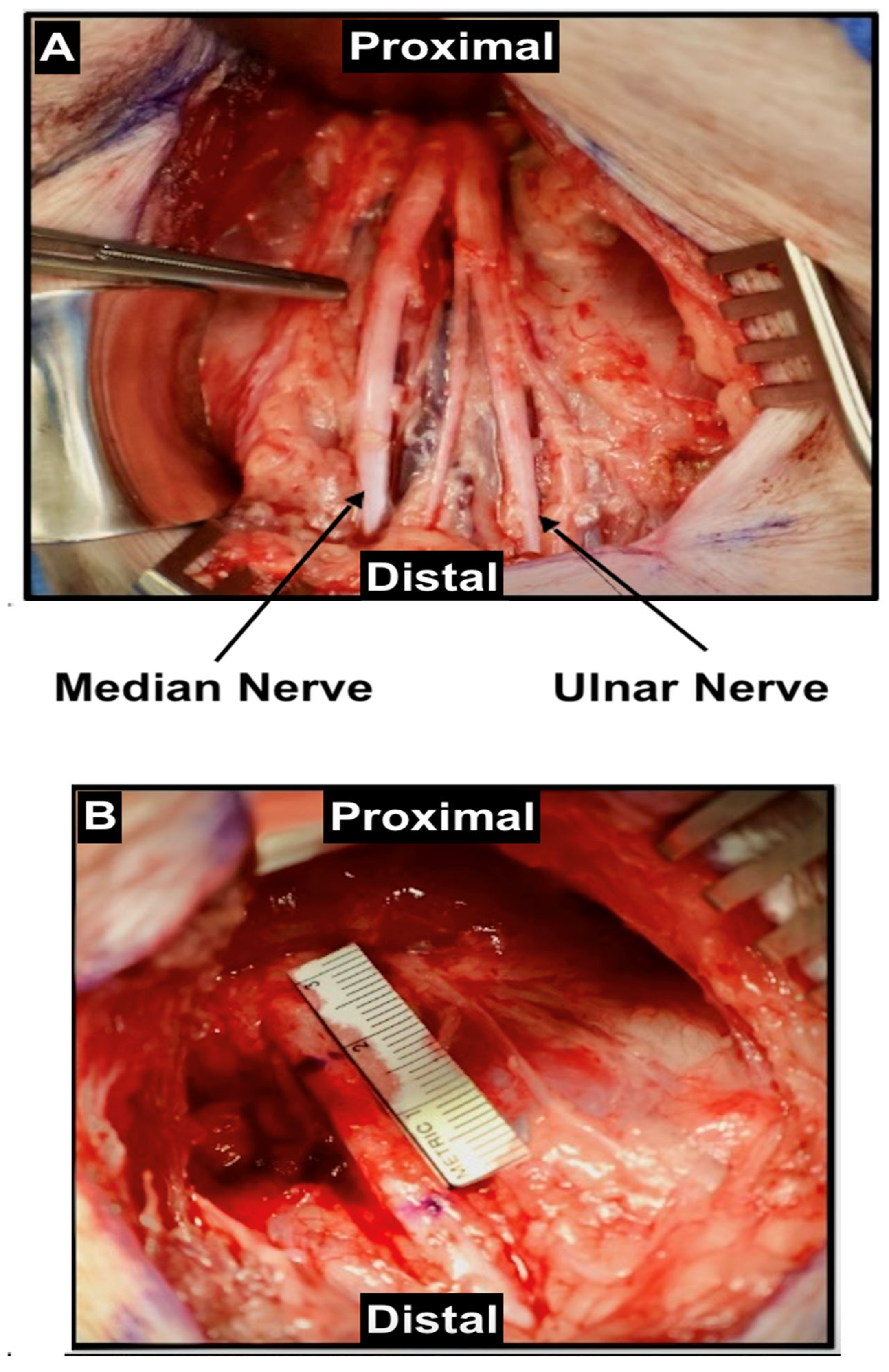
2.2. Median Nerve
2.3. Radial Nerve
2.4. Ulnar Nerve
2.5. Tibial and Peroneal Nerve
2.6. Facial Nerve
2.7. Trigeminal Nerve
2.8. Impact of Nerve Selection on Translatability
3. Species Selection for Peripheral Nerve Injury
3.1. Non-Mammalian Species
3.2. Small Animal Models
3.3. Large Animal Models
4. Methods of Peripheral Nerve Induced Injury
4.1. Crush Injury Model
4.2. Transection Model
4.3. Comparison Between Crush and Transection Models
4.4. Chronic Constriction/Ligation Model
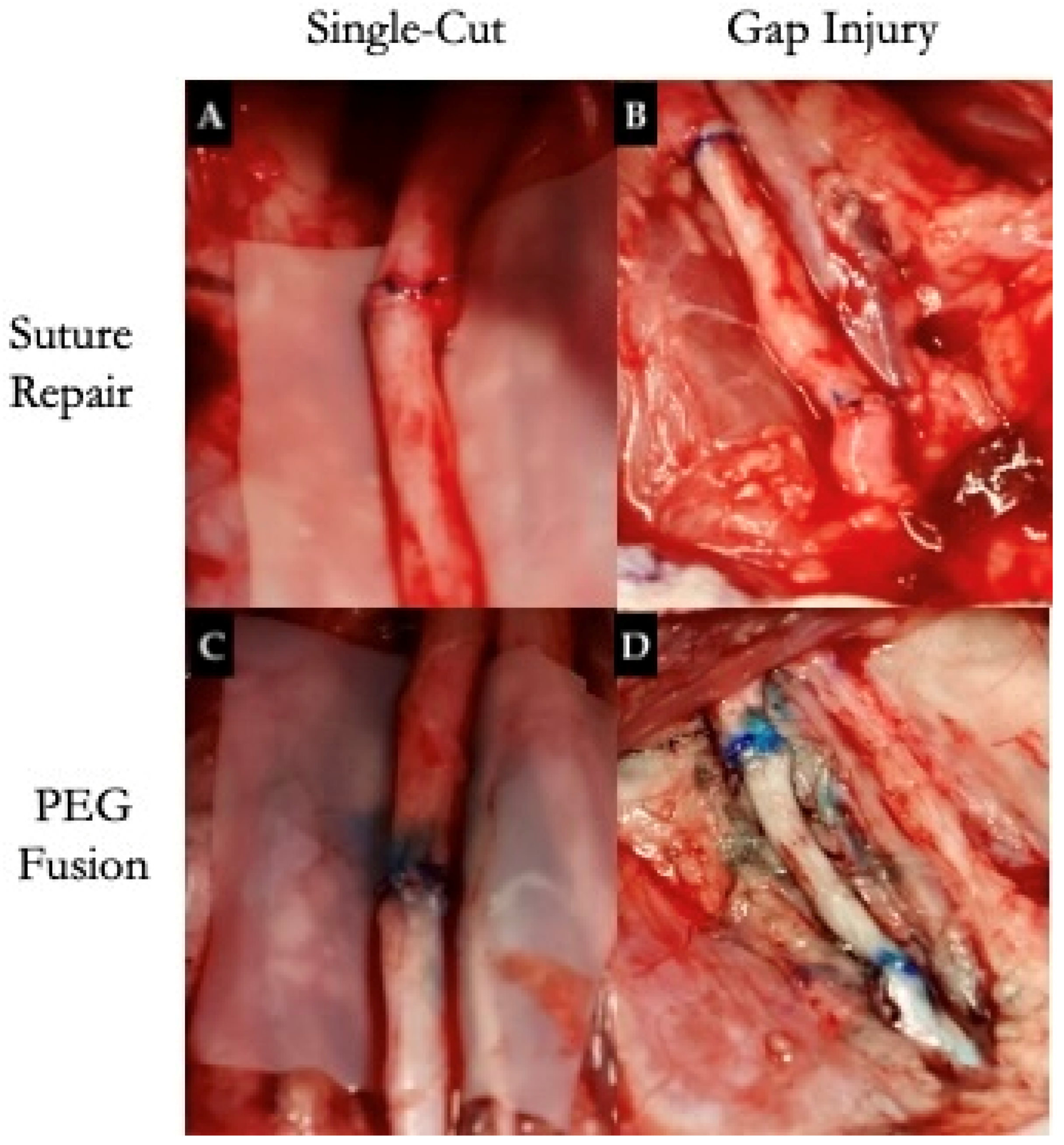
4.5. Epineurium-Preserving Injury Model
4.6. Chemical Injury Model
4.7. Ischemia-Reperfusion Injury/Tourniquet-Induced Injury Models
5. Evaluation of Peripheral Nerve Injuries in Animals
5.1. Histomorphological and Microscopic Assessments
5.2. Neuromuscular Functional Assessments
5.3. Advanced Imaging Techniques
5.4. Pain Assessments
6. Influence of Age and Sex on Animal Models
7. Therapeutic Strategies for Nerve Regeneration Informed by Animal Models
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PNI | Peripheral Nerve Injury |
| PN | Peripheral nerve |
| C. elegans, nematode | Caenorhabditis elegans |
| R. pipiens, northern leopard frog | Rana pipiens |
| LPC | Lysophosphatidylcholine |
| CMAPs | Compound muscle action potentials |
| NCV | Motor nerve conduction velocity |
| NGF | Nerve growth factor |
| MRI | Magnetic resonance imaging |
| MTR | Magnetization transfer ratio |
| DTI | Magnetic resonance diffusion tensor imaging |
| FA | Fractional anisotropy |
| ADC | Apparent diffusion coefficient |
| TMR | Targeted muscle reinnervation |
| PEG fusion | Polyethylene glycol-mediated fusion |
| GRG | Regenerative gel |
| AGRG | Antigliotic regenerative gel |
References
- Burrell, J.C.; Browne, K.D.; Dutton, J.L.; Laimo, F.A.; Das, S.; Brown, D.P.; Roberts, S.; Petrov, D.; Ali, Z.; Ledebur, H.C.; et al. A Porcine Model of Peripheral Nerve Injury Enabling Ultra-Long Regenerative Distances: Surgical Approach, Recovery Kinetics, and Clinical Relevance. Neurosurgery 2020, 87, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Muratori, L.; Ronchi, G.; Raimondo, S.; Giacobini-Robecchi, M.G.; Fornaro, M.; Geuna, S. Can Regenerated Nerve Fibers Return to Normal Size? A Long-term Post-traumatic Study of the Rat Median Nerve Crush Injury Model. Microsurgery 2012, 32, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Siwei, Q.; Ma, N.; Wang, W.; Chen, S.; Wu, Q.; Li, Y.; Yang, Z. Construction and Effect Evaluation of Different Sciatic Nerve Injury Models in Rats. Transl. Neurosci. 2022, 13, 38–51. [Google Scholar] [CrossRef]
- Jones, P.E.; Meyer, R.M.; Faillace, W.J.; Landau, M.E.; Smith, J.K.; McKay, P.L.; Nesti, L.J. Combat Injury of the Sciatic Nerve–An Institutional Experience. Mil. Med. 2018, 183, e434–e441. [Google Scholar] [CrossRef]
- An, Y.; Yan, H.-X.; Zhao, J.-N.; Yang, X.-M.; Yan, J.-T. Evaluation Methods of a Rat Sciatic Nerve Crush Injury Model. J. Integr. Neurosci. 2022, 21, 91. [Google Scholar] [CrossRef]
- Casañas, J.; Torre, J.D.L.; Soler, F.; García, F.; Rodellar, C.; Pumarola, M.; Climent, J.; Soler, R.; Orozco, L. Peripheral Nerve Regeneration after Experimental Section in Ovine Radial and Tibial Nerves Using Synthetic Nerve Grafts, Including Expanded Bone Marrow Mesenchymal Cells: Morphological and Neurophysiological Results. Injury 2014, 45, S2–S6. [Google Scholar] [CrossRef]
- Fang, Y.; Bonini, N.M. Axon Degeneration and Regeneration: Insights from Drosophila Models of Nerve Injury. Annu. Rev. Cell Dev. Biol. 2012, 28, 575–597. [Google Scholar] [CrossRef]
- Bhattacharya, M.R.C. A Nerve-Wracking Buzz: Lessons from Drosophila Models of Peripheral Neuropathy and Axon Degeneration. Front. Aging Neurosci. 2023, 15, 1166146. [Google Scholar] [CrossRef]
- Vest, M.; Guida, A.; Colombini, C.; Cordes, K.; Pena, D.; Maki, M.; Briones, M.; Antonio, S.; Hollifield, C.; Tian, E.; et al. Closing the Gap Between Mammalian and Invertebrate Peripheral Nerve Injury: Protocol for a Novel Nerve Repair. JMIR Res. Protoc. 2020, 9, e18706. [Google Scholar] [CrossRef]
- Blanco, R.E.; Rosado, J.; Padilla, J.; Del Cueto, C. Ultrastructural Studies of Dorsal Root Axons Regenerating through Adult Frog Optic and Sciatic Nerves. Microsc. Res. Tech. 1999, 46, 310–318. [Google Scholar] [CrossRef]
- Soares, L.; Parisi, M.; Bonini, N.M. Axon Injury and Regeneration in the Adult Drosophila. Sci. Rep. 2014, 4, 6199. [Google Scholar] [CrossRef] [PubMed]
- DeLeonibus, A.; Rezaei, M.; Fahradyan, V.; Silver, J.; Rampazzo, A.; Bassiri Gharb, B. A META-ANALYSIS of Functional Outcomes in Rat Sciatic Nerve Injury Models. Microsurgery 2021, 41, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Diogo, C.C.; Camassa, J.A.; Pereira, J.E.; Costa, L.M.D.; Filipe, V.; Couto, P.A.; Geuna, S.; Maurício, A.C.; Varejão, A.S. The Use of Sheep as a Model for Studying Peripheral Nerve Regeneration Following Nerve Injury: Review of the Literature. Neurol. Res. 2017, 39, 926–939. [Google Scholar] [CrossRef]
