Renal Lobulation—A Benign Macroanatomical Variation?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Covantev, S.; Mazuruc, N.; Belic, O. Renal Arteries: A Morphological and Angiographic Assessment. Online J. Health Allied Sci. 2018, 17, 9. [Google Scholar]
- Standring, S. Gray’s Anatomy E-Book: The Anatomical Basis of Clinical Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Covantev, S.; Mazuruc, N.; Belic, O. Renal Veins: Developmental Variations and Clinical Significance. Online J. Health Allied Sci. 2017, 16, 12. [Google Scholar]
- Hodson, J. The lobar structure of the kidney. Br. J. Urol. 1972, 44, 246–261. [Google Scholar] [CrossRef]
- Syed, S.A.; Joshi, R.A.; Herekar, N.G. Histogenesis of Kidney in Human Fetuses. Int. J. Recent Trends Sci. Technol. 2012, 3, 44–48. [Google Scholar]
- Mishra, S.; Dinesh, A.; Kaul, J.M. Morphological and Morpho-metrical Study of Human Renal Development during Mid-Gestation Period. J. Anat. Soc. India 2006, 55, 5–10. [Google Scholar]
- Harrison, L.H.; Flye, M.W.; Seigler, H.F. Incidence of Anatomical Variants in Renal Vasculature in the Presence of Normal Renal Function. Ann. Surg. 1978, 188, 83–89. [Google Scholar] [CrossRef]
- Lorenz, E.C.; Vrtiska, T.J.; Lieske, J.C.; Dillon, J.J.; Stegall, M.D.; Li, X.; Bergstralh, E.B.; Rule, A.D. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin. J. Am. Soc. Nephrol. 2010, 5, 431–438. [Google Scholar] [CrossRef]
