Pheromone Sensing in Mammals: A Review of the Vomeronasal System
Abstract
1. Introduction
1.1. Chemical Communication in Mammals
1.1.1. Types of Chemical Signals
Pheromones
Kairomones
1.1.2. Responsible Exocrine Organs
1.1.3. Ethology and Scent Marking
1.1.4. Chemical Signal Detection Systems
1.1.5. Olfactory Subsystems
2. Vomeronasal System
2.1. Anatomy of the Vomeronasal Organ
2.2. Neuroanatomy of the Accessory Olfactory Bulb


2.3. Olfactory Pathways
3. Specific Study of the Vomeronasal System
3.1. Capybara (Hydrochoerus hydrochaeris)
3.2. Bennett’s Wallaby (Notamacropus rufogriseus)
3.3. Meerkat (Suricata suricatta) (Figure 24)
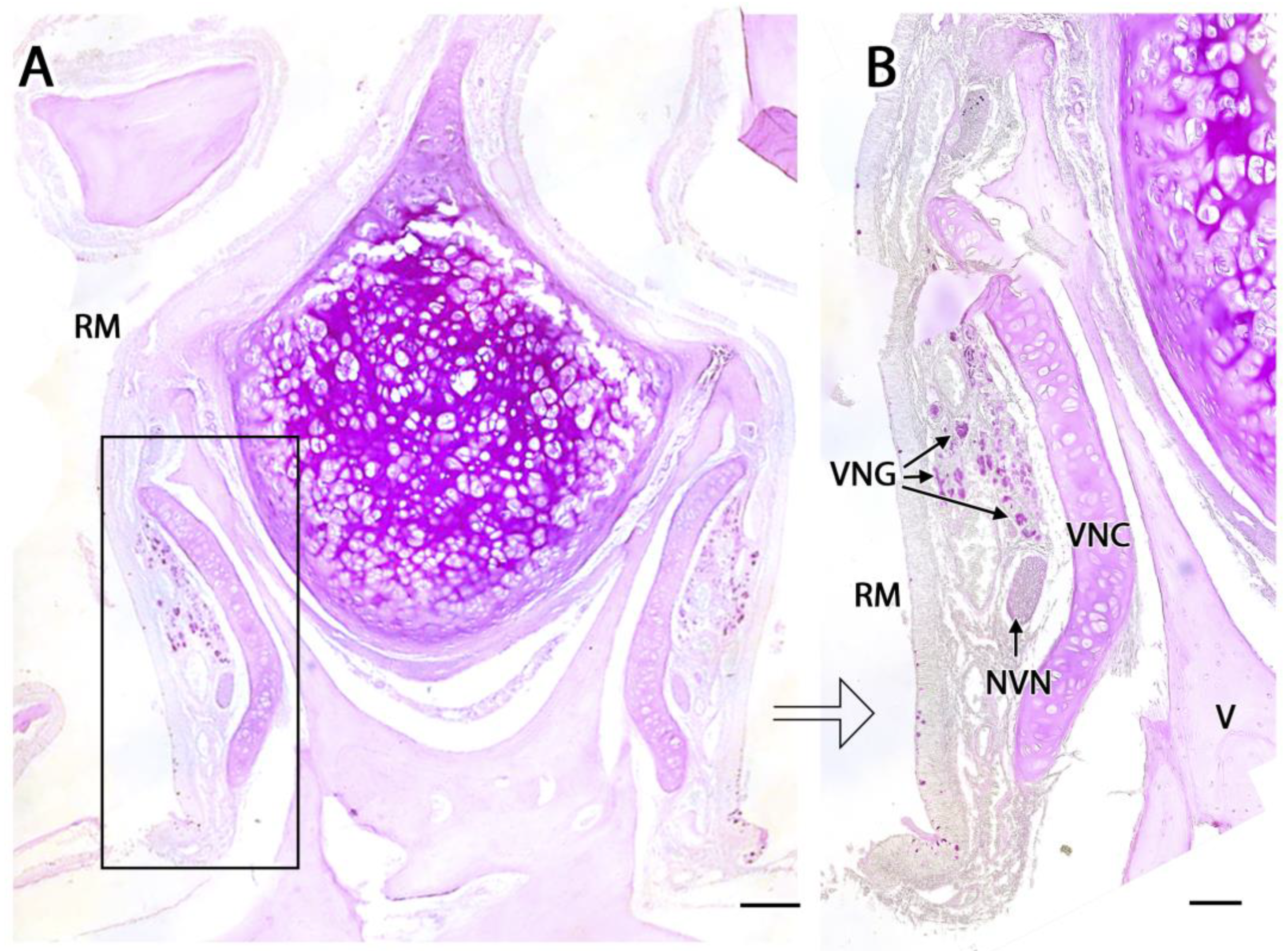
3.4. Dama gazelle (Nanger dama)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevens, M. Sensory Ecology, Behaviour, and Evolution; Oxford University Press: Oxford, UK, 2013; ISBN 978-0-19-960177-6. [Google Scholar]
- Wyatt, T.D. Pheromones and Animal Behaviour: Communication by Smell and Taste; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2003; ISBN 978-0-521-48068-0. [Google Scholar]
- Wood, W.F. Chemical Ecology: Chemical Communication in Nature. J. Chem. Educ. 1983, 60, 531. [Google Scholar] [CrossRef]
- Castañeda, M.d.L.A.; Martínez-Gómez, M.; Guevara-Guzmán, R.; Hudson, R. Chemical communication in domestic mammals. Vet. Mex. 2007, 38, 105–123. [Google Scholar]
- Kokocińska-Kusiak, A.; Woszczyło, M.; Zybala, M.; Maciocha, J.; Barłowska, K.; Dzięcioł, M. Canine Olfaction: Physiology, Behavior, and Possibilities for Practical Applications. Animals 2021, 11, 2463. [Google Scholar] [CrossRef] [PubMed]
- Iovino, M.; Messana, T.; Iovino, E.; De Pergola, G.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. Neuroendocrine Mechanisms Involved in Male Sexual and Emotional Behavior. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Beruter, J.; Beauchamp, G.K.; Muetterties, E.L. Complexity of Chemical Communication in Mammals: Urinary Components Mediating Sex Discrimination by Male Guinea Pigs. Biochem. Biophys. Res. Commun. 1973, 53, 264–271. [Google Scholar] [CrossRef]
- Wilson, E.O. Chemical Communication within Animal Species. In Chemical Ecology; Academic Press: New York, NY, USA, 1970; pp. 133–155. [Google Scholar]
- Surov, A.V.; Maltsev, A.N. Analysis of Chemical Communication in Mammals: Zoological and Ecological Aspects. Biol. Bull. Russ. Acad. Sci. 2016, 43, 1175–1183. [Google Scholar] [CrossRef]
- Villamayor, P.R.; Arana, Á.J.; Coppel, C.; Ortiz-Leal, I.; Torres, M.V.; Sanchez-Quinteiro, P.; Sánchez, L. A Comprehensive Structural, Lectin and Immunohistochemical Characterization of the Zebrafish Olfactory System. Sci. Rep. 2021, 11, 8865. [Google Scholar] [CrossRef]
- Saraiva, L.R.; Ahuja, G.; Ivandic, I.; Syed, A.S.; Marioni, J.C.; Korsching, S.I.; Logan, D.W. Molecular and Neuronal Homology between the Olfactory Systems of Zebrafish and Mouse. Sci. Rep. 2015, 5, 11487. [Google Scholar] [CrossRef]
- Meister, M. On the Dimensionality of Odor Space. eLife 2015, 4, e07865. [Google Scholar] [CrossRef]
- Mollo, E.; Garson, M.J.; Polese, G.; Amodeo, P.; Ghiselin, M.T. Taste and Smell in Aquatic and Terrestrial Environments. Nat. Prod. Rep. 2017, 34, 496–513. [Google Scholar] [CrossRef]
- Hepper, P.G.; Wells, D.L. Perinatal Olfactory Learning in the Domestic Dog. Chem. Senses 2006, 31, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Weissburg, M.J.; Ferner, M.C.; Pisut, D.P.; Smee, D.L. Ecological Consequences of Chemically Mediated Prey Perception. J. Chem. Ecol. 2002, 28, 1953–1970. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, D.A.; Lewis, W.J. Terminology of Chemical Releasing Stimuli in Intraspecific and Interspecific Interactions. J. Chem. Ecol. 1976, 2, 211–220. [Google Scholar] [CrossRef]
- Law, J.H.; Regnier, F.E. Pheromones. Annu. Rev. Biochem. 1971, 40, 533–548. [Google Scholar] [CrossRef]
- Calcagnile, M.; Tredici, S.M.; Talà, A.; Alifano, P. Bacterial Semiochemicals and Transkingdom Interactions with Insects and Plants. Insects 2019, 10, 441. [Google Scholar] [CrossRef]
- Burger, B.V. Mammalian Semiochemicals. In The Chemistry of Pheromones and Other Semiochemicals II; Schulz, S., Ed.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; Volume 240, pp. 231–278. ISBN 978-3-540-21308-6. [Google Scholar]
- Apfelbach, R.; Parsons, M.H.; Soini, H.A.; Novotny, M.V. Are Single Odorous Components of a Predator Sufficient to Elicit Defensive Behaviors in Prey Species? Front. Neurosci. 2015, 9, 263. [Google Scholar] [CrossRef]
- Dicke, M.; Sabelis, M.W. Infochemical Terminology: Based on Cost-Benefit Analysis Rather than Origin of Compounds? Funct. Ecol. 1988, 2, 131. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and Behavior. In Chemical Communication in Crustaceans; Breithaupt, T., Thiel, M., Eds.; Springer: New York, NY, USA, 2010; pp. 23–38. ISBN 978-0-387-77100-7. [Google Scholar]
- Sbarbati, A.; Osculati, F. Allelochemical Communication in Vertebrates: Kairomones, Allomones and Synomones. Cells Tissues Organs 2006, 183, 206–219. [Google Scholar] [CrossRef]
- Blum, M.S. Semiochemical Parsimony in the Arthropoda. Annu. Rev. Entomol. 1996, 41, 353–374. [Google Scholar] [CrossRef]
- Gallie, D.R.; Chang, S.C. Signal Transduction in the Carnivorous Plant Sarracenia Purpurea (Regulation of Secretory Hydrolase Expression during Development and in Response to Resources). Plant Physiol. 1997, 115, 1461–1471. [Google Scholar] [CrossRef]
- Apfelbach, R.; Blanchard, C.D.; Blanchard, R.J.; Hayes, R.A.; McGregor, I.S. The Effects of Predator Odors in Mammalian Prey Species: A Review of Field and Laboratory Studies. Neurosci. Biobehav. Rev. 2005, 29, 1123–1144. [Google Scholar] [CrossRef] [PubMed]
- Dunkelblum, E.; Mendel, Z.; Gries, G.; Gries, R.; Zegelman, L.; Hassner, A.; Mori, K. Antennal Response and Field Attraction of the Predator Elatophilus hebraicus (Hemiptera: Anthocoridae) to Sex Pheromones and Analogues of Three Matsucoccus spp. (Homoptera: Matsucoccidae). Bioorg. Med. Chem. 1996, 4, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Cavill, G.W.K.; Robertson, P.L. Ant Venoms, Attractants, and Repellents: Secretions Are Used by Ants in Attack and Defense and as Chemical Messengers in Their Social Organization. Science 1965, 149, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Mattiacci, L.; Dicke, M.; Posthumus, M.A. Induction of Parasitoid Attracting Synomone in Brussels Sprouts Plants by Feeding ofPieris Brassicae Larvae: Role of Mechanical Damage and Herbivore Elicitor. J. Chem. Ecol. 1994, 20, 2229–2247. [Google Scholar] [CrossRef] [PubMed]
- Stowe, M.K.; Turlings, T.C.; Loughrin, J.H.; Lewis, W.J.; Tumlinson, J.H. The Chemistry of Eavesdropping, Alarm, and Deceit. Proc. Natl. Acad. Sci. USA 1995, 92, 23–28. [Google Scholar] [CrossRef]
- Han, B.; Chen, Z. Behavioral and Electrophysiological Responses of Natural Enemies to Synomones from Tea Shoots and Kairomones from Tea Aphids, Toxoptera aurantii. J. Chem. Ecol. 2002, 28, 2203–2219. [Google Scholar] [CrossRef]
- Pernaa, J.; Aksela, M. Learning Organic Chemistry through a Study of Semiochemicals. J. Chem. Educ. 2011, 88, 1644–1647. [Google Scholar] [CrossRef]
- Kasinger, H.; Bauer, B.; Denzinger, J. The Meaning of Semiochemicals to the Design of Self-Organizing Systems. In Proceedings of the 2008 Second IEEE International Conference on Self-Adaptive and Self-Organizing Systems, Venezia, Italy, 20–24 October 2008; pp. 139–148. [Google Scholar]
- Karlson, P.; Lüscher, M. ‘Pheromones’: A New Term for a Class of Biologically Active Substances. Nature 1959, 183, 55–56. [Google Scholar] [CrossRef]
- Butenandt, A.; Beckmann, R.; Stamm, D.; Hecker, E. Uber Den Sexsual-Lockstoff Des Seidenspinners Bombyx Mori. Reindarstellung Und Konstitution. Z. Naturforschg. B 1959, 14, 283–284. [Google Scholar]
- Wilson, E.O. Chemical Communication in the Social Insects: Insect Societies Are Organized Principally by Complex Systems of Chemical Signals. Science 1965, 149, 1064–1071. [Google Scholar] [CrossRef]
- Conte, Y.L.; Hefetz, A. Primer Pheromones in Social Hymenoptera. Annu. Rev. Entomol. 2008, 53, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Cork, A.; Kamal, N.; Alam, S.; Choudhury, J.; Talekar, N. Pheromones and Their Applications to Insect Pest Control. Bangladesh J. Entomol. 2003, 13, 1–13. [Google Scholar]
- Liberles, S.D. Mammalian Pheromones. Annu. Rev. Physiol. 2014, 76, 151–175. [Google Scholar] [CrossRef]
- Brennan, P.A.; Zufall, F. Pheromonal Communication in Vertebrates. Nature 2006, 444, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Doty, R.L.; Moulton, D.G.; Mugford, R.A. The Pheromone Concept in Mammalian Chemical Communication: A Critique. In Mammalian Olfaction, Reproductive Processes, and Behavior; Elsevier: Amsterdam, The Netherlands, 1976; pp. 143–160. ISBN 978-0-12-221250-5. [Google Scholar]
- Brown, R.E. Mammalian Social Odors: A Critical Review. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 1979; Volume 10, pp. 103–162. ISBN 978-0-12-004510-5. [Google Scholar]
- Booth, W.D.; Signoret, J.P. Olfaction and Reproduction in Ungulates. Oxf. Rev. Reprod. Biol. 1992, 14, 263–301. [Google Scholar]
- Schaal, B.; Coureaud, G.; Langlois, D.; Giniès, C.; Sémon, E.; Perrier, G. Chemical and Behavioural Characterization of the Rabbit Mammary Pheromone. Nature 2003, 424, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Apps, P.J.; Weldon, P.J.; Kramer, M. Chemical Signals in Terrestrial Vertebrates: Search for Design Features. Nat. Prod. Rep. 2015, 32, 1131–1153. [Google Scholar] [CrossRef]
- Ferrero, D.M.; Moeller, L.M.; Osakada, T.; Horio, N.; Li, Q.; Roy, D.S.; Cichy, A.; Spehr, M.; Touhara, K.; Liberles, S.D. A Juvenile Mouse Pheromone Inhibits Sexual Behaviour through the Vomeronasal System. Nature 2013, 502, 368–371. [Google Scholar] [CrossRef]
- Lin, D.Y.; Zhang, S.-Z.; Block, E.; Katz, L.C. Encoding Social Signals in the Mouse Main Olfactory Bulb. Nature 2005, 434, 470–477. [Google Scholar] [CrossRef]
- Novotny, M.; Harvey, S.; Jemiolo, B.; Alberts, J. Synthetic Pheromones That Promote Inter-Male Aggression in Mice. Proc. Natl. Acad. Sci. USA 1985, 82, 2059–2061. [Google Scholar] [CrossRef]
- Singer, A.G.; Agosta, W.C.; O’Connell, R.J.; Pfaffmann, C.; Bowen, D.V.; Field, F.H. Dimethyl Disulfide: An Attractant Pheromone in Hamster Vaginal Secretion. Science 1976, 191, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Dorries, K.M.; Adkins-regan, E.; Halpern, B.P. Olfactory Sensitivity to the Pheromone, Androstenone, Is Sexually Dimorphic in the Pig. Physiol. Behav. 1995, 57, 255–259. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Lazar, J.; Greenwood, D.R. Olfactory Adventures of Elephantine Pheromones. Biochem. Soc. Trans. 2003, 31, 137–141. [Google Scholar] [CrossRef]
- Brown, W.L.; Eisner, T.; Whittaker, R.H. Allomones and Kairomones: Transspecific Chemical Messengers. BioScience 1970, 20, 21–22. [Google Scholar] [CrossRef]
- Lass, S.; Bittner, K. Facing Multiple Enemies: Parasitised Hosts Respond to Predator Kairomones. Oecologia 2002, 132, 344–349. [Google Scholar] [CrossRef]
- Weldon, P.J. In Defense of “Kairomone” as a Class of Chemical Releasing Stimuli. J. Chem. Ecol. 1980, 6, 719–725. [Google Scholar] [CrossRef]
- Pasteels, J.M. Is Kairomone a Valid and Useful Term? J. Chem. Ecol. 1982, 8, 1079–1081. [Google Scholar] [CrossRef]
- Ruther, J.; Meiners, T.; Steidle, J.L.M. Rich in Phenomena-Lacking in Terms. A Classification of Kairomones. Chemoecology 2002, 12, 161–167. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Pirk, C.W.W.; Yusuf, A.A.; Chailleux, A.; Mohamed, S.A.; Deletre, E. Exploring the Kairomone-Based Foraging Behaviour of Natural Enemies to Enhance Biological Control: A Review. Front. Ecol. Evol. 2021, 9, 641974. [Google Scholar] [CrossRef]
- Schonewolf, K.W.; Bell, R.; Rypstra, A.L.; Persons, M.H. Field Evidence of an Airborne Enemy-Avoidance Kairomone in Wolf Spiders. J. Chem. Ecol. 2006, 32, 1565–1576. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reinecke, A.; Ruther, J.; Tolasch, T.; Francke, W.; Hilker, M. Alcoholism in Cockchafers: Orientation of Male Melolontha melolontha towards Green Leaf Alcohols. Naturwissenschaften 2002, 89, 265–269. [Google Scholar] [CrossRef]
- Jones, R.L.; Lewis, W.J.; Bowman, M.C.; Beroza, M.; Bierl, B.A. Host-Seeking Stimulant for Parasite of Corn Earworm: Isolation, Identification, and Synthesis. Science 1971, 173, 842–843. [Google Scholar] [CrossRef]
- Acree, F.; Turner, R.B.; Gouck, H.K.; Beroza, M.; Smith, N. L-Lactic Acid: A Mosquito Attractant Isolated from Humans. Science 1968, 161, 1346–1347. [Google Scholar] [CrossRef]
- Thibout, E.; Guillot, J.F.; Ferary, S.; Limouzin, P.; Auger, J. Origin and Identification of Bacteria Which Produce Kairomones in the Frass of Acrolepiopsis assectella (Lep., Hyponomeutoidea). Experientia 1995, 51, 1073–1075. [Google Scholar] [CrossRef]
