Advances in Neuroanatomy through Brain Atlasing
Abstract
1. Introduction
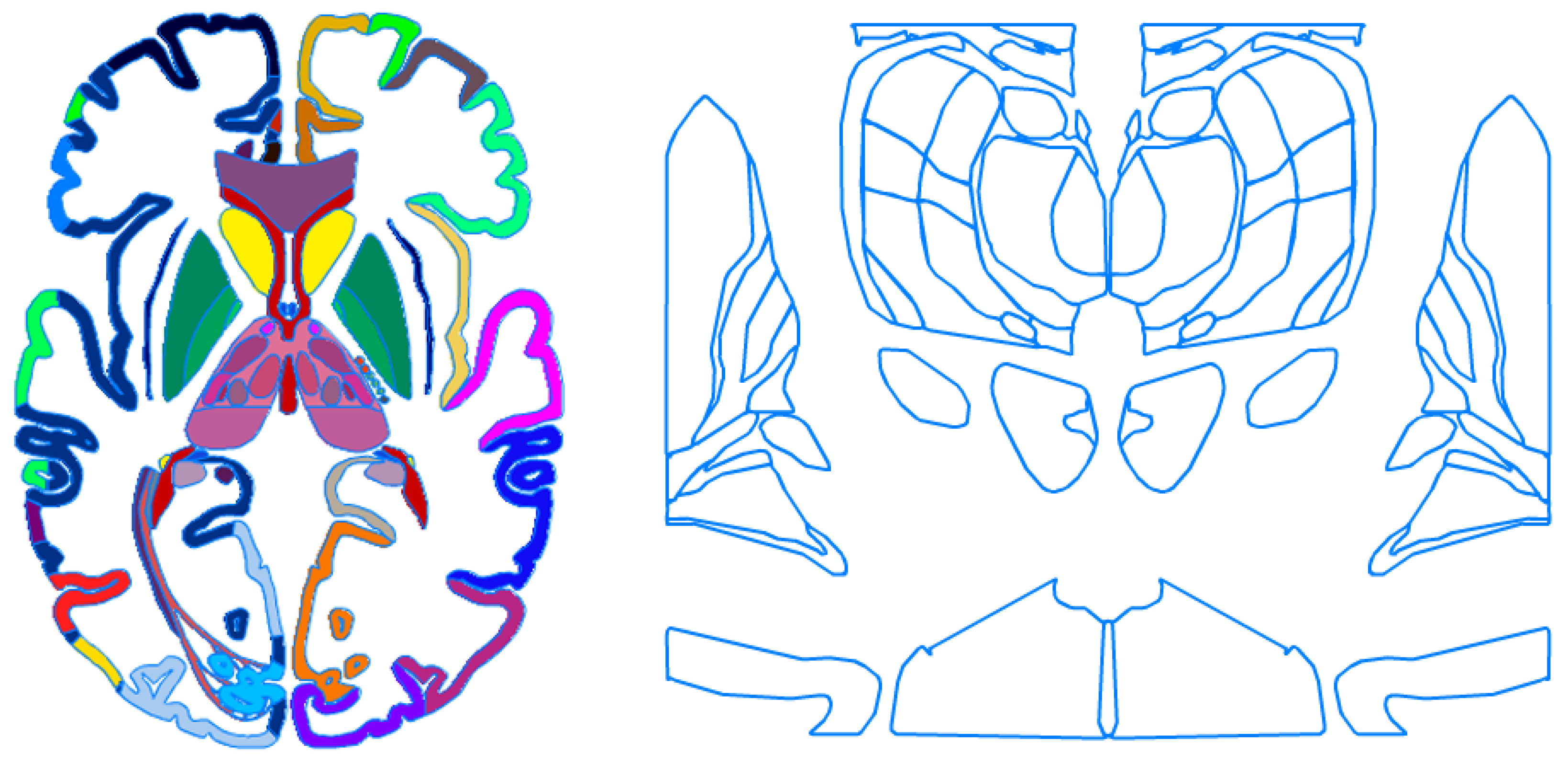
2. Evolution of Brain Atlas Concept
3. Creation of Human Brain Maps and Atlases
4. Brain Atlas-Assisted Applications
4.1. Education
4.2. Research
4.3. Clinics
5. Future Developments
6. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polyak, S. The Vertebrate Visual System; Kluever, K., Ed.; University of Chicago Press: Chicago, IL, USA, 1957. [Google Scholar]
- Schmahmann, J.D.; Pandya, D.N. Fiber Pathways of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Brodmann, K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues; Barth JA: Leipzig, Germany, 1909. [Google Scholar]
- Nowinski, W.L. Evolution of human brain atlases in terms of content, applications, functionality, and availability. Neuroinformatics 2021, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Roland, P.E.; Zilles, K. Brain atlases–a new research tool. Trends Neurosci. 1994, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.C.; Janke, A.L.; Collins, D.L.; Baillet, S. Brain templates and atlases. Neuroimage 2012, 62, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Oishi, K.; Faria, A.V.; Miller, M.I. Atlas-based neuroinformatics via MRI: Harnessing information from past clinical cases and quantitative image analysis for patient care. Annu. Rev. Biomed. Eng. 2013, 15, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Amunts, K.; Hawrylycz, M.J.; Van Essen, D.C.; Van Horn, J.D.; Harel, N.; Poline, J.B.; De Martino, F.; Bjaalie, J.G.; Dehaene-Lambertz, G.; Dehaene, S.; et al. Interoperable atlases of the human brain. Neuroimage 2014, 99, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kuan, L.; Li, Y.; Lau, C.; Feng, D.; Bernard, A.; Sunkin, S.M.; Zeng, H.; Dang, C.; Hawrylycz, M.; Ng, L. Neuroinformatics of the Allen Mouse Brain Connectivity Atlas. Methods 2015, 73, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Manton, J.D.; Ostrovsky, A.D.; Prohaska, S.; Jefferis, G.S. NBLAST: Rapid, sensitive comparison of neuronal structure and construction of neuron family databases. Neuron 2016, 91, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Chon, U.; Vanselow, D.J.; Cheng, K.C.; Kim, Y. Enhanced and unified anatomical labeling for a common mouse brain atlas. Nat. Commun. 2019, 10, 5067. [Google Scholar] [CrossRef]
- Nowinski, W.L. Towards constructing an ideal stereotactic brain atlas. Acta Neurochir. 2008, 150, 1–14. [Google Scholar] [CrossRef]
- Nowinski, W.L. Towards an architecture of a multi-purpose, user-extendable reference human brain atlas. Neuroinformatics 2022, 20, 405–426. [Google Scholar] [CrossRef]
- Campbell, A.W. Histological Studies on the Localisation of Cerebral Function; Cambridge University Press: Cambridge, UK, 1905. [Google Scholar]
- Flechsig, P. Anatomie des Menschlichen Gehirns und Rückenmarks auf Myelogenetischer Grundlage; Thieme: Leipzig, Germany, 1920. [Google Scholar]