- Kaul, A. Unusual Kidney Cancer Presentation, Case Report and Review. Proc. UCLA Healthcare 2013, 17. Available online: https://proceedings.med.ucla.edu/wp-content/uploads/2016/11/Unusual-Kidney-Cancer-Presentation-Case-Report-and-Review-edited.pdf (accessed on 6 May 2022).
- Bhatt, S.; MacLennan, G.; Dogra, V. Renal pseudotumors. AJR Am. J. Roentgenol. 2007, 188, 1380–1387. [Google Scholar] [CrossRef]
- Nazim, S.M.; Bangash, M.; Salam, B. Persistent fetal lobulation of kidney mimicking renal tumour. BMJ Case Rep. 2017, 2017, bcr-2017-219856. [Google Scholar] [CrossRef]
- Ritz, B.; Nestor, N.; Ruiz, K.; Aldawood, A.; Zdilla, M.; Klinkhachorn, P. Persistent Fetal Kidney Lobulation: Anatomy and Pathology. FASEB J. 2019, 33, 616.6. [Google Scholar] [CrossRef]
- Beales, P.L.; Elcioglu, N.; Woolf, A.S.; Parker, D.; Flinter, F.A. New criteria for improved diagnosis of Bardet-Biedl syndrome: Results of a population survey. J. Med. Genet. 1999, 36, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.J.; Green, J.S.; Fan, Y.; Bhogal, A.K.; Dicks, E.; Fernandez, B.A.; Stefanelli, M.; Murphy, C.; Cramer, B.C.; Dean, J.C.; et al. Clinical and Genetic Epidemiology of Bardet–Biedl Syndrome in Newfoundland: A 22-Year Prospective, Population-Based, Cohort Study. Am. J. Med. Genet. Part A 2005, 132, 352–360. [Google Scholar] [CrossRef]