- Schoeppner, N.M.; Relyea, R.A. Damage, Digestion, and Defence: The Roles of Alarm Cues and Kairomones for Inducing Prey Defences: Damage, Digestion, and Defence. Ecol. Lett. 2005, 8, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Ozawa, R.; Kugimiya, S.; Takabayashi, J.; Bohlmann, J. Herbivore-Induced Defense Response in a Model Legume. Two-Spotted Spider Mites Induce Emission of (E)-β-Ocimene and Transcript Accumulation of (E)-β-Ocimene Synthase in Lotus japonicus. Plant. Physiol. 2004, 135, 1976–1983. [Google Scholar] [CrossRef]
- Reinecke, A.; Ruther, J.; Mayer, C.J.; Hilker, M. Optimized Trap Lure for Male Melolontha Cockchafers. J. Appl. Entomol. 2006, 130, 171–176. [Google Scholar] [CrossRef]
- Briand, L.; Trotier, D.; Pernollet, J.-C. Aphrodisin, an Aphrodisiac Lipocalin Secreted in Hamster Vaginal Secretions. Peptides 2004, 25, 1545–1552. [Google Scholar] [CrossRef]
- Altmann, D.; Sorensen, L. Zoo Behaviour Science in the Research Centre for Vertebrate Studies of the Academy of Sciences of the German Democratic Republic (in the Animal Park of Berlin). Appl. Anim. Behav. Sci. 1987, 18, 67–81. [Google Scholar] [CrossRef]
- Salazar, I.; Fdez de Troconiz, P.; Prieto, M.D.; Cifuentes, J.M.; Quinteiro, P.S. Anatomy and Cholinergic Innervation of the Sinus Paranalis in Dogs. Anat. Histol. Embryol. 1996, 25, 49–53. [Google Scholar] [CrossRef]
- Martín, J.; Barja, I.; López, P. Chemical Scent Constituents in Feces of Wild Iberian Wolves (Canis lupus signatus). Biochem. Syst. Ecol. 2010, 38, 1096–1102. [Google Scholar] [CrossRef]
- Aviles-Rosa, E.O.; Surowiec, K.; McGlone, J. Identification of Faecal Maternal Semiochemicals in Swine (Sus scrofa) and Their Effects on Weaned Piglets. Sci. Rep. 2020, 10, 5349. [Google Scholar] [CrossRef] [PubMed]
- Ewer, R.F. Ethology of Mammals; Springer: Boston, MA, USA, 1968; ISBN 978-1-4899-4658-4. [Google Scholar]
- Bradshaw, J.W.S.; Cameron-Beaumont, C.L. The Signaling Repertoire of the Domestic Cat and Its Undomesticated Relatives. In The Domestic Cat: The Biology of Its Behaviour; Cambridge University Press: Cambridge, UK, 2000; pp. 67–93. [Google Scholar]
- Miyazaki, M.; Yamashita, T.; Suzuki, Y.; Saito, Y.; Soeta, S.; Taira, H.; Suzuki, A. A Major Urinary Protein of the Domestic Cat Regulates the Production of Felinine, a Putative Pheromone Precursor. Chem. Biol. 2006, 13, 1071–1079. [Google Scholar] [CrossRef]
- Stefańczyk-Krzymowska, S.; Krzymowski, T.; Wasowska, B.; Jana, B.; Słomiński, J. Intramuscular Injections of Male Pheromone 5 Alpha-Androstenol Change the Secretory Ovarian Function in Gilts during Sexual Maturation. Reprod. Biol. 2003, 3, 241–257. [Google Scholar]
- Booth, W.D. Sexual Dimorphism Involving Steroidal Pheromones and Their Binding Protein in the Submaxillary Salivary Gland of the Göttingen Miniature Pig. J. Endocrinol. 1984, 100, 195–202. [Google Scholar] [CrossRef]
- Pietras, R.J. Sex Pheromone Production by Preputial Gland: The Regulatory Role of Estrogen. Chem. Senses 1981, 6, 391–408. [Google Scholar] [CrossRef]
- Murphy, M.R.; Schneider, G.E. Olfactory Bulb Removal Eliminates Mating Behavior in the Male Golden Hamster. Science 1970, 167, 302–304. [Google Scholar] [CrossRef]
- Michael, R.P.; Keverne, E.B. Primate Sex Pheromones of Vaginal Origin. Nature 1970, 225, 84–85. [Google Scholar] [CrossRef]
- Goodwin, M.; Gooding, K.M.; Regnier, F. Sex Pheromone in the Dog. Science 1979, 203, 559–561. [Google Scholar] [CrossRef]
- Rivard, G.; Klemm, W.R. Two Body Fluids Containing Bovine Estrous Pheromone(s). Chem. Senses 1989, 14, 273–279. [Google Scholar] [CrossRef]
- Hayes, R.A.; Richardson, B.J.; Claus, S.C.; Wyllie, S.G. Semiochemicals and Social Signaling in the Wild European Rabbit in Australia: II. Variations in Chemical Composition of Chin Gland Secretion across Sampling Sites. J. Chem. Ecol. 2002, 28, 2613–2625. [Google Scholar] [CrossRef]
- Thiessen, D.D.; Friend, H.C.; Lindzey, G. Androgen Control of Territorial Marking in the Mongolian Gerbil. Science 1968, 160, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Müller-Schwarze, D. Pheromones in Black-Tailed Deer (Odocoileus hemionus columbianus). Anim. Behav. 1971, 19, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Müller-Schwarze, D.; Müller-Schwarze, C.; Singer, A.G.; Silverstein, R.M. Mammalian Pheromone: Identification of Active Component in the Subauricular Scent of the Male Pronghorn. Science 1974, 183, 860–862. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Krantz, K.; Aplin, R.T. Behavioural, Anatomical and Chemical Aspects of Scent Marking amongst Capybaras (Hydrochoerus hydrochaeris) (Rodentia: Caviomorpha). J. Zool. 1984, 202, 341–360. [Google Scholar] [CrossRef]
- Berüter, J.; Beauchamp, G.K.; Muetterties, E.L. Mammalian Chemical Communication: Perineal Gland Secretion of the Guinea Pig. Physiol. Zool. 1974, 47, 130–136. [Google Scholar] [CrossRef]
- Schultze-Westrum, T. Innerartliche Verständigung durch Düfte beim Gleitbeutler Petaurus breviceps papuanus Thomas (Marsupialia, Phalangeridae). Z. Vergl. Physiol. 1965, 50, 151–220. [Google Scholar] [CrossRef]
- Stumpf, P.; Künzle, H.; Welsch, U. Cutaneous Eccrine Glands of the Foot Pads of the Small Madagascan Tenrec (Echinops telfairi, Insectivora, Tenrecidae): Skin Glands in a Primitive Mammal. Cell Tissue Res. 2004, 315, 59–70. [Google Scholar] [CrossRef]
- Yager, J.A.; Hunter, D.B.; Wilson, M.R.; Allen, O.B. A Source of Cutaneous Maternal Semiochemicals in the Mink? Experientia 1988, 44, 79–81. [Google Scholar] [CrossRef]
- Izard, M.K. Pheromones and Reproduction in Domestic Animals. In Pheromones and Reproduction in Mammals; Academic Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Melrose, D.R.; Reed, H.C.B.; Patterson, R.L.S. Androgen Steroids Associated with Boar Odour as an Aid to the Detection of Oestrus in Pig Artificial Insemination. Br. Vet. J. 1971, 127, 497–502. [Google Scholar] [CrossRef]
- Gosling, L.M. A Reassessment of the Function of Scent Marking in Territories. Z. Tierpsychol. 1982, 60, 89–118. [Google Scholar] [CrossRef]
- Eisenberg, J.F.; Kleiman, D.G. Olfactory Communication in Mammals. Annu. Rev. Ecol. Syst. 1972, 3, 1–32. [Google Scholar] [CrossRef]
- Johnson, R.P. Scent Marking in Mammals. Anim. Behav. 1973, 21, 521–535. [Google Scholar] [CrossRef]
- Jones, R.B.; Nowell, N.W. A Comparison of the Aversive and Female Attractant Properties of Urine from Dominant and Subordinate Male Mice. Anim. Learn. Behav. 1974, 2, 141–144. [Google Scholar] [CrossRef]
- Hayes, R.A.; Richardson, B.J.; Wyllie, S.G. To Fix or Not to Fix: The Role of 2-Phenoxyethanol in Rabbit, Oryctolagus Cuniculus, Chin Gland Secretion. J. Chem. Ecol. 2003, 29, 1051–1064. [Google Scholar] [CrossRef]
- Wirant, S.C.; Halvorsen, K.T.; McGuire, B. Preliminary Observations on the Urinary Behaviour of Female Jack Russell Terriers in Relation to Stage of the Oestrous Cycle, Location, and Age. Appl. Anim. Behav. Sci. 2007, 106, 161–166. [Google Scholar] [CrossRef]
- Müller, C.A.; Manser, M.B. Scent-Marking and Intrasexual Competition in a Cooperative Carnivore with Low Reproductive Skew: Scent-Marking and Intrasexual Competition. Ethology 2008, 114, 174–185. [Google Scholar] [CrossRef]
- Bronson, F.H.; Whitten, W.K. Oestrus-Accelerating Pheromone of Mice: Assay, Androgen-Dependency and Presence in Bladder Urine. Reproduction 1968, 15, 131–134. [Google Scholar] [CrossRef]
- Bronson, F.H.; Caroom, D. Preputial Gland of the Male Mouse: Attractant Function. Reproduction 1971, 25, 279–282. [Google Scholar] [CrossRef]
- Seitz, E. Die Bedeutung Geruchlicher Orientierung Beim Plumplori Nycticebus coucang Boddaert 1785 (Prosimii, Lorisidae). Z. Tierpsychol. 1969, 26, 73–103. [Google Scholar] [CrossRef]
- Harrington, F.H. Urine Marking at Food and Caches in Captive Coyotes. Can. J. Zool. 1982, 60, 776–782. [Google Scholar] [CrossRef]
- Sun, L.; Müller-Schwarze, D. Sibling Recognition in the Beaver: A Field Test for Phenotype Matching. Anim. Behav. 1997, 54, 493–502. [Google Scholar] [CrossRef][Green Version]
- Jordan, N.R.; Golabek, K.A.; Apps, P.J.; Gilfillan, G.D.; McNutt, J.W. Scent-Mark Identification and Scent-Marking Behaviour in African Wild Dogs (Lycaon pictus). Ethology 2013, 119, 644–652. [Google Scholar] [CrossRef]
- Ferkin, M.H.; Johnston, R.E. Meadow Voles, Microtus Pennsylvanicus, Use Multiple Sources of Scent for Sex Recognition. Anim. Behav. 1995, 49, 37–44. [Google Scholar] [CrossRef]
- Hradecký, P. Possible Pheromonal Regulation of Reproduction in Wild Carnivores. J. Chem. Ecol. 1985, 11, 241–250. [Google Scholar] [CrossRef]
- Kiyokawa, Y.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Modulatory Role of Testosterone in Alarm Pheromone Release by Male Rats. Horm. Behav. 2004, 45, 122–127. [Google Scholar] [CrossRef]
- Boissy, A.; Terlouw, C.; Le Neindre, P. Presence of Cues from Stressed Conspecifics Increases Reactivity to Aversive Events in Cattle: Evidence for the Existence of Alarm Substances in Urine. Physiol. Behav. 1998, 63, 489–495. [Google Scholar] [CrossRef]
- Aleksiuk, M. Scent-Mound Communication, Territoriality, and Population Regulation in Beaver (Castor canadensis Kuhl). J. Mammal. 1968, 49, 759–762. [Google Scholar] [CrossRef]
- Bartecki, U.; Heymann, E.W. Field Observations on Scent-marking Behaviour in Saddle-back Tamarins, Saguinus fuscicollis (Callitrichidae, Primates). J. Zool. 1990, 220, 87–99. [Google Scholar] [CrossRef]
- Tschanz, B.; Meyer-Holzapfel, M.; Bachmann, S. Das Informationssystem Bei Braunbären1. Z. Tierpsychol. 2010, 27, 47–72. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Iwata, E.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Regional Differences of Pheromone Production in the Sebaceous Glands of Castrated Goats Treated with Testosterone. J. Vet. Med. Sci. 2000, 62, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, J.F. A Comparative Study of Sandbathing Behavior in Heteromyid Rodents. Behavior 1963, 22, 16–23. [Google Scholar] [CrossRef]
- Hayes, R.A.; Richardson, B.J.; Wyllie, S.G. Semiochemicals and Social Signaling in the Wild European Rabbit in Australia: I. Scent Profiles of Chin Gland Secretion from the Field. J. Chem. Ecol. 2002, 28, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Laska, M.; Bauer, V.; Salazar, L.T.H. Self-Anointing Behavior in Free-Ranging Spider Monkeys (Ateles geoffroyi) in Mexico. Primates 2007, 48, 160–163. [Google Scholar] [CrossRef]
- Xu, Z.; Stoddart, D.M.; Ding, H.; Zhang, J. Self-Anointing Behavior in the Rice-Field Rat, Rattus rattoides. J. Mammal. 1995, 76, 1238–1241. [Google Scholar] [CrossRef]
- Breer, H.; Fleischer, J.; Strotmann, J. Signaling in the Chemosensory Systems: The Sense of Smell: Multiple Olfactory Subsystems. Cell. Mol. Life Sci. 2006, 63, 1465–1475. [Google Scholar] [CrossRef]
- Munger, S.D. Noses within Noses. Nature 2009, 459, 521–522. [Google Scholar] [CrossRef]
- Barrios, A.W.; Sanchez Quinteiro, P.; Salazar, I. The Nasal Cavity of the Sheep and Its Olfactory Sensory Epithelium. Microsc. Res. Tech. 2014, 77, 1052–1059. [Google Scholar] [CrossRef]
- Ramón y Cajal, S. Inducciones Fisiológicas de La Morfología y Conexiones de Las Neuronas. Arch. Pedagog. Cienc. Afines 1906, 1, 216–236. [Google Scholar]
- Salazar, I.; Sanchez-Quinteiro, P.; Barrios, A.W.; López Amado, M.; Vega, J.A. Anatomy of the Olfactory Mucosa. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 164, pp. 47–65. ISBN 978-0-444-63855-7. [Google Scholar]
- McCotter, R.E. The Connection of the Vomeronasal Nerves with the Accessory Olfactory Bulb in the Opossum and Other Mammals. Anat. Rec. 1912, 6, 299–318. [Google Scholar] [CrossRef]
- Salazar, I.; Barrios, A.W.; Sánchez-Quinteiro, P. Revisiting the Vomeronasal System From an Integrated Perspective. Anat. Rec. 2016, 299, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Barrios, A.W.; Núñez, G.; Sanchez Quinteiro, P.; Salazar, I. Anatomy, Histochemistry, and Immunohistochemistry of the Olfactory Subsystems in Mice. Front. Neuroanat. 2014, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, J.; Breer, H. The Grueneberg Ganglion: A Novel Sensory System in the Nose. Histol. Histopathol. 2010, 25, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Storan, M.J.; Key, B. Septal Organ of Grüneberg Is Part of the Olfactory System. J. Comp. Neurol. 2006, 494, 834–844. [Google Scholar] [CrossRef]
- Ortiz-Leal, I.; Torres, M.V.; Villamayor, P.R.; López-Beceiro, A.; Sanchez-Quinteiro, P. The Vomeronasal Organ of Wild Canids: The Fox (Vulpes vulpes) as a Model. J. Anat. 2020, 237, 890–906. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Krosnowski, K.; Zhang, L.; Bekkerman, M.; Lin, W. Chemoreception Regulates Chemical Access to Mouse Vomeronasal Organ: Role of Solitary Chemosensory Cells. PLoS ONE 2010, 5, e11924. [Google Scholar] [CrossRef] [PubMed]