- Roballo, K.C.S.; Burns, D.T.; Ghnenis, A.B.; Osimanjiang, W.; Bushman, J.S. Long-term Neural Regeneration Following Injury to the Peroneal Branch of the Sciatic Nerve in Sheep. Eur. J. Neurosci. 2020, 52, 4385–4394. [Google Scholar] [CrossRef]
- Guo, N.; Gu, X. Sciatic Nerve Neuropathy in Cynomolgus Monkey Macaca Fascicularis: Altered Leg Usage and Peripheral Nerve Firing. J. Neurol. Neurophysiol. 2014, 05, 1000247. [Google Scholar] [CrossRef]
- Angius, D.; Wang, H.; Spinner, R.J.; Gutierrez-Cotto, Y.; Yaszemski, M.J.; Windebank, A.J. A Systematic Review of Animal Models Used to Study Nerve Regeneration in Tissue-Engineered Scaffolds. Biomaterials 2012, 33, 8034–8039. [Google Scholar] [CrossRef]
- Merolli, A.; Li, M.; Voronin, G.; Bright, L. A Sciatic Nerve Gap-Injury Model in the Rabbit. J. Mater. Sci. Mater. Med. 2022, 33, 14. [Google Scholar] [CrossRef]
- Drysch, M.; Wallner, C.; Schmidt, S.V.; Reinkemeier, F.; Wagner, J.M.; Lehnhardt, M.; Behr, B. An Optimized Low-Pressure Tourniquet Murine Hind Limb Ischemia Reperfusion Model: Inducing Acute Ischemia Reperfusion Injury in C57BL/6 Wild Type Mice. PLoS ONE 2019, 14, e0210961. [Google Scholar] [CrossRef]
- Rafee, M.; Amarpal; Kinjavdekar, P.; Aithal, H.; Wani, S.; Bhat, I. Guinea Pigs as an Animal Model for Sciatic Nerve Injury. Neural Regen. Res. 2017, 12, 452. [Google Scholar] [CrossRef]
- Wang, B.B.; Guo, C.; Sun, S.Q.; Zhang, X.N.; Li, Z.; Li, W.J.; Li, D.Z.; Schumacher, M.; Liu, S. Comparison of the Nerve Regeneration Capacity and Characteristics between Sciatic Nerve Crush and Transection Injury Models in Rats. Biomed. Environ. Sci. 2023, 36, 160–173. [Google Scholar] [CrossRef]
- Yayama, T.; Kobayashi, S.; Nakanishi, Y.; Uchida, K.; Kokubo, Y.; Miyazaki, T.; Takeno, K.; Awara, K.; Mwaka, E.S.; Iwamoto, Y.; et al. Effects of Graded Mechanical Compression of Rabbit Sciatic Nerve on Nerve Blood Flow and Electrophysiological Properties. J. Clin. Neurosci. 2010, 17, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Kotwal, P.P.; Farooque, M.; Dinda, A.K. Muscle Autografts in Nerve Gaps. Pattern of Regeneration and Myelination in Various Lengths of Graft: An Experimental Study in Guinea Pigs. J. Orthop. Sci. 2001, 6, 527–534. [Google Scholar] [CrossRef]
- Ding, F.; Wu, J.; Yang, Y.; Hu, W.; Zhu, Q.; Tang, X.; Liu, J.; Gu, X. Use of Tissue-Engineered Nerve Grafts Consisting of a Chitosan/Poly(Lactic- Co. -Glycolic Acid)-Based Scaffold Included with Bone Marrow Mesenchymal Cells for Bridging 50-Mm Dog Sciatic Nerve Gaps. Tissue Eng. Part A 2010, 16, 3779–3790. [Google Scholar] [CrossRef] [PubMed]
- Kaemmer, D.; Bozkurt, A.; Otto, J.; Junge, K.; Klink, C.; Weis, J.; Sellhaus, B.; O’Dey, D.M.; Pallua, N.; Jansen, M.; et al. Evaluation of Tissue Components in the Peripheral Nervous System Using Sirius Red Staining and Immunohistochemistry: A Comparative Study (Human, Pig, Rat). J. Neurosci. Methods 2010, 190, 112–116. [Google Scholar] [CrossRef]
- Zilic, L.; Garner, P.E.; Yu, T.; Roman, S.; Haycock, J.W.; Wilshaw, S. An Anatomical Study of Porcine Peripheral Nerve and Its Potential Use in Nerve Tissue Engineering. J. Anat. 2015, 227, 302–314. [Google Scholar] [CrossRef]
- Geuna, S. The Sciatic Nerve Injury Model in Pre-Clinical Research. J. Neurosci. Methods 2015, 243, 39–46. [Google Scholar] [CrossRef]
- Kaplan, H.M.; Mishra, P.; Kohn, J. The Overwhelming Use of Rat Models in Nerve Regeneration Research May Compromise Designs of Nerve Guidance Conduits for Humans. J. Mater. Sci. Mater. Med. 2015, 26, 226. [Google Scholar] [CrossRef]
- Li, A.; Pereira, C.; Hill, E.E.; Vukcevich, O.; Wang, A. In Vitro, In Vivo and Ex Vivo Models for Peripheral Nerve Injury andRegeneration. Curr. Neuropharmacol. 2022, 20, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, G.; Morano, M.; Fregnan, F.; Pugliese, P.; Crosio, A.; Tos, P.; Geuna, S.; Haastert-Talini, K.; Gambarotta, G. The Median Nerve Injury Model in Pre-Clinical Research–A Critical Review on Benefits and Limitations. Front. Cell. Neurosci. 2019, 13, 288. [Google Scholar] [CrossRef]
- O’Daly, A.; Rohde, C.; Brushart, T. The Topographic Specificity of Muscle Reinnervation Predicts Function. Eur. J. Neurosci. 2016, 43, 443–450. [Google Scholar] [CrossRef]
- Wang, D.; Huang, X.; Fu, G.; Gu, L.; Liu, X.; Wang, H.; Hu, J.; Yi, J.; Niu, X.; Zhu, Q. A Simple Model of Radial Nerve Injury in the Rhesus Monkey to Evaluate Peripheral Nerve Repair. Neural Regen. Res. 2014, 9, 1041–1046. [Google Scholar] [CrossRef]