- Houat, A.P.; Guimarães, C.T.S.; Takahashi, M.S.; Rodi, G.P.; Gasparetto, T.P.D.; Blasbalg, R.; Velloni, F.G. Congenital Anomalies of the Upper Urinary Tract: A Comprehensive Review. Radiographics 2021, 41, 462–486. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.F. Morphological evidence of marine adaptations in human kidneys. Med. Hypotheses 2006, 66, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.M. Congenital Anomalies of the Kidney and the Urinary Tract (CAKUT). Fetal Pediatr. Pathol. 2014, 33, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Drougas, J.G.; Barakat, R. Association of congenital abnormalities of the kidney and urinary tract with those of other organ systems in 13,775 autopsies. Child Nephrol. Urol. 1988, 9, 269–272. [Google Scholar]
- Patil, S.T.; Meshram, M.M.; Kasote, A.P. Bilateral malrotation and lobulation of kidney with altered hilar anatomy: A rare congenital variation. Surg. Radiol. Anat. 2011, 33, 941–944. [Google Scholar] [CrossRef]
- Bordei, P.; Sapte, E.; Iliescu, D. Double renal arteries originating from the aorta. Surg. Radiol. Anat. 2004, 26, 474–479. [Google Scholar] [CrossRef]
- Morishima, K.; Miyaki, T.; Ito, H. A rare case of a kidney with an widely opened hilus and supernumerary renal vessels. Kaibogaku Zasshi 1996, 71, 215–218. [Google Scholar]
- Ohtsuka, A.; Kikuta, A.; Taguchi, T.; Murakami, T. Ectopic kidney in front of the right common iliac artery and its blood vascular supply—A case report. Okajimas Folia Anat. Jpn. 1993, 70, 29–34. [Google Scholar] [CrossRef]
- Rakesh, V.; Nayak, S.; Potu, B.K.; Vollala, V.R.; Pulakunta, T. Twisted renal vessels producing an abnormal shape of the right kidney. Singap. Med. J. 2008, 49, e252–e253. [Google Scholar]