- Juilfs, D.M.; Fülle, H.J.; Zhao, A.Z.; Houslay, M.D.; Garbers, D.L.; Beavo, J.A. A Subset of Olfactory Neurons That Selectively Express cGMP-Stimulated Phosphodiesterase (PDE2) and Guanylyl Cyclase-D Define a Unique Olfactory Signal Transduction Pathway. Proc. Natl. Acad. Sci. USA 1997, 94, 3388–3395. [Google Scholar] [CrossRef]
- Larriva-Sahd, J. Cytological Organization of the Alpha Component of the Anterior Olfactory Nucleus and Olfactory Limbus. Front. Neuroanat. 2012, 6, 23. [Google Scholar] [CrossRef]
- Valverde, F.; López-Mascaraque, L.; De Carlos, J.A. Structure of the Nucleus Olfactorius Anterior of the Hedgehog (Erinaceus europaeus). J. Comp. Neurol. 1989, 279, 581–600. [Google Scholar] [CrossRef]
- Villamayor, P.R.; Cifuentes, J.M.; Quintela, L.; Barcia, R.; Sanchez-Quinteiro, P. Structural, Morphometric and Immunohistochemical Study of the Rabbit Accessory Olfactory Bulb. Brain Struct. Funct. 2020, 225, 203–226. [Google Scholar] [CrossRef]
- Ortiz-Leal, I.; Torres, M.V.; Vargas-Barroso, V.; Fidalgo, L.E.; López-Beceiro, A.M.; Larriva-Sahd, J.A.; Sánchez-Quinteiro, P. The Olfactory Limbus of the Red Fox (Vulpes vulpes). New Insights Regarding a Noncanonical Olfactory Bulb Pathway. Front. Neuroanat. 2023, 16, 1097467. [Google Scholar] [CrossRef] [PubMed]
- Menco, B. Ultrastructural Studies on Membrane, Cytoskeletal, Mucous, and Protective Compartments in Olfaction. Microsc. Res. Tech. 1992, 22, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Dryer, L.; Berghard, A. Odorant Receptors: A Plethora of G-Protein-Coupled Receptors. Trends Pharmacol. Sci. 1999, 20, 413–417. [Google Scholar] [CrossRef]
- Zhang, X.; Firestein, S. The Olfactory Receptor Gene Superfamily of the Mouse. Nat. Neurosci. 2002, 5, 124–133. [Google Scholar] [CrossRef]
- Malnic, B.; Hirono, J.; Sato, T.; Buck, L.B. Combinatorial Receptor Codes for Odors. Cell 1999, 96, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Crespo, C.; Liberia, T.; Blasco-Ibáñez, J.M.; Nácher, J.; Varea, E. Cranial Pair I: The Olfactory Nerve: The olfactory nerve. Anat. Rec. 2019, 302, 405–427. [Google Scholar] [CrossRef]
- Mombaerts, P.; Wang, F.; Dulac, C.; Chao, S.K.; Nemes, A.; Mendelsohn, M.; Edmondson, J.; Axel, R. Visualizing an Olfactory Sensory Map. Cell 1996, 87, 675–686. [Google Scholar] [CrossRef]
- Price, J.L.; Powell, T.P. Certain Observations on the Olfactory Pathway. J. Anat. 1971, 110, 105–126. [Google Scholar]
- Höfer, D.; Shin, D.; Drenckhahn, D. Identification of Cytoskeletal Markers for the Different Microvilli and Cell Types of the Rat Vomeronasal Sensory Epithelium. J. Neurocytol. 2000, 29, 147–156. [Google Scholar] [CrossRef]
- Salazar, I.; Quinteiro, P.S.; Cifuentes, J.M. Comparative Anatomy of the Vomeronasal Cartilage in Mammals: Mink, Cat, Dog, Pig, Cow and Horse. Ann. Anat. 1995, 177, 475–481. [Google Scholar] [CrossRef]
- Villamayor, P.R.; Cifuentes, J.M.; Fdz-de-Troconiz, P.; Sanchez-Quinteiro, P. Morphological and Immunohistochemical Study of the Rabbit Vomeronasal Organ. J. Anat. 2018, 233, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Wöhrmann-Repenning, A. Comparative anatomical studies of the vomeronasal complex and the rostral palate of various mammals. Gegenbaurs Morphol. Jahrb. 1984, 130, 501–530. [Google Scholar] [PubMed]
- Salazar, I.; Sánchez-Quinteiro, P.; Alemañ, N.; Prieto, D. Anatomical, Immnunohistochemical and Physiological Characteristics of the Vomeronasal Vessels in Cows and Their Possible Role in Vomeronasal Reception. J. Anat. 2008, 212, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, C.J. Neurobehavioral Evidence for the Involvement of the Vomeronasal System in Mammalian Reproduction. Neurosci. Biobehav. Rev. 1979, 3, 301–341. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Sánchez Quinteiro, P. Differential Development of Binding Sites for Four Lectins in the Vomeronasal System of Juvenile Mouse: From the Sensory Transduction Site to the First Relay Stage. Brain Res. 2003, 979, 15–26. [Google Scholar] [CrossRef]
- Ressler, K.J.; Sullivan, S.L.; Buck, L.B. A Zonal Organization of Odorant Receptor Gene Expression in the Olfactory Epithelium. Cell 1993, 73, 597–609. [Google Scholar] [CrossRef]
- Zapiec, B.; Mombaerts, P. The Zonal Organization of Odorant Receptor Gene Choice in the Main Olfactory Epithelium of the Mouse. Cell Rep. 2020, 30, 4220–4234.e5. [Google Scholar] [CrossRef]
- Trinh, K.; Storm, D.R. Vomeronasal Organ Detects Odorants in Absence of Signaling through Main Olfactory Epithelium. Nat. Neurosci. 2003, 6, 519–525. [Google Scholar] [CrossRef]
- Hohenbrink, P.; Mundy, N.I.; Zimmermann, E.; Radespiel, U. First Evidence for Functional Vomeronasal 2 Receptor Genes in Primates. Biol. Lett. 2013, 9, 20121006. [Google Scholar] [CrossRef]
- Pro-Sistiaga, P.; Mohedano-Moriano, A.; Ubeda-Bañon, I.; Del Mar Arroyo-Jimenez, M.; Marcos, P.; Artacho-Pérula, E.; Crespo, C.; Insausti, R.; Martinez-Marcos, A. Convergence of Olfactory and Vomeronasal Projections in the Rat Basal Telencephalon. J. Comp. Neurol. 2007, 504, 346–362. [Google Scholar] [CrossRef]
- Grüneberg, H. A Ganglion Probably Belonging to the N. Terminalis System in the Nasal Mucosa of the Mouse. Z. Anat. Entwickl. Gesch. 1973, 140, 39–52. [Google Scholar] [CrossRef]
- Fleischer, J.; Schwarzenbacher, K.; Breer, H. Expression of Trace Amine-Associated Receptors in the Grueneberg Ganglion. Chem. Senses 2007, 32, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, J.; Schwarzenbacher, K.; Besser, S.; Hass, N.; Breer, H. Olfactory Receptors and Signalling Elements in the Grueneberg Ganglion. J. Neurochem. 2006, 98, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Pyrski, M.; Biel, M.; Leinders-Zufall, T.; Zufall, F. Grueneberg Ganglion Neurons Are Finely Tuned Cold Sensors. J. Neurosci. 2010, 30, 7563–7568. [Google Scholar] [CrossRef]
- Rodolfo-Masera, T. Su l’esistenza di un particolare organo olfattivo nel setto nasale della cavia e di altri roditori. Arch. Ital. Anat. Embriol. 1943, 48, 157–213. [Google Scholar]
- Tian, H.; Ma, M. Molecular Organization of the Olfactory Septal Organ. J. Neurosci. 2004, 24, 8383–8390. [Google Scholar] [CrossRef]
- Weiler, E.; Farbman, A.I. The Septal Organ of the Rat During Postnatal Development. Chem. Senses 2003, 28, 581–593. [Google Scholar] [CrossRef]
- Ma, M.; Grosmaitre, X.; Iwema, C.L.; Baker, H.; Greer, C.A.; Shepherd, G.M. Olfactory Signal Transduction in the Mouse Septal Organ. J. Neurosci. 2003, 23, 317–324. [Google Scholar] [CrossRef]
- Marshall, D.A.; Maruniak, J.A. Masera’s Organ Responds to Odorants. Brain Res. 1986, 366, 329–332. [Google Scholar] [CrossRef]
- Wysocki, C.J.; Wellington, J.L.; Beauchamp, G.K. Access of Urinary Nonvolatiles to the Mammalian Vomeronasal Organ. Science 1980, 207, 781–783. [Google Scholar] [CrossRef]
- Tizzano, M.; Cristofoletti, M.; Sbarbati, A.; Finger, T.E. Expression of Taste Receptors in Solitary Chemosensory Cells of Rodent Airways. BMC Pulm. Med. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Silver, W.L.; Moulton, D.G. Chemosensitivity of Rat Nasal Trigeminal Receptors. Physiol. Behav. 1982, 28, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Filoramo, N.I.; Schwenk, K. The Mechanism of Chemical Delivery to the Vomeronasal Organs in Squamate Reptiles: A Comparative Morphological Approach. J. Exp. Zool. 2009, 311, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, D.; Kaneoya, Y.; Tonomori, W.; Kitayama, C. Histological Features and Gα olf Expression Patterns in the Nasal Cavity of Sea Turtles. J. Anat. 2023, 243, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.O.; Eisthen, H.L. Comparative Anatomy and Physiology of Chemical Senses in Amphibians. In Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates; University of California Press: Berkeley, CA, USA, 2008; ISBN 978-0-520-25278-3. [Google Scholar]
- Monti-Bloch, L.; Jennings-White, C.; Berliner, D.L. The Human Vomeronasal System: A Review. Ann. N. Y. Acad. Sci. 1998, 855, 373–389. [Google Scholar] [CrossRef]
- Tirindelli, R. Coding of Pheromones by Vomeronasal Receptors. Cell Tissue Res. 2021, 383, 367–386. [Google Scholar] [CrossRef]
- Shinohara, H.; Asano, T.; Kato, K. Differential Localization of G-Proteins Gi and Go in the Accessory Olfactory Bulb of the Rat. J. Neurosci. 1992, 12, 1275–1279. [Google Scholar] [CrossRef]
- Dulac, C.; Axel, R. A Novel Family of Genes Encoding Putative Pheromone Receptors in Mammals. Cell 1995, 83, 195–206. [Google Scholar] [CrossRef]
- Herrada, G.; Dulac, C. A Novel Family of Putative Pheromone Receptors in Mammals with a Topographically Organized and Sexually Dimorphic Distribution. Cell 1997, 90, 763–773. [Google Scholar] [CrossRef]
- Matsunami, H.; Buck, L.B. A Multigene Family Encoding a Diverse Array of Putative Pheromone Receptors in Mammals. Cell 1997, 90, 775–784. [Google Scholar] [CrossRef]
- Ryba, N.J.P.; Tirindelli, R. A New Multigene Family of Putative Pheromone Receptors. Neuron 1997, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Rivière, S.; Challet, L.; Fluegge, D.; Spehr, M.; Rodriguez, I. Formyl Peptide Receptor-like Proteins Are a Novel Family of Vomeronasal Chemosensors. Nature 2009, 459, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A. The Vomeronasal System. Cell. Mol. Life Sci. 2001, 58, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Papes, F.; Logan, D.W.; Stowers, L. The Vomeronasal Organ Mediates Interspecies Defensive Behaviors through Detection of Protein Pheromone Homologs. Cell 2010, 141, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.; Kubie, J.L. The Role of the Ophidian Vomeronasal System in Species-Typical Behavior. Trends Neurosci. 1984, 7, 472–477. [Google Scholar] [CrossRef]
- Placyk, J.S., Jr.; Graves, B.M. Prey Detection by Vomeronasal Chemoreception in a Plethodontid Salamander. J. Chem. Ecol. 2002, 28, 1017–1036. [Google Scholar] [CrossRef]
- Halpern, M.; Daniels, Y.; Zuri, I. The Role of the Vomeronasal System in Food Preferences of the Gray Short-Tailed Opossum, Monodelphis Domestica. Nutr. Metab. 2005, 2, 6. [Google Scholar] [CrossRef]
- Dagg, A.I.; Taub, A. Flehmen. Mammalia 1970, 34, 686–695. [Google Scholar] [CrossRef]
- Estes, R.D. The Role of the Vomeronasal Organ in Mammalian Reproduction. Mammalia 1972, 36, 315–341. [Google Scholar] [CrossRef]
- Hart, B.L. Flehmen Behavior and Vomeronasal Organ Function. In Chemical Signals in Vertebrates 3; Müller-Schwarze, D., Silverstein, R.M., Eds.; Springer: Boston, MA, USA, 1983; pp. 87–103. ISBN 978-1-4757-9652-0. [Google Scholar]
- Hart, L.A.; Hart, B.L. Flehmen, Osteophagia, and Other Behaviors of Giraffes (Giraffa giraffa angolensis): Vomeronasal Organ Adaptation. Animals 2023, 13, 354. [Google Scholar] [CrossRef]
- Jacobson, L. Anatomisk Beskrivelse over et myt Organ i Huusdyrenes Naese. Vet. Selsk. Skr. 1813, 2, 209–246. [Google Scholar]
- Ruysch, F. Thesaurus Anatomicus Tertius; J. Wolters: Amsterdam, Netherlands, 1703; pp. 48–49. [Google Scholar]
- Dursy, E. Zur Entwicklungsgeschichte des Kopfes des Menschen und der höheren Wirbelthiere: Mit Holzschnitten und einem Atlas von neun Kupfertafeln mit erklärendem Texte; H. Laupp: Tübingen, Germany, 1869. [Google Scholar]
- Kölliker, A. Über Die Jacobson’schen Organe Des Menschen. In Festschrift zu dem 40 jährign Professoren-Jubiläum des Herrn Franz von Rinecker 31 März 1877; Wilhelm Engelmann: Leipzig, Germany, 1877; pp. 3–11. [Google Scholar]
- Balogh, C. Das Jacobson’sche Organ des Schafes, Sitzungsberichte Der Kais. Akad. Der Wiss. Wien. 1860, 42, 449–476. [Google Scholar]
- Klein, E. Memoirs: Contributions to the Minute Anatomy of the Nasal Mucous Membrane. J. Cell Sci. 1881, 2, 98–113. [Google Scholar] [CrossRef]
- Piana, G. Contribuzioni alla conoscenza della strutture e della funzione dell’ organo di jacobson. Deusch. Zeitsch. f. Tiermedizin 1882, 7, 325. [Google Scholar]
- Retzius, G. Die riechzellen der ophidier in der riechschleimhaut und im jacobson’schen organ. Biol. Untersuch. Neue Folge 1894, 6, 48–51. [Google Scholar]
- Gudden, B.V. Experimentaluntersuchungen Über Das Peripherische Und Centrale Nervensystem. Arch. Psychiatr. Nervenkr. 1870, 2, 693–723. [Google Scholar] [CrossRef]
- Ramón y Cajal, S.R. Textura Del Lobulo Olfativo Accesorio. Rev. Micros. 1902, 1, 141–150. [Google Scholar]
- Vandenbergh, J.G. Male Odor Accelerates Female Sexual Maturation in Mice. Endocrinology 1969, 84, 658–660. [Google Scholar] [CrossRef]
- Whitten, W.K. Modification of the Oestrous Cycle of the Mouse by External Stimuli Associated with the Male. J. Endocrinol. 1956, 13, 399–404. [Google Scholar] [CrossRef]
- Powers, J.B.; Winans, S.S. Vomeronasal Organ: Critical Role in Mediating Sexual Behavior of the Male Hamster. Science 1975, 187, 961–963. [Google Scholar] [CrossRef]
- Grus, W.E.; Zhang, J. Origin and Evolution of the Vertebrate Vomeronasal System Viewed through System-Specific Genes. Bioessays 2006, 28, 709–718. [Google Scholar] [CrossRef] [PubMed]