- Scholz, T.; Pharaon, M.; Evans, G.R.D. Peripheral Nerve Anatomy for Regeneration Studies in Pigs: Feasibility of Large Animal Models. Ann. Plast. Surg. 2010, 65, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, Y.-L.; Niu, S.-P.; Zhang, P.-X.; Yin, X.-F.; Han, N.; Zhang, Y.-J.; Zhang, D.-Y.; Kou, Y.-H.; Jiang, B.-G. Repair of Long Segmental Ulnar Nerve Defects in Rats by Several Different Kinds of Nerve Transposition. Neural Regen. Res. 2019, 14, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.C.; Granja, R.; Solorzano, B.R.; Romero-Ortega, M.; Kilgard, M.P.; Rennaker, R.L.; Hays, S. Median and Ulnar Nerve Injuries Reduce Volitional Forelimb Strength in Rats. Muscle Nerve 2017, 56, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Bergmeister, K.D.; Aman, M.; Riedl, O.; Manzano-Szalai, K.; Sporer, M.E.; Salminger, S.; Aszmann, O.C. Experimental Nerve Transfer Model in the Rat Forelimb. Eur. Surg. 2016, 48, 334–341. [Google Scholar] [CrossRef]
- Vela, F.; Martínez-Chacón, G.; Ballestín, A.; Campos, J.; Sánchez-Margallo, F.; Abellán, E. Animal Models Used to Study Direct Peripheral Nerve Repair: A Systematic Review. Neural Regen. Res. 2020, 15, 491–502. [Google Scholar] [CrossRef]
- Contreras, E.; Traserra, S.; Bolívar, S.; Forés, J.; Jose-Cunilleras, E.; Delgado-Martínez, I.; García, F.; Udina, E.; Navarro, X. Repair of Long Peripheral Nerve Defects in Sheep: A Translational Model for Nerve Regeneration. Int. J. Mol. Sci. 2023, 24, 1333. [Google Scholar] [CrossRef]
- Lin, H.; Chen, D.; Hou, C. Common Peroneal Nerve Grafting to Repair the Tibial Nerve as a Salvage Procedure in the Treatment of Sciatic Nerve Injury with Long-Segment Defects. J. Reconstr. Microsurg. Open 2018, 03, e41–e45. [Google Scholar] [CrossRef]
- Zhang, S.; Han, G.; Xiong, Y.; Wang, Z.; Wang, Z.; Lai, X. Characteristics and Mechanism of Lower Limb Injury Induced by Landmine Blast: A Research in a Rabbit Model. Ulus. Travma Acil Cerrahi Derg. 2023, 29, 1335–1343. [Google Scholar] [CrossRef]
- Ali, S.A.; Stebbins, A.W.; Hanks, J.E.; Kupfer, R.A.; Hogikyan, N.D.; Feldman, E.L.; Brenner, M.J. Facial Nerve Surgery in the Rat Model to Study Axonal Inhibition and Regeneration. JoVE 2020, 59224. [Google Scholar] [CrossRef]
- Hadlock, T.; Kowaleski, J.; Lo, D.; Bermejo, R.; Zeigler, H.P.; Mackinnon, S.; Heaton, J.T. Functional Assessments of the Rodent Facial Nerve: A Synkinesis Model. Laryngoscope 2008, 118, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.-S.; Zhang, T.; Zuo, C.-X.; Zuo, Z.-F.; Li, H.; Wu, S.-X.; Wang, W.; Li, Y.-Q. An Animal Model for Trigeminal Neuralgia by Compression of the Trigeminal Nerve Root. Pain. Physician 2012, 15, 187–196. [Google Scholar]
- Harriott, A.M.; Strother, L.C.; Vila-Pueyo, M.; Holland, P.R. Animal Models of Migraine and Experimental Techniques Used to Examine Trigeminal Sensory Processing. J. Headache Pain. 2019, 20, 91. [Google Scholar] [CrossRef]
- Brace, E.J.; DiAntonio, A. Models of Axon Regeneration in Drosophila. Exp. Neurol. 2017, 287 Pt 3, 310–317. [Google Scholar] [CrossRef]
- Bremer, J.; Skinner, J.; Granato, M. A Small Molecule Screen Identifies in Vivo Modulators of Peripheral Nerve Regeneration in Zebrafish. PLoS ONE 2017, 12, e0178854. [Google Scholar] [CrossRef]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish Models for Translational Neuroscience Research: From Tank to Bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef]
- Cirrincione, A.M.; Rieger, S. Analyzing Chemotherapy-Induced Peripheral Neuropathy in Vivo Using Non-Mammalian Animal Models. Exp. Neurol. 2020, 323, 113090. [Google Scholar] [CrossRef]
- Vega-Meléndez, G.S.; Blagburn, J.M.; Blanco, R.E. Ciliary Neurotrophic Factor and Fibroblast Growth Factor Increase the Speed and Number of Regenerating Axons after Optic Nerve Injury in Adult Rana Pipiens. J. Neurosci. Res. 2014, 92, 13–23. [Google Scholar] [CrossRef]
- Gordon, T.; Borschel, G.H. The Use of the Rat as a Model for Studying Peripheral Nerve Regeneration and Sprouting after Complete and Partial Nerve Injuries. Exp. Neurol. 2017, 287, 331–347. [Google Scholar] [CrossRef]
- Mazzer, P.Y.C.N.; Barbieri, C.H.; Mazzer, N.; Fazan, V.P.S. Morphologic and Morphometric Evaluation of Experimental Acute Crush Injuries of the Sciatic Nerve of Rats. J. Neurosci. Methods 2008, 173, 249–258. [Google Scholar] [CrossRef]
- Medeiros, P.; Dos Santos, I.R.; Júnior, I.M.; Palazzo, E.; Da Silva, J.A.; Machado, H.R.; Ferreira, S.H.; Maione, S.; Coimbra, N.C.; De Freitas, R.L. An Adapted Chronic Constriction Injury of the Sciatic Nerve Produces Sensory, Affective, and Cognitive Impairments: A Peripheral Mononeuropathy Model for the Study of Comorbid Neuropsychiatric Disorders Associated with Neuropathic Pain in Rats. Pain. Med. 2021, 22, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Shim, S.W.; Zhao, A.M.; Roh, D.; Han, H.M.; Middleton, S.J.; Kim, W.; Chung, S.; Johnson, E.; Prentice, J.; et al. Long-Term Tactile Hypersensitivity after Nerve Crush Injury in Mice Is Characterized by the Persistence of Intact Sensory Axons. Pain 2023, 164, 2327–2342. [Google Scholar] [CrossRef]
- Umansky, D.; Hagen, K.M.; Chu, T.H.; Pathiyil, R.K.; Alzahrani, S.; Ousman, S.S.; Midha, R. Functional Gait Assessment Using Manual, Semi-Automated and Deep Learning Approaches Following Standardized Models of Peripheral Nerve Injury in Mice. Biomolecules 2022, 12, 1355. [Google Scholar] [CrossRef]