- Vogt, C.; Vogt, O. Allgemeinere Ergebnisse unserer Hirnforschung (English Translation: Results of our brain research in a broader context). J. Psychol. Neurol. 1919, 25, 292–398. [Google Scholar]
- Von Economo, C.; Koskinas, G.N. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen; Springer: Berlin, Germany, 1925. [Google Scholar]
- Speigel, E.A.; Wycis, H.T. Stereoencephalotomy: Part I. Methods and Stereotactic Atlas of the Human Brain; Grune and Stratton: New York, NY, USA, 1952. [Google Scholar]
- Talairach, J.; David, M.; Tournoux, P. Atlas d’Anatomie Stereotaxique des Noyaux Gris Centraux; Masson: Paris, France, 1957. [Google Scholar]
- Schaltenbrand, G.; Bailey, W. Atlas of Stereotaxy of the Human Brain; Georg Thieme Verlag: Stuttgart, Germany, 1959. [Google Scholar]
- Andrew, J.; Watkins, E.S. A Stereotaxic Atlas of the Human Thalamus and Adjacent Structures. A Variability Study; Williams and Wilkins: Baltimore, MD, USA, 1969. [Google Scholar]
- Van Buren, J.M.; Borke, R.C. Variations and Connections of the Human Thalamus; Springer: Berlin, Germany, 1972. [Google Scholar]
- Schaltenbrand, G.; Wahren, W. Atlas of Stereotaxy of the Human Brain; Georg Thieme Verlag: Stuttgart, Germany, 1977. [Google Scholar]
- Afshar, E.; Watkins, E.S.; Yap, J.C. Stereotactic Atlas of the Human Brainstem and Cerebellar Nuclei; Raven Press: New York, NY, USA, 1978. [Google Scholar]
- Talairach, J.; Tournoux, P. Co-Planar Stereotactic Atlas of the Human Brain; Thieme: Stuttgart, Germany; New York, NY, USA, 1988. [Google Scholar]
- Talairach, J.; Tournoux, P. Referentially Oriented Cerebral MRI Anatomy: Atlas of Stereotaxic Anatomical Correlations for Gray and White Matter; Thieme: Stuttgart, Germany, 1993. [Google Scholar]
- Takayoshi, M.; Hirano, A. Atlas of the Human Brain for Computerized Tomography; Igaku Shoin Medical Publishers: New York, NY, USA, 1978. [Google Scholar]
- Duvernoy, H.M. The Human Hippocampus: Atlas of Applied Anatomy; Bergman: Munich, Germany, 1988. [Google Scholar]
- Ono, M.; Kubik, S.; Abernathey, C.D. Atlas of the Cerebral Sulci; Georg Thieme Verlag/Thieme Medical Publishers: Stuttgart, Germany; New York, NY, USA, 1990. [Google Scholar]
- Orrison, W.W., Jr. Atlas of Brain Function; Thieme: New York, NY, USA, 1995. [Google Scholar]
- Duvernoy, H.M. The Human Brain Stem and Cerebellum. Surface, Structure, Vascularization, and Three-Dimensional Sectional Anatomy, with MRI; Springer: Wien, Austria; New York, NY, USA, 1995. [Google Scholar]
- Scarabino, T.; Salvolini, U.; DiSalle, F.; Duvernoy, H.; Rabischong, P. (Eds.) Atlas of Morphology and Functional Anatomy of the Brain; Springer: Berlin, Germany, 2006. [Google Scholar]
- Naidich, T.h.P.; Duvernoy, H.M.; Delman, B.N.; Sorensen, A.G.; Kollias, S.S.; Haacke, E.M. Duvernoy’s Atlas of the Human Brain Stem and Cerebellum; Springer: Wien, Austria; New York, NY, USA, 2009. [Google Scholar]
- Felten, D.L.; O’Banion, M.K.; Maida, M.E. Netter’s Atlas of Neuroscience, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Alho, E.J.L.; Grinberg, L.; Heinsen, H. Review of printed and electronic stereotactic atlases of the human brain. In Neuroimaging for Clinicians: Combining Research and Practice; Peres, J.F.P., Ed.; InTech: Rijeka, Croatia, 2011; pp. 145–172. [Google Scholar]
