1. Introduction
Femoral shaft fractures (FSFs) are significant injuries that pose considerable challenges to healthcare providers. They can occur in various age groups, resulting from high-energy trauma such as motor vehicle collisions, low-energy trauma, osteoporosis, or pathologic processes [
1]. The femoral shaft consists of a cylindrical diaphysis and FSFs result from the transmission of force through the femur, causing disruption of the cortical and trabecular bone structures.
FSFs account for a significant proportion of long bone fractures. The epidemiology of these fractures varies among different populations, with a higher incidence observed in young adults due to high-energy trauma, whereas low-energy trauma and osteoporosis-related fractures are more prevalent in elderly individuals [
1]. The clinical presentation of FSFs typically includes pain, deformity, swelling, and functional impairment. The presence of associated injuries such as vascular compromise and damage to nearby structures are important and also evaluated [
1].
A thorough understanding of the anatomy of the femoral shaft is essential for assessing fracture patterns and determining appropriate treatment strategies. FSFs can be classified based on fracture location, morphology, and associated injuries to guide treatment decisions [
2].
The pathophysiology involves a combination of bending, compression, and torsional forces, leading to fracture propagation. The severity and direction of force determine the fracture pattern, ranging from simple transverse or oblique fractures to more complex comminuted or segmental fractures. Understanding the pathophysiology aids in determining fracture stability, potential complications, and treatment options [
2].
Radiologic imaging plays a pivotal role in the diagnosis and evaluation of FSFs. Plain radiographs, including anteroposterior and lateral views, provide initial information regarding fracture characteristics, displacement, and alignment. Computed tomography (CT) scans offer detailed visualization of fracture fragments, intra-articular involvement, and associated injuries. Magnetic resonance imaging (MRI) can provide additional insights into soft tissue injuries, such as muscle or ligament damage, and can be particularly useful in assessing suspected pathologic fractures or occult injuries.
The treatment of FSFs depends on several factors, including fracture characteristics, patient age, associated injuries, and surgeon expertise. Nonoperative management options, such as skeletal traction or functional bracing, may be suitable for specific cases [
3]. Surgical interventions, including intramedullary nailing, plate fixation, or external fixation, are often required for optimal fracture stabilization and alignment [
3]. Postoperative care involves early mobilization, weight-bearing progression, and rehabilitation programs tailored to individual patients.
The prognosis of FSFs is influenced by various factors. Complications such as infection, nonunion, malunion, and compartment syndrome can impact functional outcomes and long-term prognosis [
3]. Early diagnosis, appropriate treatment, meticulous surgical techniques, and diligent postoperative care contribute to improved outcomes and reduced complications.
This research review aims to provide a comprehensive overview of FSFs, covering epidemiology, clinical presentation, anatomy, pathophysiology, classification, radiologic imaging, treatment and management, and prognosis. The novelty of this review is the focus on radiological imaging modalities in FSF diagnosis and recent advances. These key aspects are vital for accurate diagnosis, effective treatment planning, and optimal patient outcomes.
2. Epidemiology
Fractures are a common occurrence in the field of orthopedics, causing significant morbidity and requiring medical intervention. Among the various types of fractures, FSFs are of particular interest due to their distinct anatomical location and associated implications. FSFs constitute a significant proportion of long bone fractures, accounting for a substantial number of hospital admissions and emergency room visits.
The occurrence of FSFs exhibits demographic variations. These fractures predominantly affect the younger and older populations, with a bimodal age distribution [
1]. The first peak is observed in individuals aged 15–25 years, primarily resulting from high-energy trauma, such as motor vehicle accidents and sports-related injuries. The second peak occurs in individuals over 65 years old, commonly due to low-energy mechanisms, including falls from standing height or osteoporotic conditions. According to population studies conducted in Sweden and Australia, the incidence of FSFs ranges between 10 and 21 per 100,000 annually [
4,
5].
FSFs typically result from a combination of axial loading and bending forces, and certain factors contribute to an increased risk. These include male gender, young age, participation in high-impact activities, alcohol or substance abuse, and occupational hazards. Additionally, the long-term use of medications, such as bisphosphonates, and the presence of underlying medical conditions, such as osteoporosis or malignancies, may predispose individuals to a higher risk of fractures, including FSFs [
6].
In comparison with other bone fractures, FSFs tend to have distinct characteristics. FSFs are often associated with severe injuries due to the high-energy nature of the traumatic events that cause them. Consequently, they often require surgical intervention for optimal management, including intramedullary nailing or external fixation.
FSFs can result from both traumatic and non-traumatic causes. Traumatic fractures typically occur due to direct or indirect forces applied to the femur, resulting in excessive stress and fracture. Common mechanisms include motor vehicle accidents, sports injuries, falls, and industrial accidents. Non-traumatic causes, such as pathological fractures, are associated with underlying bone diseases, including osteoporosis, metastatic malignancies, and primary bone tumors. Other causes of FSFs include osteoporosis and the long-term use of medications, such as bisphosphonates [
7].
In terms of clinical outcomes, FSFs can have a significant impact on functional mobility and quality of life. They are associated with prolonged hospital stays, increased risk of complications (e.g., infection, nonunion, malunion), and higher healthcare costs [
1]. Rehabilitation and long-term follow-up are crucial for achieving successful outcomes and restoring pre-injury function.
FSFs represent a significant burden in orthopedic practice, with a higher prevalence compared with other long bone fractures. Further research and surveillance are ongoing to monitor trends in FSFs and develop targeted interventions to reduce their incidence and improve patient outcomes.
3. Clinical Presentation
One of the hallmark symptoms of a femoral shaft fracture is severe pain localized in the thigh region. The pain is often exacerbated by movement and weight bearing, leading to an inability to walk or bear weight on the affected leg. The intensity of pain can vary, but it is generally described as deep and constant.
FSFs are often accompanied by localized swelling around the fracture site. The swelling may appear rapidly due to soft tissue damage and bleeding. In addition, bruising or ecchymosis may be visible due to the extravasation of blood into the surrounding tissues.
In some cases, FSFs can result in visible deformity or angulation of the affected limb. The fracture may cause the leg to appear shortened, rotated, or in an abnormal alignment, which can be noticeable upon visual inspection [
8]. FSFs significantly impact the mobility and function of the affected limb. Patients typically experience a loss of function, including the inability to actively move the leg, perform weight-bearing activities, or perform routine activities of daily living that involve lower limb movements. Depending on the severity and nature of the fracture, neurovascular compromise may occur. This can manifest as paresthesia (abnormal sensations), numbness, or motor weakness in the leg.
Clinical presentation may vary based on open or closed fractures and pathological fractures [
9]. Open fractures, where the fracture site communicates with the external environment, may exhibit additional signs of injury, such as a visible wound, bone protrusion, or exposure of bone fragments. In cases where FSFs occur due to underlying bone pathologies (e.g., metastatic bone lesions), the clinical presentation may be atypical and symptoms may be less severe. These fractures are often associated with a history of previous malignancy.