- Hammers, D.W.; Matheny, R.W.; Sell, C.; Adamo, M.L.; Walters, T.J.; Estep, J.S.; Farrar, R.P. Impairment of IGF-I Expression and Anabolic Signaling Following Ischemia/Reperfusion in Skeletal Muscle of Old Mice. Exp. Gerontol. 2011, 46, 265–272. [Google Scholar] [CrossRef]
- Bonheur, J.A.; Albadawi, H.; Patton, G.M.; Watkins, M.T. A Noninvasive Murine Model of Hind Limb Ischemia-Reperfusion Injury. J. Surg. Res. 2004, 116, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Baugher, W.H.; Stamp, W.G. Epiphyseal Transplant in Amputations. Effects on Overgrowth in a Rabbit Model. Clin. Orthop. Relat. Res. 1978, 285–288. [Google Scholar]
- Huang, H.; Chen, L.; Zhang, H.; Li, S.; Liu, P.; Zhao, T.; Li, C. Autophagy Promotes Peripheral Nerve Regeneration and Motor Recovery Following Sciatic Nerve Crush Injury in Rats. J. Mol. Neurosci. 2016, 58, 416–423. [Google Scholar] [CrossRef]
- Kosacka, J.; Nowicki, M.; Blüher, M.; Baum, P.; Stockinger, M.; Toyka, K.V.; Klöting, I.; Stumvoll, M.; Serke, H.; Bechmann, I.; et al. Increased Autophagy in Peripheral Nerves May Protect Wistar Ottawa Karlsburg W Rats against Neuropathy. Exp. Neurol. 2013, 250, 125–135. [Google Scholar] [CrossRef]
- Alvites, R.; Lopes, B.; Sousa, P.; Sousa, A.C.; Coelho, A.; Moreira, A.; Rêma, A.; Atayde, L.; Mendonça, C.; Luís, A.L.; et al. Ultrasound Landmarks in the Approach to the Common Peroneal Nerve in a Sheep Model—Application in Peripheral Nerve Regeneration. Life 2023, 13, 1919. [Google Scholar] [CrossRef]
- Xue, C.; Hu, N.; Gu, Y.; Yang, Y.; Liu, Y.; Liu, J.; Ding, F.; Gu, X. Joint Use of a Chitosan/PLGA Scaffold and MSCs to Bridge an Extra Large Gap in Dog Sciatic Nerve. Neurorehabil Neural Repair. 2012, 26, 96–106. [Google Scholar] [CrossRef]
- Attar, B.M.; Zalzali, H.; Razavi, M.; Ghoreishian, M.; Rezaei, M. Effectiveness of Fibrin Adhesive in Facial Nerve Anastomosis in Dogs Compared With Standard Microsuturing Technique. J. Oral. Maxillofac. Surg. 2012, 70, 2427–2432. [Google Scholar] [CrossRef]
- Archibald, S.J.; Krarup, C.; Shefner, J.; Li, S.; Madison, R.D. A Collagen-based Nerve Guide Conduit for Peripheral Nerve Repair: An Electrophysiological Study of Nerve Regeneration in Rodents and Nonhuman Primates. J. Comp. Neurol. 1991, 306, 685–696. [Google Scholar] [CrossRef]
- Yao, Y.; Cui, Y.; Zhao, Y.; Xiao, Z.; Li, X.; Han, S.; Chen, B.; Fang, Y.; Wang, P.; Pan, J.; et al. Efect of Longitudinally Oriented Collagen Conduit Combined with Nerve Growth Factor on Nerve Regeneration after Dog Sciatic Nerve Injury. J. Biomed. Mater. Res. 2018, 106, 2131–2139. [Google Scholar] [CrossRef]
- Lu, Q.; Gu, L.; Jiang, L.; Qin, B.; Fu, G.; Li, X.; Yang, J.; Huang, X.; Yang, Y.; Zhu, Q.; et al. The Upper Brachial Plexus Defect Model in Rhesus Monkeys: A Cadaveric Feasibility Study. NeuroReport 2013, 24, 884–888. [Google Scholar] [CrossRef]
- Hu, N.; Wu, H.; Xue, C.; Gong, Y.; Wu, J.; Xiao, Z.; Yang, Y.; Ding, F.; Gu, X. Long-Term Outcome of the Repair of 50 Mm Long Median Nerve Defects in Rhesus Monkeys with Marrow Mesenchymal Stem Cells-Containing, Chitosan-Based Tissue Engineered Nerve Grafts. Biomaterials 2013, 34, 100–111. [Google Scholar] [CrossRef]
- Lopes, B.; Coelho, A.; Alvites, R.; Sousa, A.C.; Sousa, P.; Moreira, A.; Atayde, L.; Salgado, A.; Geuna, S.; Maurício, A.C. Animal Models in Peripheral Nerve Transection Studies: A Systematic Review on Study Design and Outcomes Assessment. Regen. Med. 2024, 19, 189–203. [Google Scholar] [CrossRef]
- Hanna, A.S.; Hellenbrand, D.J.; Schomberg, D.T.; Salamat, S.M.; Loh, M.; Wheeler, L.; Hanna, B.; Ozaydin, B.; Meudt, J.; Shanmuganayagam, D. Brachial Plexus Anatomy in the Miniature Swine as Compared to Human. J. Anat. 2022, 240, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Hort-Legrand, C.; Noah, L.; Mériguet, E.; Mésangeau, D. Motor and Sensory Nerve Conduction Velocities in Yucatan Minipigs. Lab. Anim. 2006, 40, 53–57. [Google Scholar] [CrossRef]
- Sufan, W.; Suzuki, Y.; Tanihara, M.; Ohnishi, K.; Suzuki, K.; Endo, K.; Nishimura, Y. Sciatic Nerve Regeneration through Alginate with Tubulation or Nontubulation Repair in Cat. J. Neurotrauma 2001, 18, 329–338. [Google Scholar] [CrossRef]
- Bisby, M.A.; Pollock, B. Increased Regeneration Rate in Peripheral Nerve Axons Following Double Lesions: Enhancement of the Conditioning Lesion Phenomenon. J. Neurobiol. 1983, 14, 467–472. [Google Scholar] [CrossRef]
- Forman, D.S.; Wood, D.K.; DeSilva, S. Rate of Regeneration of Sensory Axons in Transected Rat Sciatic Nerve Repaired with Epineurial Sutures. J. Neurol. Sci. 1979, 44, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, J.; Kanje, M. The Initial Period of Peripheral Nerve Regeneration and the Importance of the Local Environment for the Conditioning Lesion Effect. Brain Res. 1990, 529, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. BioMed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Mohanty, C.; Bhat, D.; Devi, B. Use of Animal Models in Peripheral Nerve Surgery and Research. Neurol. India 2019, 67, S100–S105. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian Degeneration: Gaining Perspective on Inflammatory Events after Peripheral Nerve Injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef]
