Microencapsulated Jaboticaba Berry (M. cauliflora) Juice Improves Storage Stability and In Vitro Bioaccessibility of Polyphenols
Abstract
1. Introduction
2. Materials and Methods
2.1. Microencapsulation by Spray Dryer
2.2. Characterization of the Microencapsulated Jaboticaba
2.2.1. Proximate Composition and Sugar Profile Analyses
2.2.2. Water Activity
2.2.3. Solubility
2.2.4. Hygroscopicity
2.2.5. Colorimetric Analysis
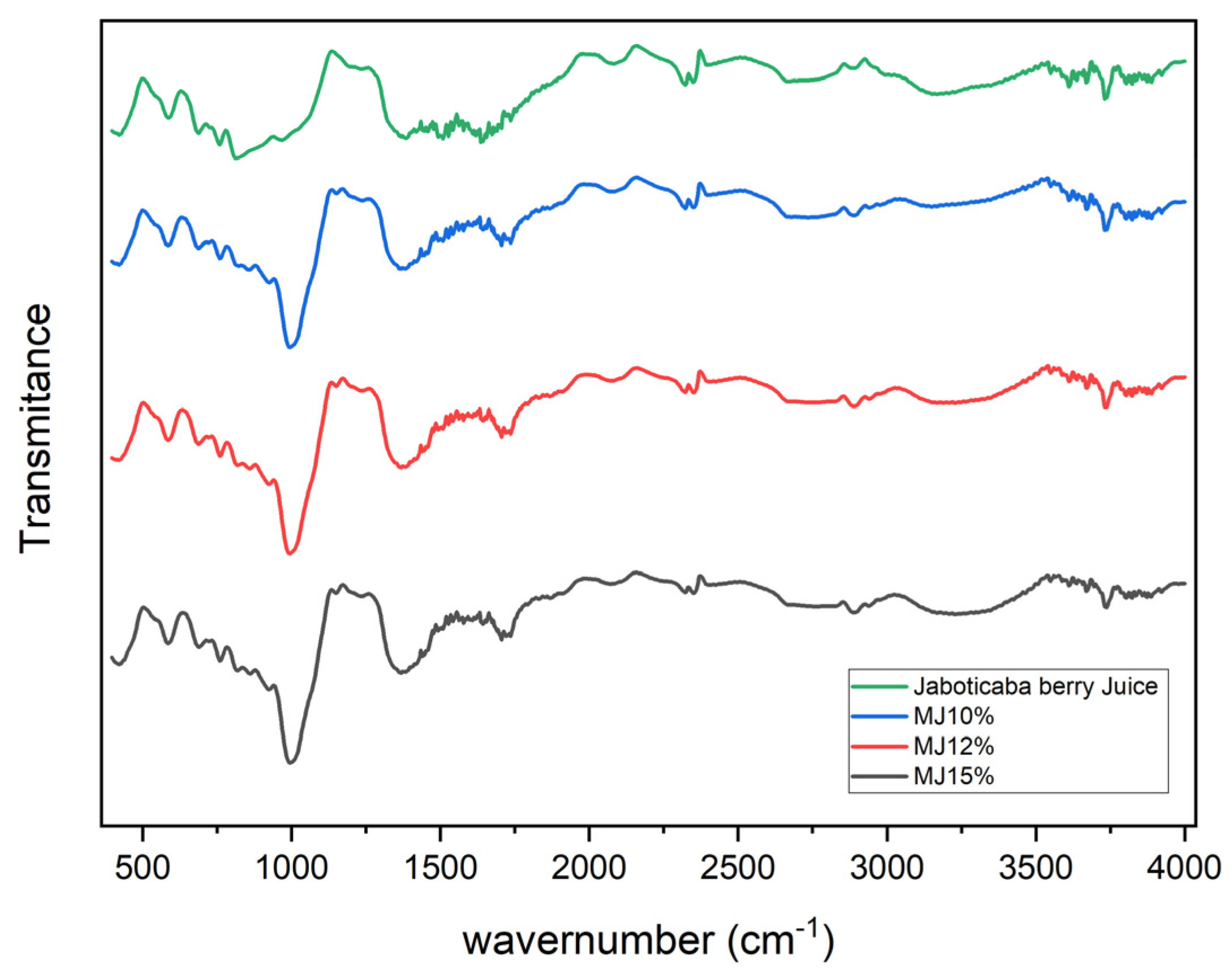
2.2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.7. Particle Size
2.3. Determination of Bioactive Compounds and Antioxidant Activity
2.3.1. Analysis of Total Polyphenols
2.3.2. Analysis of Antioxidant Capacity
2.3.3. Storage Stability Procedures of Total Polyphenols and Antioxidant Capacity
2.3.4. Simulated In Vitro Gastrointestinal Digestion
2.4. Statistical Analysis
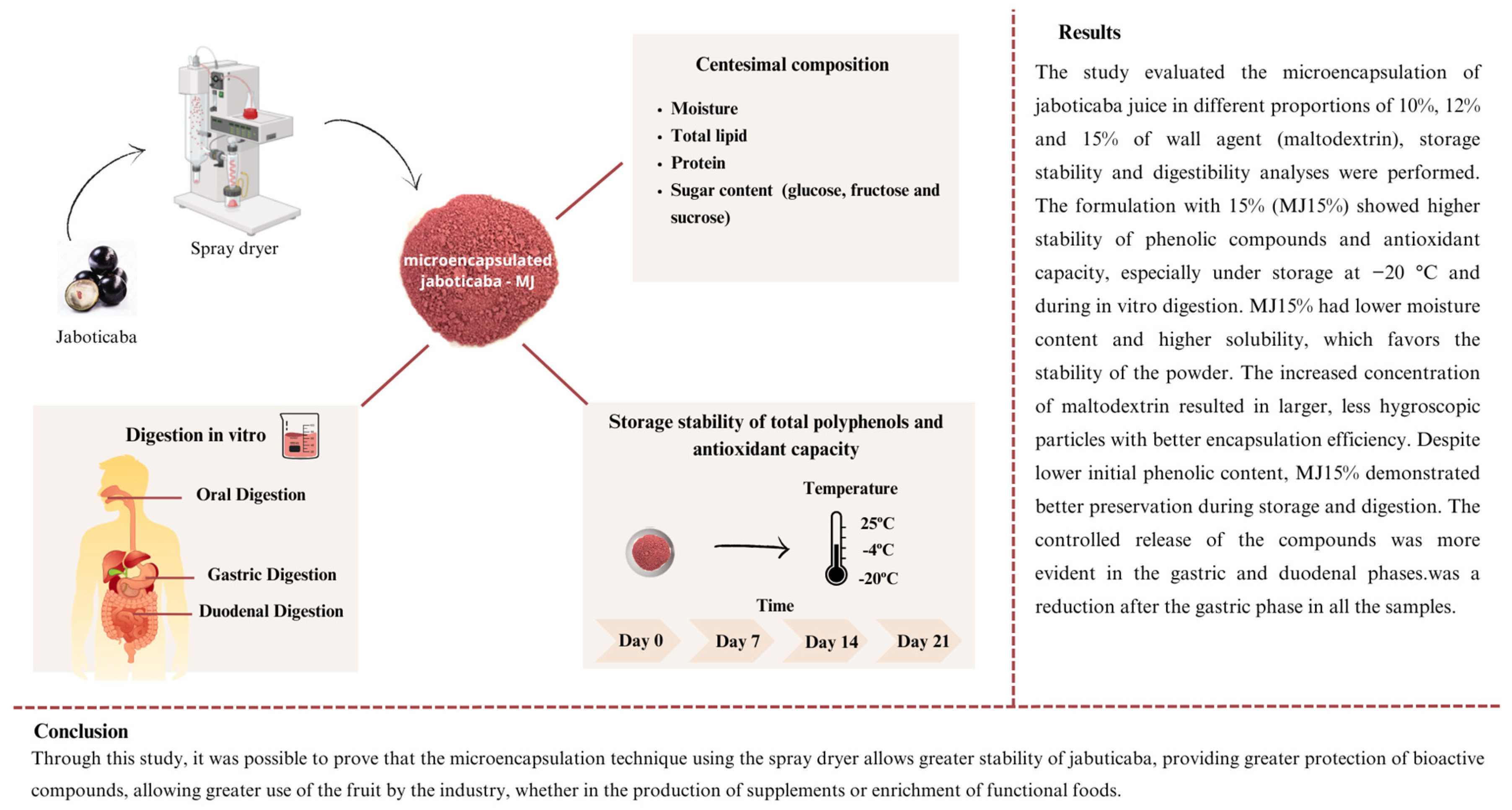
3. Results
3.1. Proximate Composition and Sugar Content of Microencapsulated Jaboticaba Berry Juice
3.2. Water Activity (aw)
3.3. Solubility
3.4. Hygroscopicity
3.5. Colorimetric Analysis
3.6. Fourier Transform Infrared (FTIR) Spectroscopy
3.7. Particle Size
3.8. Total Bioactive Compounds and Antioxidant Activity of Microencapsulated Jaboticaba Berry Juice
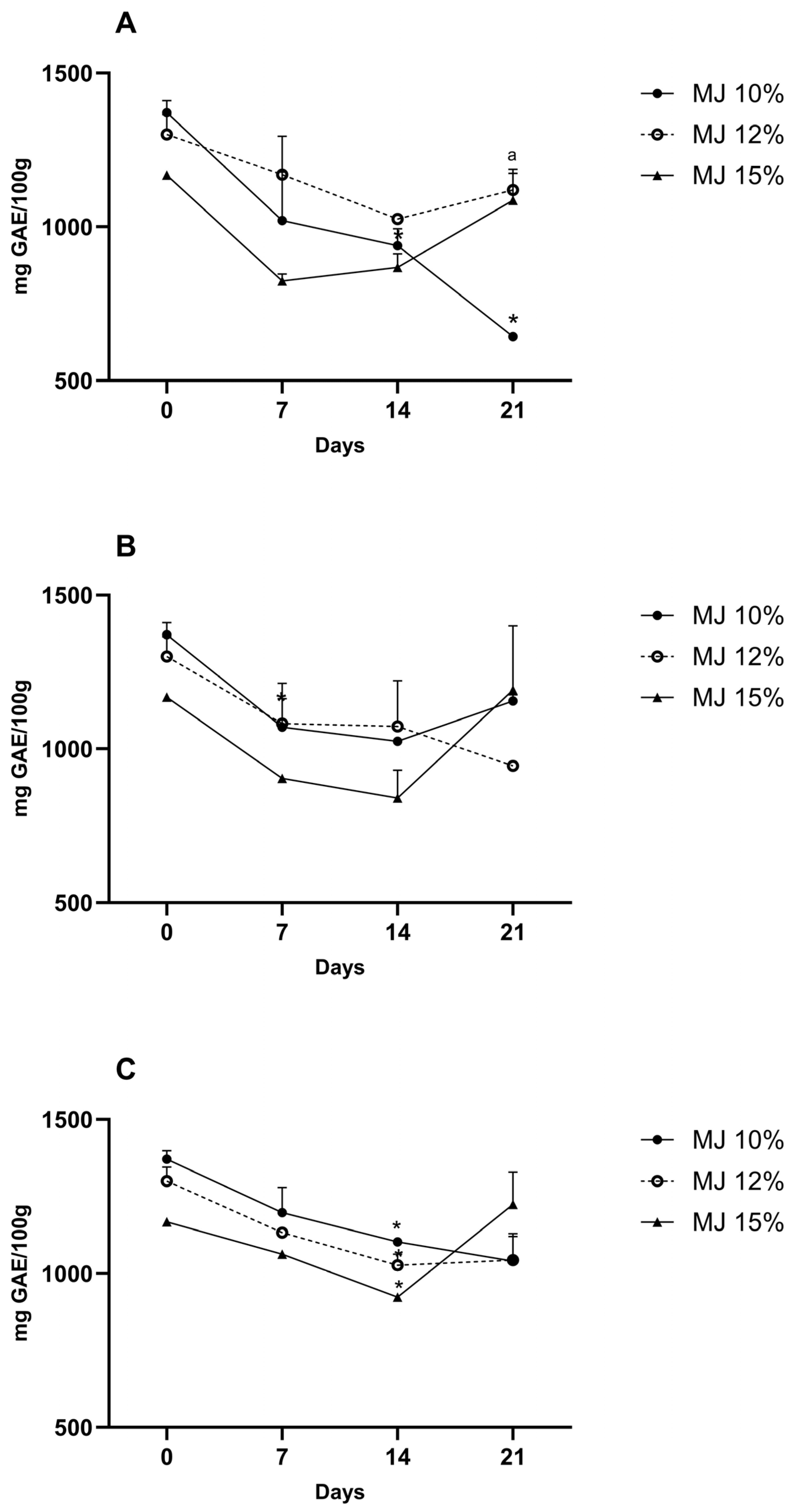
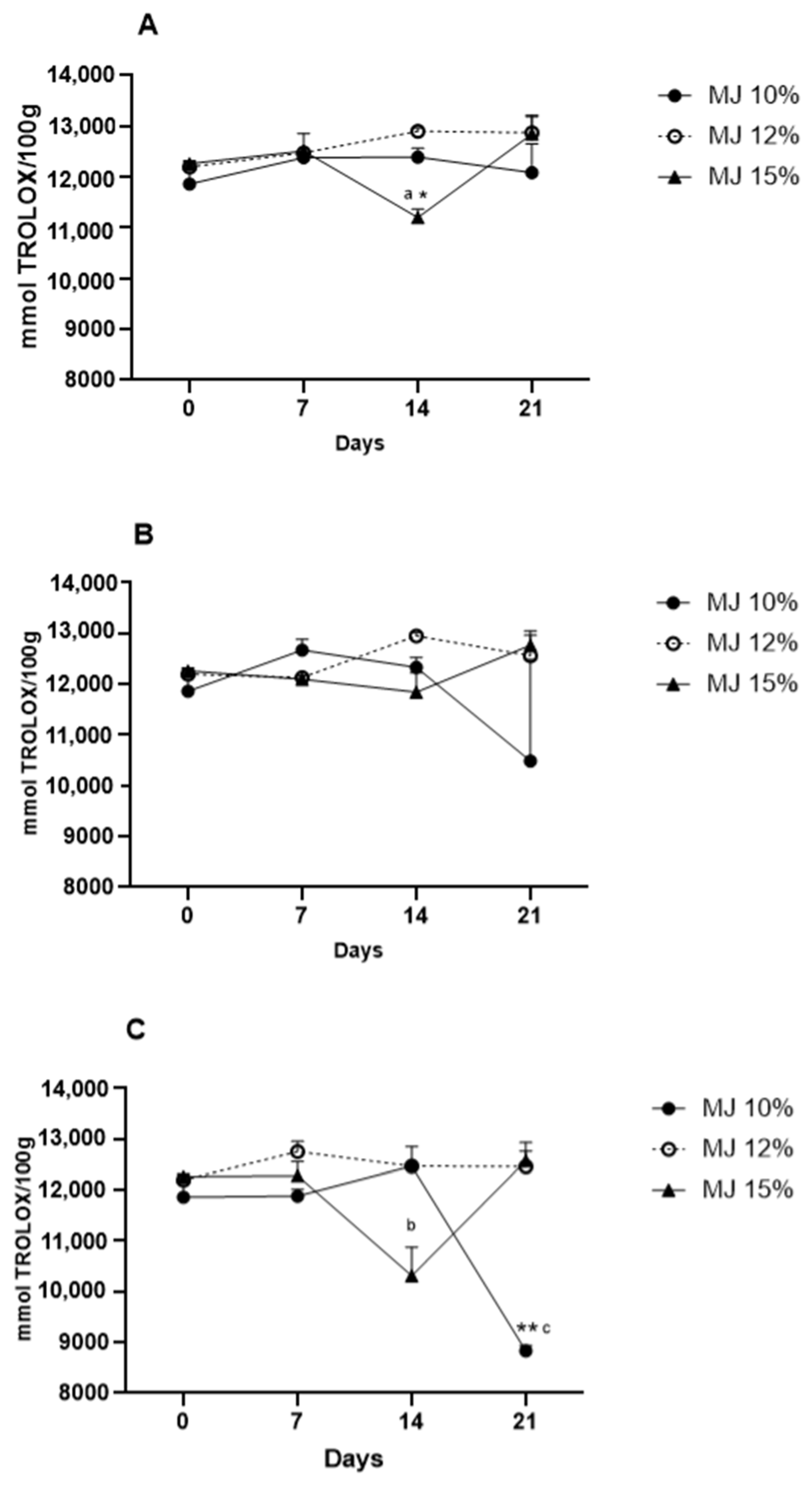
3.9. Storage Stability of Microencapsulated Jaboticaba Berry Juice
3.9.1. Total Phenolic Content
3.9.2. Total Antioxidant Capacity
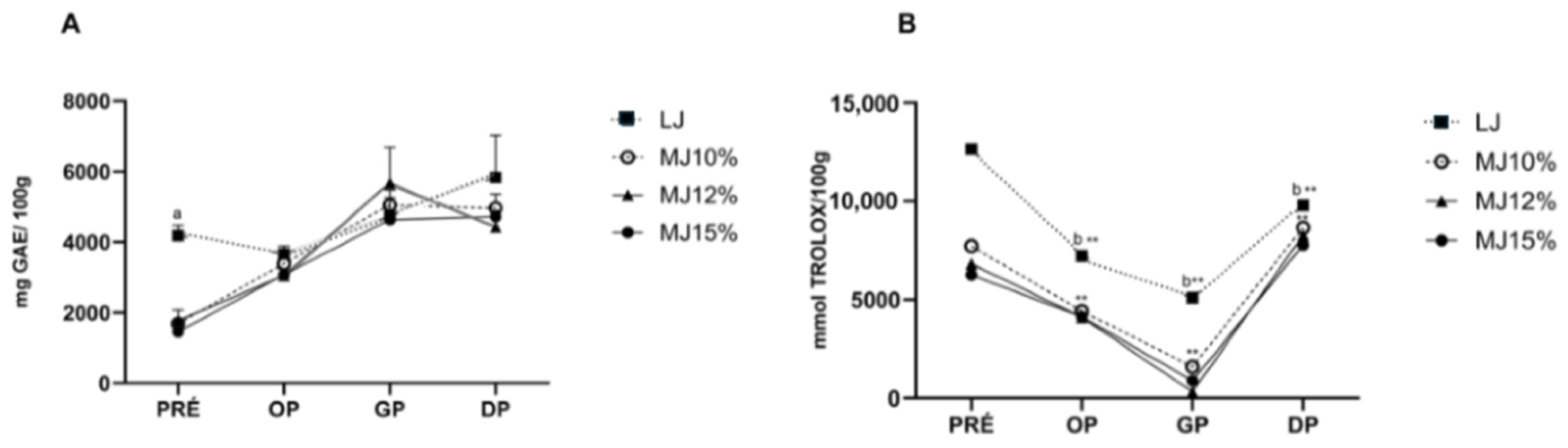
3.10. Simulated In Vitro Digestibility