- González, A. Lungfishes, like Tetrapods, Possess a Vomeronasal System. Front. Neuroanat. 2010, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Nakamuta, S.; Nakamuta, N.; Taniguchi, K.; Taniguchi, K. Histological and Ultrastructural Characteristics of the Primordial Vomeronasal Organ in Lungfish. Anat. Rec. 2012, 295, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Nakamuta, S.; Yamamoto, Y.; Miyazaki, M.; Sakuma, A.; Nikaido, M.; Nakamuta, N. Type 1 Vomeronasal Receptors Expressed in the Olfactory Organs of Two African Lungfish, Protopterus annectens and Protopterus amphibius. J. Comp. Neurol. 2023, 531, 116–131. [Google Scholar] [CrossRef]
- Wittmer, C.; Nowack, C. Epithelial Crypts: A Complex and Enigmatic Olfactory Organ in African and South American Lungfish (Lepidosireniformes, Dipnoi). J. Morphol. 2017, 278, 791–800. [Google Scholar] [CrossRef]
- Swaney, W.T.; Keverne, E.B. The Evolution of Pheromonal Communication. Behav. Brain Res. 2009, 200, 239–247. [Google Scholar] [CrossRef]
- Pihlström, H. Comparative Anatomy and Physiology of Chemical Senses in Aquatic Mammals. In Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates; Thewissen, J.G.M., Ed.; University of California Press: Berkeley, CA, USA, 2008; ISBN 978-0-520-25278-3. [Google Scholar]
- Suzuki, H.; Nishida, H.; Kondo, H.; Yoda, R.; Iwata, T.; Nakayama, K.; Enomoto, T.; Wu, J.; Moriya-Ito, K.; Miyazaki, M.; et al. A Single Pheromone Receptor Gene Conserved across 400 Million Years of Vertebrate Evolution. Mol. Biol. Evol. 2018, 35, 2928–2939. [Google Scholar] [CrossRef]
- Breathnach, A.S. The Cetacean Central Nervous System. Biol. Rev. 1960, 35, 187–230. [Google Scholar] [CrossRef]
- McGowen, M.R.; Clark, C.; Gatesy, J. The Vestigial Olfactory Receptor Subgenome of Odontocete Whales: Phylogenetic Congruence between Gene-Tree Reconciliation and Supermatrix Methods. Syst. Biol. 2008, 57, 574–590. [Google Scholar] [CrossRef]
- Kishida, T. Olfaction of Aquatic Amniotes. Cell Tissue Res. 2021, 383, 353–365. [Google Scholar] [CrossRef]
- Halpern, M. Nasal Chemical Senses in Reptiles: Structure and Function. In Hormones, Brain, and Behaviour. Biology of the Reptilia; University of Chicago Press: Chicago, IL, USA, 1992; Volume 18, pp. 423–523. [Google Scholar]
- Schwenk, K. Of Tongues and Noses: Chemoreception in Lizards and Snakes. Trends Ecol. Evol. 1995, 10, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, D.; Yamamoto, Y.; Nakamuta, N.; Taniguchi, K.; Taniguchi, K. Lectin Histochemical Studies on the Olfactory Epithelium and Vomeronasal Organ in the Japanese Striped Snake, Elaphe Quadrivirgata. J. Morphol. 2010, 271, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, D.; Yamamoto, Y.; Nakamuta, N.; Taniguchi, K.; Taniguchi, K. Seasonal Changes in the Histochemical Properties of the Olfactory Epithelium and Vomeronasal Organ in the Japanese Striped Snake, Elaphe Quadrivirgata: Seasonal Changes in Snake Olfactory Organs. Anat. Histol. Embryol. 2012, 41, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, T.; Matsuzaki, O. Odor Responses of the Vomeronasal System in Reeve’s Turtle, Geoclemys reevesii. Brain Behav. Evol. 1993, 41, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.S. Studies on the Comparative Embryology of the Reptilian Nose. Bull. Mus. Comp. Zool. Harv. Coll. 1959, 120, 261–275. [Google Scholar]
- Parsons, T.S. Evolution of the Nasal Structure in the Lower Tetrapods. Am. Zool. 1967, 7, 397–413. [Google Scholar] [CrossRef]
- Houck, L.D. Pheromone Communication in Amphibians and Reptiles. Annu. Rev. Physiol. 2009, 71, 161–176. [Google Scholar] [CrossRef]
- Eisthen, H.L. Presence of the Vomeronasal System in Aquatic Salamanders. Philos. Trans. R. Soc. Lond. B 2000, 355, 1209–1213. [Google Scholar] [CrossRef]
- Silva, L.; Antunes, A. Vomeronasal Receptors in Vertebrates and the Evolution of Pheromone Detection. Annu. Rev. Anim. Biosci. 2017, 5, 353–370. [Google Scholar] [CrossRef]
- Bhatnagar, K.P.; Meisami, E. Vomeronasal Organ in Bats and Primates: Extremes of Structural Variability and Its Phylogenetic Implications. Microsc. Res. Tech. 1998, 43, 465–475. [Google Scholar] [CrossRef]
- Suárez, R.; Fernández-Aburto, P.; Manger, P.R.; Mpodozis, J. Deterioration of the Gαo Vomeronasal Pathway in Sexually Dimorphic Mammals. PLoS ONE 2011, 6, e26436. [Google Scholar] [CrossRef]
- Kondoh, D.; Watanabe, K.; Nishihara, K.; Ono, Y.S.; Nakamura, K.G.; Yuhara, K.; Tomikawa, S.; Sugimoto, M.; Kobayashi, S.; Horiuchi, N.; et al. Histological Properties of Main and Accessory Olfactory Bulbs in the Common Hippopotamus. Brain Behav. Evol. 2017, 90, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, J.; Korzekwa, A.; Kawai, Y.K.; Robstad, C.A.; Rosell, F.; Kondoh, D. The Vomeronasal System in Semiaquatic Beavers. J. Anat. 2022, 241, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Grus, W.E.; Shi, P.; Zhang, J. Largest Vertebrate Vomeronasal Type 1 Receptor Gene Repertoire in the Semiaquatic Platypus. Mol. Biol. Evol. 2007, 24, 2153–2157. [Google Scholar] [CrossRef][Green Version]
- Cartmill, M. Rethinking Primate Origins: The Characteristic Primate Traits Cannot Be Explained Simply as Adaptations to Arboreal Life. Science 1974, 184, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Heesy, C.P.; Ross, C.F. Evolution of Activity Patterns and Chromatic Vision in Primates: Morphometrics, Genetics and Cladistics. J. Hum. Evol. 2001, 40, 111–149. [Google Scholar] [CrossRef]
- Wang, G.; Shi, P.; Zhu, Z.; Zhang, Y.-P. More Functional V1R Genes Occur in Nest-Living and Nocturnal Terricolous Mammals. Genom. Biol. Evol. 2010, 2, 277–283. [Google Scholar] [CrossRef]
- Garrett, E.C.; Dennis, J.C.; Bhatnagar, K.P.; Durham, E.L.; Burrows, A.M.; Bonar, C.J.; Steckler, N.K.; Morrison, E.E.; Smith, T.D. The Vomeronasal Complex of Nocturnal Strepsirhines and Implications for the Ancestral Condition in Primates: Vomeronasal Complex of Nocturnal Strepsirhines. Anat. Rec. 2013, 296, 1881–1894. [Google Scholar] [CrossRef]
- Smith, T.D.; Garrett, E.C.; Bhatnagar, K.P.; Bonar, C.J.; Bruening, A.E.; Dennis, J.C.; Kinznger, J.H.; Johnson, E.W.; Morrison, E.E. The Vomeronasal Organ of New World Monkeys (Platyrrhini). Anat. Rec. 2011, 294, 2158–2178. [Google Scholar] [CrossRef]
- Smith, T.D.; Siegel, M.I.; Bonar, C.J.; Bhatnagar, K.P.; Mooney, M.P.; Burrows, A.M.; Smith, M.A.; Maico, L.M. The Existence of the Vomeronasal Organ in Postnatal Chimpanzees and Evidence for Its Homology with That of Humans. J. Anat. 2001, 198, 77–82. [Google Scholar] [CrossRef]
- Smith, T.D.; Siegel, M.I.; Bhatnagar, K.P. Reappraisal of the Vomeronasal System of Catarrhine Primates: Ontogeny, Morphology, Functionality, and Persisting Questions. Anat. Rec. 2001, 265, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Boehm, N.; Roos, J.; Gasser, B. Luteinizing Hormone-Releasing Hormone (LHRH)-Expressing Cells in the Nasal Septum of Human Fetuses. Dev. Brain Res. 1994, 82, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kjær, I.; Hansen, B.F. The Human Vomeronasal Organ: Prenatal Developmental Stages and Distribution of Luteinizing Hormone-Releasing Hormone. Eur. J. Oral Sci. 1996, 104, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, T. The Development of the Olfactory and the Accessory Olfactory Formations in Human Embryos and Fetuses. J. Comp. Neurol. 1940, 73, 431–468. [Google Scholar] [CrossRef]
- Moran, D.T.; Jafek, B.W.; Rowley, J.C. The Vomeronasal (Jacobson’s) Organ in Man: Ultrastructure and Frequency of Occurence. J. Steroid Biochem. Mol. Biol. 1991, 39, 545–552. [Google Scholar] [CrossRef]
- Hazem, A.G.; Ahmed, A.T.; Tantawy, A.M.; Diaa, M.H.; Hesham, M.S. The Vomeronasal (Jacobson’s) Organ in Adult Humans: Frequency of Occurrence and Enzymatic Study. Acta Otolaryngol. 1998, 118, 409–412. [Google Scholar] [CrossRef]
- Witt, M.; Hummel, T. Vomeronasal Versus Olfactory Epithelium: Is There a Cellular Basis for Human Vomeronasal Perception? Int. Rev. Cytol. 2006, 248, 209–259. [Google Scholar] [CrossRef]
- Meredith, M. Human Vomeronasal Organ Function: A Critical Review of Best and Worst Cases. Chem. Senses 2001, 26, 433–445. [Google Scholar] [CrossRef]
- Zhang, J.; Webb, D.M. Evolutionary Deterioration of the Vomeronasal Pheromone Transduction Pathway in Catarrhine Primates. Proc. Natl. Acad. Sci. USA 2003, 100, 8337–8341. [Google Scholar] [CrossRef]
- Smith, T.D.; Laitman, J.T.; Bhatnagar, K.P. The Shrinking Anthropoid Nose, the Human Vomeronasal Organ, and the Language of Anatomical Reduction: The Shrinking Anthropoid Nose. Anat. Rec. 2014, 297, 2196–2204. [Google Scholar] [CrossRef]
- Negus, V.E. The Organ of Jacobson. J. Anat. 1956, 90, 515–519. [Google Scholar] [PubMed]
- Salazar, I.; Sanchez-Quinteiro, P. Supporting Tissue and Vasculature of the Mammalian Vomeronasal Organ: The Rat as a Model. Microsc. Res. Tech. 1998, 41, 492–505. [Google Scholar] [CrossRef]
- Døving, K.B.; Trotier, D. Structure and Function of the Vomeronasal Organ. J. Exp. Biol. 1998, 201, 2913–2925. [Google Scholar] [CrossRef] [PubMed]
- Menco, B.P.M. Ultrastructural Aspects of Olfactory Signaling. Chem. Senses 1997, 22, 295–311. [Google Scholar] [CrossRef]
- Menco, B.P.M.; Carr, V.M.; Ezeh, P.I.; Liman, E.R.; Yankova, M.P. Ultrastructural Localization of G-Proteins and the Channel Protein TRP2 to Microvilli of Rat Vomeronasal Receptor Cells. J. Comp. Neurol. 2001, 438, 468–489. [Google Scholar] [CrossRef]
- Salazar, I.; Sanchez-Quinteiro, P.; Cifuentes, J.M.; De Troconiz, P.F. General Organization of the Perinatal and Adult Accessory Olfactory Bulb in Mice. Anat. Rec. 2006, 288, 1009–1025. [Google Scholar] [CrossRef]
- Jungblut, L.D.; Reiss, J.O.; Pozzi, A.G. Olfactory Subsystems in the Peripheral Olfactory Organ of Anuran Amphibians. Cell Tissue Res. 2021, 383, 289–299. [Google Scholar] [CrossRef]
- Mendoza, A.S.; Kühnel, W. Morphological Evidence for a Direct Innervation of the Mouse Vomeronasal Glands. Cell Tissue Res. 1987, 247, 457–459. [Google Scholar] [CrossRef]
- Takami, S.; Getchell, M.L.; Getchell, T.V. Resolution of Sensory and Mucoid Glycoconjugates with Terminal α-Galactose Residues in the Mucomicrovillar Complex of the Vomeronasal Sensory Epithelium by Dual Confocal Laser Scanning Microscopy. Cell Tissue Res. 1995, 280, 211–216. [Google Scholar] [CrossRef]
- Kondoh, D.; Tomiyasu, J.; Itakura, R.; Sugahara, M.; Yanagawa, M.; Watanabe, K.; Alviola, P.A.; Yap, S.A.; Cosico, E.A.; Cruz, F.A.; et al. Comparative Histological Studies on Properties of Polysaccharides Secreted by Vomeronasal Glands of Eight Laurasiatheria Species. Acta Histochem. 2020, 122, 151515. [Google Scholar] [CrossRef]
- Salazar, I.; Sánchez Quinteiro, P.; Cifuentes, J.M.; Fernández, P.; Lombardero, M. Distribution of the Arterial Supply to the Vomeronasal Organ in the Cat. Anat. Rec. 1997, 247, 129–136. [Google Scholar] [CrossRef]
- Meredith, M.; O’Connell, R.J. Efferent Control of Stimulus Access to the Hamster Vomeronasal Organ. J. Physiol. 1979, 286, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R. Autonomic Innervation of the Vomeronasal Organ of the Cat. Physiol. Behav. 1982, 28, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M. Chronic Recording of Vomeronasal Pump Activation in Awake Behaving Hamsters. Physiol. Behav. 1994, 56, 345–354. [Google Scholar] [CrossRef]
- Halpern, M.; Shapiro, L.S.; Jia, C. Heterogeneity in the Accessory Olfactory System. Chem. Senses 1998, 23, 477–481. [Google Scholar] [CrossRef][Green Version]
- Weiß, E.; Kretschmer, D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol. 2018, 39, 815–829. [Google Scholar] [CrossRef]
- Villamayor, P.R.; Robledo, D.; Fernández, C.; Gullón, J.; Quintela, L.; Sánchez-Quinteiro, P.; Martínez, P. Analysis of the Vomeronasal Organ Transcriptome Reveals Variable Gene Expression Depending on Age and Function in Rabbits. Genomics 2021, 113, 2240–2252. [Google Scholar] [CrossRef]
- Liberles, S.D.; Horowitz, L.F.; Kuang, D.; Contos, J.J.; Wilson, K.L.; Siltberg-Liberles, J.; Liberles, D.A.; Buck, L.B. Formyl Peptide Receptors Are Candidate Chemosensory Receptors in the Vomeronasal Organ. Proc. Natl. Acad. Sci. USA 2009, 106, 9842–9847. [Google Scholar] [CrossRef]
- Bufe, B.; Teuchert, Y.; Schmid, A.; Pyrski, M.; Pérez-Gómez, A.; Eisenbeis, J.; Timm, T.; Ishii, T.; Lochnit, G.; Bischoff, M.; et al. Bacterial MgrB Peptide Activates Chemoreceptor Fpr3 in Mouse Accessory Olfactory System and Drives Avoidance Behaviour. Nat. Commun. 2019, 10, 4889. [Google Scholar] [CrossRef]
- Swain, S.L. T Cell Subsets and the Recognition of MHC Class. Immunol. Rev. 1983, 74, 129–142. [Google Scholar] [CrossRef]
- Singh, P. Chemosensation and Genetic Individuality. Reproduction 2001, 121, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A.; Kendrick, K.M. Mammalian Social Odours: Attraction and Individual Recognition. Philos. Trans. R. Soc. B 2006, 361, 2061–2078. [Google Scholar] [CrossRef] [PubMed]