- Tajima, K.; Tohyama, K.; Ide, C.; Abe, M. Regeneration through Nerve Allografts in the Cynomolgus Monkey (Macaca Fascicularis). J. Bone Jt. Surg. Am. 1991, 73, 172–185. [Google Scholar] [CrossRef]
- Dash, H.; Kononov, A.; Prayson, R.A.; Petras, S.; Browne, E.Z. Evaluation of Nerve Recovery From Minimal-Duration Crush Injury. Ann. Plast. Surg. 1996, 37, 526–531. [Google Scholar] [CrossRef]
- Lee, J.I.; Wandling, G.; Talukder, M.A.H.; Elfar, J.; Govindappa, P.K. A Novel Standardized Peripheral Nerve Transection Method and a Novel Digital Pressure Sensor Device Construction for Peripheral Nerve Crush Injury. BIO-Protoc. 2022, 12, e4350. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef]
- Wu, W.; Niu, Y.; Kong, X.; Liu, D.; Long, X.; Shu, S.; Su, X.; Wang, B.; Liu, X.; Ma, Y.; et al. Application of Diffusion Tensor Imaging in Quantitatively Monitoring Chronic Constriction Injury of Rabbit Sciatic Nerves: Correlation with Histological and Functional Changes. Br. J. Radiol. 2018, 91, 20170414. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Xie, Y.-K. A Peripheral Mononeuropathy in Rat That Produces Disorders of Pain Sensation like Those Seen in Man. Pain. 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Wang, C.; Chen, P.; Lin, D.; Chen, Y.; Lv, B.; Zheng, K.; Lin, X.; Wu, Z. Effects of Varying Degrees of Ligation in a Neuropathic Pain Model Induced by Chronic Constriction Injury. Life Sci. 2021, 276, 119441. [Google Scholar] [CrossRef]
- Crawford, R.S.; Hashmi, F.F.; Jones, J.E.; Albadawi, H.; McCormack, M.; Eberlin, K.; Entabi, F.; Atkins, M.D.; Conrad, M.F.; Austen, W.G.; et al. A Novel Model of Acute Murine Hindlimb Ischemia. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H830–H837. [Google Scholar] [CrossRef]
- Heemskerk, A.M.; Drost, M.R.; Van Bochove, G.S.; Van Oosterhout, M.F.M.; Nicolay, K.; Strijkers, G.J. DTI-based Assessment of Ischemia-reperfusion in Mouse Skeletal Muscle. Magn. Reson. Med. 2006, 56, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Vignaud, A.; Hourde, C.; Medja, F.; Agbulut, O.; Butler-Browne, G.; Ferry, A. Impaired Skeletal Muscle Repair after Ischemia-Reperfusion Injury in Mice. J. Biomed. Biotechnol. 2010, 2010, 724914. [Google Scholar] [CrossRef]
- Bain, J.R.; Mackinnon, S.E.; Hunter, D.A. Functional Evaluation of Complete Sciatic, Peroneal, and Posterior Tibial Nerve Lesions in the Rat. Plast. Reconstr. Surg. 1989, 83, 129–136. [Google Scholar] [CrossRef]
- Wood, M.D.; Kemp, S.W.P.; Weber, C.; Borschel, G.H.; Gordon, T. Outcome Measures of Peripheral Nerve Regeneration. Ann. Anat.-Anat. Anz. 2011, 193, 321–333. [Google Scholar] [CrossRef]
- Di Scipio, F.; Raimondo, S.; Tos, P.; Geuna, S. A Simple Protocol for Paraffin-Embedded Myelin Sheath Staining with Osmium Tetroxide for Light Microscope Observation. Microsc. Res. Tech. 2008, 71, 497–502. [Google Scholar] [CrossRef]
- Wang, H.; Sorenson, E.J.; Spinner, R.J.; Windebank, A.J. Electrophysiologic Findings and Grip Strength after Nerve Injuries in the Rat Forelimb. Muscle Nerve 2008, 38, 1254–1265. [Google Scholar] [CrossRef]
- Heinzel, J.; Längle, G.; Oberhauser, V.; Hausner, T.; Kolbenschlag, J.; Prahm, C.; Grillari, J.; Hercher, D. Use of the CatWalk Gait Analysis System to Assess Functional Recovery in Rodent Models of Peripheral Nerve Injury–a Systematic Review. J. Neurosci. Methods 2020, 345, 108889. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Deumens, R.; Scheffel, J.; O’Dey, D.M.; Weis, J.; Joosten, E.A.; Führmann, T.; Brook, G.A.; Pallua, N. CatWalk Gait Analysis in Assessment of Functional Recovery after Sciatic Nerve Injury. J. Neurosci. Methods 2008, 173, 91–98. [Google Scholar] [CrossRef] [PubMed]
- De Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An Index of the Functional Condition of Rat Sciatic Nerve Based on Measurements Made from Walking Tracks. Exp. Neurol. 1982, 77, 634–643. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Chen, J.; Wang, X.; Liu, Q.; Liang, B. Magnetic Resonance Imaging Evaluation of Acute Crush Injury of Rabbit Sciatic Nerve: Correlation with Histology. Can. Assoc. Radiol. J. 2008, 59, 123–130. [Google Scholar]
- Zhu, Y.; Jin, Z.; Luo, Y.; Wang, Y.; Peng, N.; Peng, J.; Wang, Y.; Yu, B.; Lu, C.; Zhang, S. Evaluation of the Crushed Sciatic Nerve and Denervated Muscle with Multimodality Ultrasound Techniques: An Animal Study. Ultrasound Med. Biol. 2020, 46, 377–392. [Google Scholar] [CrossRef]
- Giorgetti, E.; Obrecht, M.; Ronco, M.; Panesar, M.; Lambert, C.; Accart, N.; Doelemeyer, A.; Nash, M.; Bidinosti, M.; Beckmann, N. Magnetic Resonance Imaging as a Biomarker in Rodent Peripheral Nerve Injury Models Reveals an Age-Related Impairment of Nerve Regeneration. Sci. Rep. 2019, 9, 13508. [Google Scholar] [CrossRef]