3.10.1. Effects on Total Phenolic Content
3.10.2. Effects of Total Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Inada, K.O.P.; Oliveira, A.A.; Revorêdo, T.B.; Martins, A.B.N.; Lacerda, E.C.Q.; Freire, A.S.; Braz, B.F.; Santelli, R.E.; Torres, A.G.; Perrone, D.; et al. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J. Funct. Foods 2015, 17, 422–433. [Google Scholar] [CrossRef]
- Salomão, L.C.C.; Siqueira, D.L.; Aquino, L.C.R. Jaboticaba—Myrciaria spp. In Exotic Fruits Reference Guide; Rodrigues, S., Silva, E.O., de Brito, E.D., Eds.; Academic Press: Amsterdam, The Netherlands; Elsevier: Amsterdam, The Netherlands, 2018; pp. 237–244. [Google Scholar]
- Albuquerque, B.R.; Pinela, J.; Barros, L.; Oliveira, M.B.P.; Ferreira, I.C. Anthocyanin-rich extract of jabuticaba epicarp as a natural colorant: Optimization of heat- and ultrasound-assisted extractions and application in a bakery product. Food Chem. 2020, 316, 126364. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.G.S.; Silva, A.P.S.; Cunha, R.X.; Fonseca, C.S.M.; Araújo, T.F.S.; Campos, J.K.L.; Nascimento, W.M.; Araújo, H.D.A.; Silva, J.P.R.; Tavares, J.F.; et al. Anti-inflammatory, hypoglycemic, hypolipidemic, and analgesic activities of Plinia cauliflora (Mart.) Kausel (Brazilian grape) epicarp. J. Ethnopharmacol. 2021, 268, 113611. [Google Scholar] [CrossRef] [PubMed]
- Dragano, N.R.V.; Marques, A.Y.C.; Cintra, D.E.C.; Solon, C.; Morari, J.; Leite-Legatti, A.V.; Velloso, L.A.; Maróstica-Júnior, M.R. Freeze-dried jaboticaba peel powder improves insulin sensitivity in high-fat-fed mice. Br. J. Nutr. 2013, 110, 447–455. [Google Scholar] [CrossRef]