In children and young adults, FSFs may be associated with high-energy trauma and often involve other concomitant injuries. Clinical signs may include significant pain, swelling, deformity, and an inability to bear weight. In older adults, FSFs may result from low-energy mechanisms, such as falls from standing height. These fractures may exhibit less obvious deformity but are frequently associated with significant pain, swelling, and functional impairment due to underlying osteoporosis and comorbidities.
Clinical assessment, including a thorough history, physical examination, and radiographic evaluation, is crucial for the accurate diagnosis and appropriate management of FSFs. Prompt and accurate diagnosis of FSFs enables appropriate intervention, leading to optimal patient outcomes and improved quality of life.
4. Anatomy
Anatomy plays an important role in the accurate diagnosis and effective management of FSFs. The femoral shaft, located between the hip and knee joints, is a crucial weight-bearing structure. The femoral shaft, also known as the diaphysis, is a long, cylindrical bone that exhibits specific morphological features. The proximal end consists of the femoral head, neck, and greater and lesser trochanters. Beginning at the inferior border of the lesser trochanter, the femoral shaft ends just proximal to the condyles [
10]. The shaft itself is divided into three main regions: the proximal third, the middle third, and the distal third. The cortical and trabecular bone characteristics influence fracture stability, healing, and fixation techniques.
The blood supply to the femoral shaft is essential for fracture healing. The primary arterial supply for the femoral shaft is derived from the deep femoral artery, which gives rise to the nutrient artery and multiple muscular branches [
11]. These vessels form an intricate network of anastomoses, ensuring adequate blood flow to the femoral shaft. A disruption in the blood supply can lead to complications such as avascular necrosis and delayed fracture healing.
Various muscles and tendons surround the femoral shaft, contributing to its stability and biomechanical functions. The major muscle groups include the quadriceps femoris, adductors, and hamstrings. These muscle attachments play a critical role in fracture reduction and maintenance of alignment during the healing process.
The femoral shaft possesses several anatomical landmarks that aid in fracture classification, reduction, and fixation. The greater and lesser trochanters, linea aspera, and intercondylar notch are crucial reference points for assessing fracture displacement and angulation [
12]. Additionally, the distal femoral condyles and epicondyles serve as landmarks for determining the location and orientation of distal femoral fractures.
Radiographic evaluation, including anteroposterior and lateral views, is the initial diagnostic modality. Computed tomography (CT) scans provide detailed information about fracture morphology, displacement, and comminution. Magnetic resonance imaging (MRI) aids in assessing soft tissue injuries, including associated ligamentous or meniscal damage.
The type of treatment and management of FSFs rely on anatomical considerations. FSFs are associated with potential complications, including nonunion, malunion, infection, neurovascular injuries, and joint stiffness. Rehabilitation protocols focus on early mobilization, muscle strengthening, and functional restoration to achieve optimal outcomes.
5. Types of Femoral Shaft Fractures
Accurate classification of FSFs is crucial for effective treatment planning and predicting outcomes. Understanding the types of FSFs, their classification systems, and associated characteristics aids healthcare professionals in making informed decisions regarding treatment and management.
Based on fracture characteristics, the types of FSFs include transverse, oblique, spiral, comminuted, segmental, and open fractures [
2]. These are highlighted in
Table 1.
These fractures are associated with different causes. Transverse fractures are typically caused by a direct blow or axial loading force, often commonly associated with high-energy trauma. Oblique fractures result from a combination of axial and rotational forces and can be stable or unstable, depending on the amount of comminution. Spiral fractures are often associated with rotational forces such as those encountered in sports injuries or twisting falls. Comminuted fractures are usually caused by high-energy trauma or pathological conditions and are usually unstable. Segmental fractures also often occur due to high-energy trauma and are associated with a higher risk of complications, including nonunion. Finally, open fractures require immediate attention due to the increased risk of infection and soft tissue complications.
The type of femoral shaft fracture has clinical implications for treatment planning, surgical approach selection, and fracture stabilization techniques. Stable fractures may be treated non-operatively with casting or bracing, whereas unstable fractures typically require surgical intervention, such as intramedullary nailing, plate osteosynthesis, or external fixation [
13].
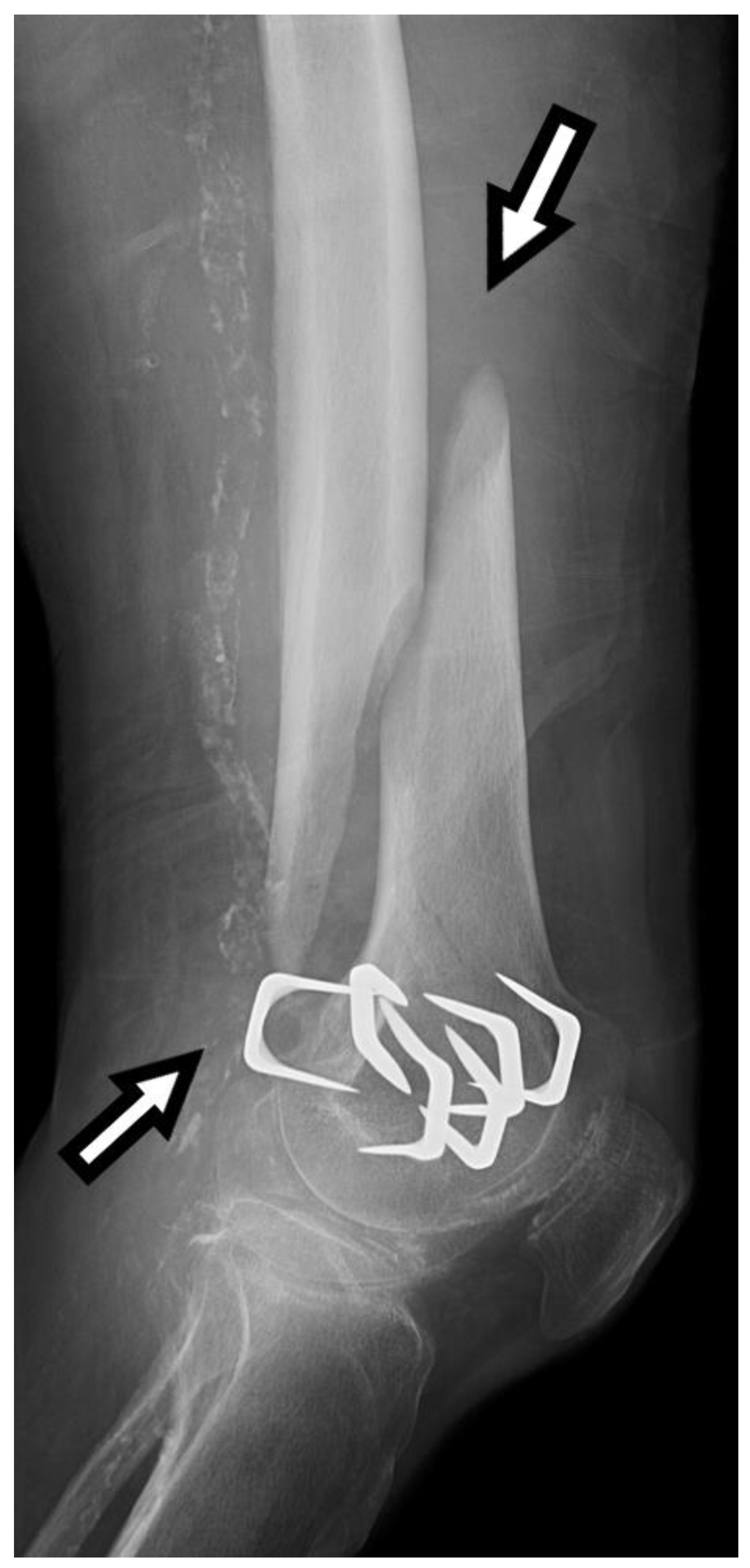
Figure 1, for example, shows a spiral fracture that requires surgical intervention.