- Ruff, J.S.; Nelson, A.C.; Kubinak, J.L.; Potts, W.K. MHC Signaling during Social Communication. In Self and Nonself; Advances in Experimental Medicine and Biology; López-Larrea, C., Ed.; Springer: New York, NY, USA, 2012; Volume 738, pp. 290–313. ISBN 978-1-4614-1679-1. [Google Scholar]
- Yamazaki, K.; Beauchamp, G.K. Genetic Basis for MHC-Dependent Mate Choice. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2007; Volume 59, pp. 129–145. ISBN 978-0-12-017660-1. [Google Scholar]
- Leinders-Zufall, T.; Brennan, P.; Widmayer, P.; Shivalingappa, P.C.; Maul-Pavicic, A.; Jäger, M.; Li, X.-H.; Breer, H.; Zufall, F.; Boehm, T. MHC Class I Peptides as Chemosensory Signals in the Vomeronasal Organ. Science 2004, 306, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Konjević, D.; Erman, V.; Bujanić, M.; Svetličić, I.; Arbanasić, H.; Lubura Strunjak, S.; Galov, A. Wild Boar (Sus scrofa)—Fascioloides Magna Interaction from the Perspective of the MHC Genes. Pathogens 2022, 11, 1359. [Google Scholar] [CrossRef]
- Milinski, M.; Griffiths, S.; Wegner, K.M.; Reusch, T.B.H.; Haas-Assenbaum, A.; Boehm, T. Mate Choice Decisions of Stickleback Females Predictably Modified by MHC Peptide Ligands. Proc. Natl. Acad. Sci. USA 2005, 102, 4414–4418. [Google Scholar] [CrossRef]
- Leinders-Zufall, T.; Lane, A.P.; Puche, A.C.; Ma, W.; Novotny, M.V.; Shipley, M.T.; Zufall, F. Ultrasensitive Pheromone Detection by Mammalian Vomeronasal Neurons. Nature 2000, 405, 792–796. [Google Scholar] [CrossRef]
- Spehr, M.; Kelliher, K.R.; Li, X.-H.; Boehm, T.; Leinders-Zufall, T.; Zufall, F. Essential Role of the Main Olfactory System in Social Recognition of Major Histocompatibility Complex Peptide Ligands. J. Neurosci. 2006, 26, 1961–1970. [Google Scholar] [CrossRef]
- Liman, E.R.; Corey, D.P.; Dulac, C. TRP2: A Candidate Transduction Channel for Mammalian Pheromone Sensory Signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 5791–5796. [Google Scholar] [CrossRef]
- Leypold, B.G.; Yu, C.R.; Leinders-Zufall, T.; Kim, M.M.; Zufall, F.; Axel, R. Altered Sexual and Social Behaviors in Trp2 Mutant Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 6376–6381. [Google Scholar] [CrossRef]
- Stowers, L.; Holy, T.E.; Meister, M.; Dulac, C.; Koentges, G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science 2002, 295, 1493–1500. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Li, Q.; Li, B.; Larkin, D.M.; Lee, C.; Storz, J.F.; Antunes, A.; Greenwold, M.J.; Meredith, R.W.; et al. Comparative Genomics Reveals Insights into Avian Genome Evolution and Adaptation. Science 2014, 346, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhang, J. Comparative Genomic Analysis Identifies an Evolutionary Shift of Vomeronasal Receptor Gene Repertoires in the Vertebrate Transition from Water to Land. Genome Res. 2007, 17, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Massa, H.F.; Hsu, L.; Trask, B.J. Extreme Variability among Mammalian V1R Gene Families. Genome Res. 2010, 20, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.; Greer, C.A.; Mok, M.Y.; Mombaerts, P. A Putative Pheromone Receptor Gene Expressed in Human Olfactory Mucosa. Nat. Genet. 2000, 26, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.J.; Kelliher, K.R. Complementary Roles of the Main and Accessory Olfactory Systems in Mammalian Mate Recognition. Annu. Rev. Physiol. 2009, 71, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Omura, M.; Mombaerts, P. Trpc2-Expressing Sensory Neurons in the Main Olfactory Epithelium of the Mouse. Cell Rep. 2014, 8, 583–595. [Google Scholar] [CrossRef]
- Omura, M.; Mombaerts, P. Trpc2-Expressing Sensory Neurons in the Mouse Main Olfactory Epithelium of Type B Express the Soluble Guanylate Cyclase Gucy1b2. Mol. Cell. Neurosci. 2015, 65, 114–124. [Google Scholar] [CrossRef]
- Schneider, N.Y.; Fletcher, T.P.; Shaw, G.; Renfree, M.B. Goα Expression in the Vomeronasal Organ and Olfactory Bulb of the Tammar Wallaby. Chem. Senses 2012, 37, 567–577. [Google Scholar] [CrossRef][Green Version]
- Suárez, R.; Mpodozis, J. Heterogeneities of Size and Sexual Dimorphism between the Subdomains of the Lateral-Innervated Accessory Olfactory Bulb (AOB) of Octodon degus (Rodentia: Hystricognathi). Behav. Brain Res. 2009, 198, 306–312. [Google Scholar] [CrossRef]
- Halpern, M.; Shapiro, L.S.; Jia, C. Differential Localization of G Proteins in the Opossum Vomeronasal System. Brain Res. 1995, 677, 157–161. [Google Scholar] [CrossRef]
- Young, J.M.; Trask, B.J. V2R Gene Families Degenerated in Primates, Dog and Cow, but Expanded in Opossum. Trends Genet. 2007, 23, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Cifuentes, J.M.; Sánchez-Quinteiro, P. Morphological and Immunohistochemical Features of the Vomeronasal System in Dogs. Anat. Rec. 2013, 296, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Moriya-Ito, K.; Hayakawa, T.; Suzuki, H.; Hagino-Yamagishi, K.; Nikaido, M. Evolution of Vomeronasal Receptor 1 (V1R) Genes in the Common Marmoset (Callithrix jacchus). Gene 2018, 642, 343–353. [Google Scholar] [CrossRef]
- Kishimoto, J.; Keverne, E.B.; Emson, P.C. Calretinin, Calbindin-D28k and Parvalbumin-like Immunoreactivity in Mouse Chemoreceptor Neurons. Brain Res. 1993, 610, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Verhaagen, J.; Oestreicher, A.; Gispen, W.; Margolis, F. The Expression of the Growth Associated Protein B50/GAP43 in the Olfactory System of Neonatal and Adult Rats. J. Neurosci. 1989, 9, 683–691. [Google Scholar] [CrossRef]
- Salazar, I.; Sánchez Quinteiro, P. Lectin Binding Patterns in the Vomeronasal Organ and Accessory Olfactory Bulb of the Rat. Anat. Embryol. 1998, 198, 331–339. [Google Scholar] [CrossRef]
- Ichikawa, M.; Osada, T.; Ikai, A. Bandeiraea Simplicifolia Lectin I and Vicia Villosa Agglutinin Bind Specifically to the Vomeronasal Axons in the Accessory Olfactory Bulb of the Rat. Neurosci. Res. 1992, 13, 73–79. [Google Scholar] [CrossRef]
- Ibarra-Soria, X.; Levitin, M.O.; Saraiva, L.R.; Logan, D.W. The Olfactory Transcriptomes of Mice. PLoS Genet. 2014, 10, e1004593. [Google Scholar] [CrossRef]
- Francia, S.; Silvotti, L.; Ghirardi, F.; Catzeflis, F.; Percudani, R.; Tirindelli, R. Evolution of Spatially Coexpressed Families of Type-2 Vomeronasal Receptors in Rodents. Genom. Biol. Evol. 2015, 7, 272–285. [Google Scholar] [CrossRef]
- Yohe, L.R.; Davies, K.T.J.; Rossiter, S.J.; Dávalos, L.M. Expressed Vomeronasal Type-1 Receptors (V1rs) in Bats Uncover Conserved Sequences Underlying Social Chemical Signaling. Genom. Biol. Evol. 2019, 11, 2741–2749. [Google Scholar] [CrossRef]
- Haga, S.; Hattori, T.; Sato, T.; Sato, K.; Matsuda, S.; Kobayakawa, R.; Sakano, H.; Yoshihara, Y.; Kikusui, T.; Touhara, K. The Male Mouse Pheromone ESP1 Enhances Female Sexual Receptive Behaviour through a Specific Vomeronasal Receptor. Nature 2010, 466, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Mendes, T.; Antunes, A. Acquisition of Social Behavior in Mammalian Lineages Is Related with Duplication Events of FPR Genes. Genomics 2020, 112, 2778–2783. [Google Scholar] [CrossRef] [PubMed]
- Oboti, L.; Ibarra-Soria, X.; Pérez-Gómez, A.; Schmid, A.; Pyrski, M.; Paschek, N.; Kircher, S.; Logan, D.W.; Leinders-Zufall, T.; Zufall, F.; et al. Pregnancy and Estrogen Enhance Neural Progenitor-Cell Proliferation in the Vomeronasal Sensory Epithelium. BMC Biol. 2015, 13, 104. [Google Scholar] [CrossRef]
- Villamayor, P.R.; Gullón, J.; Quintela, L.; Sánchez-Quinteiro, P.; Martínez, P.; Robledo, D. Sex Separation Unveils the Functional Plasticity of the Vomeronasal Organ in Rabbits. Front. Mol. Neurosci. 2022, 15, 1034254. [Google Scholar] [CrossRef]
- Segovia, S.; Guillamón, A. Effects of Sex Steroids on the Development of the Vomeronasal Organ in the Rat. Dev. Brain Res. 1982, 5, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, A.; Segovia, S. Sex Differences in the Vomeronasal System. Brain Res. Bull. 1997, 44, 377–382. [Google Scholar] [CrossRef]
- Maico, L.M.; Burrows, A.M.; Mooney, M.P.; Siegel, M.I.; Bhatnagar, K.P.; Smith, T.D. Size of the Vomeronasal Organ in Wild Microtus with Different Mating Strategies. Acta Biol. Hung. 2003, 54, 263–274. [Google Scholar] [CrossRef]
- Tai, F.D.; Wang, T.Z.; Zhang, Y.H.; Sun, R.Y. Sexual Dimorphism of the Vomeronasal Organ and the Accessory Olfactory Bulb of the Mandarin Vole Microtus mandarinus and the Reed Vole M. fortis. Acta Theriol. 2004, 49, 33–42. [Google Scholar] [CrossRef]
- Peretto, P.; Giachino, C.; Panzica, G.; Fasolo, A. Sexually Dimorphic Neurogenesis Is Topographically Matched with the Anterior Accessory Olfactory Bulb of the Adult Rat. Cell Tissue Res. 2001, 306, 385–389. [Google Scholar] [CrossRef]
- Broom, R. A Contribution to the Comparative Anatomy of the Mammalian Organ of Jacobson. Trans. R. Soc. Edinb. 1900, 39, 231–255. [Google Scholar] [CrossRef]
- Wöhrmann-Repenning, A. Phylogenetic aspects of the Jacobson’s organ and nasopalatine duct topography in insectivores, primates, Tupaia and Didelphis. Anat. Anz. 1984, 157, 137–149. [Google Scholar] [PubMed]
- Sánchez-Villagra, M.R. Ontogenetic and Phylogenetic Transformations of the Vomeronasal Complex and Nasal Floor Elements in Marsupial Mammals. Zool. J. Linn. Soc. 2001, 131, 459–479. [Google Scholar] [CrossRef][Green Version]
- Vaccarezza, O.L.; Sepich, L.N.; Tramezzani, J.H. The Vomeronasal Organ of the Rat. J. Anat. 1981, 132, 167–185. [Google Scholar] [PubMed]
- Mechin, V.; Pageat, P.; Teruel, E.; Asproni, P. Histological and Immunohistochemical Characterization of Vomeronasal Organ Aging in Mice. Animals 2021, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Weiler, E.; McCulloch, M.A.; Farbman, A.I. Proliferation in the Vomeronasal Organ of the Rat during Postnatal Development. Eur. J. Neurosci. 1999, 11, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, P.; Benedetto, A.; Tirindelli, R.; Fasolo, A. Proliferation and Migration of Receptor Neurons in the Vomeronasal Organ of the Adult Mouse. Dev. Brain Res. 2000, 123, 33–40. [Google Scholar] [CrossRef]
- Ibokwe, C.; Okpe, G.C. Morphological Studies Of Vomeronasal Organ In The Wild Juvenile Red-Flanked Duiker Cephalophus Rufilatus (GRAY 1864). Anim. Res. Int. 2009, 6, 932–937. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Yang, C.; He, Y. Histological Structure of the Vomeronasal Organ and Accessory Olfactory Bulb and the Seasonal Changes of Olfactory Bulb C-Fos Expression in Spermophilus dauricus. Acta Theriol. Sin. 2021, 41, 685. [Google Scholar] [CrossRef]
- Oikawa, T.; Shimamura, K.; Saito, T.R.; Taniguchi, K. Fine Structure of the Vomeronasal Organ in the Chinchilla (Chinchilla laniger). Exp. Anim. 1994, 43, 487–497. [Google Scholar] [CrossRef]
- Jurcisek, J.A.; Durbin, J.E.; Kusewitt, D.F.; Bakaletz, L.O. Anatomy of the Nasal Cavity in the Chinchilla. Cells Tissues Organs 2003, 174, 136–152. [Google Scholar] [CrossRef]
- Smith, T.D.; Alport, L.J.; Burrows, A.M.; Bhatnagar, K.P.; Dennis, J.C.; Tuladhar, P.; Morrison, E.E. Perinatal Size and Maturation of the Olfactory and Vomeronasal Neuroepithelia in Lorisoids and Lemuroids. Am. J. Primatol. 2007, 69, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.C.; Stilwell, N.K.; Smith, T.D.; Park, T.J.; Bhatnagar, K.P.; Morrison, E.E. Is the Mole Rat Vomeronasal Organ Functional? Anat. Rec. 2020, 303, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Luckhaus, G. Light and electron microscopic findings in the epithelial lamina of the vomeronasal organ of the rabbit. Anat. Anz. 1969, 124, 477–489. [Google Scholar] [PubMed]
- Mahdy, E.; El Behery, E.; Mohamed, S. Comparative Morpho-Histological Analysis on the Vomeronasal Organ and the Accessory Olfactory Bulb in Balady Dogs (Canis familiaris) and New Zealand Rabbits (Oryctolagus cuniculus). J. Adv. Vet. Res. 2019, 6, 506. [Google Scholar] [CrossRef] [PubMed]
- Elgayar, S.A.M.; Eltony, S.A.; Othman, M.A. Morphology of Non-Sensory Epithelium during Post-Natal Development of the Rabbit Vomeronasal Organ. Anat. Histol. Embryol. 2014, 43, 282–293. [Google Scholar] [CrossRef]
- Schneider, N.Y.; Fletcher, T.P.; Shaw, G.; Renfree, M.B. The Vomeronasal Organ of the Tammar Wallaby. J. Anat. 2008, 213, 93–105. [Google Scholar] [CrossRef]
- Poran, N.S. Vomeronasal Organ and Its Associated Structures in the opossum Monodelphis domestica. Microsc. Res. Tech. 1998, 43, 500–510. [Google Scholar] [CrossRef]
- Schneider, N.Y. The Development of the Olfactory Organs in Newly Hatched Monotremes and Neonate Marsupials: Olfaction in Monotremes and Marsupials. J. Anat. 2011, 219, 229–242. [Google Scholar] [CrossRef]
- Ashwell, K.W.S. Development of the Olfactory Pathways in Platypus and Echidna. Brain Behav. Evol. 2012, 79, 45–56. [Google Scholar] [CrossRef]