- Plaza, M.; Batista, Â.G.; Cazarin, C.B.B.; Sandahl, M.; Turner, C.; Östman, E.; Junior, M.R.M. Characterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: A pilot clinical study. Food Chem. 2016, 211, 185–197. [Google Scholar] [CrossRef]
- De Andrade, C.R.M.; Jr Silva, E.L.C.; da Matta, M.F.B.; Castier, M.B.; Rosa, M.L.G.; Gomes, M.B. Vascular or chronological age: Which is the better marker to estimate the cardiovascular risk in patients with type 1 diabetes? Acta Diabetol. 2016, 53, 925–933. [Google Scholar] [CrossRef]
- Calloni, C.; Agnol, R.D.; Martínez, L.S.; Marcon, F.d.S.; Moura, S.; Salvador, M. Jaboticaba (Plinia trunciflora (O. Berg) Kausel) fruit reduces oxidative stress in human fibroblasts cells (MRC-5). Food Res. Int. 2015, 70, 15–22. [Google Scholar] [CrossRef]
- Ferreira, P.R.; Marins, J.C.B.; de Oliveira, L.L.; Bastos, D.S.S.; Júnior, D.T.S.; da Silva, C.D.; Fontes, E.A.F. Beverage based on whey permeate with phenolic extract of jabuticaba peel: A pilot study on effects on muscle and oxidative stress in trained individuals. J. Funct. Foods 2020, 65, 103749. [Google Scholar] [CrossRef]
- Geraldi, M.V.; Cazarin, C.B.B.; Cristianini, M.; Vasques, A.C.J.; Geloneze, B.; Júnior, M.R.M. Jabuticaba juice improves postprandial glucagon-like peptide-1 and antioxidant status in healthy adults: A randomised crossover trial. Br. J. Nutr. 2021, 128, 1545–1554. [Google Scholar] [CrossRef]