6. Classification Systems
FSFs are common orthopedic injuries that can vary in their presentation and characteristics. Accurate classification of FSFs is essential for treatment and management planning and predicting outcomes.
Several classification systems have been developed to categorize FSFs based on their anatomical features and fracture characteristics. The most commonly used classification systems include the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification and the Winquist–Hansen classification, which is used for traumatic fractures [
2].
The AO/OTA classification system categorizes FSFs based on fracture location, morphology, and fracture pattern. It classifies fractures into three major groups: type A (extra-articular), type B (partial articular), and type C (complete articular) [
2]. Each major group is further subdivided based on the specific fracture characteristics, such as fracture location and pattern, described in
Table 2.
The AO/OTA classification system provides a standardized framework for categorizing FSFs based on their anatomical features and fracture characteristics, facilitating communication among healthcare professionals and guiding treatment decisions.
For traumatic fractures, the Winquist–Hansen classification system focuses on the amount of cortical comminution and fracture stability. It categorizes fractures into four types: type I (no comminution), type II (comminution ≤ 50% of the cortical width), type III (comminution > 50% of the cortical width), and type IV (segmental comminution with a complete butterfly fragment), displayed in
Table 3 [
2].
7. Pathophysiology
FSFs are significant orthopedic injuries that result from the disruption of the structural integrity of the femoral diaphysis. The pathophysiology of FSFs involves a complex interplay of biomechanical forces, bone structure, and tissue response.
FSFs can occur due to both high-energy and low-energy trauma. Fracture propagation in FSFs occurs through a sequence of events. Initial forces applied to the femoral shaft cause microstructural damage, including microfractures and disruption of the bone matrix [
12]. These microfractures coalesce, leading to the propagation of the fracture line. The fracture line can extend longitudinally, transversely, or obliquely, depending on the direction and magnitude of the applied forces. Fracture propagation is influenced by bone quality, the rate and direction of force application, and the presence of pre-existing bone pathologies.
The bone healing process in FSFs involves a series of overlapping stages, including inflammation, repair, and remodeling. Immediately following fracture, a hematoma forms at the fracture site, initiating an inflammatory response [
12]. Inflammatory cells release cytokines and growth factors, attracting mesenchymal cells and osteoblasts to the fracture site. These cells promote the formation of a fibrocartilaginous callus, which gradually undergoes mineralization, leading to the formation of a bony callus [
12]. Finally, the bony callus is remodeled and the fracture site is restored to its pre-injury state through a process of bone resorption and deposition.
FSFs can be associated with various complications, including nonunion, delayed union, malunion, infection, and neurovascular injuries [
13]. Nonunion refers to the failure of the fractured bone to heal within the expected timeframe. Delayed union is characterized by a prolonged healing process, whereas malunion refers to improper alignment or rotation of the fractured segments during healing. Infection can occur due to contamination at the time of injury or following surgical intervention. Neurovascular injuries, although relatively rare, can result from the initial trauma or iatrogenic causes during fracture management.
Several factors influence the healing process and the development of complications in FSFs. These factors include the extent of soft tissue injury, fracture stability, fracture gap, bone quality, patient age, systemic conditions (such as smoking and diabetes), and the presence of associated injuries [
14]. Adequate reduction, stable fixation, and proper alignment of the fracture fragments are crucial for optimal healing and minimizing complications.
8. Imaging Modalities and Features
8.1. Radiographs
Radiographs play an essential role in the diagnosis of FSFs, providing important information for accurate classification, treatment planning, and monitoring of fracture healing. Radiographs, particularly anteroposterior (AP) and lateral views of the femur, are the primary imaging modalities used in diagnosing FSFs. AP views provide an overall assessment of fracture alignment and the extent of displacement, whereas lateral views help evaluate rotational deformities and the presence of oblique or spiral fractures. Additional imaging views, such as oblique or traction views, may be obtained to further assess complex fracture patterns or to identify subtle fractures.
The primary radiographic finding in FSFs is the presence of a visible fracture line traversing the femoral diaphysis. The fracture line may vary in its appearance, ranging from a transverse or oblique pattern to a spiral or comminuted pattern depending on the mechanism and forces involved.
Figure 2 demonstrates an example of a spiral fracture.
Radiographs enable the assessment of fracture displacement and angulation. Displacement refers to the separation of fracture fragments, whereas angulation refers to the deviation of the fracture line from the anatomical axis of the femur. The degree of displacement and angulation can provide important information regarding fracture stability and treatment considerations.
The evaluation of FSFs includes assessing alignment and rotation. Radiographs allow for the measurement of the mechanical axis, which passes through the center of the femoral head and the midpoint of the knee joint. Deviation from this axis indicates malalignment. Additionally, rotational deformities, such as internal or external rotation, may be apparent on radiographs.
Radiographs reveal cortical interruption, indicating a break in the continuity of the femoral shaft cortex. This finding is particularly evident in displaced fractures. Over time, as the fracture heals, callus formation may be observed as new bone formation around the fracture site. The presence and characteristics of callus provide information on fracture healing progression [
12].
FSFs may be associated with concurrent injuries, such as joint dislocations, fractures of adjacent bones (e.g., hip, knee), or soft tissue injuries. Radiographs can identify and evaluate these associated injuries, aiding in comprehensive management planning. Furthermore, radiographs are often utilized to monitor the postoperative management of FSFs.
Although radiographs are invaluable in diagnosing FSFs, they have certain limitations. Some fractures, particularly hairline or non-displaced fractures, may not be readily visible on initial radiographs. In such cases, additional imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI) may be necessary to confirm the diagnosis or evaluate associated injuries. Moreover, radiographs may not provide detailed information about intra-articular involvement or subtle fractures in complex cases. Early diagnosis and treatment of FSFs has excellent healing outcomes [
15]. Nevertheless, radiographs of FSFs are crucial for orthopedic practitioners and other healthcare professionals in order to provide optimal care and achieve favorable patient outcomes.