- Nowinski, W.L.; Fang, A.; Nguyen, B.T.; Raphel, J.K.; Jagannathan, L.; Raghavan, R.; Bryan, R.N.; Miller, G. Multiple brain atlas database and atlas-based neuroimaging system. Comput. Aided Surg. 1997, 2, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, W.L.; Bryan, R.N.; Raghavan, R. The Electronic Clinical Brain Atlas. Multiplanar Navigation of the Human Brain; Thieme: New York, NY, USA, 1997. [Google Scholar]
- Nowinski, W.L.; Thirunavuukarasuu, A.; Kennedy, D.N. Brain Atlas for Functional Imaging. Clinical and Research Applications; Thieme: New York, NY, USA, 2000. [Google Scholar]
- Nowinski, W.L.; Thirunavuukarasuu, A.; Bryan, R.N. The Cerefy Atlas of Brain Anatomy. An Introduction to Reading Radiological Scans for Students, Teachers, and Researchers; Thieme: New York, NY, USA, 2002. [Google Scholar]
- Nowinski, W.L.; Thirunavuukarasuu, A. The Cerefy Clinical Brain Atlas on CD-ROM; Thieme: New York, NY, USA, 2004. [Google Scholar]
- Nowinski, W.L. Anatomical and probabilistic functional atlases in stereotactic and functional neurosurgery. In Textbook of Stereotactic and Functional Neurosurgery, 2nd ed.; Lozano, A., Gildenberg, P., Tasker, R., Eds.; Springer: Berlin, Germany, 2009; pp. 395–441. [Google Scholar]
- Mandal, P.K.; Mahajan, R.; Dinov, I.D. Structural brain atlases: Design, rationale, and applications in normal and pathological cohorts. J. Alzheimers Dis. 2012, 31 (Suppl. 3), S169–S188. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, W.L.; Chua, B.C.; Qian, G.Y.; Marchenko, Y.; Puspitasari, F.; Nowinska, N.G.; Knopp, M.V. The Human Brain in 1492 Pieces: Structure, Vasculature, and Tracts; Thieme: New York, NY, USA, 2011. [Google Scholar]
- Nowinski, W.L.; Chua, B.C. The Human Brain in 1969 Pieces: Structure, Vasculature, Tracts, Cranial Nerves, Systems, Head Muscles, and Glands (Version 2.0); Thieme: New York, NY, USA, 2014. [Google Scholar]
- Rohlfing, T.; Zahr, N.M.; Sullivan, E.V.; Pfefferbaum, A. The SRI24 multichannel atlas of normal adult human brain structure. Hum. Brain Mapp. 2010, 31, 798–819. [Google Scholar] [CrossRef]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Conner, A.K.; Glenn, C.A.; Sali, G.; McCoy, T.M.; Battiste, J.D.; O’Donoghue, D.L.; Sughrue, M.E. A connectomic atlas of the human cerebrum chapter 1, introduction, methods, and significance. Oper. Neurosurg. 2018, 15, S1–S9. [Google Scholar] [CrossRef]
- Briggs, R.G.; Conner, A.K.; Baker, C.M.; Burks, J.D.; Glenn, C.A.; Sali, G.; Battiste, J.D.; O’Donoghue, D.L.; Sughrue, M.E. A connectomic atlas of the human cerebrum-Chapter 18, The Connectional Anatomy of Human Brain Networks. Oper. Neurosurg. 2018, 15 (Suppl. 1), S470–S480. [Google Scholar] [CrossRef]
- Mori, S.; Wakana, S.; Nagae-Poetscher, L.M.; van Zijl, P.C. MRI Atlas of Human White Matter; Elsevier: Amsterdam, The Netherlands.