- Fidelis, M.; Santos, J.S.; Escher, G.B.; Rocha, R.S.; Cruz, A.G.; Cruz, T.M.; Marques, M.B.; Nunes, J.B.; do Carmo, M.A.V.D.; de Almeida, L.A.; et al. Polyphenols of jabuticaba [Myrciaria jaboticaba (Vell.) O.Berg] seeds incorporated in a yogurt model exert antioxidant activity and modulate gut microbiota of 1,2-dimethylhydrazine-induced colon cancer in rats. Food Chem. 2021, 334, 127565. [Google Scholar] [CrossRef]
- Freitas-Sá, D.G.C.; Souza, R.C.; Araújo, M.C.P.; Mattos, G.B.L.S.; Pacheco, S.; Godoy, R.L.O. Effect of jabuticaba (Myrciaria jaboticaba (Vell) O. Berg) and jamelão (Syzygium cumini (L.) Skeels) peel powders as colorants on color-flavor congruence and acceptability of yogurts. LWT–Food Sci. Technol. 2018, 96, 215–221. [Google Scholar] [CrossRef]
- Baldin, J.C.; Michelin, E.C.; Polizer, Y.J.; Rodrigues, I.; de Godoy, S.H.S.; Fregonesi, R.P.; Pires, M.A.; Carvalho, L.T.; Fávaro-Trindade, C.S.; de Lima, C.G.; et al. Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Sci. 2016, 118, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.d.C.S.; Asquieri, E.R.; Batista, R.D.; de Morais, C.C.; Ascheri, D.P.R.; de Macêdo, I.Y.L.; Gil, E.d.S. Microencapsulation of jabuticaba extracts (Myrciaria cauliflora): Evaluation of their bioactive and thermal properties in cassava starch biscuits. LWT 2021, 137, 110460. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Januário, J.G.B.; Santos, S.S.; Bergamasco, R.; Madrona, G.S. Microcapsules of ‘jaboticaba’ byproduct: Storage stability and application in gelatin. Rev. Bras. Eng. Agrícola Ambiental 2018, 22, 424–429. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Wang, C.-Y.; Chen, B.-Y. Effects of high-pressure processing and thermal pasteurization on quality and microbiological safety of jabuticaba (Myrciaria cauliflora) juice during cold storage. J. Food Sci. Technol. 2020, 57, 3334–3344. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Leite, I.B.; Martins, A.B.N.; Fialho, E.; Tomás-Barberán, F.A.; Perrone, D.; Monteiro, M. Jaboticaba berry: A comprehensive review on its polyphenol composition, health effects, metabolism, and the development of food products. Food Res. Int. 2021, 147, 110518. [Google Scholar] [CrossRef]
- Teixeira, G.H.; Durigan, J.F.; Santos, L.O.; Hojo, E.T.; Cunha Júnior, L.C. Changes in the quality of jaboticaba fruit (Myriciaria jaboticaba (Vell) Berg. cv. Sabara) stored under different oxygen concentrations. J. Sci. Food Agric. 2011, 91, 2844–2849. [Google Scholar] [CrossRef]
- Wu, S.-B.; Dastmalchi, K.; Long, C.; Kennelly, E.J. Metabolite profiling of jaboticaba (Myrciaria cauliflora) and other dark-colored fruit juices. J. Agric. Food Chem. 2012, 60, 7513–7525. [Google Scholar] [CrossRef]
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.H.; Nichelle, S.M.; Klein, B.; Rodrigues, R.F.; Junior, M.R.M.; Fonseca, B.d.S.; de Menezes, C.R.; et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 2020, 65, 103714. [Google Scholar] [CrossRef]