- Kondoh, D.; Tanaka, Y.; Kawai, Y.K.; Mineshige, T.; Watanabe, K.; Kobayashi, Y. Morphological and Histological Features of the Vomeronasal Organ in African Pygmy Hedgehog (Atelerix albiventris). Animals 2021, 11, 1462. [Google Scholar] [CrossRef]
- Aland, R.C.; Gosden, E.; Bradley, A.J. Seasonal Morphometry of the Vomeronasal Organ in the Marsupial Mouse, Antechinus subtropicus. J. Morphol. 2016, 277, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.C.; Allgier, J.G.; Desouza, L.S.; Eward, W.C.; Morrison, E.E. Immunohistochemistry of the Canine Vomeronasal Organ. J. Anat. 2003, 203, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Sanchez Quinteiro, P.; Cifuentes, J.M.; Garcia Caballero, T. The Vomeronasal Organ of the Cat. J. Anat. 1996, 188 Pt 2, 445–454. [Google Scholar]
- Tomiyasu, J.; Kondoh, D.; Sakamoto, H.; Matsumoto, N.; Sasaki, M.; Kitamura, N.; Haneda, S.; Matsui, M. Morphological and Histological Features of the Vomeronasal Organ in the Brown Bear. J. Anat. 2017, 231, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, K.R.; Baum, M.J.; Meredith, M. The Ferret’s Vomeronasal Organ and Accessory Olfactory Bulb: Effect of Hormone Manipulation in Adult Males and Females. Anat. Rec. 2001, 263, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Lombardero, M.; Sánchez-Quinteiro, P.; Roel, P.; Cifuentes, J.M. Origin and Regional Distribution of the Arterial Vessels of the Vomeronasal Organ in the Sheep. A Methodological Investigation with Scanning Electron Microscopy and Cutting-Grinding Technique. Ann. Anat. 1998, 180, 181–187. [Google Scholar] [CrossRef]
- Salazar, I.; Quinteiro, P.S.; Cifuentes, J.M. The Soft-Tissue Components of the Vomeronasal Organ in Pigs, Cows and Horses. Anat. Histol. Embryol. 1997, 26, 179–186. [Google Scholar] [CrossRef]
- Salazar, I.; Quinteiro, P.S.; Alemañ, N.; Cifuentes, J.M.; Troconiz, P.F. Diversity of the Vomeronasal System in Mammals: The Singularities of the Sheep Model. Microsc. Res. Tech. 2007, 70, 752–762. [Google Scholar] [CrossRef]
- Ichikawa, M.; Shin, T.; Kang, M.S. Fine Structure of the Vomeronasal Sensory Epithelium of Korean Goats (Capra Hircus). J. Rep. Dev. 1999, 45, 81–89. [Google Scholar] [CrossRef]
- Yang, W.; Choi, Y.; Park, C.; Lee, K.-H.; Ahn, M.; Kang, W.; Heo, S.-D.; Kim, J.; Shin, T. Histological and Lectin Histochemical Studies in the Vomeronasal Organ of the Korean Black Goat, Capra hircus coreanae. Acta Histochem. 2021, 123, 151684. [Google Scholar] [CrossRef]
- Park, C.; Ahn, M.; Lee, J.-Y.; Lee, S.; Yun, Y.; Lim, Y.-K.; Taniguchi, K.; Shin, T. A Morphological Study of the Vomeronasal Organ and the Accessory Olfactory Bulb in the Korean Roe Deer, Capreolus pygargus. Acta Histochem. 2014, 116, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Vedin, V.; Eriksson, B.; Berghard, A. Organization of the Chemosensory Neuroepithelium of the Vomeronasal Organ of the Scandinavian Moose Alces alces. Brain Res. 2010, 1306, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, D.; Nakamura, K.G.; Ono, Y.S.; Yuhara, K.; Bando, G.; Watanabe, K.; Horiuchi, N.; Kobayashi, Y.; Sasaki, M.; Kitamura, N. Histological Features of the Vomeronasal Organ in the Giraffe, Giraffa camelopardalis. Microsc. Res. Tech. 2017, 80, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.D.; Bhatnagar, K.P.; Shimp, K.L.; Kinzinger, J.H.; Bonar, C.J.; Burrows, A.M.; Mooney, M.P.; Siegel, M.I. Histological Definition of the Vomeronasal Organ in Humans and Chimpanzees, with a Comparison to Other Primates. Anat. Rec. 2002, 267, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Meisami, E.; Bhatnagar, K.P. Structure and Diversity in Mammalian Accessory Olfactory Bulb. Microsc. Res. Tech. 1998, 43, 476–499. [Google Scholar] [CrossRef]
- Takami, S.; Graziadei, P.P.C. Morphological Complexity of the Glomerulus in the Rat Accessory Olfactory Bulb—A Golgi Study. Brain Res. 1990, 510, 339–342. [Google Scholar] [CrossRef]
- Nakajima, M.; Tsuruta, M.; Mori, H.; Nishikawa, C.; Okuyama, S.; Furukawa, Y. A Comparative Study of Axon-Surrounding Cells in the Two Nasal Nerve Tracts from Mouse Olfactory Epithelium and Vomeronasal Organ. Brain Res. 2013, 1503, 16–23. [Google Scholar] [CrossRef]
- Vassar, R.; Chao, S.K.; Sitcheran, R.; Nuñez, J.M.; Vosshall, L.B.; Axel, R. Topographic Organization of Sensory Projections to the Olfactory Bulb. Cell 1994, 79, 981–991. [Google Scholar] [CrossRef]
- Del Punta, K.; Puche, A.; Adams, N.C.; Rodriguez, I.; Mombaerts, P. A Divergent Pattern of Sensory Axonal Projections Is Rendered Convergent by Second-Order Neurons in the Accessory Olfactory Bulb. Neuron 2002, 35, 1057–1066. [Google Scholar] [CrossRef]
- Crosby, E.C.; Humphrey, T. Studies of the Vertebrate Telencephalon. II. The Nuclear Pattern of the Anterior Olfactory Nucleus, Tuberculum Olfactorium and the Amygdaloid Complex in Adult Man. J. Comp. Neurol. 1941, 74, 309–352. [Google Scholar] [CrossRef]
- Allison, A.C. The Structure of the Olfactory Bulb and Its Relationship to the Olfactory Pathways in the Rabbit and the Rat. J. Comp. Neurol. 1953, 98, 309–353. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Sánchez-Quinteiro, P. A Detailed Morphological Study of the Vomeronasal Organ and the Accessory Olfactory Bulb of Cats. Microsc. Res. Tech. 2011, 74, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Larriva-Sahd, J. The Accessory Olfactory Bulb in the Adult Rat: A Cytological Study of Its Cell Types, Neuropil, Neuronal Modules, and Interactions with the Main Olfactory System. J. Comp. Neurol. 2008, 510, 309–350. [Google Scholar] [CrossRef] [PubMed]
- Switzer, R.C., III; Johnson, J.I.; Kirsch, J.A.W. Phylogeny Through Brain Traits. Brain Behav. Evol. 1980, 17, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.; Towle, A.C.; Margolis, F.L. Differential Afferent Regulation of Dopaminergic and GABAergic Neurons in the Mouse Main Olfactory Bulb. Brain Res. 1988, 450, 69–80. [Google Scholar] [CrossRef]
- Gouda, M.; Matsutani, S.; Senba, E.; Tohyama, M. Peptidergic Granule Cell Populations in the Rat Main and Accessory Olfactory Bulb. Brain Res. 1990, 512, 339–342. [Google Scholar] [CrossRef]
- McLean, J.H.; Shipley, M.T. Neuroanatomical Substrates of Olfaction. In Science of Olfaction; Serby, M.J., Chobor, K.L., Eds.; Springer: New York, NY, USA, 1992; pp. 126–171. ISBN 978-1-4612-7690-6. [Google Scholar]
- Frahm, H.D.; Stephan, H.; Baron, G. Comparison of Brain Structure Volumes in Insectivora and Primates. V. Area Striata (AS). J. Hirnforsch. 1984, 25, 537–557. [Google Scholar]
- Bonfanti, L.; Peretto, P.; Merighi, A.; Fasolo, A. Newly-Generated Cells from the Rostral Migratory Stream in the Accessory Olfactory Bulb of the Adult Rat. Neuroscience 1997, 81, 489–502. [Google Scholar] [CrossRef]
- Defteralı, Ç.; Moreno-Estellés, M.; Crespo, C.; Díaz-Guerra, E.; Díaz-Moreno, M.; Vergaño-Vera, E.; Nieto-Estévez, V.; Hurtado-Chong, A.; Consiglio, A.; Mira, H.; et al. Neural Stem Cells in the Adult Olfactory Bulb Core Generate Mature Neurons In Vivo. Stem Cells 2021, 39, 1253–1269. [Google Scholar] [CrossRef]
- Inaki, K.; Nishimura, S.; Nakashiba, T.; Itohara, S.; Yoshihara, Y. Laminar Organization of the Developing Lateral Olfactory Tract Revealed by Differential Expression of Cell Recognition Molecules. J. Comp. Neurol. 2004, 479, 243–256. [Google Scholar] [CrossRef]
- Broadwell, R.D. Olfactory Relationships of the Telencephlaon and Diencephalon in the Rabbit. 1. An Autoradiographic Study of the Efferent Connections of the Main and Accessory Olfactory Bulbs. J. Comp. Neurol. 1975, 163, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Schwob, J.E.; Price, J.L. The Development of Axonal Connections in the Central Olfactory System of Rats. J. Comp. Neurol. 1984, 223, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Takami, S.; Fernandez, G.D.; Graziadei, P.P.C. The Morphology of GABA-Immunoreactive Neurons in the Accessory Olfactory Bulb of Rats. Brain Res. 1992, 588, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Takami, S.; Graziadei, P.P.C. Light Microscopic Golgi Study of Mitral/Tufted Cells in the Accessory Olfactory Bulb of the Adult Rat. J. Comp. Neurol. 1991, 311, 65–83. [Google Scholar] [CrossRef]
- Hinds, J.W. Autoradiographic Study of Histogenesis in the Mouse Olfactory Bulb I. Time of Origin of Neurons and Neuroglia. J. Comp. Neurol. 1968, 134, 287–304. [Google Scholar] [CrossRef]
- Rall, W.; Shepherd, G.M.; Reese, T.S.; Brightman, M.W. Dendrodendritic Synaptic Pathway for Inhibition in the Olfactory Bulb. Exp. Neurol. 1966, 14, 44–56. [Google Scholar] [CrossRef]
- Martín-López, E.; Corona, R.; López-Mascaraque, L. Postnatal Characterization of Cells in the Accessory Olfactory Bulb of Wild Type and Reeler Mice. Front. Neuroanat. 2012, 6, 15. [Google Scholar] [CrossRef]
- Jia, C.; Chen, W.R.; Shepherd, G.M. Synaptic Organization and Neurotransmitters in the Rat Accessory Olfactory Bulb. J. Neurophysiol. 1999, 81, 345–355. [Google Scholar] [CrossRef]
- Del Punta, K. Sequence Diversity and Genomic Organization of Vomeronasal Receptor Genes in the Mouse. Genom. Res. 2000, 10, 1958–1967. [Google Scholar] [CrossRef]
- Segovia, S.; Orensanz, L.M.; Valencia, A.; Guillamón, A. Effects of Sex Steroids on the Development of the Accessory Olfactory Bulb in the Rat: A Volumetric Study. Dev. Brain Res. 1984, 16, 312–314. [Google Scholar] [CrossRef]
- Yokosuka, M. Histological Properties of the Glomerular Layer in the Mouse Accessory Olfactory Bulb. Exp. Anim. 2012, 61, 13–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frahm, H.D.; Bhatnagar, K.P. Comparative Morphology of the Accessory Olfactory Bulb in Bats. J. Anat. 1980, 130, 349–365. [Google Scholar] [PubMed]
- Jia, C.; Halpern, M. Calbindin D28k, Parvalbumin, and Calretinin Immunoreactivity in the Main and Accessory Olfactory Bulbs of the Gray Short-Tailed Opossum, Monodelphis domestica. J. Morphol. 2004, 259, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Leal, I.; Torres, M.V.; Villamayor, P.R.; Fidalgo, L.E.; López-Beceiro, A.; Sanchez-Quinteiro, P. Can Domestication Shape Canidae Brain Morphology? The Accessory Olfactory Bulb of the Red Fox as a Case in Point. Ann. Anat. 2022, 240, 151881. [Google Scholar] [CrossRef]
- Bird, D.J.; Jacquemetton, C.; Buelow, S.A.; Evans, A.W.; Van Valkenburgh, B. Domesticating Olfaction: Dog Breeds, Including Scent Hounds, Have Reduced Cribriform Plate Morphology Relative to Wolves. Anat. Rec. 2021, 304, 139–153. [Google Scholar] [CrossRef]
- Bird, D.J.; Murphy, W.J.; Fox-Rosales, L.; Hamid, I.; Eagle, R.A.; Van Valkenburgh, B. Olfaction Written in Bone: Cribriform Plate Size Parallels Olfactory Receptor Gene Repertoires in Mammalia. Proc. R. Soc. B 2018, 285, 20180100. [Google Scholar] [CrossRef]
- Wynn, E.H.; Sánchez-Andrade, G.; Carss, K.J.; Logan, D.W. Genomic Variation in the Vomeronasal Receptor Gene Repertoires of Inbred Mice. BMC Genom. 2012, 13, 415. [Google Scholar] [CrossRef]
- Ortiz-Leal, I.; Torres, M.V.; López-Callejo, L.N.; Fidalgo, L.E.; López-Beceiro, A.; Sanchez-Quinteiro, P. Comparative Neuroanatomical Study of the Main Olfactory Bulb in Domestic and Wild Canids: Dog, Wolf and Red Fox. Animals 2022, 12, 1079. [Google Scholar] [CrossRef]
- Dzięcioł, M.; Podgórski, P.; Stańczyk, E.; Szumny, A.; Woszczyło, M.; Pieczewska, B.; Niżański, W.; Nicpoń, J.; Wrzosek, M.A. MRI Features of the Vomeronasal Organ in Dogs (Canis familiaris). Front. Vet. Sci. 2020, 7, 159. [Google Scholar] [CrossRef]
- Muñiz-de Miguel, S.; Barreiro-Vázquez, J.D.; Sánchez-Quinteiro, P.; Ortiz-Leal, I.; González-Martínez, Á. Behavioural Disorder in a Dog with Congenital Agenesis of the Vomeronasal Organ and the Septum Pellucidum. Vet. Rec. Case Rep. 2023, 11, e571. [Google Scholar] [CrossRef]
- Pageat, P.; Gaultier, E. Current Research in Canine and Feline Pheromones. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, I.; Masucci, M.; Cozzi, A.; Teruel, E.; Navarra, M.; Cirmi, S.; Pennisi, M.G.; Siracusa, C. Effects of a Novel Gel Formulation of Dog Appeasing Pheromone (DAP) on Behavioral and Physiological Stress Responses in Dogs Undergoing Clinical Examination. Animals 2022, 12, 2472. [Google Scholar] [CrossRef] [PubMed]
- Asproni, P.; Cozzi, A.; Verin, R.; Lafont-Lecuelle, C.; Bienboire-Frosini, C.; Poli, A.; Pageat, P. Pathology and Behaviour in Feline Medicine: Investigating the Link between Vomeronasalitis and Aggression. J. Feline Med. Surg. 2016, 18, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Asproni, P.; Mainau, E.; Cozzi, A.; Carreras, R.; Bienboire-Frosini, C.; Teruel, E.; Pageat, P. Is There a Link between Vomeronasalitis and Aggression in Stable Social Groups of Female Pigs? Animals 2022, 12, 303. [Google Scholar] [CrossRef]
- Mechin, V.; Asproni, P.; Bienboire-Frosini, C.; Cozzi, A.; Chabaud, C.; Arroub, S.; Mainau, E.; Nagnan-Le Meillour, P.; Pageat, P. Inflammation Interferes with Chemoreception in Pigs by Altering the Neuronal Layout of the Vomeronasal Sensory Epithelium. Front. Vet. Sci. 2022, 9, 936838. [Google Scholar] [CrossRef]