8.2. CT Scans
CT scans have emerged as valuable imaging tools in the diagnosis and management of FSFs. They provide detailed anatomical information, enhance fracture characterization, and aid in surgical planning, leading to improved treatment outcomes and patient care.
CT imaging allows precise evaluation of fracture displacement and angulation. Accurate measurement of displacement and angulation aids in fracture classification and guides treatment decisions, particularly in complex fractures requiring surgical intervention.
FSFs can sometimes extend into the adjacent joints, such as the hip or knee. CT scans can accurately identify intra-articular extension, providing important information for surgical planning and determining the need for additional interventions, such as joint stabilization or arthroplasty.
FSFs can also be accompanied by associated injuries, such as vascular injury, nerve injury, or fractures of adjacent bones. CT imaging allows for the detection and evaluation of these associated injuries, enabling a comprehensive assessment of the extent of trauma and guiding appropriate treatment strategies.
Detailed visualization of fracture characteristics aids in selecting appropriate surgical approaches, determining the optimal fixation technique, and ensuring accurate alignment and reduction of fracture fragments.
CT scans are valuable when radiographs fail to provide a definitive diagnosis, especially in cases of suspected fractures with negative or equivocal findings. CT scans are particularly useful in evaluating complex fracture patterns, such as comminuted fractures, segmental fractures, or fractures involving the proximal or distal metaphysis. A research study showed that CT detected fracture lines not seen on radiographs due to overlying callus and showed higher accuracy in detecting incomplete union [
16]. CT scans excel in assessing the involvement of the hip or knee joint in FSFs. They depict articular surface fractures, joint incongruity, and associated intra-articular loose bodies, influencing treatment strategies and surgical planning.
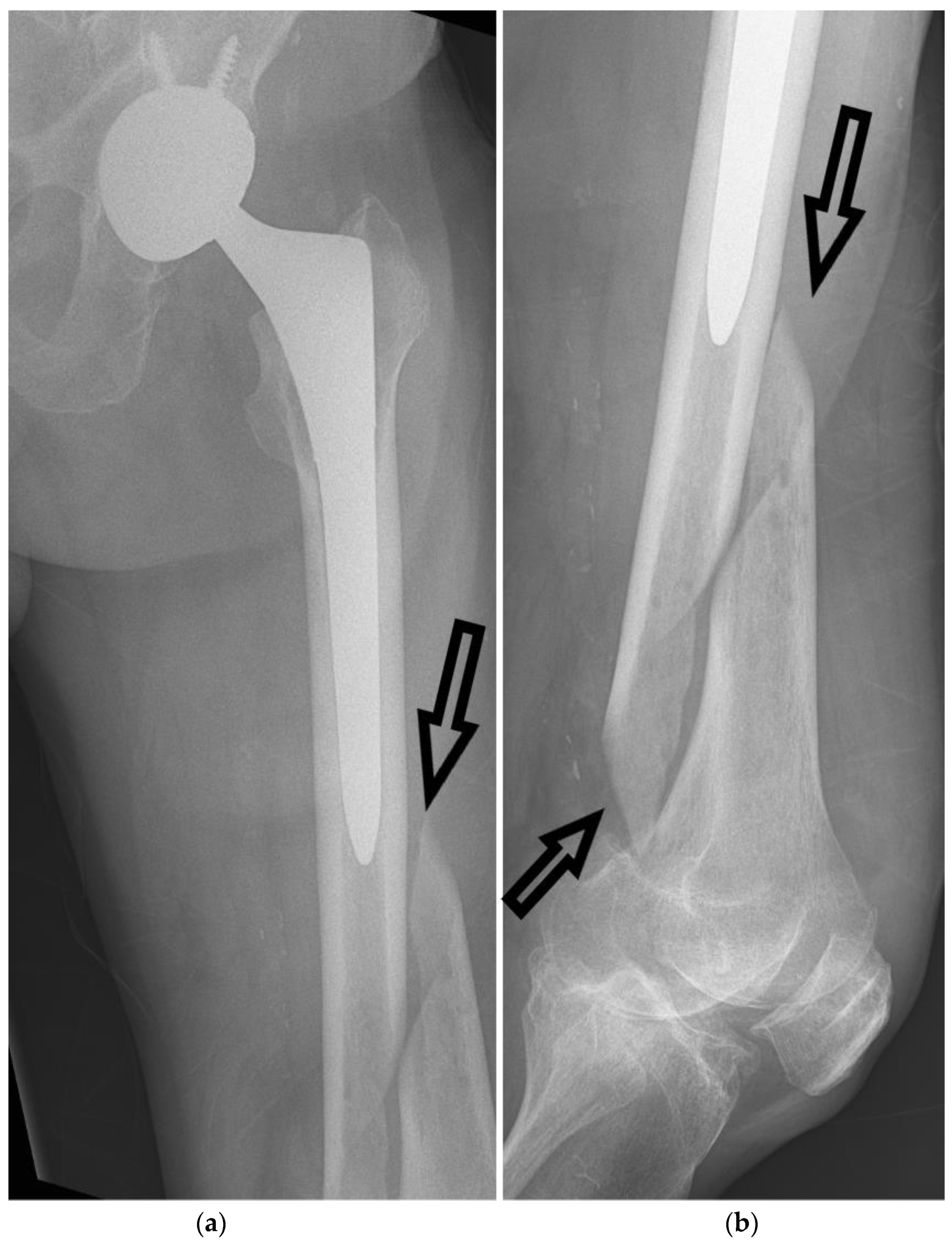
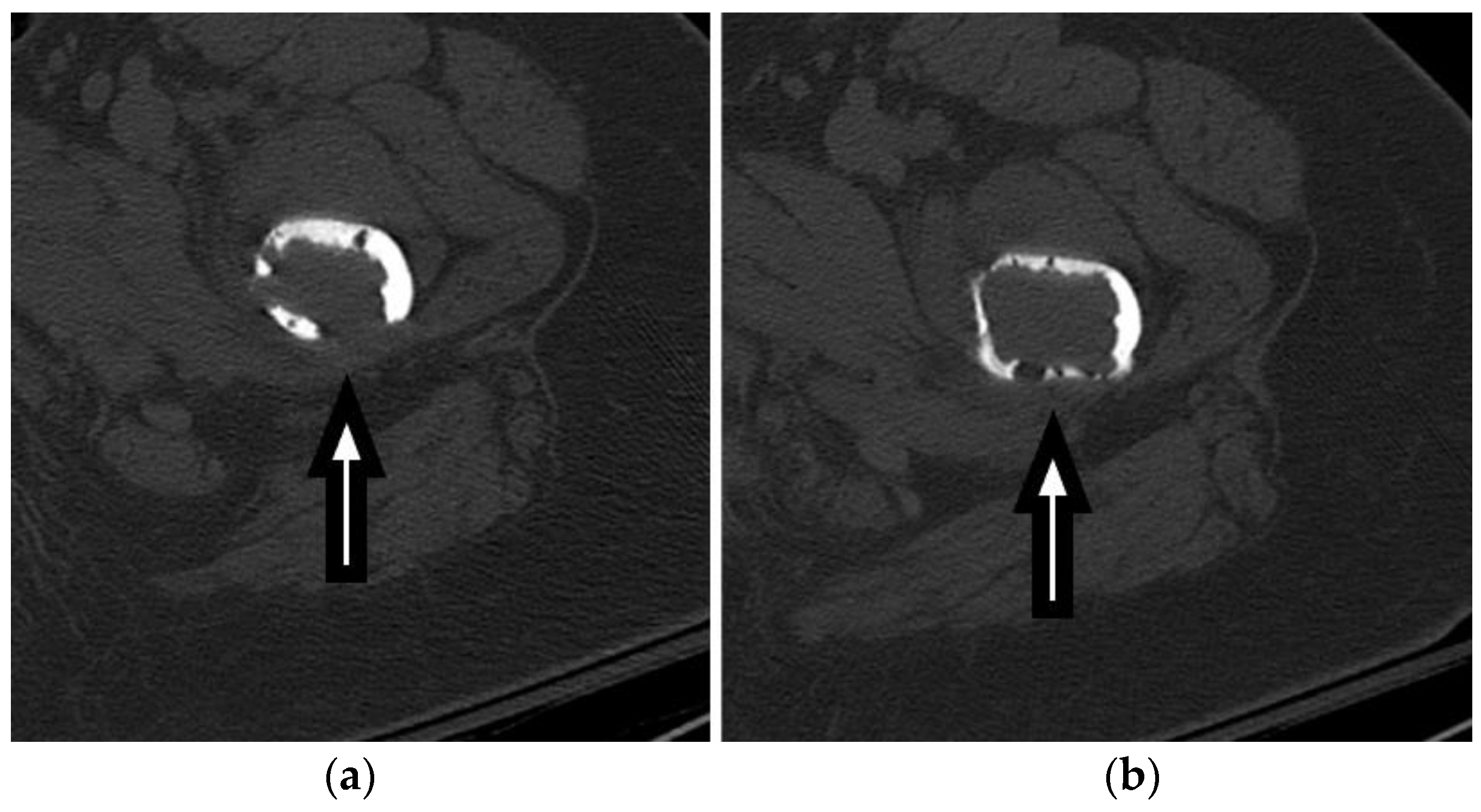
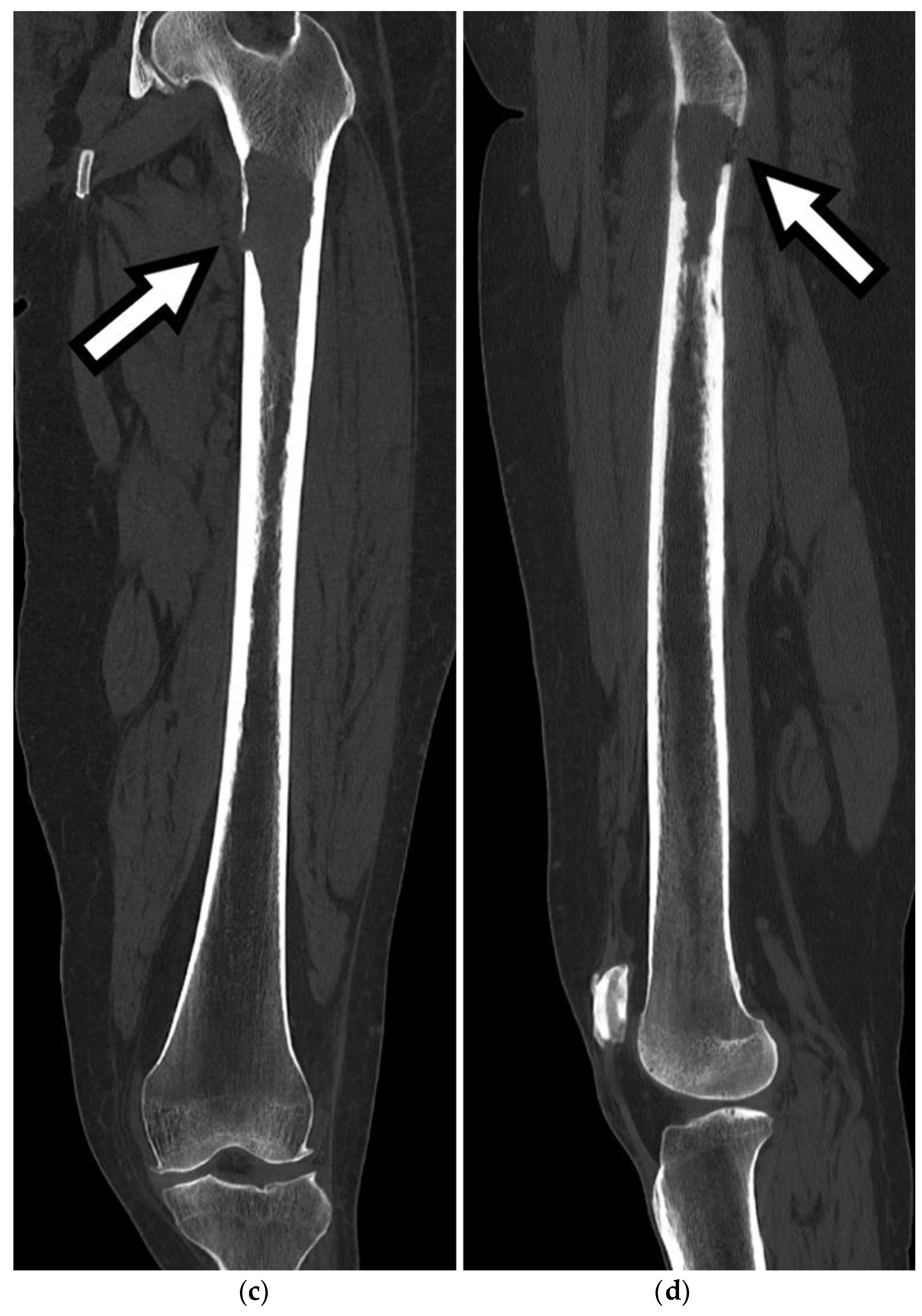
CT imaging plays a vital role in the evaluation and management of FSFs by providing detailed information on the fracture pattern, displacement, intra-articular extension, and associated injuries, as seen in
Figure 3 and
Figure 4. Furthermore,
Figure 5 demonstrates an example of a 3D reconstruction CT for surgical planning. The precise visualization of these key CT findings assists orthopedic surgeons in fracture classification, treatment planning, and surgical decision making. Incorporating CT imaging into the diagnostic workup of FSFs enhances the accuracy of diagnosis and contributes to improved patient outcomes.