- Martins, A.B.N.; Canto, M.; Perrone, D.; Monteiro, M. Chemical, Microbiological and Sensory Stability of Steam Extracted Jaboticaba (Myrciaria jaboticaba) Juice. Foods 2021, 10, 732. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Li, D.; Li, L. Effects of extraction methods on the stability and antioxidant activity of phenolic compounds from plant materials: A review. Food Chem. 2022, 377, 131965. [Google Scholar]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Bakowska-Barczak, A.M.; Kolodziejczyl, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/microencapsulation of anthocyanins; a systematic review and meta-analysis. Food Res. Int. 2020, 132, 109077. [Google Scholar] [CrossRef]
- Souza, A.C.P.; Gurak, P.D.; Marczak, L.D.F. Maltodextrin, pectin and soy protein isolate as carrier agents in the encapsulation of anthocyanins-rich extract from jaboticaba pomace. Food Bioprod. Process. 2017, 102, 186–194. [Google Scholar] [CrossRef]
- Verma, A.; Singh, S.V. Spray drying of fruit and vegetable juices–a review. Crit. Rev. Food Sci. Nutr. 2015, 55, 701–719. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Goula, A.; Adamopoulos, K. A method for pomegranate seed application in food industries: Seed oil encapsulation. Food Bioprod. Process. 2012, 90, 639–652. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chokkalingam, K.; Swedlund, P. The effect of processing conditions on the microencapsulation of fish oil by spray drying. J. Food Eng. 2007, 85, 527–535. [Google Scholar]
- Merrill, A.L.; Watt, B.K. Energy Value of Foods: Basis and Derivation; USDA Agriculture Handbook No. 74; Agricultural Research Service, United States Department of Agriculture: Washington, DC, USA, 1973.