- Sun, C.; Hou, Z.; Hong, G.; Wan, Q.; Li, X. In Vivo Evaluation of Sciatic Nerve Crush Injury Using Diffusion Tensor Imaging: Correlation With Nerve Function and Histology. J. Comput. Assist. Tomogr. 2014, 38, 790–796. [Google Scholar] [CrossRef]
- Yamasaki, T.; Fujiwara, H.; Oda, R.; Mikami, Y.; Ikeda, T.; Nagae, M.; Shirai, T.; Morisaki, S.; Ikoma, K.; Masugi-Tokita, M.; et al. In Vivo Evaluation of Rabbit Sciatic Nerve Regeneration with Diffusion Tensor Imaging (DTI): Correlations with Histology and Behavior. Magn. Reson. Imaging 2015, 33, 95–101. [Google Scholar] [CrossRef]
- Farinas, A.F.; Pollins, A.C.; Stephanides, M.; O’Neill, D.; Al-Kassis, S.; Esteve, I.V.M.; Colazo, J.M.; Keller, P.R.; Rankin, T.; Wormer, B.A.; et al. Diffusion Tensor Tractography to Visualize Axonal Outgrowth and Regeneration in a 4-Cm Reverse Autograft Sciatic Nerve Rabbit Injury Model. Neurol. Res. 2019, 41, 257–264. [Google Scholar] [CrossRef]
- Ricci, V.; Ricci, C.; Cocco, G.; Gervasoni, F.; Donati, D.; Farì, G.; Özçakar, L. Histopathology and High-Resolution Ultrasound Imaging for Peripheral Nerve (Injuries). J. Neurol. 2022, 269, 3663–3675. [Google Scholar] [CrossRef] [PubMed]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of Facial Expressions of Pain in the Laboratory Mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lichtman, J.W. Motor Axon Regeneration and Muscle Reinnervation in Young Adult and Aged Animals. J. Neurosci. 2013, 33, 19480–19491. [Google Scholar] [CrossRef]
- Maita, K.C.; Garcia, J.P.; Avila, F.R.; Torres-Guzman, R.A.; Ho, O.; Chini, C.C.S.; Chini, E.N.; Forte, A.J. Evaluation of the Aging Effect on Peripheral Nerve Regeneration: A Systematic Review. J. Surg. Res. 2023, 288, 329–340. [Google Scholar] [CrossRef]
- Brown, T.J.; Khan, T.; Jones, K.J. Androgen Induced Acceleration of Functional Recovery after Rat Sciatic Nerve Injury. Restor. Neurol. Neurosci. 1999, 15, 289–295. [Google Scholar] [CrossRef]
- Azarkish, F.; Armin, F.; Parvar, A.A.A.; Dehghani, A. The Influence of Renal Ischemia-Reperfusion Injury on Remote Organs: The Histological Brain Changes in Male and Female Rats. Brain Circ. 2021, 7, 194–200. [Google Scholar] [CrossRef]
- Syu, W.-Z.; Hueng, D.-Y.; Chen, W.-L.; Chan, J.Y.-H.; Chen, S.-G.; Huang, S.-M. Adipose-Derived Neural Stem Cells Combined with Acellular Dermal Matrix as a Neural Conduit Enhances Peripheral Nerve Repair. Cell Transplant. 2019, 28, 1220–1230. [Google Scholar] [CrossRef]
- Lavorato, A.; Raimondo, S.; Boido, M.; Muratori, L.; Durante, G.; Cofano, F.; Vincitorio, F.; Petrone, S.; Titolo, P.; Tartara, F.; et al. Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. Int. J. Mol. Sci. 2021, 22, 572. [Google Scholar] [CrossRef]
- Daeschler, S.C.; Feinberg, K.; Harhaus, L.; Kneser, U.; Gordon, T.; Borschel, G.H. Advancing Nerve Regeneration: Translational Perspectives of Tacrolimus (FK506). Int. J. Mol. Sci. 2023, 24, 12771. [Google Scholar] [CrossRef]
- Kim, J.; Choi, Y.E.; Kim, J.H.; Lee, S.H.; Oh, S.; Kim, S.H. Nerve Repair and Orthodromic and Antidromic Nerve Grafts: An Experimental Comparative Study in Rabbit. Biomed. Res. Int. 2020, 2020, 5046832. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Dai, J.; Jin, S.; Huang, J.; Guo, C.; Wang, C.; Pang, L.; Wang, Y. A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury. Sci. Rep. 2017, 7, 3541. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, M.D.; Wenke, J.C. Mechanisms by Which Hydrogen Sulfide Attenuates Muscle Function Following Ischemia–Reperfusion Injury: Effects on Akt Signaling, Mitochondrial Function, and Apoptosis. J. Transl. Med. 2019, 17, 33. [Google Scholar] [CrossRef]
- Ball, C.J.; Reiffel, A.J.; Chintalapani, S.; Kim, M.; Spector, J.A.; King, M.R. Hydrogen Sulfide Reduces Neutrophil Recruitment in Hind-Limb Ischemia-Reperfusion Injury in an L-Selectin and ADAM-17–Dependent Manner. Plast. Reconstr. Surg. 2013, 131, 487–497. [Google Scholar] [CrossRef]
- Kurlander, D.E.; Wee, C.; Chepla, K.J.; Lineberry, K.D.; Long, T.C.; Gillis, J.A.; Valerio, I.L.; Khouri, J.S. TMRpni: Combining Two Peripheral Nerve Management Techniques. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e3132. [Google Scholar] [CrossRef]
- Hooper, R.C.; Cederna, P.S.; Brown, D.L.; Haase, S.C.; Waljee, J.F.; Egeland, B.M.; Kelley, B.P.; Kung, T.A. Regenerative Peripheral Nerve Interfaces for the Management of Symptomatic Hand and Digital Neuromas. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2792. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Barlow, A.K.; Hargrove, L.J.; Dumanian, G.A. Targeted Muscle Reinnervation for the Upper and Lower Extremity. Tech. Orthop. 2017, 32, 109–116. [Google Scholar] [CrossRef]
- Frost, C.; Salous, A.; Ketheeswaran, S.; Ngaage, L.M.; Hanwright, P.J.; Ghergherehchi, C.; Tuffaha, S.; Vaidya, D.; Bittner, G.D.; Brandacher, G.; et al. Polyethylene Glycol Fusion Restores Axonal Continuity and Improves Return of Function in a Rat Median Nerve Denervation Model. Plast. Reconstr. Surg. 2023. [Google Scholar] [CrossRef]