- Nowinski, W.L.; Chua, B.C.; Yang, G.L.; Qian, G.Y. Three-dimensional interactive human brain atlas of white matter tracts. Neuroinformatics 2012, 10, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, D.C. Cartography and connectomes. Neuron 2013, 80, 775–790. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.J.; Yacoub, E.; Ugurbil, K. The WU-Minn Human Connectome Project: An overview. NeuroImage 2013, 80, 62–79. [Google Scholar] [CrossRef]
- Huck, J.; Wanner, Y.; Fan, A.P.; Jäger, A.T.; Grahl, S.; Schneider, U.; Villringer, A.; Steele, C.J.; Tardif, C.L.; Bazin, P.L.; et al. High resolution atlas of the venous brain vasculature from 7 T quantitative susceptibility maps. Brain Struct. Funct. 2019, 224, 2467–2485. [Google Scholar] [CrossRef]
- Nowinski, W.L.; Thirunavuukarasuu, A.; Volkau, I.; Marchenko, Y.; Runge, V.M. The Cerefy Atlas of Cerebral Vasculature; Thieme: New York, NY, USA, 2009. [Google Scholar]
- Nowinski WL, A. Thirunnavuukarasuu, Volkau I, Marchenko Y, Aminah B, Puspitasaari F, Runge VM. A three-dimensional interactive atlas of cerebral arterial variants. Neuroinformatics 2009, 7, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, W.L.; Johnson, A.; Chua, B.C.; Nowinska, N.G. Three-dimensional interactive and stereotactic atlas of cranial nerves and nuclei correlated with surface neuroanatomy, vasculature and magnetic resonance imaging. J. Neurosci. Methods 2012, 206, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Sunkin, S.M.; Ng, L.; Lau, C.; Dolbeare, T.; Gilbert, T.L.; Thompson, C.L.; Hawrylycz, M.; Dang, C. Allen Brain Atlas: An integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 2013, 41, D996–D1008. [Google Scholar] [CrossRef] [PubMed]
- Kanton, S.; Boyle, M.J.; He, Z.; Santel, M.; Weigert, A.; Sanchís-Calleja, F.; Guijarro, P.; Sidow, L.; Fleck, J.S.; Han, D.; et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 2019, 574, 418–422. [Google Scholar] [CrossRef]
- James, G.A.; Hazaroglu, O.; Bush, K.A. A human brain atlas derived via n-cut parcellation of resting-state and task-based fMRI data. Magn. Reason. Imaging 2016, 34, 209–218. [Google Scholar] [CrossRef]
- Yelnik, J.; Bardinet, E.; Dormont, D.; Malandain, G.; Ourselin, S.; Tandé, D.; Karachi, C.; Ayache, N.; Cornu, P.; Agid, Y. A three-dimensional, histological and deformable atlas of human basal ganglia. I. Atlas construction based on immunohistochemical and MRI data. NeuroImage 2007, 34, 618–638. [Google Scholar] [CrossRef]
- Nowinski, W.L.; Qian, G.; Bhanu Prakash, K.N.; Thirunavuukarasuu, A.; Hu, Q.M.; Ivanov, N.; Parimal, A.S.; Runge, V.M.; Beauchamp, N.J. Analysis of ischemic stroke MR images by means of brain atlases of anatomy and blood supply territories. Acad. Radiol. 2006, 13, 1025–1034. [Google Scholar] [CrossRef]
- Arsiwalla, X.D.; Zucca, R.; Betella, A.; Martinez, E.; Dalmazzo, D.; Omedas, P.; Deco, G.; Verschure, P.F. Network dynamics with BrainX(3): A large-scale simulation of the human brain network with real-time interaction. Front. Neuroinform. 2015, 9, 2. [Google Scholar] [CrossRef]
- Fan, L.; Li, H.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.R.; et al. The human brainnetome atlas: A new brain atlas based on connectional architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef]
- McGrath, H.; Zaveri, H.P.; Collins, E.; Jafar, T.; Chishti, O.; Obaid, S.; Ksendzovsky, A.; Wu, K.; Papademetris, X.; Spencer, D.D. High-resolution cortical parcellation based on conserved brain landmarks for localization of multimodal data to the nearest centimeter. Sci. Rep. 2022, 12, 18778. [Google Scholar] [CrossRef]
- Amunts, K.; Lenzen, M.; Friederici, A.D.; Schleicher, A.; Morosan, P.; Palomero-Gallagher, N.; Zilles, K. Broca’s region: Novel organizational principles and multiple receptor mapping. PLoS Biol. 2010, 8, e1000489. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A multi-modal parcellation of human cerebral cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Federative Committee on Anatomical Terminology (FCAT). Terminologia Anatomica, International Anatomical Terminology; Thieme: Stuttgart, Germany; New York, NY, USA, 1988. [Google Scholar]
- Federative International Programme for Anatomical Terminology. Terminologia Neuroanatomica. 2017. Available online: https://fipat.library.dal.ca (accessed on 28 December 2022).
- Bowden, D.M.; Song, E.; Kosheleva, J.; Dubach, M.F. NeuroNames: An ontology for the BrainInfo portal to neuroscience on the web. Neuroinformatics 2012, 10, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Haendel, M.A.; Balhoff, J.P.; Bastian, F.B.; Blackburn, D.C.; Blake, J.A.; Bradford, Y.; Comte, A.; Dahdul, W.M.; Dececchi, T.A.; Druzinsky, R.E.; et al. Unification of multi-species vertebrate anatomy ontologies for comparative biology in Uberon. J. Biomed. Semant. 2014, 5, 21. [Google Scholar] [CrossRef]