- Duarte-Delgado, D.; Narváez-Cuenca, C.-E.; Restrepo-Sánchez, L.-P.; Kushalappa, A.; Mosquera-Vásquez, T. Development and validation of a liquid chromatographic method to quantify sucrose, glucose, and fructose in tubers of Solanum tuberosum Group Phureja. J. Chromatogr. B 2015, 975, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Lin, X.; Xu, X.-R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J.R. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vinic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Corleto, K.A.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Storage stability of dietary nitrate and phenolic compounds in beetroot (Beta vulgaris) and arugula (Eruca sativa) juices. J. Food Sci. 2018, 83, 1237–1248. [Google Scholar] [CrossRef]
- Morgado, M.; de Oliveira, G.V.; Vasconcellos, J.; Monteiro, M.L.; Conte-Junior, C.; Pierucci, A.P.T.R.; Alvares, T.S. Development of a beetroot-based nutritional gel containing high content of bioaccessible dietary nitrate and antioxidants. Int. J. Food Sci. Nutr. 2016, 67, 153–160. [Google Scholar] [CrossRef]
- Nunes, G.L.; Boaventura, B.C.B.; Pinto, S.S.; Verruck, S.; Murakami, F.S.; Prudêncio, E.S. Amboni RDM C. Microencapsulation of freeze-concentrated Ilex paraguariensis extract by spray drying. J Food Eng. 2015, 151, 60–68. [Google Scholar] [CrossRef]
- Özbek, Z.A.; Ergönül, P.G. Influence of Wall Material Composition on Microencapsulation Efficiency of Cold Pressed Pumpkin Seed Oil by Freeze-Drying. Nov. Tech. Nutr. Food Sci. 2018, 3, 236–239. [Google Scholar]
- Alezandro, M.R.; Granato, D.; Genovese, M.I. Jaboticaba (Myrciaria jaboticaba (Vell.) Berg), a Brazilian grape-like fruit, improves plasma lipid profile in streptozotocin-mediated oxidative stress in diabetic rats. Food Res. Int. 2013, 54, 650–659. [Google Scholar] [CrossRef]
- Laureanti, E.J.G.; Paiva, T.S.; Jorge, L.M.d.M.; Jorge, R.M.M. Microencapsulation of bioactive compound extracts using maltodextrin and gum arabic by spray and freeze-drying techniques. Int. J. Biol. Macromol. 2023, 253, 126969. [Google Scholar] [CrossRef]
- Premi, M.; Sharma, H.K. Effect of different combinations of maltodextrin, gum arabic and whey protein concentrate on the encapsulation behavior and oxidative stability of spray-dried drumstick (Moringa oleifera) oil. Int J Biol Macromol. 2017, 105, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; de Oliveira, I.R.N. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; Guerrero, P.; de la Caba, K. Characterization of agar/soy protein biocomposite films: Effect of agar on the extruded pellets and compression moulded films. Carbohydr. Polym. 2016, 151, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Meena, G.S.; Singh, A.K.; Gupta, V.K.; Gujral, H.K.; Malhotra, R.K. Heat stability of high protein ultrafiltration retentate: Effect of concentration factor and stabilizing salts. Indian J. Dairy Sci. 2021, 74, 8–17. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Santos, S.S.D.; Paraíso, C.M.; Costa, S.C.D.; Ogawa, C.Y.L.; Sato, F.; Madrona, G.S. Recovery of bioactive compounds from an agro-industrial waste: Extraction, microencapsulation, and characterization of jaboticaba (Myrciaria cauliflora Berg) pomace as a source of antioxidant. An. Acad. Bras. Ciências 2022, 94, e20191372. [Google Scholar] [CrossRef]