- Ballesteros, L.E.; Saldarriaga, V.; Ramirez, L.M. Morphologic evaluation of the renal veins: A study with autopsy material from Colombian subjects. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2014, 55, 77–81. [Google Scholar]
- Miclăuş, G.D.; Sas, I.; Joseph, S.C.; Matusz, P.; Pleş, H.; Tubbs, R.S.; Loukas, M. Seven renal arteries: A case report using MDCT angiography. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2014, 55 (Suppl. S3), 1181–1184. [Google Scholar]
- Covanțev, S.; Mazuruc, N.; Belic, O. An unusual case of colon vascularization by the inferior mesenteric artery. J. Vasc. Bras. 2017, 16, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Adachi, B. Das Arteriensystem der Japaner II; Maruzen Publishing Co.: Tokyo, Japan, 1928; pp. 73–78. [Google Scholar]
- Matusz, P.; Miclaus, G.; Ples, H. Study of the renal additional arteries on 1000 CT angiography continuous series. Clin. Anat. 2011, 24, 408. [Google Scholar]
- Matusz, P.; Miclăuş, G.D.; Banciu, C.D.; Sas, I.; Joseph, S.C.; Pirtea, L.C.; Tubbs, R.S.; Loukas, M. Congenital solitary kidney with multiple renal arteries: Case report using MDCT angiography. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2015, 56 (Suppl. S2), 823–826. [Google Scholar]
- Favorito, L.A.; Lobo, M.L.P.; Fernandes, A.V.; Gallo, C.M.; Sampaio, F.J.B. Kidney surface development in human fetuses: Study applied to radiological diagnosis. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2022, 48, 930–936. [Google Scholar] [CrossRef]