- Rosse, C.; Mejino, J.L., Jr. A reference ontology for biomedical informatics: The Foundational Model of Anatomy. J. Biomed. Inform. 2003, 36, 478–500. [Google Scholar] [CrossRef]
- Börner, K.; Teichmann, S.A.; Quardokus, E.M.; Gee, J.C.; Browne, K.; Osumi-Sutherland, D.; Herr BW 2nd Bueckle, A.; Paul, H.; Haniffa, M.; Jardine, L.; et al. Anatomical structures, cell types and biomarkers of the Human Reference Atlas. Nat. Cell Biol. 2021, 23, 1117–1128. [Google Scholar] [CrossRef]
- Liang, P.; Shi, L.; Chen, N.; Luo, Y.; Wang, X.; Liu, K.; Mok, V.C.; Chu, W.C.; Wang, D.; Li, K. Construction of brain atlases based on a multi-center MRI dataset of 2020 Chinese adults. Sci. Rep. 2015, 5, 18216. [Google Scholar] [CrossRef]
- Figley, T.D.; Mortazavi Moghadam, B.; Bhullar, N.; Kornelsen, J.; Courtney, S.M.; Figley, C.R. Probabilistic white matter atlases of human auditory, basal ganglia, language, precuneus, sensorimotor, visual and visuospatial networks. Front. Hum. Neurosci. 2017, 11, 306. [Google Scholar] [CrossRef]
- Diedrichsen, J.; Balsters, J.H.; Flavell, J.; Cussans, E.; Ramnani, N. A probabilistic MR atlas of the human cerebellum. NeuroImage 2009, 46, 39–46. [Google Scholar] [CrossRef]
- Pauli, W.M.; Nili, A.N.; Tyszka, J.M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data 2018, 5, 180063. [Google Scholar] [CrossRef]
- Shattuck, D.W.; Mirza, M.; Adisetiyo, V.; Hojatkashani, C.; Salamon, G.; Narr, K.L.; Poldrack, R.A.; Bilder, R.M. Toga AW Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage 2008, 39, 1064–1080. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ma, T.; Ceritoglu, C.; Li, Y.; Chotiyanonta, J.; Hou, Z.; Hsu, J.; Xu, X.; Brown, T.; Miller, M.I.; et al. Resource atlases for multi-atlas brain segmentations with multiple ontology levels based on T1-weighted MRI. Neuroimage 2016, 125, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Hoehne, K.H. VOXEL-MAN, Part 1, Brain and Skull; Version 2.0.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Cho, Z.H.; Kim, Y.B.; Han, J.Y.; Min, H.K.; Kim, K.N.; Choi, S.H.; Veklerov, E.; Shepp, L.A. New brain atlas—Mapping the human brain in vivo with 7.0 T MRI and comparison with postmortem histology: Will these images change modern medicine? Int. J. Imaging Syst. Technol. 2008, 18, 2–8. [Google Scholar] [CrossRef]
- Liu, Y.; D’Haese, P.F.; Newton, A.T.; Dawant, B.M. Generation of human thalamus atlases from 7 T data and application to intrathalamic nuclei segmentation in clinical 3 T T1-weighted images. Magn. Reason. Imaging 2020, 65, 114–128. [Google Scholar] [CrossRef]
- Nowinski, W.L.; Chua, B.C.; Thaung, T.S.L.; Wut Yi, S.H. The Human Brain, Head and Neck in 2953 Pieces; Thieme: New York, NY, USA, 2015; Available online: http://www.thieme.com/nowinski/ (accessed on 2 January 2023).
- Saygin, Z.M.; Kliemann, D.; Iglesias, J.E.; van der Kouwe, A.J.W.; Boyd, E.; Reuter, M.; Stevens, A.; Van Leemput, K.; McKee, A.; Frosch, M.P.; et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: Manual segmentation to automatic atlas. Neuroimage 2017, 155, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Schira, M.M.; Isherwood, Z.J.; Kassem, M.; Barth, M.; Shaw, T.B.; Roberts, M.M.; Paxinos, G. HumanBrainAtlas: An in vivo MRI dataset for detailed segmentations. bioRxiv 2022. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Avants, B.B.; Pluta, J.; Das, S.; Minkoff, D.; Mechanic-Hamilton, D.; Glynn, S.; Pickup, S.; Liu, W.; Gee, J.C.; et al. A high-resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4. T. NeuroImage 2009, 44, 385–398. [Google Scholar] [CrossRef]
- Oishi, K.; Linda Chang, L.; Huang, H. Baby brain atlases. NeuroImage 2019, 185, 865–880. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, H.; Cronin, M.J.; He, N.; Yan, F.; Liu, C. Longitudinal atlas for normative human brain development and aging over the lifespan using quantitative susceptibility mapping. Neuroimage 2018, 171, 176–189. [Google Scholar] [CrossRef]
- Zuo, X.N.; He, Y.; Betzel, R.F.; Colcombe, S.; Sporns, O.; Milham, M.P. Human connectomics across the life span. Trends Cogn. Sci. 2017, 21, 32–45. [Google Scholar] [CrossRef]
- Kuklisova-Murgasova, M.; Aljabar, P.; Srinivasan, L.; Counsell, S.J.; Doria, V.; Serag, A.; Gousias, I.S.; Boardman, J.P.; Rutherford, M.A.; Edwards, A.D.; et al. A dynamic 4D probabilistic atlas of the developing brain. Neuroimage 2011, 54, 2750–2763. [Google Scholar] [CrossRef] [PubMed]