8.3. MRI Scans
Magnetic resonance imaging (MRI) is a valuable imaging modality for the evaluation of FSFs, providing detailed visualization of soft tissues and bone marrow. MRI offers excellent soft tissue contrast and can detect associated injuries, evaluate the extent of muscle and ligamentous damage, and assess the healing process.
Importantly, MRI imaging plays a complementary role to radiographs and CT scans in the evaluation of FSFs, particularly in cases where soft tissue injuries or stress fractures are suspected. Furthermore, a research study found that MRIs can provide more accurate detection of insufficiency fractures, such as in the pelvis and proximal femur, than CTs [
17]. T1-weighted images may show a subtle interruption of the normal low signal intensity cortex, whereas T2-weighted images may reveal a hyperintense signal representing the fracture line and surrounding bone marrow edema.
MRI is highly sensitive in detecting bone marrow edema associated with FSFs. Edema appears as areas of increased signal intensity on fluid-sensitive sequences, such as T2-weighted or short tau inversion recovery (STIR) images. Bone marrow edema reflects the underlying bone injury and can help assess the extent of the fracture and associated bone contusions.
Furthermore, MRI is particularly valuable in assessing soft tissue injuries associated with FSFs. It can reveal injuries to the surrounding muscles, tendons, and ligaments. Edema and signal alterations within the muscles, such as myotendinous junction disruption or muscle strains, can be visualized. Ligamentous injuries, such as tears or sprains of the surrounding knee or hip ligaments, can also be identified.
Follow-up MRI examinations can help assess the healing process of FSFs. Serial MRIs can monitor the resolution of bone marrow edema, the formation of callus, and the progression of healing. These findings aid in determining the timing of weight-bearing progression and assessing the adequacy of fracture healing.
Pathologic FSFs pose unique diagnostic challenges due to their association with underlying bone pathology. Magnetic resonance imaging (MRI) has emerged as a valuable imaging modality in the diagnosis of pathologic fractures, offering excellent soft tissue contrast and the ability to detect underlying bone abnormalities. MRI is highly sensitive in detecting underlying bone abnormalities that contribute to the development of pathologic fractures, including bone tumors, metastatic lesions, osteomyelitis, or stress fractures [
18]. It can help differentiate between benign and malignant lesions and provide information about tumor size, extent, and the involvement of adjacent structures, as seen in
Figure 6.
9. Treatment and Management
The management of FSFs has evolved over time, with various treatment options available, including both nonoperative and operative approaches.
Skeletal traction is an initial management option used to stabilize FSFs temporarily. It involves the use of traction pins or wires inserted into the femur and attachment to an external traction system. Skeletal traction can provide pain relief, allow fracture alignment, and facilitate soft tissue healing. However, it is typically reserved for specific indications, such as polytrauma patients or those with significant comorbidities.
Functional bracing is a nonoperative treatment option suitable for certain FSFs. It involves the application of a rigid or semi-rigid brace that allows early mobilization while providing stability to the fractured bone. Functional bracing is commonly used for isolated, closed fractures with minimal displacement or angulation. It enables early weight bearing.
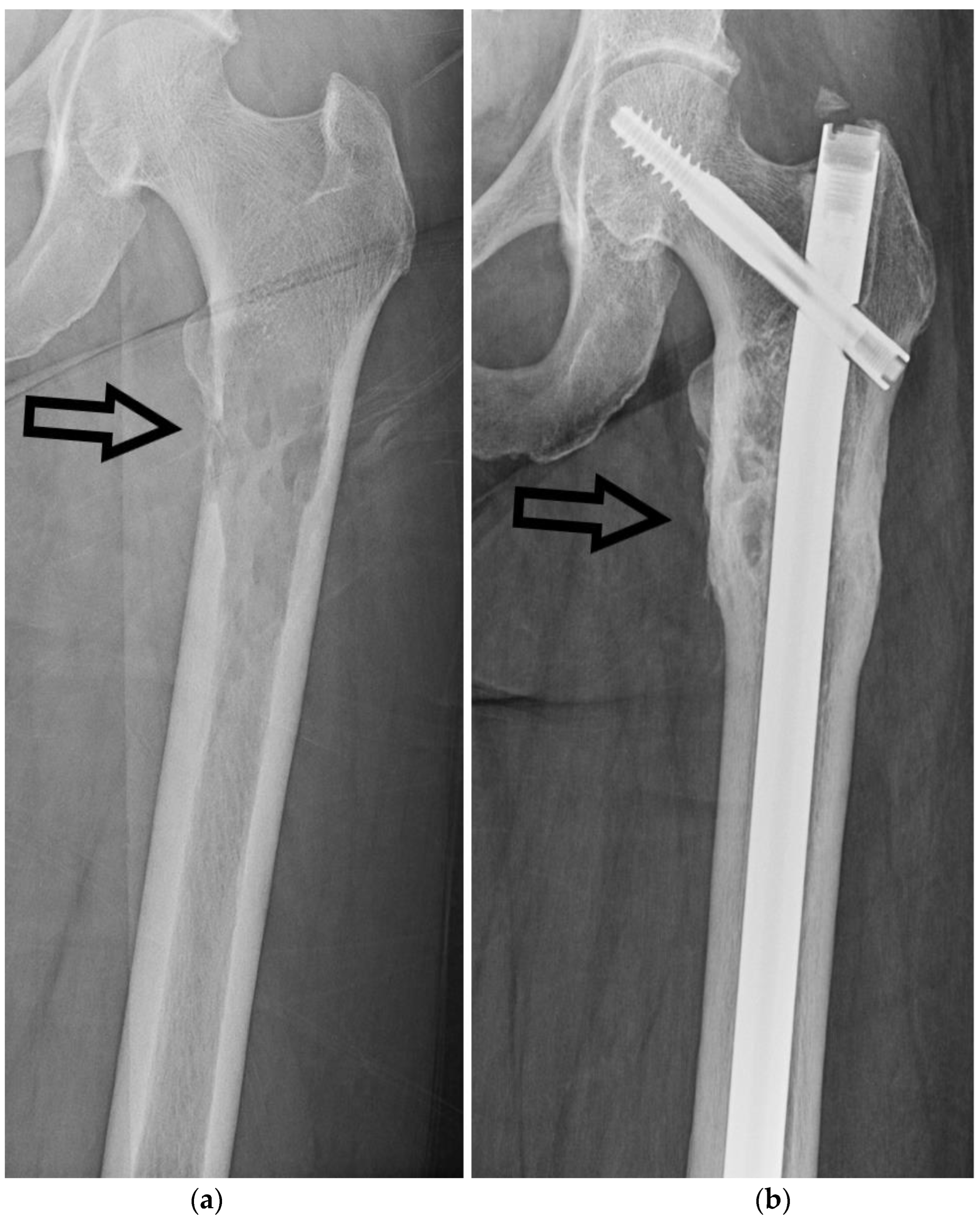
Intramedullary nailing is the gold standard for the surgical treatment of FSFs [
19]. It involves the insertion of a nail into the medullary canal of the femur, providing stable fixation, as seen in
Figure 7. This technique allows early mobilization, promotes fracture union, and minimizes soft tissue disruption. Intramedullary nailing is suitable for most FSFs, including simple, comminuted, and segmental fractures.