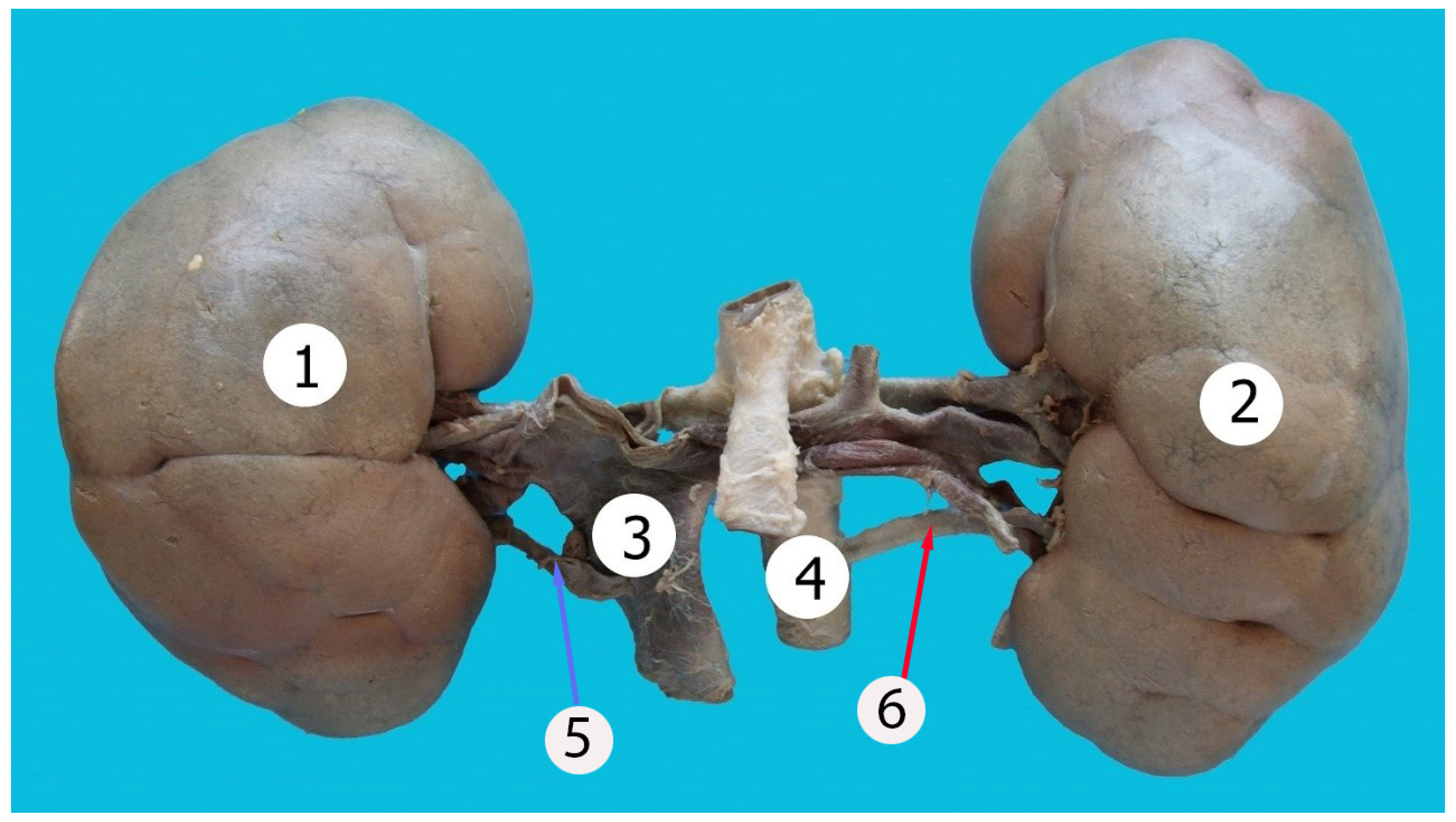
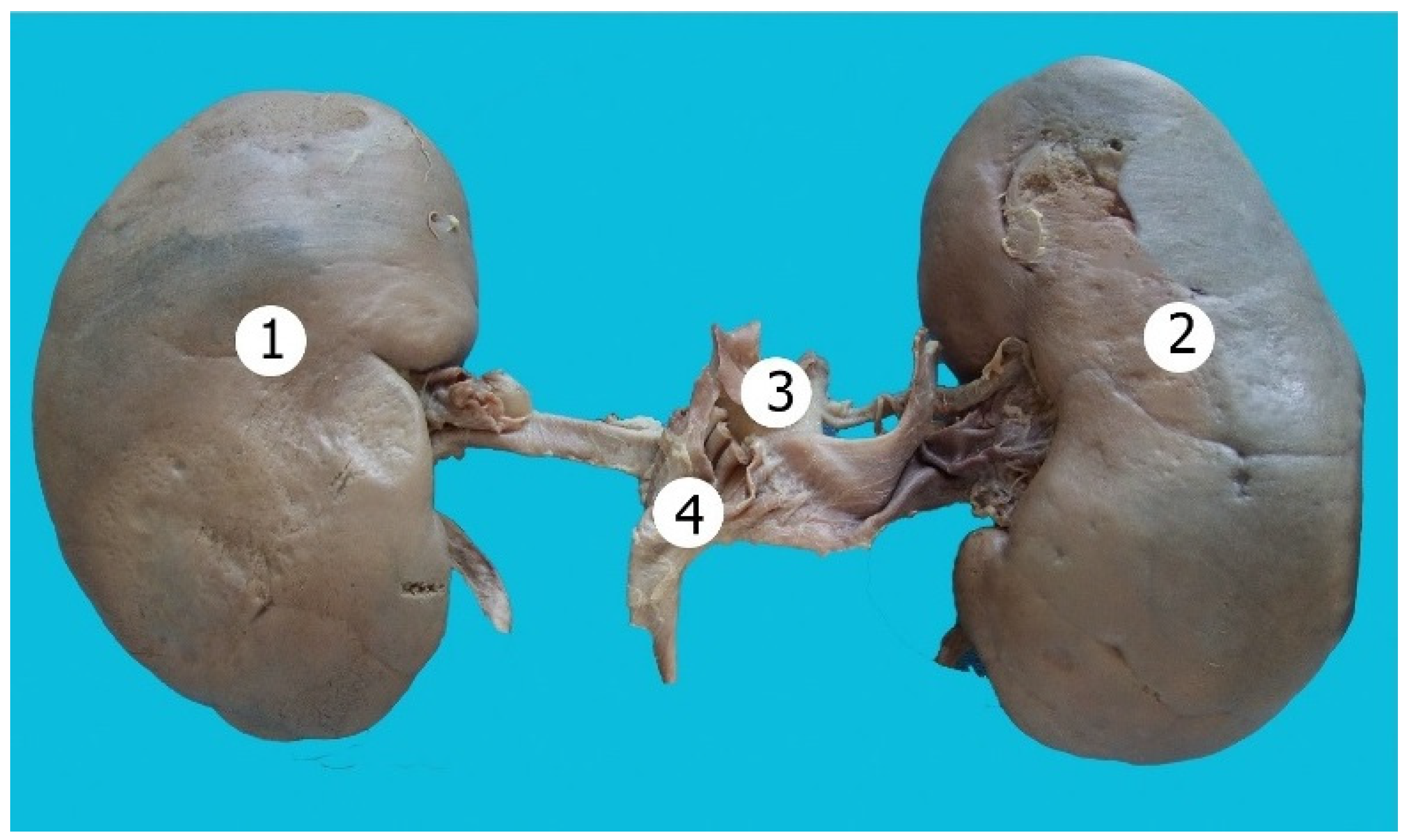
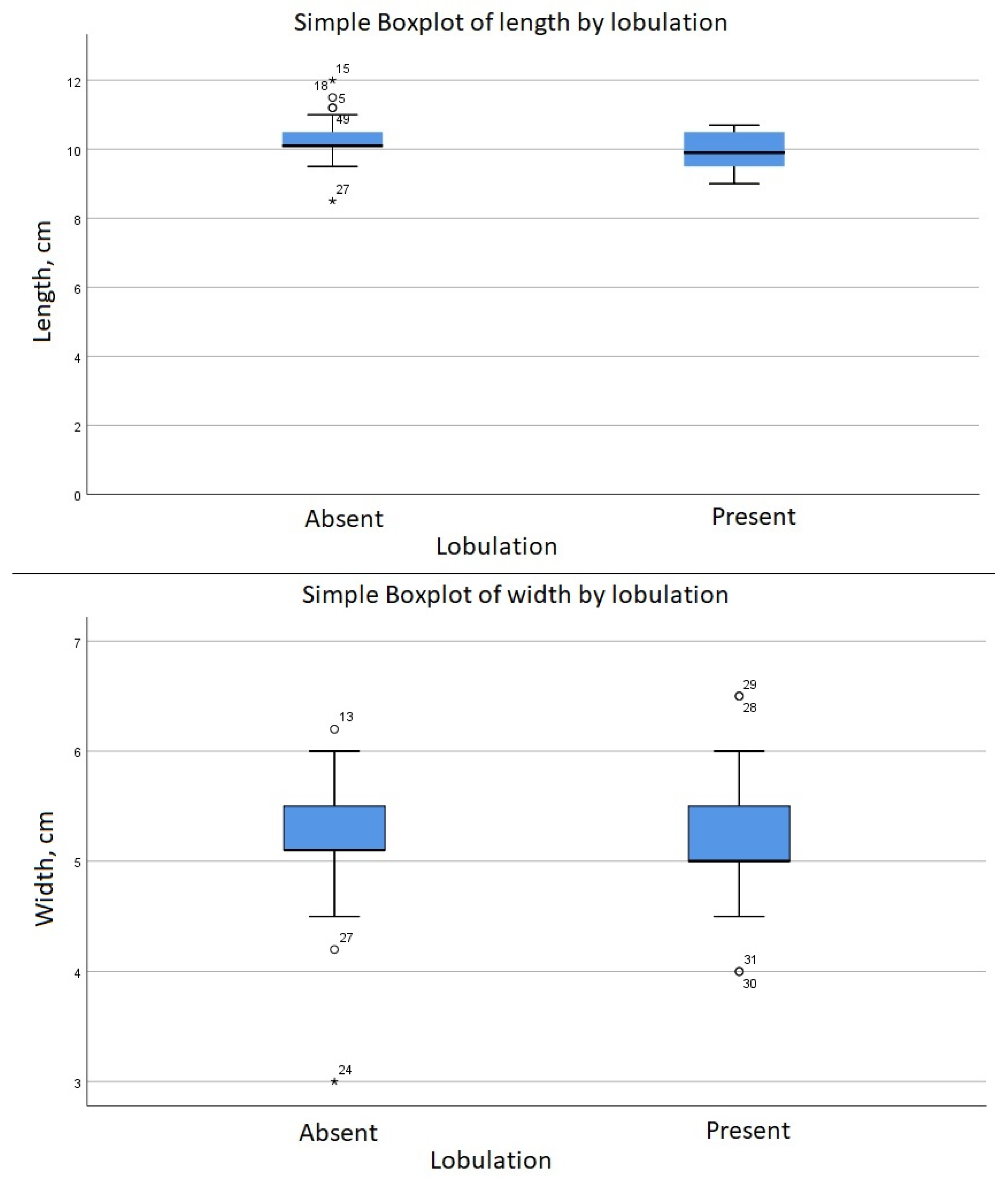
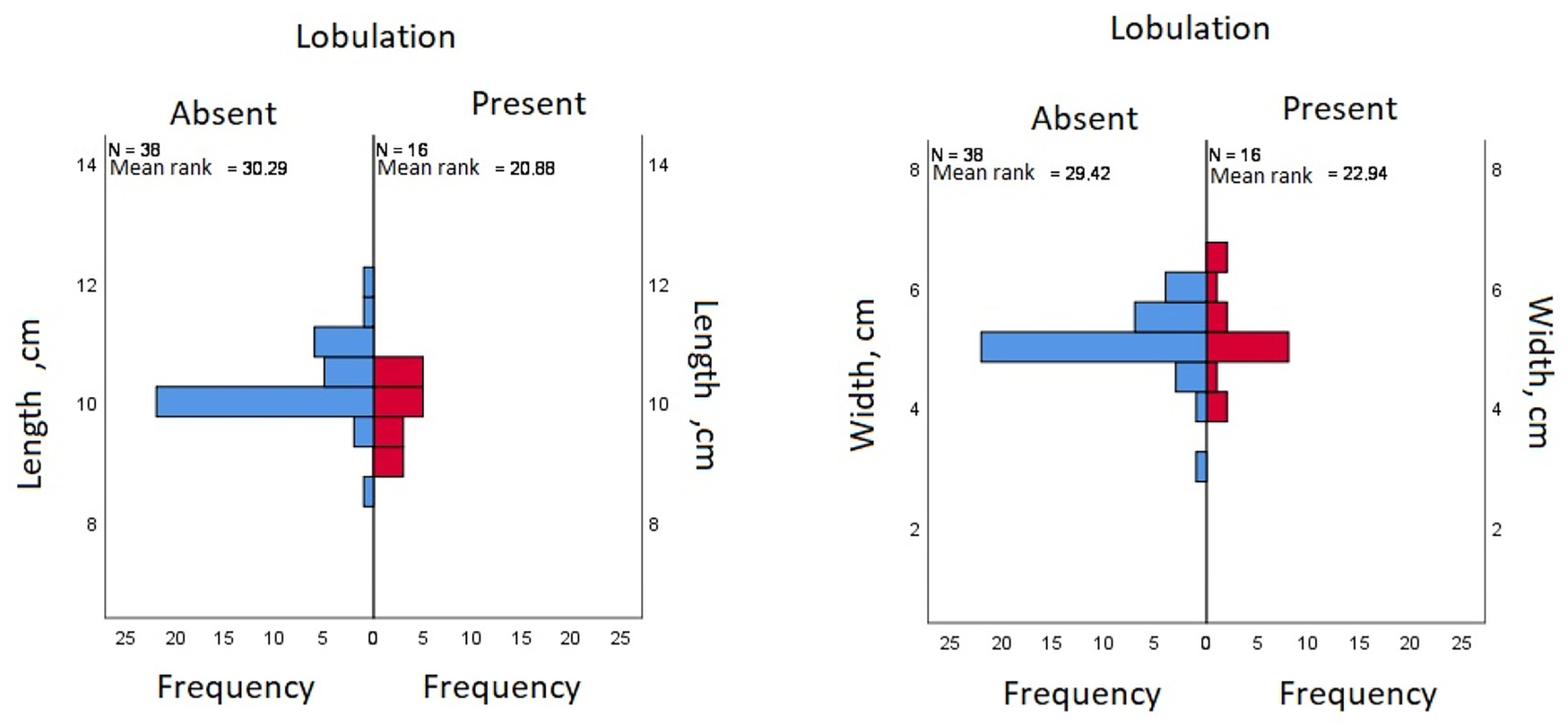
| Lobulation | ||||
|---|---|---|---|---|
| Absent | Present | |||
| Length, cm | Mean (SD) | 10.29 (0.61) | 9.89 (0.6) | |
| Median (IQR) | 10 (1) | 10 (1) | ||
| Width, cm | Mean (SD) | 5.14 | 5.16 | |
| Median (IQR) | 5 (1) | 5 (1) | ||
| Vascular anomalies | Absent | Count (%) | 26 (66.7) | 13 (33.3) |
| 95% CI | 51.1, 79.9 | 20.1, 48.9 | ||
| Present | Count (%) | 12 (80.0) | 3 (20.0) | |
| 95% CI | 55.6, 94.0 | 6.0, 44.4 | ||
| Number of major calyces | Two | Count (%) | 13 (34.2) | 2 (12.5) |
| 95% CI | 20.7, 50.0 | 2.7, 34.4 | ||
| Three | Count (%) | 17 (44.7) | 12 (75.0) | |
| 95% CI | 29.8, 60.4 | 50.9, 90.9 | ||
| Four | Count (%) | 8 (21.1) | 2 (12.5) | |
| 95% CI | 10.5, 35.8 | 2.7, 34.4 | ||
| Sex | Female | Count (%) | 14 (73.7) | 5 (26.3) |
| 95% CI | 51.6, 89.2 | 10.8, 48.4 | ||
| Male | Count (%) | 24 (68.6) | 11 (31.4) | |
| 95% CI | 52.2, 82.0 | 18.0, 47.8 | ||
| Polar artery | Absent | Count (%) | 32 (82.1) | 7 (17.9) |
| 95% CI | 68.0, 91.6 | 8.4, 32.0 | ||
| Present | Count (%) | 6 (40.0) | 9 (60.0) | |
| 95% CI | 18.8, 64.7 | 35.3, 81.2 | ||
| Position of the anatomical structures (vein, artery, pelvis) | Abnormal | Count (%) | 19 (70.4) | 8 (29.6) |
| 95% CI | 51.8, 84.9 | 15.1, 48.2 | ||
| Normal | Count (%) | 19 (70.4) | 8 (29.6) | |