- Mechin, V.; Pageat, P.; Boutry, M.; Teruel, E.; Portalier, C.; Asproni, P. Does the Environmental Air Impact the Condition of the Vomeronasal Organ? A Mouse Model for Intensive Farming. Animals 2023, 13, 1902. [Google Scholar] [CrossRef]
- Salazar, I.; Cifuentes, J.M.; Quinteiro, P.S.; Caballero, G. The Vomeronasal System of the Mink, Mustela Vison. I. The Vomeronasal Organ. Funct. Dev. Morphol. 1994, 4, 113–117. [Google Scholar]
- Salazar, I.; Quinteiro, P.S.; Cifuentes, J.M.; Lombardero, M. The Accessory Olfactory Bulb of the Mink, Mustela Vison: A Morphological and Lectin Histochemical Study. Anat. Histol. Embryol. 1998, 27, 297–300. [Google Scholar] [CrossRef]
- Salazar, I.; Lombardero, M.; Alemañ, N.; Sánchez Quinteiro, P. Development of the Vomeronasal Receptor Epithelium and the Accessory Olfactory Bulb in Sheep. Microsc. Res. Tech. 2003, 61, 438–447. [Google Scholar] [CrossRef]
- Tirindelli, R.; Mucignat-Caretta, C.; Ryba, N.J.P. Molecular Aspects of Pheromonal Communication via the Vomeronasal Organ of Mammals. Trends Neurosci. 1998, 21, 482–486. [Google Scholar] [CrossRef]
- Alonso, J.R.; Briñón, J.G.; Crespo, C.; Bravo, I.G.; Arévalo, R.; Aijón, J. Chemical Organization of the Macaque Monkey Olfactory Bulb: II. Calretinin, Calbindin D-28k, Parvalbumin, and Neurocalcin Immunoreactivity: Calcium-Binding Proteins in the Monkey OB. J. Comp. Neurol. 2001, 432, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Briñón, J.G.; Weruaga, E.; Crespo, C.; Porteros, A.; Arévalo, R.; Aijón, J.; Alonso, J.R. Calretinin-, Neurocalcin-, and Parvalbumin-Immunoreactive Elements in the Olfactory Bulb of the Hedgehog (Erinaceus europaeus). J. Comp. Neurol. 2001, 429, 554–570. [Google Scholar] [CrossRef] [PubMed]
- Weruaga, E. A Sexually Dimorphic Group of Atypical Glomeruli in the Mouse Olfactory Bulb. Chem. Senses 2001, 26, 7–15. [Google Scholar] [CrossRef][Green Version]
- Smithson, L.J.; Kawaja, M.D. A Comparative Examination of Biomarkers for Olfactory Ensheathing Cells in Cats and Guinea Pigs. Brain Res. 2009, 1284, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Dehmelt, L.; Halpain, S. The MAP2/Tau Family of Microtubule-Associated Proteins. Genome Biol. 2005, 6, 204. [Google Scholar] [CrossRef]
- Benowitz, L.I.; Perrone-Bizzozero, N.I.; Neve, R.L.; Rodriguez, W. Chapter 26 GAP-43 as a Marker for Structural Plasticity in the Mature CNS. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1990; Volume 86, pp. 309–320. ISBN 978-0-444-81121-9. [Google Scholar]
- Shapiro, L.S.; Roland, R.M.; Halpern, M. Development of Olfactory Marker Protein and N-CAM Expression in Chemosensory Systems of the Opossum, Monodelphis domestica. J. Morphol. 1997, 234, 109–129. [Google Scholar] [CrossRef]
- Mugnaini, E.; Oertel, W.H.; Wouterlood, F.F. Immunocytochemical Localization of GABA Neurons and Dopamine Neurons in the Rat Main and Accessory Olfactory Bulbs. Neurosci. Lett. 1984, 47, 221–226. [Google Scholar] [CrossRef]
- Le Jeune, H.; Aubert, I.; Jourdan, F.; Quirion, R. Comparative Laminar Distribution of Various Autoradiographic Cholinergic Markers in Adult Rat Main Olfactory Bulb. J. Chem. Neuroanat. 1995, 9, 99–112. [Google Scholar] [CrossRef]
- Woo, C.C.; Leon, M. Distribution and Development of β-Adrenergic Receptors in the Rat Olfactory Bulb. J. Comp. Neurol. 1995, 352, 1–10. [Google Scholar] [CrossRef]
- Huang, Z.; Thiebaud, N.; Fadool, D.A. Differential Serotonergic Modulation across the Main and Accessory Olfactory Bulbs: Differential Serotonin Modulation in Olfactory System. J. Physiol. 2017, 595, 3515–3533. [Google Scholar] [CrossRef]
- Matsutani, S.; Senba, E.; Tohyama, M. Neuropeptide- and Neurotransmitter-Related Immunoreactivities in the Developing Rat Olfactory Bulb. J. Comp. Neurol. 1988, 272, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, L.-M.; Alm, P.; Wharton, J.; Polak, J.M. Protein Gene Product 9.5 (PGP 9.5): A New Neuronal Marker Visualizing the Whole Uterine Innervation and Pregnancy-Induced and Developmental Changes in the Guinea pig. Histochemistry 1988, 90, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nacher, J.; Crespo, C.; McEwen, B.S. Doublecortin Expression in the Adult Rat Telencephalon: Doublecortin Expression in the Adult Rat. Eur. J. Neurosci. 2001, 14, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Takami, S.; Graziadei, P.P.C.; Ichikawa, M. The Differential Staining Patterns of Two Lectins in the Accessory Olfactory Bulb of the Rat. Brain Res. 1992, 598, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Nii, Y.; Ogawa, K. Subdivisions of the Accessory Olfactory Bulb, as Demonstrated by Lectin-Histochemistry in the Golden Hamster. Neurosci. Lett. 1993, 158, 185–188. [Google Scholar] [CrossRef]
- Shapiro, L.S.; Halpern, M.; Ee, P.-L. Lectin Histochemical Identification of Carbohydrate Moieties in Opossum Chemosensory Systems during Development, with Special Emphasis on VVA-Identified Subdivisions in the Accessory Olfactory Bulb. J. Morphol. 1995, 224, 331–349. [Google Scholar] [CrossRef]
- Hovis, K.R.; Ramnath, R.; Dahlen, J.E.; Romanova, A.L.; LaRocca, G.; Bier, M.E.; Urban, N.N. Activity Regulates Functional Connectivity from the Vomeronasal Organ to the Accessory Olfactory Bulb. J. Neurosci. 2012, 32, 7907–7916. [Google Scholar] [CrossRef]
- Raisman, G. An Experimental Study of the Projection of the Amygdala to the Accessory Olfactory Bulb and Its Relationship to the Concept of a Dual Olfactory System. Exp. Brain Res. 1972, 14, 395–408. [Google Scholar] [CrossRef]
- Scalia, F.; Winans, S.S. The Differential Projections of the Olfactory Bulb and Accessory Olfactory Bulb in Mammals. J. Comp. Neurol. 1975, 161, 31–55. [Google Scholar] [CrossRef]
- Winans, S.S.; Scalia, F. Amygdaloid Nucleus: New Afferent Input from the Vomeronasal Organ. Science 1970, 170, 330–332. [Google Scholar] [CrossRef]
- Shipley, M.T.; Adamek, G.D. The Connections of the Mouse Olfactory Bulb: A Study Using Orthograde and Retrograde Transport of Wheat Germ Agglutinin Conjugated to Horseradish Peroxidase. Brain Res. Bull. 1984, 12, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Dulac, C.; Wagner, S. Genetic Analysis of Brain Circuits Underlying Pheromone Signaling. Annu. Rev. Genet. 2006, 40, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Price, J.L. Beyond the Primary Olfactory Cortex: Olfactory-Related Areas in the Neocortex, Thalamus and Hypothalamus. Chem. Senses 1985, 10, 239–258. [Google Scholar] [CrossRef]
- Courtiol, E.; Wilson, D.A. The Olfactory Thalamus: Unanswered Questions about the Role of the Mediodorsal Thalamic Nucleus in Olfaction. Front. Neural Circuits 2015, 9, 49. [Google Scholar] [CrossRef]
- Cádiz-Moretti, B.; Otero-García, M.; Martínez-García, F.; Lanuza, E. Afferent Projections to the Different Medial Amygdala Subdivisions: A Retrograde Tracing Study in the Mouse. Brain Struct. Funct. 2016, 221, 1033–1065. [Google Scholar] [CrossRef]
- Devor, M. Fiber Trajectories of Olfactory Bulb Efferents in the Hamster. J. Comp. Neurol. 1976, 166, 31–47. [Google Scholar] [CrossRef]
- Martinez-Marcos, A. On the Organization of Olfactory and Vomeronasal Cortices. Prog. Neurobiol. 2009, 87, 21–30. [Google Scholar] [CrossRef]
- Gutiérrez-Castellanos, N.; Pardo-Bellver, C.; Martínez-García, F.; Lanuza, E. The Vomeronasal Cortex—Afferent and Efferent Projections of the Posteromedial Cortical Nucleus of the Amygdala in Mice. Eur. J. Neurosci. 2014, 39, 141–158. [Google Scholar] [CrossRef]
- Stowers, L.; Liberles, S.D. State-Dependent Responses to Sex Pheromones in Mouse. Curr. Opin. Neurobiol. 2016, 38, 74–79. [Google Scholar] [CrossRef]
- Pardo-Bellver, C.; Cádiz-Moretti, B.; Novejarque, A.; Martínez-García, F.; Lanuza, E. Differential Efferent Projections of the Anterior, Posteroventral, and Posterodorsal Subdivisions of the Medial Amygdala in Mice. Front. Neuroanat. 2012, 6, 33. [Google Scholar] [CrossRef]
- Lo, L.; Anderson, D.J. A Cre-Dependent, Anterograde Transsynaptic Viral Tracer for Mapping Output Pathways of Genetically Marked Neurons. Neuron 2011, 72, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Trotier, D. Vomeronasal Organ and Human Pheromones. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Cadiz, B.; Martinez-Garcia, F.; Lanuza, E. Neural Substrate to Associate Odorants and Pheromones: Convergence of Projections from the Main and Accessory Olfactory Bulbs in Mice. In Chemical Signals in Vertebrates 12; Springer: New York, NY, USA, 2013; pp. 3–16. ISBN 978-1-4614-5926-2. [Google Scholar]
- Mohrhardt, J.; Nagel, M.; Fleck, D.; Ben-Shaul, Y.; Spehr, M. Signal Detection and Coding in the Accessory Olfactory System. Chem. Senses 2018, 43, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Záborszky, L.; Carlsen, J.; Brashear, H.R.; Heimer, L. Cholinergic and GABAergic Afferents to the Olfactory Bulb in the Rat with Special Emphasis on the Projection Neurons in the Nucleus of the Horizontal Limb of the Diagonal Band. J. Comp. Neurol. 1986, 243, 488–509. [Google Scholar] [CrossRef]
- De Saint Jan, D. Target-Specific Control of Olfactory Bulb Periglomerular Cells by GABAergic and Cholinergic Basal Forebrain Inputs. eLife 2022, 11, e71965. [Google Scholar] [CrossRef]
- Liberia, T.; Blasco Ibáñez, J.M.; Nácher, J.; Varea, E.; Lanciego, J.L.; Crespo, C. Synaptic Connectivity of the Cholinergic Axons in the Olfactory Bulb of the Cynomolgus Monkey. Front. Neuroanat. 2015, 9, 28. [Google Scholar] [CrossRef][Green Version]
- Böhm, E.; Brunert, D.; Rothermel, M. Input Dependent Modulation of Olfactory Bulb Activity by HDB GABAergic Projections. Sci. Rep. 2020, 10, 10696. [Google Scholar] [CrossRef]
- Gomez, D.M.; Newman, S.W. Differential Projections of the Anterior and Posterior Regions of the Medial Amygdaloid Nucleus in the Syrian Hamster. J. Comp. Neurol. 1992, 317, 195–218. [Google Scholar] [CrossRef]
- Fan, S.; Luo, M. The Organization of Feedback Projections in a Pathway Important for Processing Pheromonal Signals. Neuroscience 2009, 161, 489–500. [Google Scholar] [CrossRef]
- Inbar, T.; Davis, R.; Bergan, J.F. A Sex-specific Feedback Projection from Aromatase-expressing Neurons in the Medial Amygdala to the Accessory Olfactory Bulb. J. Comp. Neurol. 2022, 530, 648–655. [Google Scholar] [CrossRef]
- Pardo-Bellver, C.; Vila-Martin, M.E.; Martínez-Bellver, S.; Villafranca-Faus, M.; Teruel-Sanchis, A.; Savarelli-Balsamo, C.A.; Drabik, S.M.; Martínez-Ricós, J.; Cervera-Ferri, A.; Martínez-García, F.; et al. Neural Activity Patterns in the Chemosensory Network Encoding Vomeronasal and Olfactory Information in Mice. Front. Neuroanat. 2022, 16, 988015. [Google Scholar] [CrossRef] [PubMed]
- Keverne, E.B.; Brennan, P.A. Olfactory Recognition Memory. J. Physiol. 1996, 90, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A.; Kendrick, K.M.; Keverne, E.B. Neurotransmitter Release in the Accessory Olfactory Bulb during and after the Formation of an Olfactory Memory in Mice. Neuroscience 1995, 69, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A. The Nose Knows Who’s Who: Chemosensory Individuality and Mate Recognition in Mice. Horm. Behav. 2004, 46, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kaba, H. Muscarinic Receptor Type 1 (M1) Stimulation, Probably through KCNQ/Kv7 Channel Closure, Increases Spontaneous GABA Release at the Dendrodendritic Synapse in the Mouse Accessory Olfactory Bulb. Brain Res. 2010, 1339, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Mainen, Z.F. Early Events in Olfactory Processing. Annu. Rev. Neurosci. 2006, 29, 163–201. [Google Scholar] [CrossRef]
- Grus, W.E.; Zhang, J. Origin of the Genetic Components of the Vomeronasal System in the Common Ancestor of All Extant Vertebrates. Mol. Biol. Evol. 2009, 26, 407–419. [Google Scholar] [CrossRef]
- Smith, J.; Hurst, J.L.; Barnard, C.J. Comparing Behaviour in Wild and Laboratory Strains of the House Mouse: Levels of Comparison and Functional Inference. Behav. Process. 1994, 32, 79–86. [Google Scholar] [CrossRef]
- Catania, K.C. Underwater “sniffing” by Semi-Aquatic Mammals. Nature 2006, 444, 1024–1025. [Google Scholar] [CrossRef]
- Johnson, E.W.; Rasmussen, L. Morphological Characteristics of the Vomeronasal Organ of the Newborn Asian Elephant (Elephas maximus). Anat. Rec. 2002, 267, 252–259. [Google Scholar] [CrossRef]
- Garrosa, M.; Gayoso, M.J.; Esteban, F.J. Prenatal Development of the Mammalian Vomeronasal Organ. Microsc. Res. Tech. 1998, 41, 456–470. [Google Scholar] [CrossRef]