- Du, J.; Chen, H.; Qing, L.; Yang, X.; Jia, X. Biomimetic Neural Scaffolds: A Crucial Step towards Optimal Peripheral Nerve Regeneration. Biomater. Sci. 2018, 6, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Mankavi, F.; Ibrahim, R.; Wang, H. Advances in Biomimetic Nerve Guidance Conduits for Peripheral Nerve Regeneration. Nanomaterials 2023, 13, 2528. [Google Scholar] [CrossRef]
- Rochkind, S.; Almog, M.; Meilin, S.; Nevo, Z. Reviving Matrix for Nerve Reconstruction in Rabbit Model of Chronic Peripheral Nerve Injury With Massive Loss Defect. Front. Surg. 2020, 7, 609638. [Google Scholar] [CrossRef]
- Martinez De Albornoz, P.; Delgado, P.J.; Forriol, F.; Maffulli, N. Non-Surgical Therapies for Peripheral Nerve Injury. Br. Med. Bull. 2011, 100, 73–100. [Google Scholar] [CrossRef]
| Small Animals | Strengths | Limitations | Studies |
|---|---|---|---|
| Rats (Sprague Dawley, Wistar, Long Evans, and Lewis) |
|
| [2,3,5,12,20,26,27,49,50,51] |
| Mice (C57BL/6J, C57/Bl6, B6, and 129SF2/J) |
|
| [18,52,53,54,55] |
| Guinea Pigs (Cavia porcellus and Duncan-Hartley) |
|
| [19,22] |
| Rabbits (New Zealand White and Japanese White) |
|
| [16,17,21,56] |
| Large Animals | Strengths | Limitations | Studies |
|---|---|---|---|
| Sheep (Ovis aries, Merino, or Suffolk breed) |
|
| [6,13,14,59] |
| Porcine (Yucatan Minipigs, German House Pig, and Yorkshire Pigs) |
|
| [1,24,25] |
| Canine (Male Beagle Dogs and Dogs (18–24 kg; breed not specified) |
|
| [23,60,61,63] |
| Monkey (Macaca fascicularis or Cynomolgus Rhesus) |
|
| [15,31,62,64,65] |
| Peripheral Nerve Injury Models | Strengths | Limitations |
|---|---|---|
| Crush |
|
|
| Transection |
|
|
| Chronic Constriction |
|
|
| Chemical Damage |
|
|
| Ischemia-Reperfusion |
|
|
| Reference | Title | Animal Model | Technique | Main Outcome |
|---|---|---|---|---|
| Burrell et al. (2020) [1] | A Porcine Model of Peripheral Nerve Injury Enabling Ultra-Long Regenerative Distances: Surgical Approach, Recovery Kinetics, and Clinical Relevance (October 2020) | Yucatan Minipigs | Repair of common peroneal nerve (CPN) and deep peroneal nerve (DPN) long segmental defects and repair using a 5cm saphenous or sural nerve autograft. |
|
| Siwei (2022) [3] | Construction and Effect Evaluation of Different Sciatic Nerve Injury Models in Rats | Sprague Dawley Rats | Transverse, clamp, keep epineurium and axon cutting, and chemical damage. |
|
| Merolli (2022) [17] | A sciatic nerve gap-injury model in the rabbit | New Zealand White Rabbits | Transection with artificial nerve guides. |
|
| Muratori (2012) [2] | Can Regenerated Nerve Fibers Return to Normal Size? A Long-Term Post-Traumatic Study of the Rat Median Nerve Crush Injury Model | Female Wistar Rats | Crush median nerve via non-serrated clamp. |
|
| Drysch (2019) [18] | An Optimized Low-Pressure Tourniquet Murine Hind Limb Ischemia Reperfusion Mode: Inducing Acute Ischemia Reperfusion Injury in C57BL/6 Wild Type Mice | C57BL/6J mice | Tourniquet and artery clamping. |
|
| An (2022) [5] | Evaluation methods of a rat sciatic nerve crush injury model | Sprague–Dawley (SD) male rats | Crush injury. |
|
| Casañas (2014) [6] | Peripheral nerve regeneration after experimental section in ovine radial and tibial nerves using synthetic nerve grafts, including expanded bone marrow mesenchymal cells: morphological and neurophysiological results | Sheep | Synthetic nerve grafts (bone marrow mesenchymal cells) using radial and tibial nerve. |
|
| Roballo (2020) [14] | Long-term neural regeneration following injury to the peroneal branch of the sciatic nerve in sheep | Merino or Suffolk breed sheep | Bisection, 5 cm reverse autograft, and sham surgery. |
|
| Guo (2014) [15] | Sciatic Nerve Neuropathy in Cynomolgus Monkey Macaca Fascicularis: Altered Leg Usage and Peripheral Nerve Firing | Macaca fascicularis Monkeys | Mild injury to sciatic nerve via incomplete constriction with ligature. |
|
| Alvites (2021) [59] | Establishment of a Sheep Model for Hind Limb Peripheral Nerve Injury: Common Peroneal Nerve | Ovis aries Sheep | Surgical protocol for common peroneal nerve, including baseline controls using crush injuries and neurotmesis, with repair variables (end-to-end, nerve guidance conduit, and axonotmesis). |
|
| Rafee (2017) [19] | Guinea Pigs as an Animal Model for Sciatic Nerve Injury | Cavia porcellus Guinea Pigs | Crush injury. |
|
| Yayama (2010) [21] | Effect of Graded Mechanical Compression of Rabbit Sciatic Nerve on Nerve Blood Flow and Electrophysiological Properties | Japanese white rabbits | Clamped with a custom compressor to investigate the relationship between compressive force on the nerve and (i) intraneural blood flow and (ii) compound nerve action potentials. |
|
| Rao (2001) [22] | Muscle autografts in nerve gaps. Pattern of regeneration and myelination in various lengths of graft: an experimental study in guinea pigs | Duncan-Hartley guinea pigs | Evaluated different autograft graft lengths. |