| 95% CI | 51.8, 84.9 | 15.1, 48.2 | ||
| Kidney | Right | Count (%) | 15 (65.2) | 8 (34.8) |
| 95% CI | 44.9, 82.0 | 18.0, 55.1 | ||
| Left | Count (%) | 23 (74.2) | 8 (25.8) | |
| 95% CI | 57.1, 87.0 | 13.0, 42.9 | ||
| Variables in the Equation | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | |||
| Lower | Upper | ||||||||
| Step 1 | Polar artery | 1.93 | 0.67 | 8.2 | 1 | 0.004 | 6.86 | 1.84 | 25.61 |
| Constant | −1.52 | 0.42 | 13.27 | 1 | <0.001 | 0.22 | |||
| Step 2 | Kidney length | −1.85 | 0.7 | 6.92 | 1 | 0.009 | 0.16 | 0.04 | 0.62 |
| Polar artery | 2.64 | 0.83 | 10.24 | 1 | 0.001 | 14.01 | 2.78 | 70.56 | |
| Constant | 16.87 | 6.92 | 5.94 | 1 | 0.015 | 21,153,792.17 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covantsev, S.; Arnaut, O.; Mulaeva, K.; Belic, O. Renal Lobulation—A Benign Macroanatomical Variation? Anatomia 2023, 2, 336-345. https://doi.org/10.3390/anatomia2040030
Covantsev S, Arnaut O, Mulaeva K, Belic O. Renal Lobulation—A Benign Macroanatomical Variation? Anatomia. 2023; 2(4):336-345. https://doi.org/10.3390/anatomia2040030
Chicago/Turabian StyleCovantsev, Serghei, Oleg Arnaut, Karina Mulaeva, and Olga Belic. 2023. "Renal Lobulation—A Benign Macroanatomical Variation?" Anatomia 2, no. 4: 336-345. https://doi.org/10.3390/anatomia2040030
APA StyleCovantsev, S., Arnaut, O., Mulaeva, K., & Belic, O. (2023). Renal Lobulation—A Benign Macroanatomical Variation? Anatomia, 2(4), 336-345. https://doi.org/10.3390/anatomia2040030