- Amunts, K.; Lepage, C.; Borgeat, L.; Mohlberg, H.; Dickscheid, T.; Rousseau, M.É.; Bludau, S.; Bazin, P.L.; Lewis, L.B.; Oros-Peusquens, A.M.; et al. Bigbrain: An ultrahigh-resolution 3D human brain model. Science 2013, 340, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Royall, J.J.; Sunkin, S.M.; Ng, L.; Facer, B.A.; Lesnar, P.; Guillozet-Bongaarts, A.; McMurray, B.; Szafer, A.; Dolbeare, T.A.; et al. Comprehensive cellular-resolution atlas of the adult human brain. J. Comp. Neurol. 2016, 524, 3127–3481. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.R.; Geschwind, D.H.; Kriegstein, A.R.; Ngai, J.; Osten, P.; Polioudakis, D.; Regev, A.; Sestan, N.; Wickersham, I.R.; Zeng, H. The BRAIN Initiative Cell Census Consortium: Lessons learned toward generating a Comprehensive Brain Cell Atlas. Neuron 2017, 96, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Hawrylycz, M.J.; Lein, E.S.; Guillozet-Bongaarts, A.L.; Shen, E.H.; Ng, L.; Miller, J.A.; van de Lagemaat, L.N.; Smith, K.A.; Ebbert, A.; Riley, Z.L.; et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 2012, 489, 391–399. [Google Scholar] [CrossRef]
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A high-resolution in vivo atlas of the human brain’s serotonin system. J. Neurosci. 2017, 37, 120–128. [Google Scholar] [CrossRef]
- McKetney, J.; Runde, R.M.; Hebert, A.S.; Salamat, S.; Roy, S.; Coon, J.J. Proteomic atlas of the human brain in Alzheimer’s Disease. J. Proteome Res. 2019, 18, 1380–1391. [Google Scholar] [CrossRef]
- Thompson, P.M.; Mega, M.S.; Woods, R.P.; Zoumalan, C.I.; Lindshield, C.J.; Blanton, R.E.; Moussai, J.; Holmes, C.J.; Cummings, J.L.; Toga, A.W. Cortical change in Alzheimer’s disease detected with a disease-specific population-based brain atlas. Cereb. Cortex 2001, 11, 1–16. [Google Scholar] [CrossRef]
- Mega, M.S.; Dinov, I.D.; Mazziotta, J.C.; Manese, M.; Thompson, P.M.; Lindshield, C.; Moussai, J.; Tran, N.; Olsen, K.; Zoumalan, C.I.; et al. Automated brain tissue assessment in the elderly and demented population: Construction and validation of a sub-volume probabilistic brain atlas. NeuroImage 2005, 26, 1009–1018. [Google Scholar] [CrossRef]
- de Haan, B.; Karnath, H.O. ‘Whose atlas I use, his song I sing?’–The impact of anatomical atlases on fiber tract contributions to cognitive deficits after stroke. Neuroimage 2017, 163, 301–309. [Google Scholar] [CrossRef]
- Nowinski, W.L.; Gupta, V.; Qian, G.; Ambrosius, W.; Kazmierski, R. Population-based stroke atlas for outcome prediction: Method and preliminary results for ischemic stroke from CT. PLoS ONE 2014, 9, e102048. [Google Scholar] [CrossRef] [PubMed]
- Parisot, S.; Darlix, A.; Baumann, C.; Zouaoui, S.; Yordanova, Y.; Blonski, M.; Rigau, V.; Chemouny, S.; Taillandier, L.; Bauchet, L.; et al. A Probabilistic Atlas of Diffuse WHO Grade II Glioma Locations in the Brain. PLoS ONE 2021, 11, e0144200. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Scholly, J.; Triebkorn, P.; Sip, V.; Medina Villalon, S.; Woodman, M.M.; Le Troter, A.; Guye, M.; Bartolomei, F.; Jirsa, V. VEP atlas: An anatomic and functional human brain atlas dedicated to epilepsy patients. J. Neurosci. Methods 2021, 348, 108983. [Google Scholar] [CrossRef] [PubMed]
- Toga, A.W.; Thompson, P.M. Brain atlases of normal and diseased populations. Int. Rev. Neurobiol. 2005, 66, 1–54. [Google Scholar] [PubMed]
- Makowski, C.; van der Meer, D.; Dong, W.; Wang, H.; Wu, Y.; Zou, J.; Liu, C.; Rosenthal, S.B.; Hagler, D.J., Jr.; Fan, C.C.; et al. Discovery of genomic loci of the human cerebral cortex using genetically informed brain atlases. Science 2022, 375, 522–528. [Google Scholar] [CrossRef]
- Tang, Y.; Hojatkashani, C.; Dinov, I.D.; Sun, B.; Fan, L.; Lin, X.; Qi, H.; Hua, X.; Liu, S.; Toga, A.W. The construction of a Chinese MRI brain atlas: A morphometric comparison study between Chinese and Caucasian cohorts. Neuroimage 2010, 51, 33–41. [Google Scholar] [CrossRef]
- Bhalerao, G.V.; Parlikar, R.; Agrawal, R.; Shivakumar, V.; Kalmady, S.V.; Rao, N.P.; Agarwal, S.M.; Narayanaswamy, J.C.; Reddy, Y.C.J.; Venkatasubramanian, G. Construction of population-specific Indian MRI brain template: Morphometric comparison with Chinese and Caucasian templates. Asian J. Psychiatr. 2018, 35, 93–100. [Google Scholar] [CrossRef]
- Nowinski, W.L. Towards the holistic, reference and extendable atlas of the human brain, head and neck. Brain Inform. 2015, 2, 65–76. [Google Scholar] [CrossRef]
- Nowinski, W.L. Computational and mathematical methods in brain atlasing. Neuroradiol. J. 2017, 30, 520–534. [Google Scholar] [CrossRef]
- Nowinski, W.L. Visualization and interaction in the atlas of the human brain, head and neck. Mach. Graph. Vis. 2014, 23, 3–10. [Google Scholar] [CrossRef]
- Abarca-Olivas, J.; González-López, P.; Fernández-Cornejo, V.; Verdú-Martínez, I.; Martorell-Llobregat, C.; Baldoncini, M.; Campero, A. 3D stereoscopic view in neurosurgical anatomy: Compilation of basic methods. World Neurosurg. 2022, 163, e593–e609. [Google Scholar] [CrossRef] [PubMed]