|
| Ding (2010) [23] | Use of Tissue-Engineered Nerve Grafts Consisting of a Chitosan/Poly (lactic-co-glycolic acid)-Based Scaffold Included with Bone Marrow Mesenchymal Cells for Bridging 50-mm Dog Sciatic Nerve Gaps | Male Beagle dogs | Chitosan/PLGA-based neural scaffold combined with autologous bone marrow mesenchymal stem cells (MSCs). |
|
| Kaemmer (2010) [24] | Evaluation of tissue components in the peripheral nervous system using Sirius red staining and immunohistochemistry: A comparative study (human, pig, rat) | Human, Rat (Lewis inbred rats), Pig (German house) | Evaluation of collagen (stroma) and nerve fibers (parenchyma) in different species. |
|
| Attar (2012) [61] | Effectiveness of Fibrin Adhesive in Facial Nerve Anastomosis in Dogs Compared with Standard Microsuturing Technique | Dogs (18–24 kg; breed not specified) | Fibrin glue for peripheral nerve anastomosis. |
|
| Zilic (2015) [25] | An Anatomical Study of Porcine Peripheral Nerve and Its Potential Use in Nerve Tissue Engineering | Rats (Wistar) vs. Porcine (Yorkshire Pigs) | Dissection and quantification of the ECM components. |
|
| Wang (2014) [31] | A Simple Model of Radial Nerve Injury in the Rhesus Monkey to Evaluate Peripheral Nerve Repair | Rhesus Monkeys | 2.5 cm radial nerve lesions. |
|
| Mazzer (2008) [50] | Morphologic and morphometric evaluation of experimental acute crush injuries of the sciatic nerve of rats | Wistar Rats | Histological and morphometric analysis of a 5 mm intermediate segment after 10-min dead-weight machine application. |
|
| Wang (2023) [20] | Comparison of the Nerve Regeneration Capacity and Characteristics between Sciatic Nerve Crush and Transection Injury Models in Rats | Sprague Dawley Rats | Crush or transection injury followed by surgical repair. |
|
| Medeiros (2021) [51] | An Adapted Chronic Constriction Injury of the Sciatic Nerve Produces Sensory, Affective, and Cognitive Impairments: A Peripheral Mononeuropathy Model for the Study of Comorbid Neuropsychiatric Disorders Associated with Neuropathic Pain in Rats | Wistar Rats | Chronic constriction injury (CCI) model with four loose ligatures vs. a single ligature. |
|
| Kim (2023) [52] | Long-term tactile hypersensitivity after nerve crush injury in mice is characterized by the persistence of intact sensory axons | C57BL/6J mice | Complete or incomplete crush injury. |
|
| Umansky (2022) [53] | Functional Gait Assessment Using Manual, Semi-Automated and Deep Learning Approaches Following Standardized Models of Peripheral Nerve Injury in Mice | C57/Bl6 mice | Crush or stretch–crush injury. |
|
| Bonheur (2004) [55] | A Noninvasive Murine Model of Hind Limb Ischemia-Reperfusion Injury | B6,129SF2/J mice | Ischemia-reperfusion injury. |
|
| Archibald (1991) [62] | A Collagen-Based Nerve Guide Conduit for Peripheral Nerve Repair: An Electrophysiological Study of Nerve Regeneration in Rodents and Nonhuman Primates | Macaca fascicularis monkeys, Long Evans rats | Rats: Sciatic nerve transection and repair by (1) direct microsurgical suture, (2) 4 mm autograft, or (3) entubulation repair with collagen-based nerve guide conduits.Monkey: The median nerve was transected 2 cm above the wrist and repaired with either a 4 mm nerve autograft or a collagen-based nerve guide conduit, leaving a 4 mm gap between the nerve ends. |
|
| Blanco (1999) [10] | Ultrastructural Studies of Dorsal Root Axons Regenerating Through Adult Frog Optic and Sciatic Nerves | R. pipiens frog | Optic nerve grafts in frogs were used to test CNS glial permissiveness to sensory neurons, compared to sciatic nerve grafts. |
|
| Vega-Melendez (2014) [48] | Ciliary Neurotrophic Factor and Fibroblast Growth Factor Increase the Speed and Number of Regenerating Axons After Optic Nerve Injury in Adult Rana pipiens | R. pipiens frog | Effect of neurotrophins on nerve regeneration after optic nerve crush injury. |
|
| Luo (2022) [42] | An animal model for trigeminal neuralgia by compression of the trigeminal nerve root | Sprague Dawley rats | Chronic compression of the trigeminal nerve. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluta, N.A.; Gaviria, M.; Sabbag, C.M.; Hill, S. Advancements in Peripheral Nerve Injury Research Using Lab Animals. Anatomia 2025, 4, 8. https://doi.org/10.3390/anatomia4020008
Pluta NA, Gaviria M, Sabbag CM, Hill S. Advancements in Peripheral Nerve Injury Research Using Lab Animals. Anatomia. 2025; 4(2):8. https://doi.org/10.3390/anatomia4020008
Chicago/Turabian StylePluta, Natalia A., Manuela Gaviria, Casey M. Sabbag, and Shauna Hill. 2025. "Advancements in Peripheral Nerve Injury Research Using Lab Animals" Anatomia 4, no. 2: 8. https://doi.org/10.3390/anatomia4020008
APA StylePluta, N. A., Gaviria, M., Sabbag, C. M., & Hill, S. (2025). Advancements in Peripheral Nerve Injury Research Using Lab Animals. Anatomia, 4(2), 8. https://doi.org/10.3390/anatomia4020008