- Sugai, T.; Sugitani, M.; Onoda, N. Subdivisions of the Guinea-Pig Accessory Olfactory Bulb Revealed by the Combined Method with Immunohistochemistry, Electrophysiological, and Optical Recordings. Neuroscience 1997, 79, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Fieni, F. Apical and Basal Neurones Isolated from the Mouse Vomeronasal Organ Differ for Voltage-Dependent Currents. J. Physiol. 2003, 552, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.V.; Ortiz-Leal, I.; Villamayor, P.R.; Ferreiro, A.; Rois, J.L.; Sanchez-Quinteiro, P. The Vomeronasal System of the Newborn Capybara: A Morphological and Immunohistochemical Study. Sci. Rep. 2020, 10, 13304. [Google Scholar] [CrossRef]
- Salazar, I.; Lombardero, M.; Cifuentes, J.M.; Quinteiro, P.S.; Aleman, N. Morphogenesis and Growth of the Soft Tissue and Cartilage of the Vomeronasal Organ in Pigs. J. Anat. 2003, 202, 503–514. [Google Scholar] [CrossRef]
- Salazar, I.; Sánchez Quinteiro, P.; Lombardero, M.; Aleman, N.; Fernández de Trocóniz, P. The Prenatal Maturity of the Accessory Olfactory Bulb in Pigs. Chem. Senses 2004, 29, 3–11. [Google Scholar] [CrossRef][Green Version]
- Park, J.; Lee, W.; Jeong, C.; Kim, H.; Taniguchi, K.; Shin, T. Developmental Changes Affecting Lectin Binding in the Vomeronasal Organ of Domestic Pigs, Sus scrofa. Acta Histochem. 2012, 114, 24–30. [Google Scholar] [CrossRef]
- Suárez, R.; Santibáñez, R.; Parra, D.; Coppi, A.A.; Abrahão, L.M.B.; Sasahara, T.H.C.; Mpodozis, J. Shared and Differential Traits in the Accessory Olfactory Bulb of Caviomorph Rodents with Particular Reference to the Semiaquatic Capybara: The AOB of Capybaras and Other Caviomorphs. J. Anat. 2011, 218, 558–565. [Google Scholar] [CrossRef]
- Fernández-Aburto, P.; Delgado, S.E.; Sobrero, R.; Mpodozis, J. Can Social Behaviour Drive Accessory Olfactory Bulb Asymmetries? Sister Species of Caviomorph Rodents as a Case in Point. J. Anat. 2020, 236, 612–621. [Google Scholar] [CrossRef]
- Malz, C.R.; Knabe, W.; Kuhn, H.-J. Calretinin Immunoreactivity in the Prenatally Developing Olfactory Systems of the Tree Shrew Tupaia belangeri. Anat. Embryol. 2002, 205, 83–97. [Google Scholar] [CrossRef]
- Brunjes, P.C.; Jazaeri, A.; Sutherland, M.J. Olfactory Bulb Organization and Development in Monodelphis domestica (Grey Short-Tailed Opossum). J. Comp. Neurol. 1992, 320, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Sanchez-Quinteiro, P.; Lombardero, M.; Cifuentes, J.M. Histochemical Identification of Carbohydrate Moieties in the Accessory Olfactory Bulb of the Mouse Using a Panel of Lectins. Chem. Senses 2001, 26, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Bojsen-Møller, F. Topography of the Nasal Glands in Rats and Some Other Mammals. Anat. Rec. 1964, 150, 11–24. [Google Scholar] [CrossRef]
- Pastor, L.M.; Frutos, M.J.; Graña, L.; Ramos, D.; Gallego-Huidobro, J.; Calvo, A. Histochemical Study of Glycoconjugates in the Nasal Mucosa of the Rat and Guinea pig. Histochem. J. 1992, 24, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Roslinski, D.L.; Bhatnagar, K.P.; Burrows, A.M.; Smith, T.D. Comparative Morphology and Histochemistry of Glands Associated with the Vomeronasal Organ in Humans, Mouse Lemurs, and Voles. Anat. Rec. 2000, 260, 92–101. [Google Scholar] [CrossRef]
- Schwanzel-Fukuda, M.; Pfaff, D.W. Origin of Luteinizing Hormone-Releasing Hormone Neurons. Nature 1989, 338, 161–164. [Google Scholar] [CrossRef]
- Schwanzel-Fukuda, M.; Pfaff, D.W. The Migration of Luteinizing Hormone-Releasing Hormone (LHRH) Neurons from the Medial Alfactory Placode into the Medial Basal Forebrain. Experientia 1990, 46, 956–962. [Google Scholar] [CrossRef]
- Schwanzel-Fukuda, M.; Pfaff, D.W. Migration of LHRH-Immunoreactive Neurons from the Olfactory Placode Rationalizes Olfacto-Hormonal Relationships. J. Steroid Biochem. Mol. Biol. 1991, 39, 565–572. [Google Scholar] [CrossRef]
- Schwanzel-Fukuda, M.; Pfaff, D.W. Luteinizing Hormone-Releasing Hormone (LHRH) and Neural Cell Adhesion Molecule (NCAM)-Immunoreactivity in Development of the Forebrain and Reproductive System. Ann. Endocrinol. 1994, 55, 235–241. [Google Scholar]
- Skeen, L.C.; Hall, W.C. Efferent Projections of the Main and the Accessory Olfactory Bulb in the Tree Shrew (Tupaia glis). J. Comp. Neurol. 1977, 172, 1–35. [Google Scholar] [CrossRef]
- Stephan, H. Der Bulbus Olfatorius Accessorius Bei Insektivoren Und Primaten. Cells Tissues Organs 1965, 62, 215–253. [Google Scholar] [CrossRef]
- Schneider, N.Y.; Fletcher, T.P.; Shaw, G.; Renfree, M.B. The Olfactory System of the Tammar Wallaby Is Developed at Birth and Directs the Neonate to Its Mother’s Pouch Odours. Reproduction 2009, 138, 849–857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wettschureck, N.; Offermanns, S. Mammalian G Proteins and Their Cell Type Specific Functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, D.; Kawai, Y.K.; Watanabe, K.; Muranishi, Y. Artiodactyl Livestock Species Have a Uniform Vomeronasal System with a Vomeronasal Type 1 Receptor (V1R) Pathway. Tissue Cell 2022, 77, 101863. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.V.; Ortiz-Leal, I.; Villamayor, P.R.; Ferreiro, A.; Rois, J.L.; Sanchez-Quinteiro, P. Does a Third Intermediate Model for the Vomeronasal Processing of Information Exist? Insights from the Macropodid Neuroanatomy. Brain. Struct. Funct. 2022, 227, 881–899. [Google Scholar] [CrossRef]
- Kondoh, D.; Kamikawa, A.; Sasaki, M.; Kitamura, N. Localization of A1-2 Fucose Glycan in the Mouse Olfactory Pathway. Cells Tissues Organs 2017, 203, 20–28. [Google Scholar] [CrossRef]
- Keller, L.-A.; Niedermeier, S.; Claassen, L.; Popp, A. Comparative Lectin Histochemistry on the Murine Respiratory Tract and Primary Olfactory Pathway Using a Fully Automated Staining Procedure. Acta Histochem. 2022, 124, 151877. [Google Scholar] [CrossRef]
- Salazar, I.; Sanchez-Quinteiro, P.; Lombardero, M.; Cifuentes, J.M. A Descriptive and Comparative Lectin Histochemical Study of the Vomeronasal System in Pigs and Sheep. J. Anat. 2000, 196, 15–22. [Google Scholar] [CrossRef]
- Mogi, K.; Sakurai, K.; Ichimaru, T.; Ohkura, S.; Mori, Y.; Okamura, H. Structure and Chemical Organization of the Accessory Olfactory Bulb in the Goat. Anat. Rec. 2007, 290, 301–310. [Google Scholar] [CrossRef]
- Sweet, G. Memoirs: Contributions to Our Knowledge of the Anatomy of Notoryctes Typhlops, Stirling. J. Cell Sci. 1906, 2, 547–572. [Google Scholar] [CrossRef]
- Broom, R. On the Organ of Jacobson and Some Other Structures in the Nose of Cænolestes. By R. BROOM, D.Sc., P.R.S., C.M.Z.S. Proc. Zool. Soc. Lond. 1926, 96, 419–424. [Google Scholar] [CrossRef]
- Bock, P.; Rohn, K.; Beineke, A.; Baumgärtner, W.; Wewetzer, K. Site-Specific Population Dynamics and Variable Olfactory Marker Protein Expression in the Postnatal Canine Olfactory Epithelium. J. Anat. 2009, 215, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Cifuentes, J.M.; Quinteiro, P.S.; Caballero, T.G. Structural, Morphometric, and Immunohistological Study of the Accessory Olfactory Bulb in the Dog. Anat. Rec. 1994, 240, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.V.; Ortiz-Leal, I.; Ferreiro, A.; Rois, J.L.; Sanchez-Quinteiro, P. Neuroanatomical and Immunohistological Study of the Main and Accessory Olfactory Bulbs of the Meerkat (Suricata suricatta). Animals 2021, 12, 91. [Google Scholar] [CrossRef]
- O’Riain, M.J.; Bennett, N.C.; Brotherton, P.N.M.; McIlrath, G.; Clutton-Brock, T.H. Reproductive Suppression and Inbreeding Avoidance in Wild Populations of Co-Operatively Breeding Meerkats (Suricata suricatta). Behav. Ecol. Sociobiol. 2000, 48, 471–477. [Google Scholar] [CrossRef]
- Leclaire, S.; Nielsen, J.F.; Thavarajah, N.K.; Manser, M.; Clutton-Brock, T.H. Odour-Based Kin Discrimination in the Cooperatively Breeding Meerkat. Biol. Lett. 2013, 9, 20121054. [Google Scholar] [CrossRef]
- Leclaire, S.; Jacob, S.; Greene, L.K.; Dubay, G.R.; Drea, C.M. Social Odours Covary with Bacterial Community in the Anal Secretions of Wild Meerkats. Sci. Rep. 2017, 7, 3240. [Google Scholar] [CrossRef]
- Grus, W.E.; Zhang, J. Rapid Turnover and Species-Specificity of Vomeronasal Pheromone Receptor Genes in Mice and Rats. Gene 2004, 340, 303–312. [Google Scholar] [CrossRef]
- Salazar, I.; Sánchez-Quinteiro, P. The Risk of Extrapolation in Neuroanatomy: The Case of the Mammalian Accessory Olfactory Bulb. Front. Neuroanat. 2009, 3, 22. [Google Scholar] [CrossRef]
- Nakajima, T.; Sakaue, M.; Kato, M.; Saito, S.; Ogawa, K.; Taniguchi, K. Immunohistochemical and Enzyme-Histochemical Study on the Accessory Olfactory Bulb of the Dog. Anat. Rec. 1998, 252, 393–402. [Google Scholar] [CrossRef]
- Hughes, L. Meerkats: Essential Wildlife; Character-19: 2020. Available online: https://www.amazon.com/Meerkats-Essential-Wildlife-Lisa-Hughes-ebook/dp/B08GY42Z76 (accessed on 13 October 2023).
- Broom, R. On the Organ of Jacobson in the Hyrax. J. Anat. Physiol. 1898, 32, 709–713. [Google Scholar] [PubMed]
- Yohe, L.R.; Krell, N.T. An Updated Synthesis of and Outstanding Questions in the Olfactory and Vomeronasal Systems in Bats: Genetics Asks Questions Only Anatomy Can Answer. Anat. Rec. 2023, 306, 2765–2780. [Google Scholar] [CrossRef] [PubMed]
- Wöhrmann-Repenning, A. Functional Aspects of the Vomeronasal Complex in Mammals. Zool. Jb. Anat. 1991, 121, 71–80. [Google Scholar]
- Shnayder, L.; Schwanzel-Fukuda, M.; Halpern, M. Differential OMP Expression in Opossum Accessory Olfactory Bulb. NeuroReport 1993, 5, 193–196. [Google Scholar] [CrossRef]
- Kondoh, D.; Sasaki, M.; Kitamura, N. Age-Dependent Decrease in Glomeruli and Receptor Cells Containing A1–2 Fucose Glycan in the Mouse Main Olfactory System but Not in the Vomeronasal System. Cell Tissue Res. 2018, 373, 361–366. [Google Scholar] [CrossRef]
- Tiwari, R.P.; Ahmed, A.; Mishra, G.K. Role of Pheromones and Biostimulation in Animal Reproduction—An Overview. J. Vet. Sci. Tech. 2014, 3, 15–20. [Google Scholar]
- Rekwot, P.I.; Ogwu, D.; Oyedipe, E.O.; Sekoni, V.O. The Role of Pheromones and Biostimulation in Animal Reproduction. Anim. Reprod. Sci. 2001, 65, 157–170. [Google Scholar] [CrossRef]
- Landaeta-Hernández, A.J.; Ungerfeld, R.; Chenoweth, P.J. Biostimulation and Pheromones in Livestock: A Review. Anim. Reprod. Sci. 2023, 248, 107154. [Google Scholar] [CrossRef]
- Torres, M.V.; Ortiz-Leal, I.; Ferreiro, A.; Rois, J.L.; Sanchez-Quinteiro, P. Immunohistological Study of the Unexplored Vomeronasal Organ of an Endangered Mammal, the Dama Gazelle (Nanger dama). Microsc. Res. Tech. 2023, 86, 1206–1233. [Google Scholar] [CrossRef]
- Hart, B.L.; Hart, L.A.; Maina, J.N. Alteration in Vomeronasal System Anatomy in Alcelaphine Antelopes: Correlation with Alteration in Chemosensory Investigation. Physiol. Behav. 1988, 42, 155–162. [Google Scholar] [CrossRef]
- Meredith, M.; Marques, D.M.; O’Connell, R.J.; Stern, F.L. Vomeronasal Pump: Significance for Male Hamster Sexual Behavior. Science 1980, 207, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Krishna, N.S.R.; Getchell, M.L.; Margolis, F.L.; Getchell, T.V. Differential Expression of Vomeromodulin and Odorant-Binding Protein, Putative Pheromone and Odorant Transporters, in the Developing Rat Nasal Chemosensory Mucosae. J. Neurosci. Res. 1995, 40, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Takigami, S.; Mori, Y.; Ichikawa, M. Projection Pattern of Vomeronasal Neurons to the Accessory Olfactory Bulb in Goats. Chem. Senses 2000, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Akaogi, S.; Nakamuta, S.; Tsujimoto, T.; Nakamuta, N. Characteristics of Olfactory Organs in Sika Deer (Cervus nippon). Jpn. J. Zoo Wildl. Med. 2019, 24, 115–122. [Google Scholar] [CrossRef]
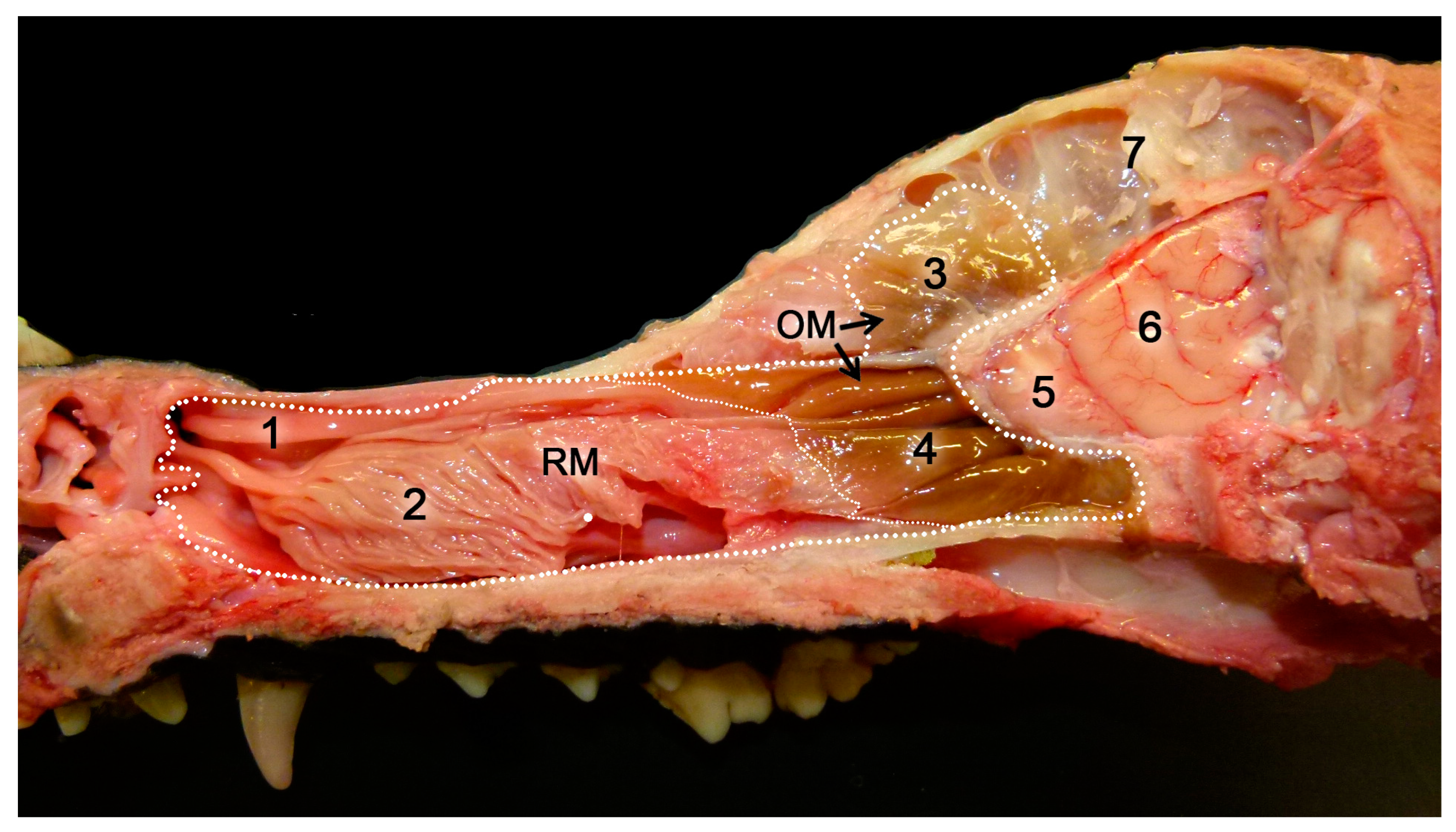
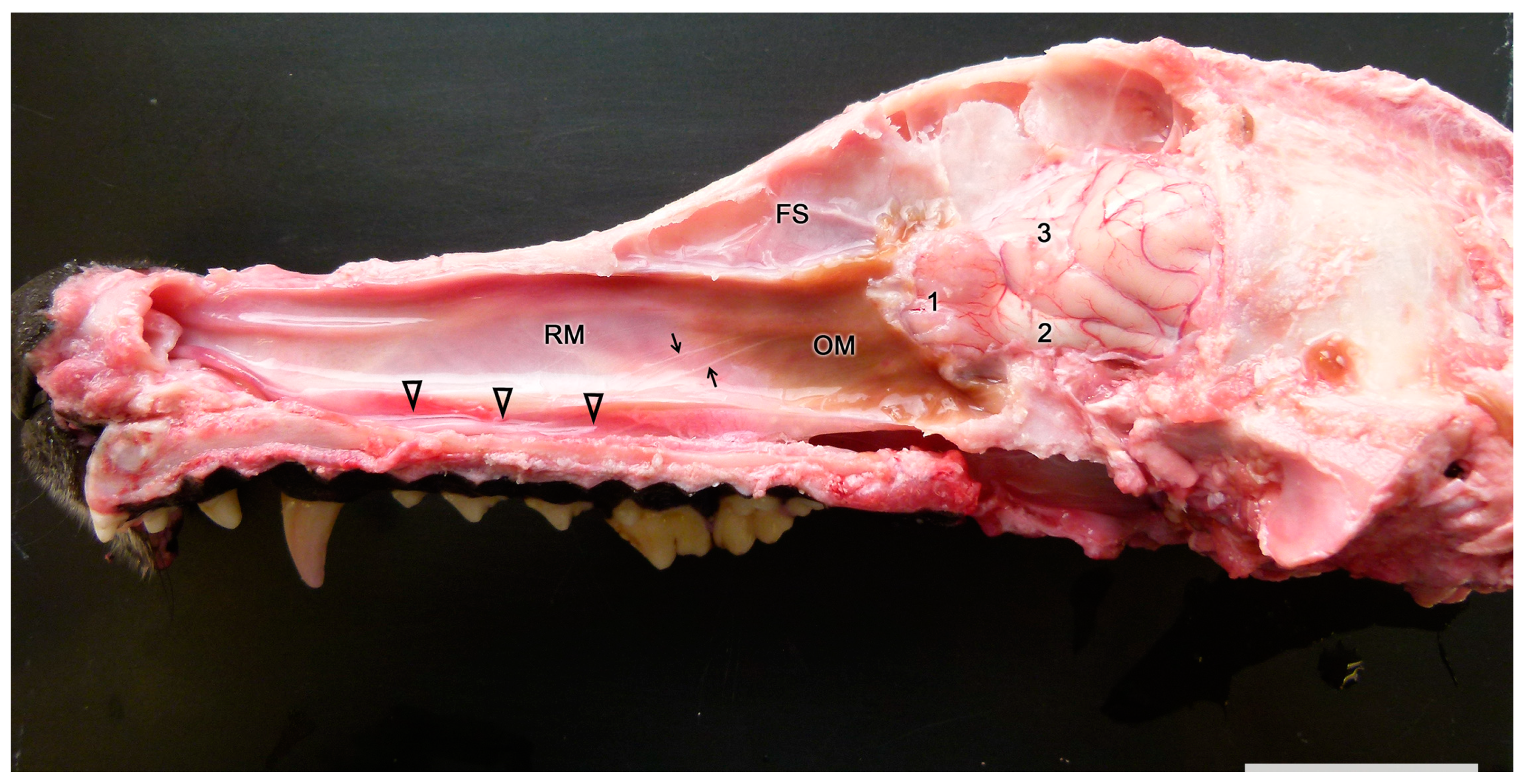

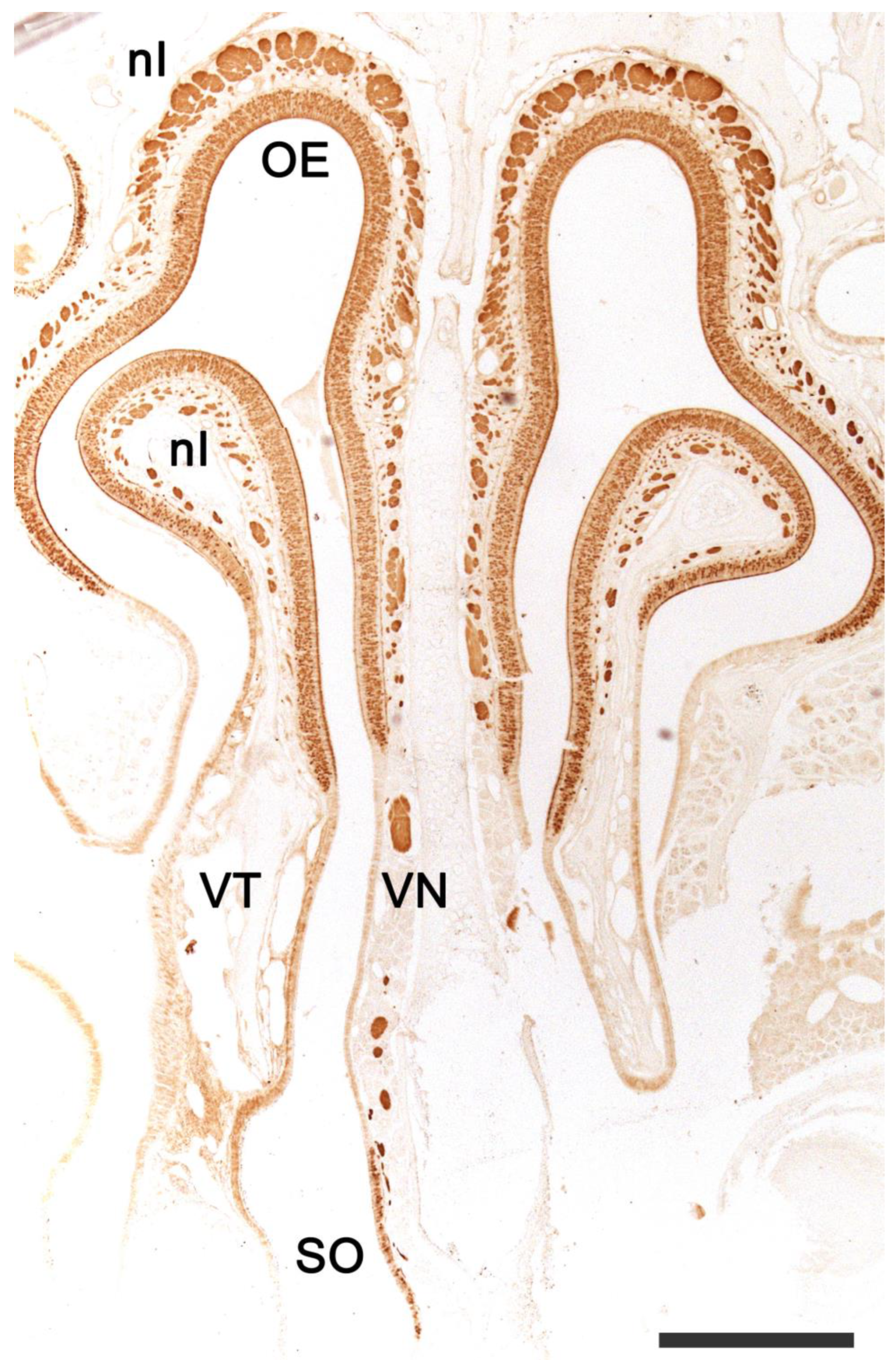

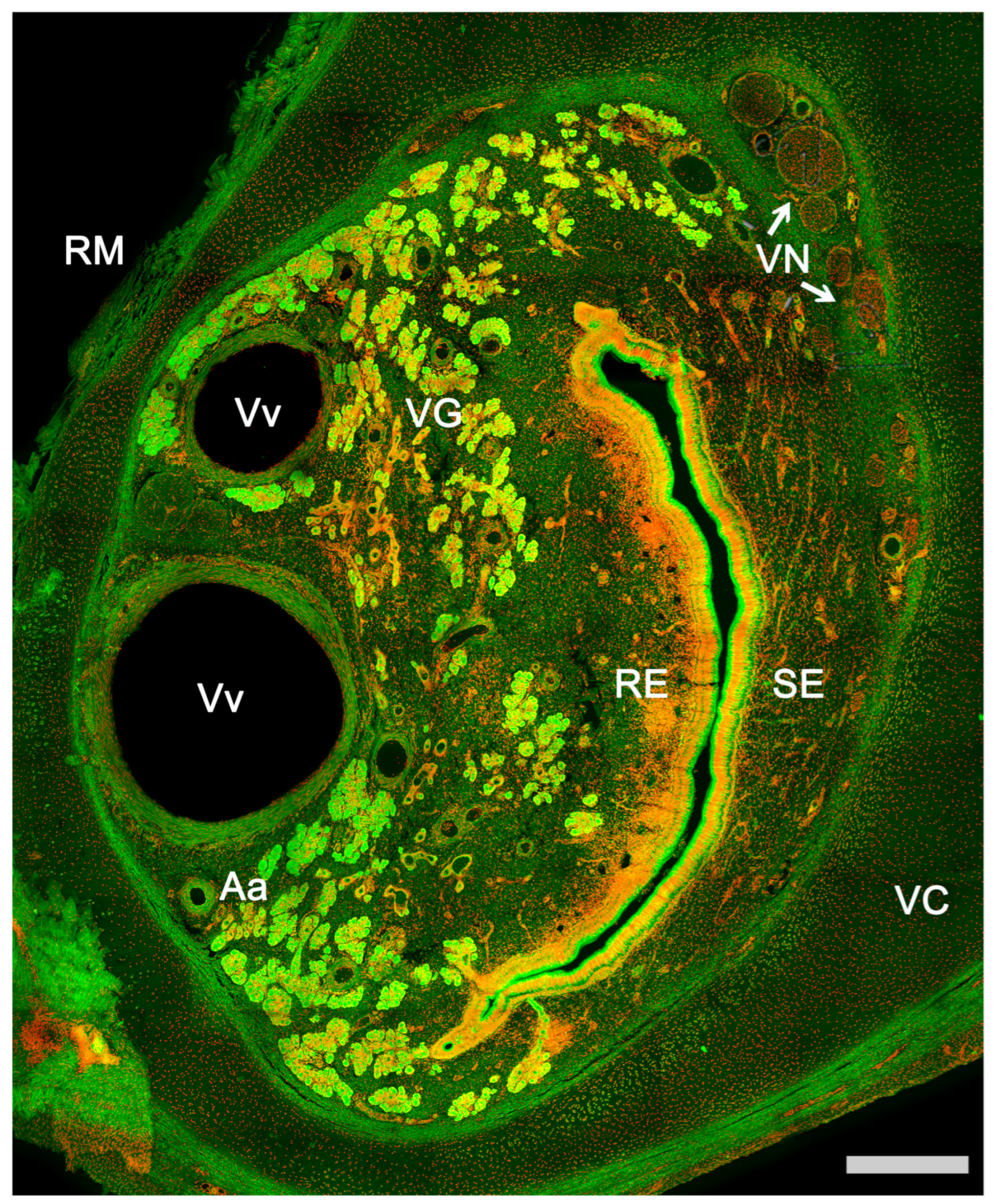
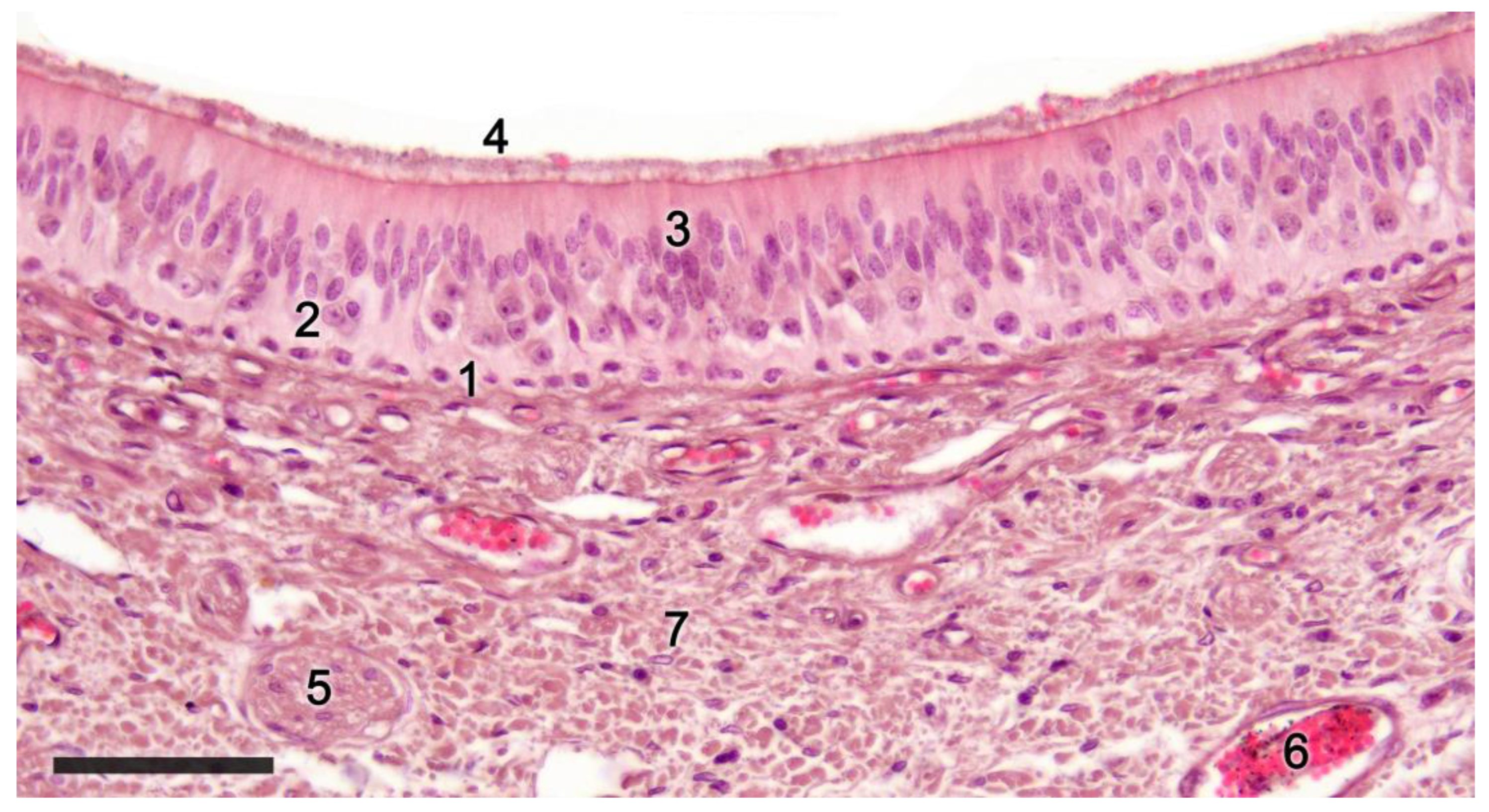
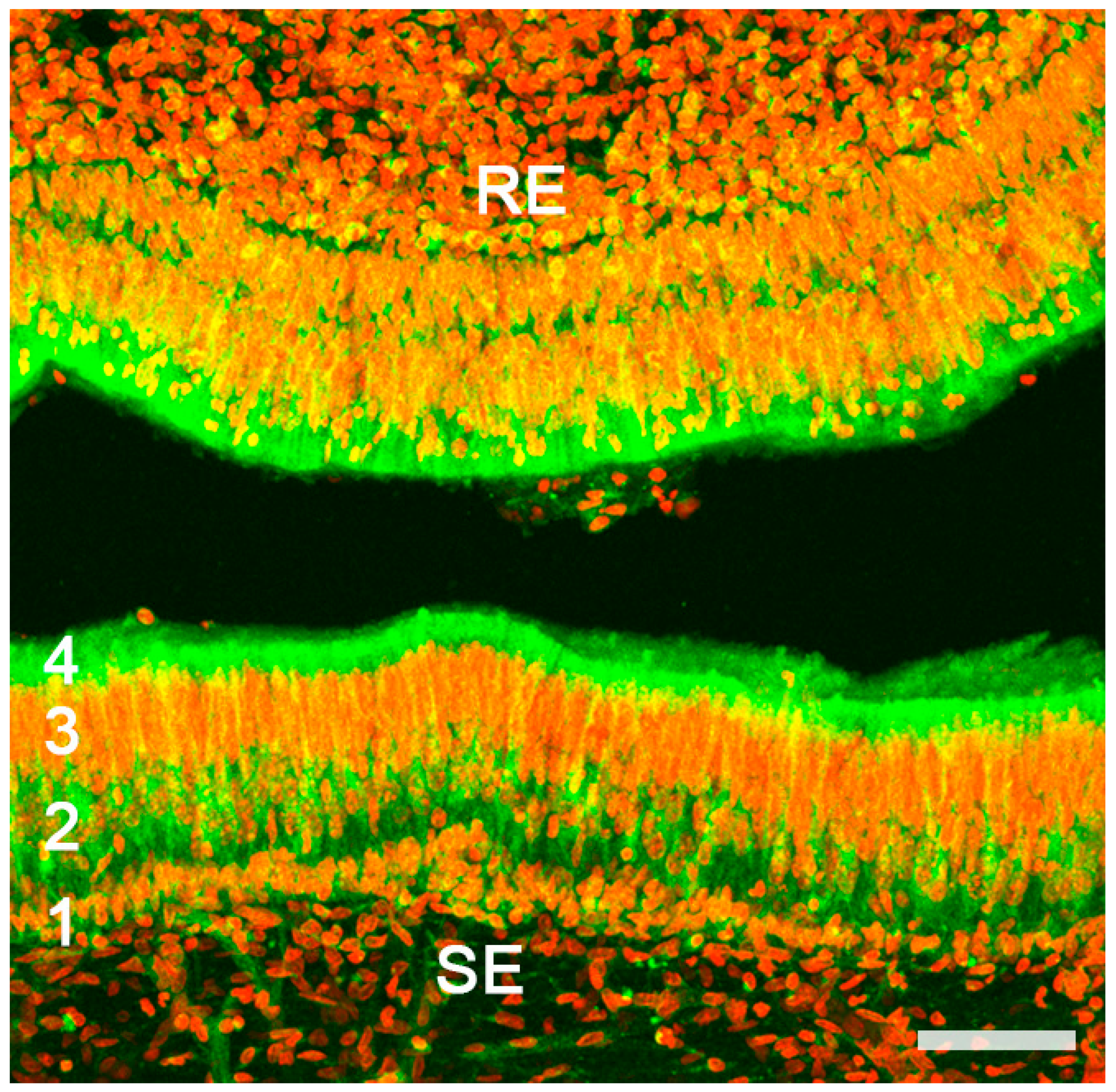
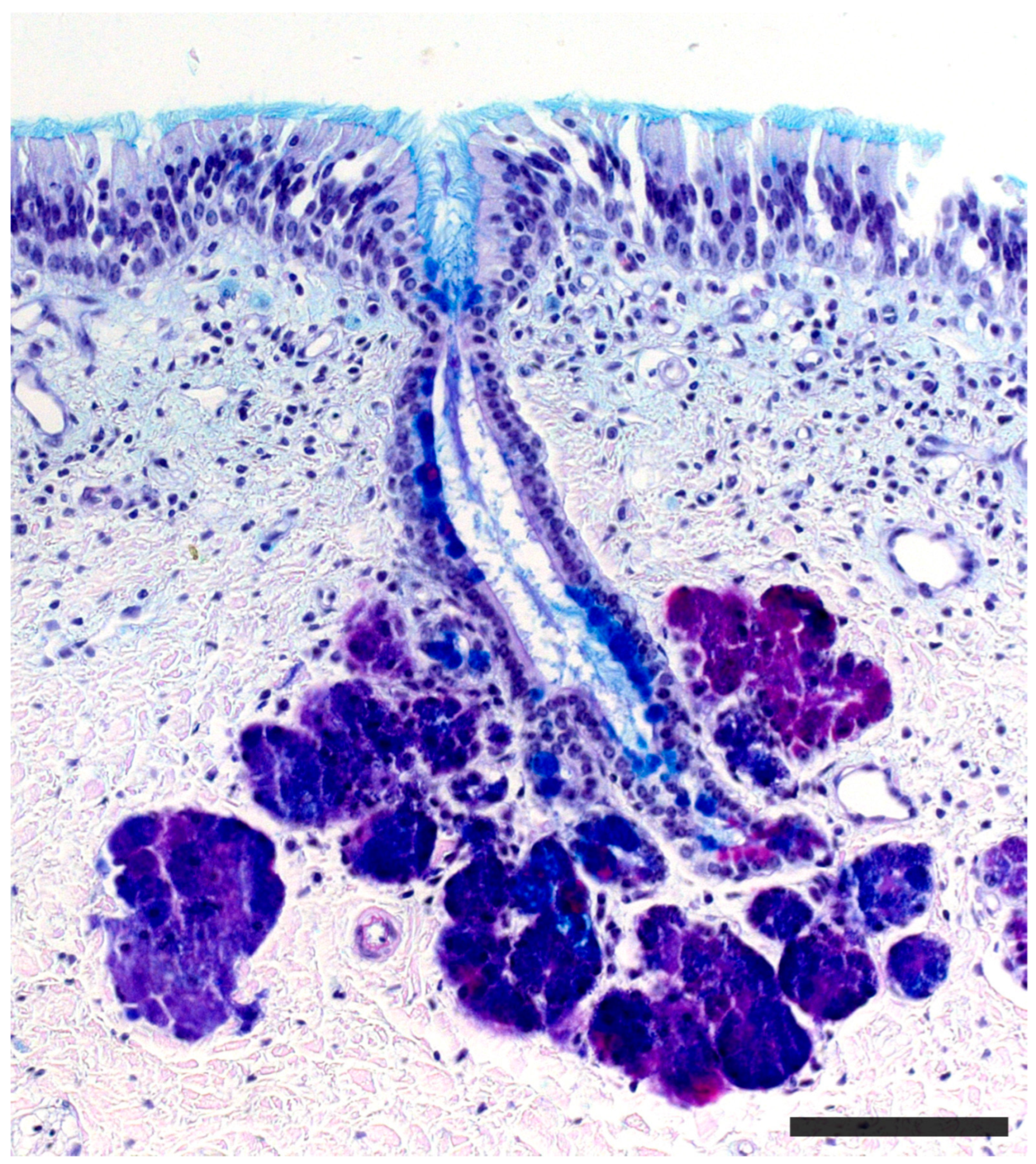
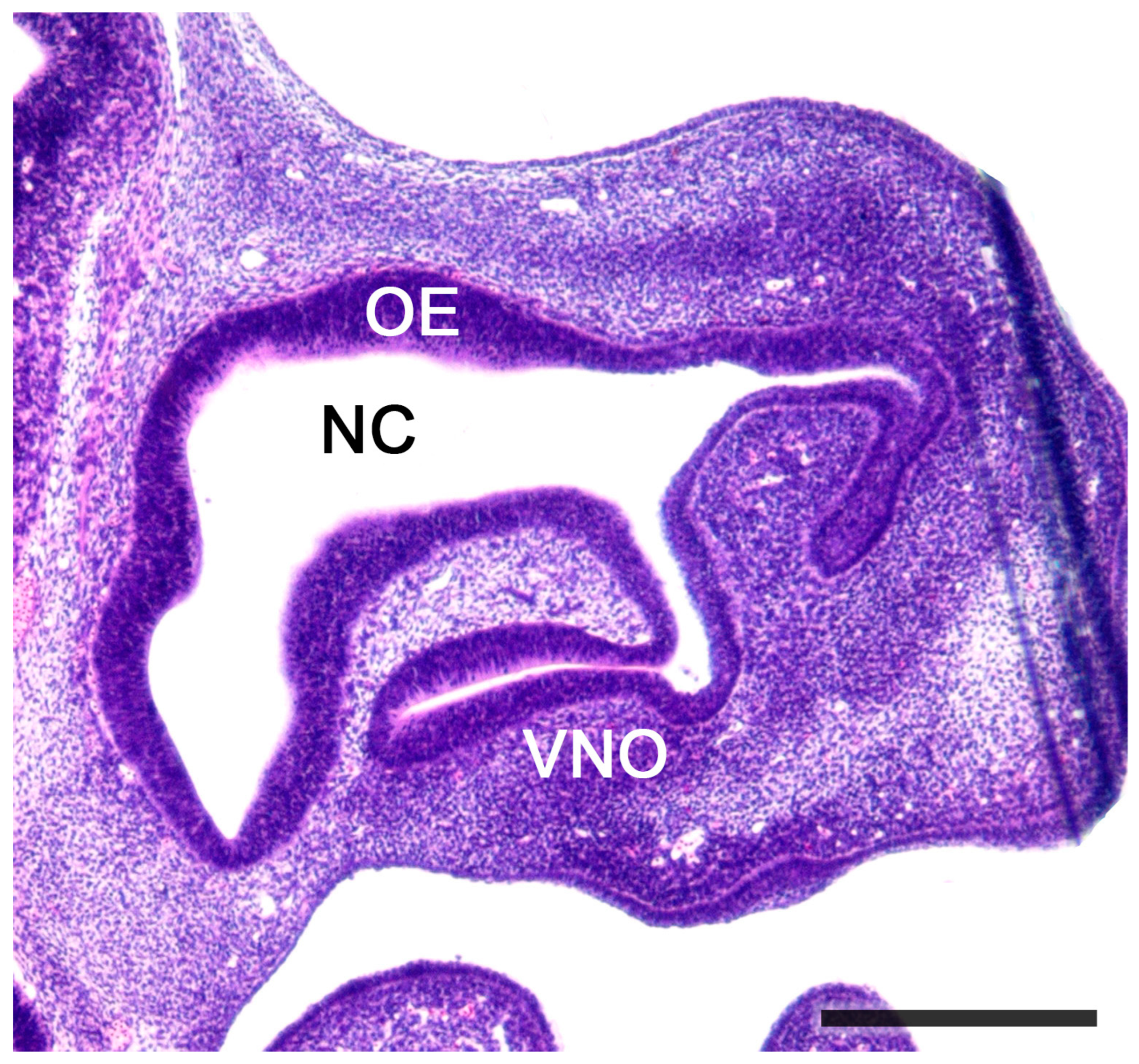

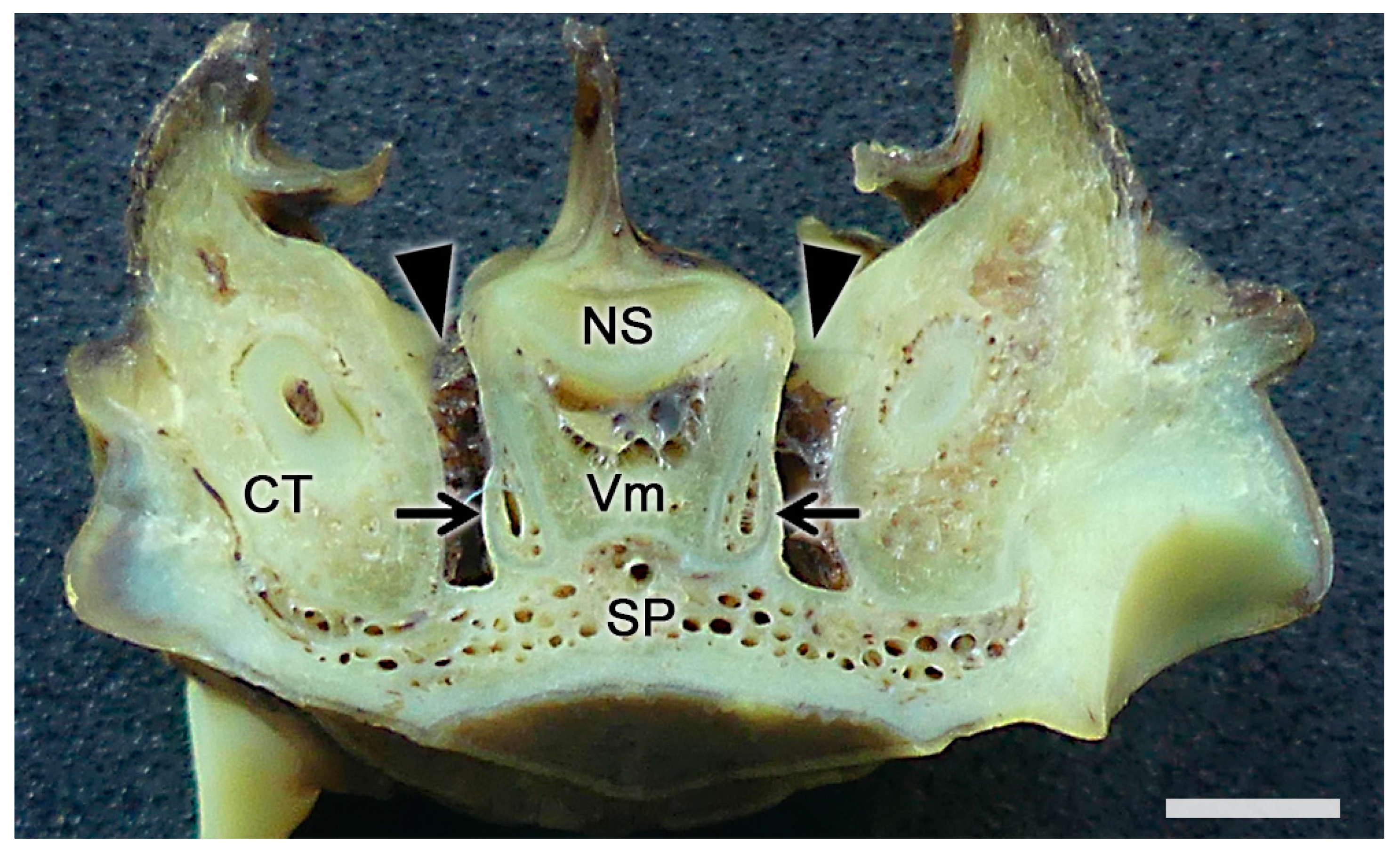

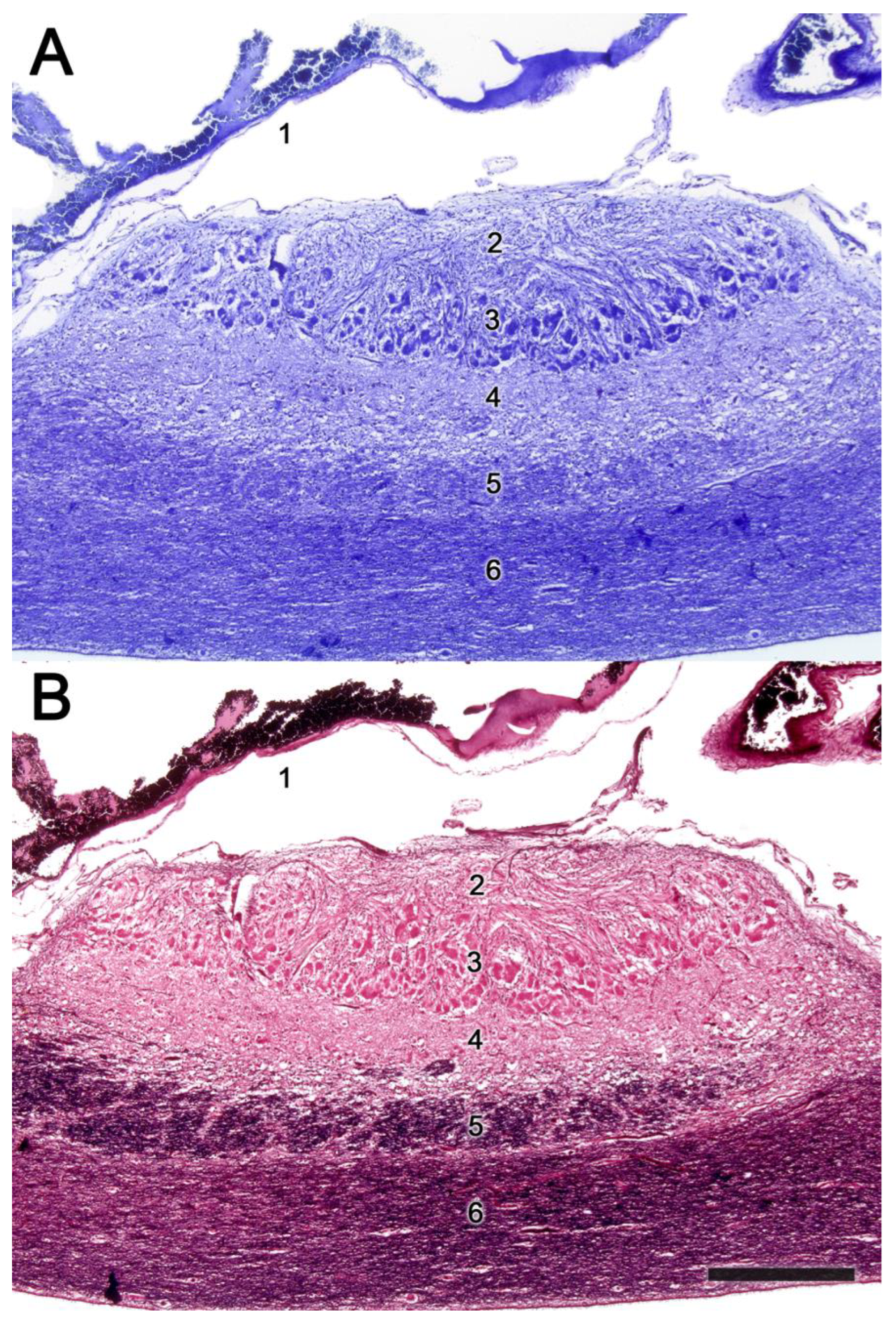




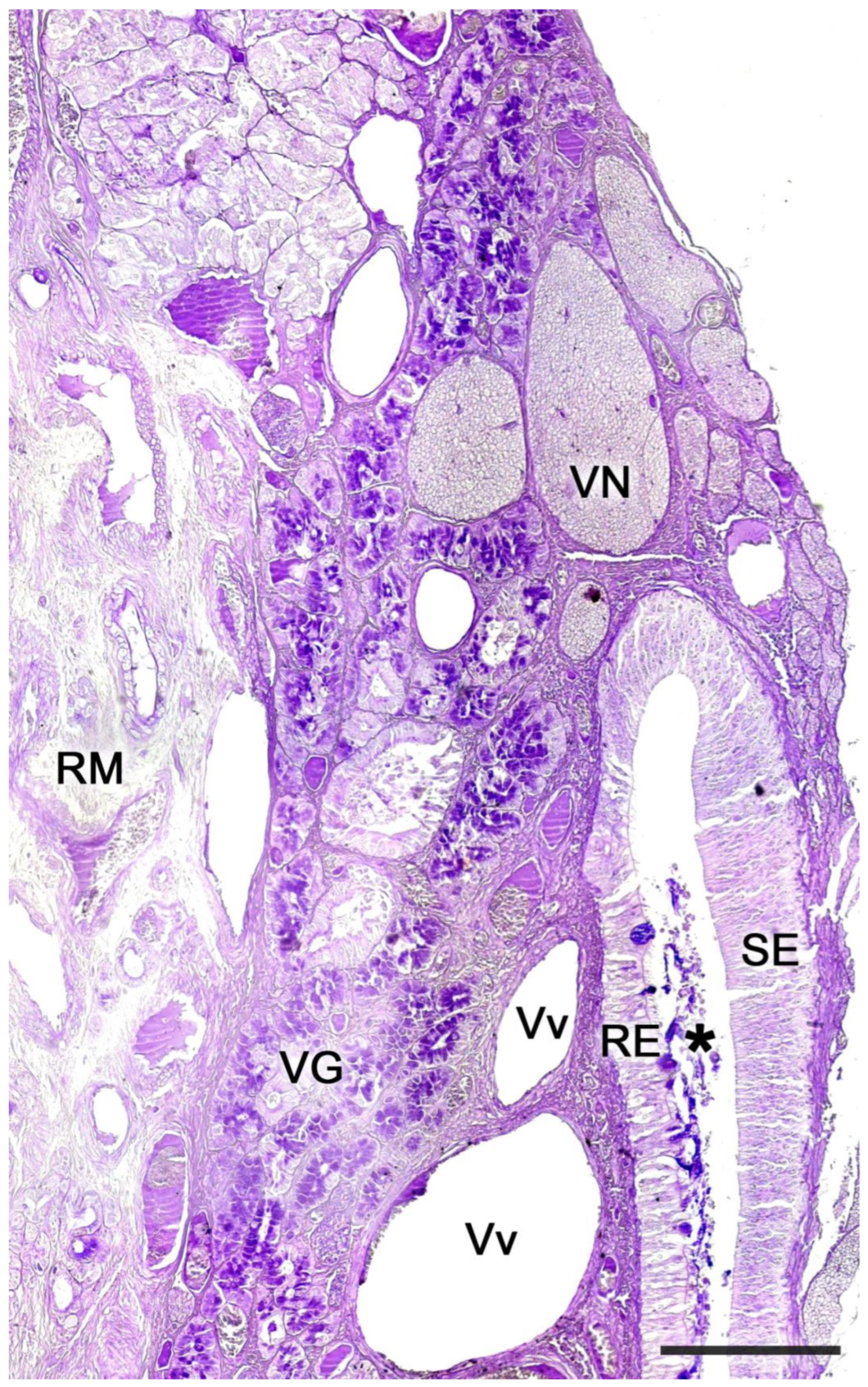
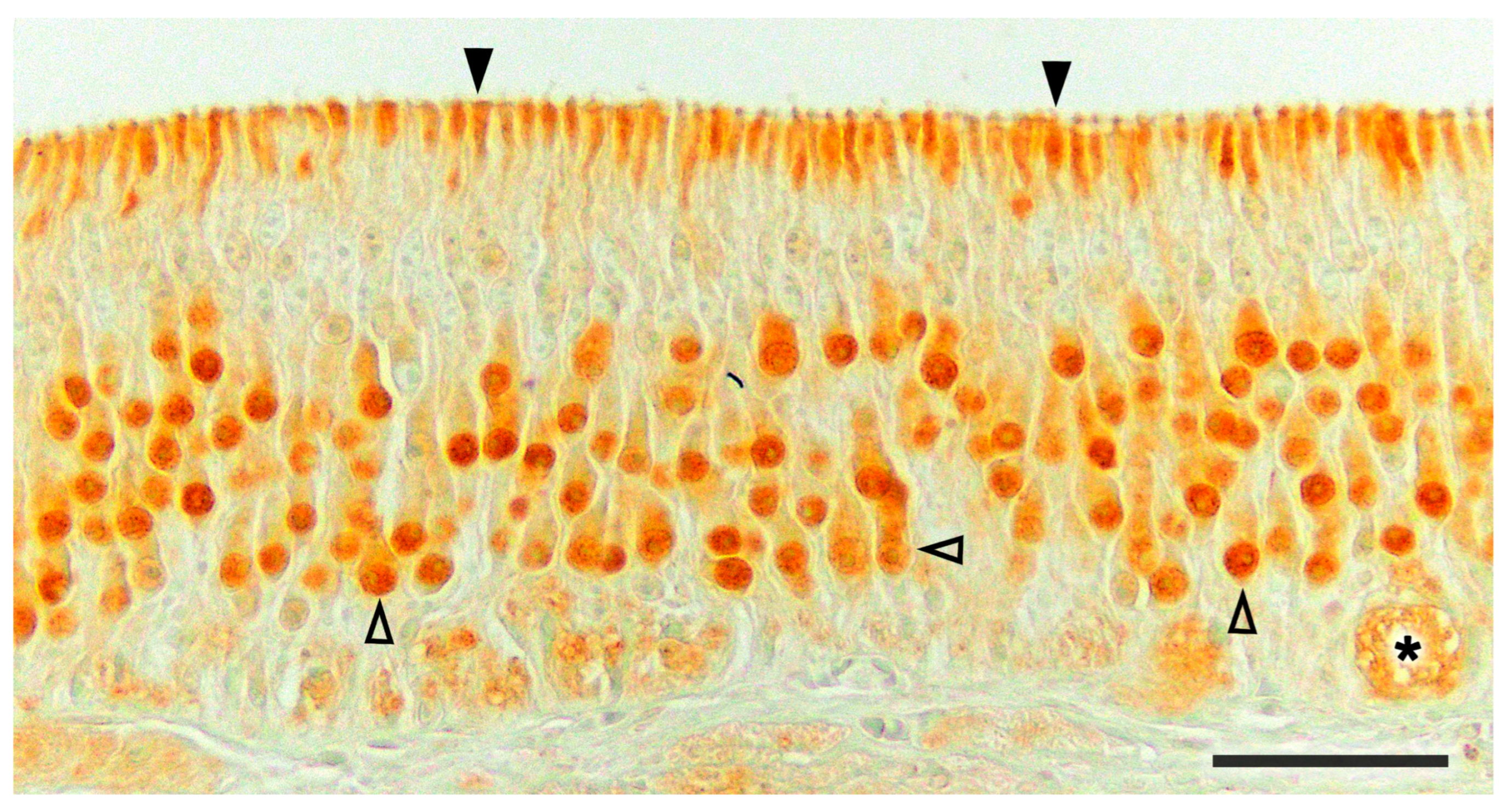
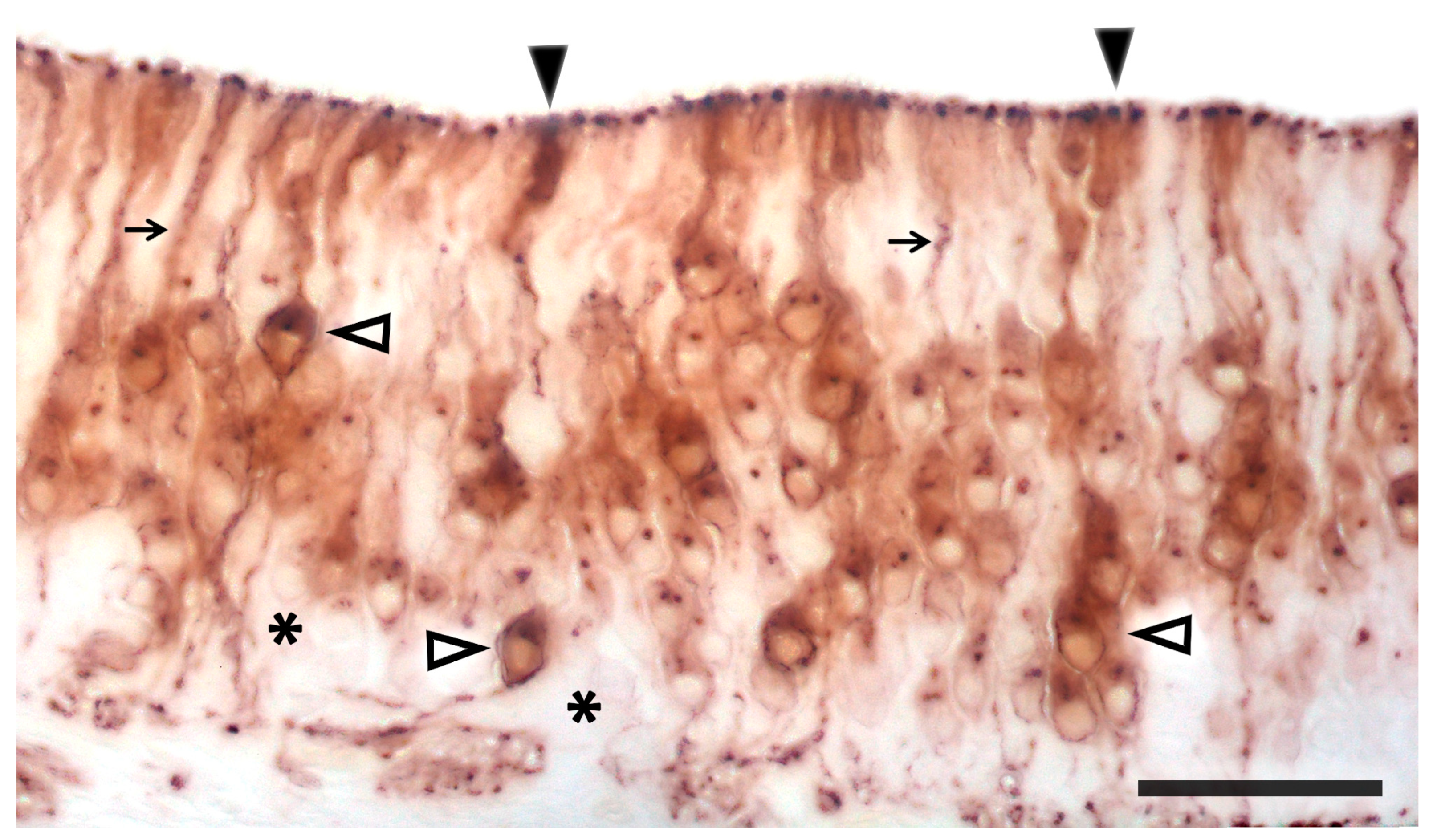
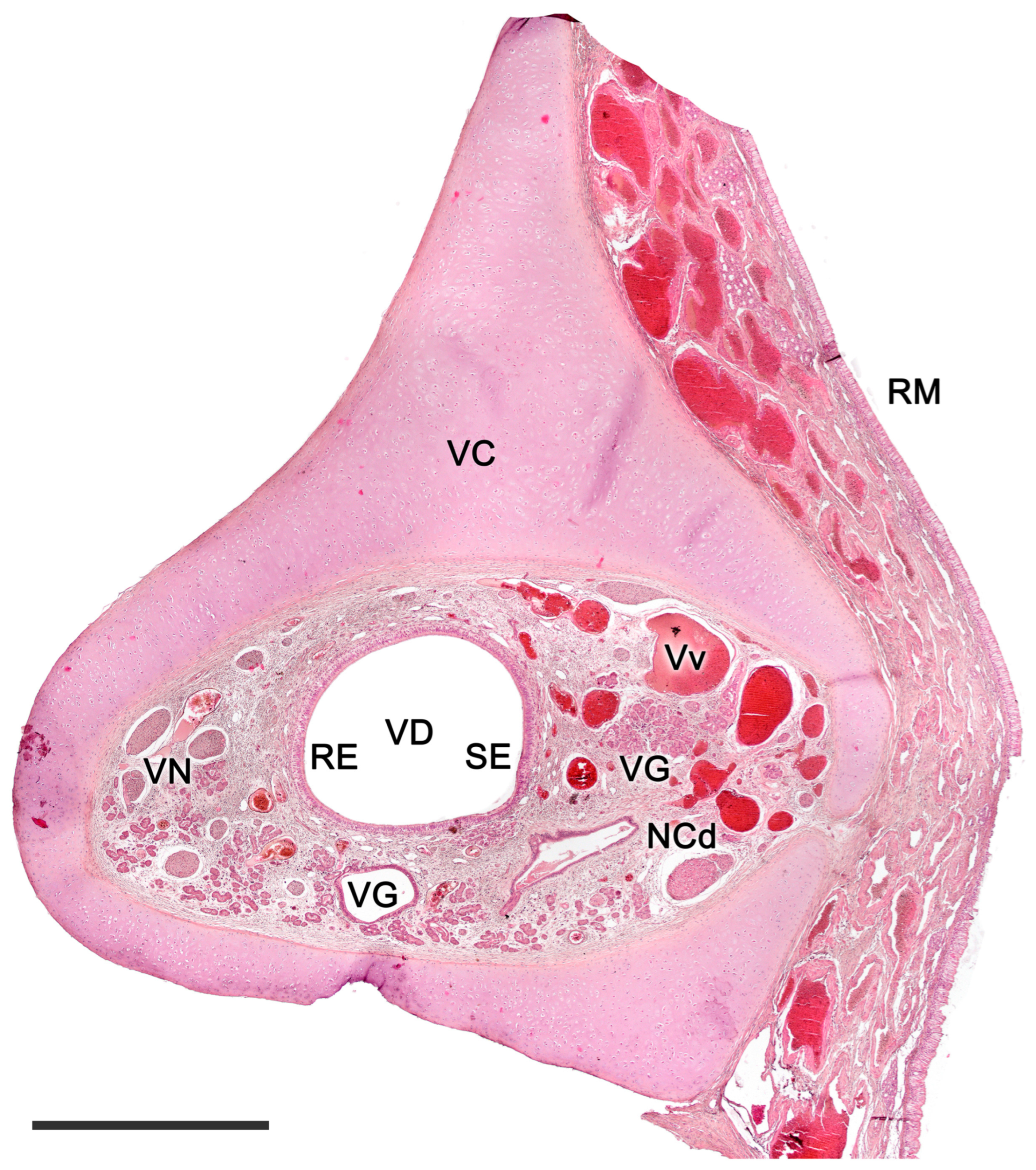

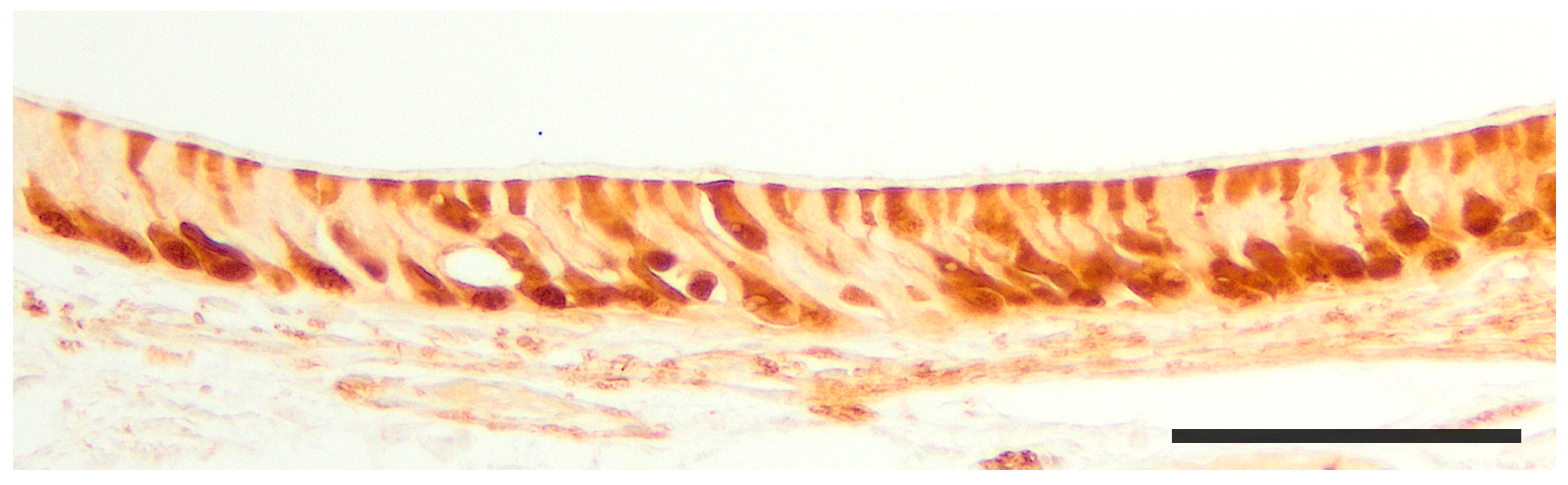
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, M.V.; Ortiz-Leal, I.; Sanchez-Quinteiro, P. Pheromone Sensing in Mammals: A Review of the Vomeronasal System. Anatomia 2023, 2, 346-413. https://doi.org/10.3390/anatomia2040031
Torres MV, Ortiz-Leal I, Sanchez-Quinteiro P. Pheromone Sensing in Mammals: A Review of the Vomeronasal System. Anatomia. 2023; 2(4):346-413. https://doi.org/10.3390/anatomia2040031
Chicago/Turabian StyleTorres, Mateo V., Irene Ortiz-Leal, and Pablo Sanchez-Quinteiro. 2023. "Pheromone Sensing in Mammals: A Review of the Vomeronasal System" Anatomia 2, no. 4: 346-413. https://doi.org/10.3390/anatomia2040031
APA StyleTorres, M. V., Ortiz-Leal, I., & Sanchez-Quinteiro, P. (2023). Pheromone Sensing in Mammals: A Review of the Vomeronasal System. Anatomia, 2(4), 346-413. https://doi.org/10.3390/anatomia2040031