- Sundsten, J.W.; Brinkley, J.F.; Eno, K.; Prothero, J. The Digital Anatomist. Interactive Brain Atlas. CD ROM for the Macintosh; University of Washington: Seattle, WA, USA, 1994. [Google Scholar]
- A.D.A.M. A.D.A.M Animated Dissection of Anatomy for Medicine. User’s Guide; A.D.A.M. Inc.: Atlanta, GA, USA, 1996. [Google Scholar]
- Berkovitz, B.; Kirsch, C.; Moxham, B.; Alusi, G.; Cheeseman, T. Interactive Head & Neck.; Primal Pictures Ltd.: London, UK, 2003. [Google Scholar]
- Nowinski, W.L. 3D atlas of the brain, head and neck in 2953 pieces. Neuroinformatics 2017, 15, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, W.L.; Chua, B.C.; Qian, G.Y.; Nowinska, N.G. The human brain in 1700 pieces: Design and development of a three-dimensional, interactive and reference atlas. J. Neurosci. Methods 2012, 204, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, W.L.; AThirunavuukarasuu, A.; Ananthasubramaniam, A.; Chua, B.C.; Qian, G.; Nowinska, N.G.; Marchenko, Y.; Volkau, I. Automatic testing and assessment of neuroanatomy using a digital brain atlas: Method and development of computer- and mobile-based applications. Anat. Sci. Educ. 2009, 2, 244–252. [Google Scholar] [CrossRef]
- Nowinski, W.L. NOWinBRAIN: A large, systematic, and extendable repository of 3D reconstructed images of a living human brain cum head and neck. J. Digit. Imaging 2022, 35, 98–114. [Google Scholar] [CrossRef]
- Nowinski, W.L. Bridging neuroradiology and neuroanatomy: NOWinBRAIN–a repository with sequences of correlated and labeled planar-surface neuroimages. Neuroradiol. J. 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/35702757/ (accessed on 2 January 2023). [CrossRef]
- Nowinski, W.L. NOWinBRAIN 3D neuroimage repository: Exploring the human brain via systematic and stereotactic dissections. Neurosci. Inform. 2022, 2, 100085. [Google Scholar] [CrossRef]
- Nowinski, W.L. On the definition, construction, and presentation of the human cerebral sulci: A morphology-based approach. J. Anat. 2022. [Google Scholar] [CrossRef]
- Hess, A.; Hinz, R.; Keliris, G.A.; Boehm-Sturm, P. On the usage of brain atlases in neuroimaging research. Mol. Imaging Biol. 2018, 20, 742–749. [Google Scholar] [CrossRef]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- Aljabar, P.; Heckemann, R.A.; Hammers, A.; Hajnal, J.V.; Rueckert, D. Multi-atlas based segmentation of brain images: Atlas selection and its effect on accuracy. Neuroimage 2009, 46, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Artaechevarria, X.; Munoz-Barrutia, A.; Ortiz-de Solorzano, C. Combination strategies in multi-atlas image segmentation: Application to brain MR data. IEEE Trans. Med. Imaging 2009, 28, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Labra, N.; Guevara, P.; Duclap, D.; Houenou, J.; Poupon, C.; Mangin, J.F.; Figueroa, M. Fast automatic segmentation of white matter streamlines based on a multi-subject bundle atlas. Neuroinformatics 2017, 15, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Kutten, K.; Ceritoglu, C.; Li, Y.; Kang, N.; Hsu, J.T.; Qiao, Y.; Wei, H.; Liu, C.; et al. Multi-atlas tool for automated segmentation of brain gray matter nuclei and quantification of their magnetic susceptibility. Neuroimage 2019, 191, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Lötjönen, J.M.; Wolz, R.; Koikkalainen, J.R.; Thurfjell, L.; Waldemar, G.; Soininen, H.; Rueckert, D.; Alzheimer’s Disease Neuroimaging Initiative. Fast and robust multi-atlas segmentation of brain magnetic resonance images. NeuroImage 2010, 49, 2352–2365. [Google Scholar] [CrossRef]
- Zaffino, P.; Ciardo, D.; Raudaschl, P.; Fritscher, K.; Ricotti, R.; Alterio, D.; Marvaso, G.; Fodor, C.; Baroni, G.; Amato, F.; et al. Multi atlas based segmentation: Should we prefer the best atlas group over the group of best atlases? Phys. Med. Biol. 2018, 63, 12NT01. [Google Scholar] [CrossRef]
- Bjerke, I.E.; Øvsthus, M.; Papp, E.A.; Yates, S.C.; Silvestri, L.; Fiorilli, J.; Pennartz, C.M.A.; Pavone, F.S.; Puchades, M.A.; Leergaard, T.B.; et al. Data integration through brain atlasing: Human Brain Project tools and strategies. Eur. Psychiatry 2018, 50, 70–76. [Google Scholar] [CrossRef]
- Nowinski, W.L. Computerized brain atlases for surgery of movement disorders. Semin. Neurosurg. 2001, 12, 183–194. [Google Scholar] [CrossRef]
- Nowinski, W.L.; Yang, G.L.; Yeo, T.T. Computer-aided stereotactic functional neurosurgery enhanced by the use of the multiple brain atlas database. IEEE Trans. Med. Imaging 2000, 19, 62–69. [Google Scholar] [CrossRef]