- Moser, P.; Telis, V.R.N.; de Andrade Neves, N.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef]
| MJ 10% | MJ 12% | MJ 15% | |
|---|---|---|---|
| Proximate composition | |||
| Moisture (%) | 12.67 ± 0.26 | 9.50 ± 1.55 | 8.73 ± 0.27 |
| Ash (%) | 1.85 ± 0.01 | 1.45 ± 0.01 | 1.45 ± 0.04 |
| Protein (%) | 4.09 ± 0.23 | 2.78 ± 0.34 | 2.79 ± 0.24 |
| Lipid (%) | 0.65 ± 0.07 | 0.65 ± 0.07 | 0.6 ± 0.00 |
| Carbohydrate (%) | 80.75 ± 0.03 | 85.63 ± 1.82 | 86.43 ± 0.47 |
| Sugars | |||
| Sucrose (g/100 g) | 0.014 ± 0.00 | 0.019 ± 0.001 | 0.024 ± 0.002 |
| Glucose (g/100 g) | 17.38 ± 0.90 | 20.08 ± 2.49 | 22.83 ± 3.35 |
| Fructose (g/100 g) | 16.29 ± 0.68 | 21.39 ± 4.91 | 26.94 ± 1.70 |
| MJ 10% | MJ 12% | MJ 15% | |
|---|---|---|---|
| Solubility (%) | 75.00 ± 6.83 | 78.00 ± 5.16 | 85.00 ± 6.00 |
| Hygroscopicity (%) | 26.50 ± 0.71 | 25.00 ± 2.83 | 23.50 ± 2.12 |
| Water Activity (aw) | 0.30 ± 0.01 | 0.31 ± 0.02 | 0.28 ± 0.01 |
| Jaboticaba Berry Juice | MJ 10% | MJ 12% | MJ 15% | |
|---|---|---|---|---|
| L* | 33.18 ± 0.92 a | 65.74 ± 0.32 a | 69.19 ± 2.30 a | 76.34 ± 1.67 |
| a* | 40.81 ± 2.39 | 23.47 ± 0.17 b | 19.23 ± 0.92 b,c | 16.55 ± 0.11 b,c |
| b* | 17.72 ± 1.53 | 12.40 ± 0.04 a,b | 10.88 ± 0.16 a,b | 8.61 ± 0.10 b |
| c* | 44.49 ± 2.8 | 26.57 ± 0.13 b | 22.10 ± 0.87 b | 18.68 ± 0.15 b |
| h° | 23.44 ± 0.6 | 27.85 ± 0.26 b | 29.53 ± 0.91 a,b | 27.73 ± 0.54 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Muros Amaral Barcellos, T.; Volino-Souza, M.; Lelis, C.A.; Conte Junior, C.A.; da Silveira Alvares, T. Microencapsulated Jaboticaba Berry (M. cauliflora) Juice Improves Storage Stability and In Vitro Bioaccessibility of Polyphenols. Appl. Biosci. 2025, 4, 31. https://doi.org/10.3390/applbiosci4030031
de Muros Amaral Barcellos T, Volino-Souza M, Lelis CA, Conte Junior CA, da Silveira Alvares T. Microencapsulated Jaboticaba Berry (M. cauliflora) Juice Improves Storage Stability and In Vitro Bioaccessibility of Polyphenols. Applied Biosciences. 2025; 4(3):31. https://doi.org/10.3390/applbiosci4030031
Chicago/Turabian Stylede Muros Amaral Barcellos, Tatiana, Mônica Volino-Souza, Carini Aparecida Lelis, Carlos Adam Conte Junior, and Thiago da Silveira Alvares. 2025. "Microencapsulated Jaboticaba Berry (M. cauliflora) Juice Improves Storage Stability and In Vitro Bioaccessibility of Polyphenols" Applied Biosciences 4, no. 3: 31. https://doi.org/10.3390/applbiosci4030031
APA Stylede Muros Amaral Barcellos, T., Volino-Souza, M., Lelis, C. A., Conte Junior, C. A., & da Silveira Alvares, T. (2025). Microencapsulated Jaboticaba Berry (M. cauliflora) Juice Improves Storage Stability and In Vitro Bioaccessibility of Polyphenols. Applied Biosciences, 4(3), 31. https://doi.org/10.3390/applbiosci4030031