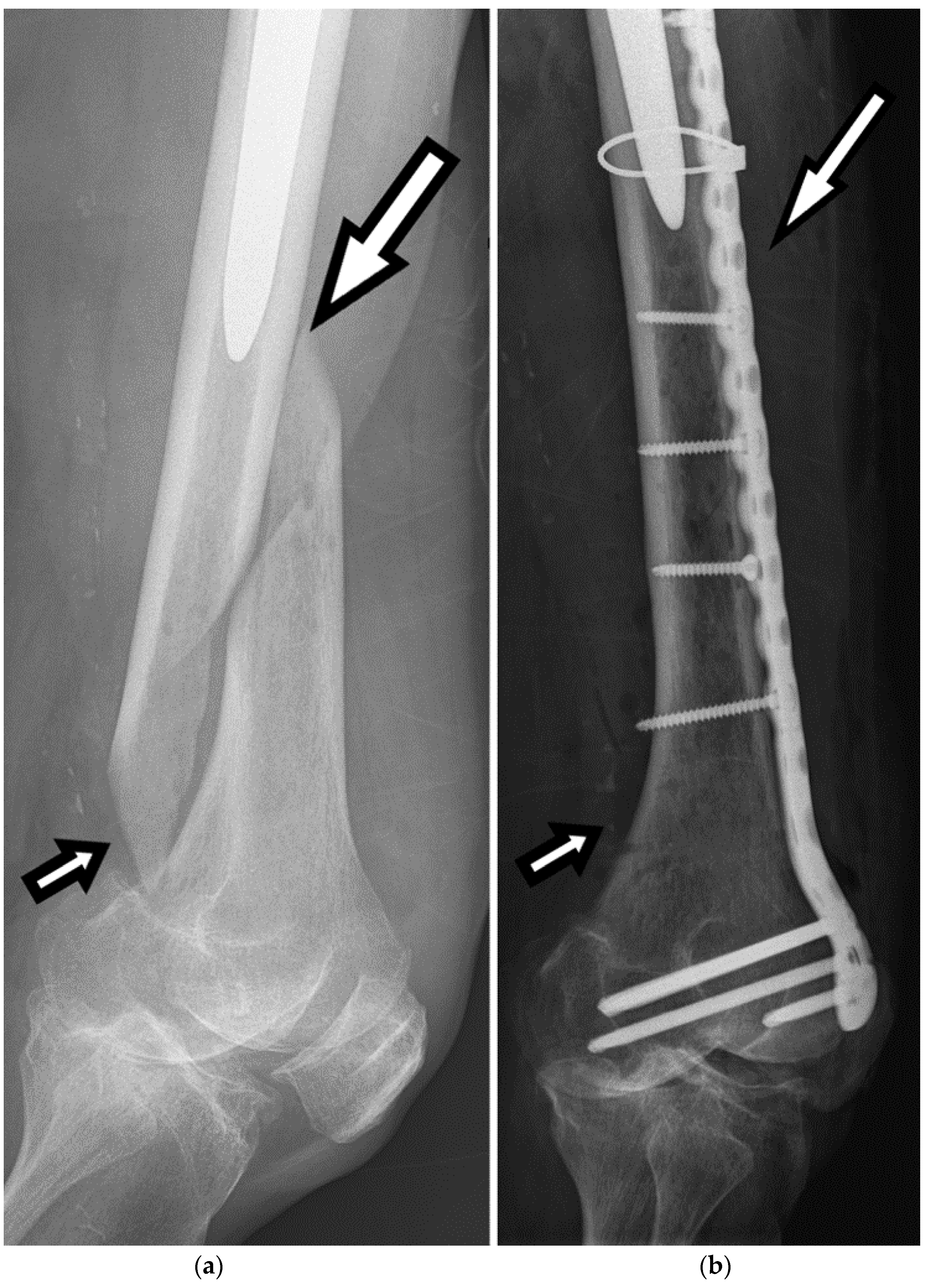
Plate fixation involves the application of plates and screws to stabilize FSFs. It is commonly used in specific scenarios, such as open fractures, fractures with significant soft tissue injury, or when intramedullary nailing is not feasible. Plate fixation provides rigid fixation, allows for direct fracture reduction, and facilitates biological healing, as seen in
Figure 8. It may be preferred in certain fracture patterns or when there is a need for anatomical alignment. Furthermore, a research study found that double locking plate fixation was effective in treating nonunions of the femoral shaft [
20].
External fixation is an alternative surgical treatment option for FSFs. It involves the use of pins or wires placed outside the skin and connected to an external frame. External fixation allows for temporary stabilization, particularly in cases of open fractures, polytrauma, or severe soft tissue injuries. It serves as a damage control measure and provides stability until definitive fixation can be performed.
Early mobilization and weight bearing are important components of postoperative care for FSFs. Depending on the fracture stability and fixation method, progressive weight-bearing and rehabilitation protocols are implemented to promote functional recovery and prevent complications such as joint stiffness and muscle atrophy.
Close monitoring and regular follow-up are crucial during the postoperative period. Radiographic assessments are performed to evaluate fracture healing, implant position, and potential complications. Clinical evaluations ensure early detection and management of any issues related to wound healing, infection, or implant failure.
10. Prognosis
FSFs are significant injuries that can have a considerable impact on a patient’s functional outcome and quality of life. The prognosis of FSFs depends on several factors, including fracture characteristics, patient demographics, associated injuries, treatment modality, and postoperative care, which are crucial for patient counseling, treatment planning, and optimizing long-term results.
The fracture pattern plays a significant role in the prognosis of FSFs. Closed fractures have a more favorable prognosis compared with open fractures [
21]. Fracture characteristics such as obliquity, displacement, and degree of comminution impact the healing potential and functional outcomes. The presence of associated injuries, such as neurovascular compromise or soft tissue injuries, can influence the prognosis of FSFs. Concomitant injuries may require additional interventions, delay fracture healing, or affect overall functional recovery.
Patient demographics, including age and overall health status, can influence the prognosis of FSFs. Elderly patients and those with preexisting medical conditions may experience slower healing, increased complications, and reduced functional outcomes compared with younger, healthier individuals. The choice of treatment modality can significantly impact the prognosis of FSFs as well. Surgical interventions, such as intramedullary nailing or plate fixation, generally provide more stable fixation and earlier mobilization, leading to improved outcomes.