- Nowinski, W.L.; Chua, B.C.; Volkau, I.; Puspitasari, F.; Marchenko, Y.; Runge, V.M.; Knopp, M.V. Simulation and assessment of cerebrovascular damage in deep brain stimulation using a stereotactic atlas of vasculature and structure derived from multiple 3T and 7T scans. J. Neurosurg. 2010, 113, 1234–1241. [Google Scholar] [CrossRef]
- Benabid, A.L.; Nowinski, W.L. Intraoperative robotics for the practice of neurosurgery: A surgeon’s perspective. In The Operating Room for the 21th Century; Apuzzo, M.L., Ed.; American Association of Neurological Surgeons: Rolling Meadows, IL, USA, 2003; pp. 103–118. [Google Scholar]
- Nowinski, W.L. Usefulness of brain atlases in neuroradiology: Current status and future potential. Neuroradiol. J. 2016, 29, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, W.L. Human brain atlases in stroke management. Neuroinformatics 2020, 18, 549–567. [Google Scholar] [CrossRef]
- Nowinski, W.L.; Chua, B.C.; Wut Yi, S.H. 3D Atlas of Neurologic Disorders; Thieme: New York, NY, USA, 2014. [Google Scholar]
- Nowinski, W.L.; Chua, B.C. Bridging neuroanatomy, neuroradiology and neurology: Three-dimensional interactive atlas of neurological disorders. Neuroradiol. J. 2013, 26, 252–262. [Google Scholar] [CrossRef]
- Sim, K.; Yang, G.L.; Loh, D.; Poon, L.Y.; Sitoh, Y.Y.; Verma, S.; Keefe, R.; Collinson, S.; Chong, S.A.; Heckers, S.; et al. White matter abnormalities and neurocognitive deficits associated with the passivity phenomenon in schizophrenia: A diffusion tensor imaging study. Psychiatry Res. 2009, 172, 121–127. [Google Scholar] [CrossRef]
- Assaf, Y.; Alexander, D.C.; Jones, D.K.; Bizzi, A.; Behrens, T.E.; Clark, C.A.; Cohen, Y.; Dyrby, T.B.; Huppi, P.S.; Knoesche, T.R.; et al. The CONNECT project: Combining macro- and micro-structure. Neuroimage 2013, 80, 273–282. [Google Scholar] [CrossRef]
- Jiang, T. Brainnetome: A new -ome to understand the brain and its disorders. Neuroimage 2013, 80, 263–272. [Google Scholar] [CrossRef]
- BRAIN Working Group. 2014. BRAIN 2025. A Scientific Vision. NIH. Available online: https://www.braininitiative.nih.gov/pdf/BRAIN2025_508C.pdf (accessed on 2 January 2023).
- Jorgenson, L.A.; Newsome, W.T.; Anderson, D.J.; Bargmann, C.I.; Brown, E.N.; Deisseroth, K.; Donoghue, J.P.; Hudson, K.L.; Ling, G.S.; MacLeish, P.R.; et al. The BRAINInitiative: Developing technology to catalyse neuroscience discovery. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140164. [Google Scholar] [CrossRef]
- Markram, H.; Muller, E.; Ramaswamy, S.; Reimann, M.W.; Abdellah, M.; Sanchez, C.A.; Ailamaki, A.; Alonso-Nanclares, L.; Antille, N.; Arsever, S.; et al. Reconstruction and simulation of neocortical microcircuitry. Cell 2015, 163, 456–492. [Google Scholar] [CrossRef]
- Amunts, K.; Ebell, C.; Muller, J.; Telefont, M.; Knoll, A.; Lippert, T. The Human Brain Project: Creating a European research infrastructure to decode the human brain. Neuron 2016, 92, 574–581. [Google Scholar] [CrossRef]
- Sadato, N.; Morita, K.; Kasai, K.; Fukushi, T.; Nakamura, K.; Nakazawa, E.; Okano, H.; Okabe, S. Neuroethical issues of the Brain/MINDS Project of Japan. Neuron 2019, 101, 385–389. [Google Scholar] [CrossRef]
- Chin, A.L.; Yang, S.M.; Chen, H.H.; Li, M.T.; Lee, T.T.; Chen, Y.J.; Lee, T.K.; Petibois, C.; Cai, X.; Low, C.M.; et al. A synchrotron X-ray imaging strategy to map large animal brains. Chin. J. Phys. 2020, 65, 24–32. [Google Scholar] [CrossRef]
- Chen, S.; He, Z.; Han, X.; He, X.; Li, R.; Zhu, H.; Zhao, D.; Dai, C.; Zhang, Y.; Lu, Z.; et al. How big data and high-performance computing drive brain science. Genom. Proteom. Bioinform. 2019, 17, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Ament, S.A.; Adkins, R.S.; Carter, R.; Chrysostomou, E.; Colantuoni, C.; Crabtree, J.; Creasy, H.H.; Degatano, K.; Felix, V.; Gandt, P.; et al. The Neuroscience Multi-Omic Archive: A BRAIN Initiative resource for single-cell transcriptomic and epigenomic data from the mammalian brain. Nucleic Acids Res. 2022, 9, 2022. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.A.; Ljungquist, B.; Ascoli, G.A. Efficient metadata mining of web-accessible neural morphologies. Prog. Biophys. Mol. Biol. 2022, 168, 94–102. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowinski, W.L. Advances in Neuroanatomy through Brain Atlasing. Anatomia 2023, 2, 28-42. https://doi.org/10.3390/anatomia2010004
Nowinski WL. Advances in Neuroanatomy through Brain Atlasing. Anatomia. 2023; 2(1):28-42. https://doi.org/10.3390/anatomia2010004
Chicago/Turabian StyleNowinski, Wieslaw L. 2023. "Advances in Neuroanatomy through Brain Atlasing" Anatomia 2, no. 1: 28-42. https://doi.org/10.3390/anatomia2010004
APA StyleNowinski, W. L. (2023). Advances in Neuroanatomy through Brain Atlasing. Anatomia, 2(1), 28-42. https://doi.org/10.3390/anatomia2010004