There are potential complications including malunion, which refers to the healing of the fracture in a suboptimal alignment, whereas nonunion is the failure of the fracture to heal. These complications can lead to functional limitations, limb-length discrepancies, and long-term disability. In a case–control study, it was concluded that tobacco use, open fracture, and delayed weight bearing are risk factors for nonunion after intramedullary nailing of FSFs [
21].
Infection is a potential complication following the surgical treatment of FSFs. Deep infections, such as osteomyelitis, can result in prolonged hospitalization, additional surgeries, and impaired functional outcomes. Delayed union refers to a prolonged healing process where the fracture takes longer than expected to heal. This can result in delayed return to function and increased morbidity. Compartment syndrome, although relatively rare, is a severe complication associated with FSFs. Prompt recognition and treatment are crucial to prevent tissue necrosis, nerve damage, and long-term disability.
Fracture stability, achieved through appropriate surgical fixation, is a significant prognostic factor for FSFs. Stable fixation allows early mobilization, promotes fracture healing, and reduces the risk of complications. Prompt diagnosis and the timely initiation of treatment play a crucial role in the prognosis of FSFs. In a cohort study involving 216 trauma centers, delayed treatment can lead to increased morbidity, greater risk for pulmonary embolisms, longer hospital stays, higher complication rates, and poorer functional outcomes [
22]. Patient compliance with postoperative instructions, including weight-bearing restrictions and rehabilitation protocols, is vital for optimal outcomes [
21]. Active participation in physical therapy and rehabilitation programs can enhance functional recovery and improve long-term prognosis.
11. Conclusions
FSFs are significant injuries that affect many. Utilizing the anatomy, classification, pathophysiology, imaging modalities, treatment and management options, and prognosis factors of FSFs can aid healthcare professionals in improving patient care and managing these fractures effectively.
The anatomy is crucial in interpreting fracture patterns, selecting appropriate treatment approaches, and evaluating potential complications. FSFs can be classified based on several systems, including the AO/OTA classification and the Winquist–Hansen classification. Radiographic imaging plays a vital role in the diagnosis and evaluation of FSFs. Initial evaluation usually involves plain radiographs, including anteroposterior and lateral views. These provide information about fracture location, displacement, angulation, and associated injuries. CT scans offer enhanced visualization of fracture details, especially in complex or intra-articular fractures. MRI can provide valuable insights into soft tissue involvement, such as muscle injuries or neurovascular compromise, particularly in cases of complex fractures or suspected pathologic fractures.
Nonoperative management options include skeletal traction or functional bracing, whereas surgical interventions typically involve intramedullary nailing, plate fixation, or external fixation. Postoperative care includes early mobilization, weight-bearing progression, and rehabilitation programs tailored to each patient’s needs. The prognosis of FSFs is influenced by fracture pattern, associated injuries, treatment modality, and patient compliance with rehabilitation protocols. Complications such as malunion, nonunion, infection, and compartment syndrome can affect functional outcomes and long-term prognosis. Prompt diagnosis, appropriate treatment, and diligent postoperative care can improve outcomes and minimize complications. With advances in diagnostic and treatment modalities, healthcare professionals can optimize patient care and achieve favorable outcomes in cases of FSFs.
12. Future Directions
Radiologic diagnosis and imaging play a crucial role in the evaluation and management of FSFs. Advancements in imaging technology have significantly improved the accuracy and precision of diagnosing and characterizing these fractures. The future directions of radiologic diagnosis and imaging in FSFs include emerging techniques and technologies that hold promise in enhancing diagnostic capabilities and patient outcomes.
Three-dimensional (3D) imaging techniques, such as cone-beam computed tomography (CBCT) and digital tomosynthesis, offer volumetric imaging capabilities that provide a more comprehensive visualization of fracture patterns and intra-articular involvement. One research study demonstrated how one CBCT image can be used to calculate malalignment of a complex femoral shaft fracture [
23]. These techniques have the potential to improve fracture classification accuracy, surgical planning, and treatment outcomes.
Advanced imaging modalities, such as dual-energy CT (DECT), are an emerging technology that allows for improved tissue characterization and material decomposition. DECT can differentiate between bone and soft tissues more accurately, facilitating the identification of subtle fractures and associated soft tissue injuries. Diffusion-weighted imaging (DWI), an MRI technique, provides information on tissue cellularity and perfusion. It can aid in evaluating the viability of bone fragments and assessing the extent of bone marrow edema, which is valuable in determining fracture stability and predicting healing potential. A retrospective study showed that using DWI with conventional MRI can increase the specificity and diagnostic accuracy while maintaining the sensitivity in distinguishing between traumatic fractures from metastases and pathologic fractures [
24].
Quantitative imaging techniques, such as quantitative computed tomography (QCT) and dual-energy X-ray absorptiometry (DXA), enable the assessment of bone mineral density and mechanical properties. These techniques can aid in identifying patients at risk of fractures, monitoring the fracture healing progress, and optimizing treatment strategies. One research study used QCT to measure morphologic features of the hip and femoral neck, and it concluded that patients with severe osteoporosis with a thin cortical bone of the diaphysis were more likely to have trochanteric fractures than femoral neck fractures [
25].
The integration of AI and machine learning algorithms has the potential to revolutionize radiologic diagnosis and imaging in FSFs. AI algorithms can assist in automated fracture detection, fracture classification, and treatment planning. Machine learning models can learn from large datasets to improve fracture identification and enhance diagnostic accuracy. A study utilized AI such as deep learning to increase identification of high-risk patients for osteoporosis and fracture risk using radiographs [
26]. Another study demonstrated an AI diagnostic algorithm increasing the diagnosis of femoral intertrochanteric fractures, serving as a clinical aid to healthcare professionals [
27]. Artificial intelligence has been shown to be effective in aiding the diagnosis of various types of fractures [
28,
29,
30,
31].
Point-of-care ultrasound is a portable and real-time imaging modality that can be used for rapid assessment and triage of FSFs. It offers real-time visualization of fractures, evaluation of soft tissue injuries, and can guide interventions, especially in resource-limited settings or pre-hospital care. A study showed that in the pediatric patient population, surveillance ultrasonography can reduce radiation exposure to children with femoral shaft fracture closed reductions without increasing the complication rate [
32].
The future of radiologic diagnosis and imaging in FSFs holds great promise with advancements in technology and techniques. Three-dimensional imaging, advanced modalities such as DECT and DWI, quantitative imaging, AI and machine learning, and POCUS are emerging as potential game changers in the field. These developments have the potential to improve fracture characterization, treatment planning, and patient outcomes. Continued research, technological innovations, and interdisciplinary collaborations will pave the way for the implementation of these future directions in clinical practice to improve patient outcomes.