Linear DNA–Chitosan Nanoparticles: Formulation Challenges and Transfection Efficiency in Lung Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fluorescent Chitosan (FITC-CS)
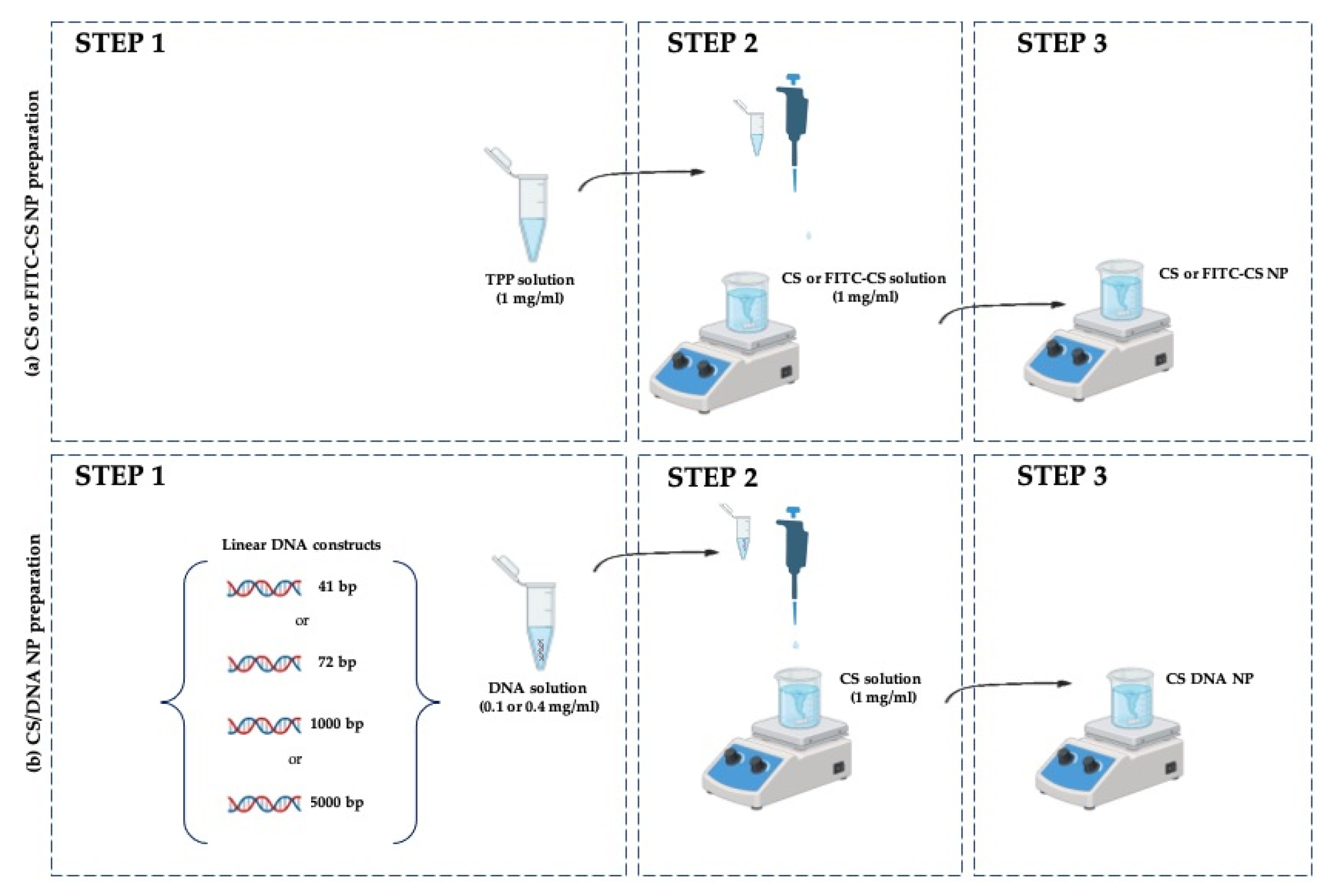
2.3. Preparation of Reference Blank (CS NP) and Chitosan–DNA Nanoparticles (CS/DNA NPs)
2.4. Physical–Chemical Characterization of Nanoparticles
2.4.1. NP Size Distribution and Zeta Potential Analysis
2.4.2. Entrapment Efficiency (EE) of CS-DNA NPs
2.5. Biological Evaluation
2.5.1. Cell Culture Condition
2.5.2. Cell Viability of NPs
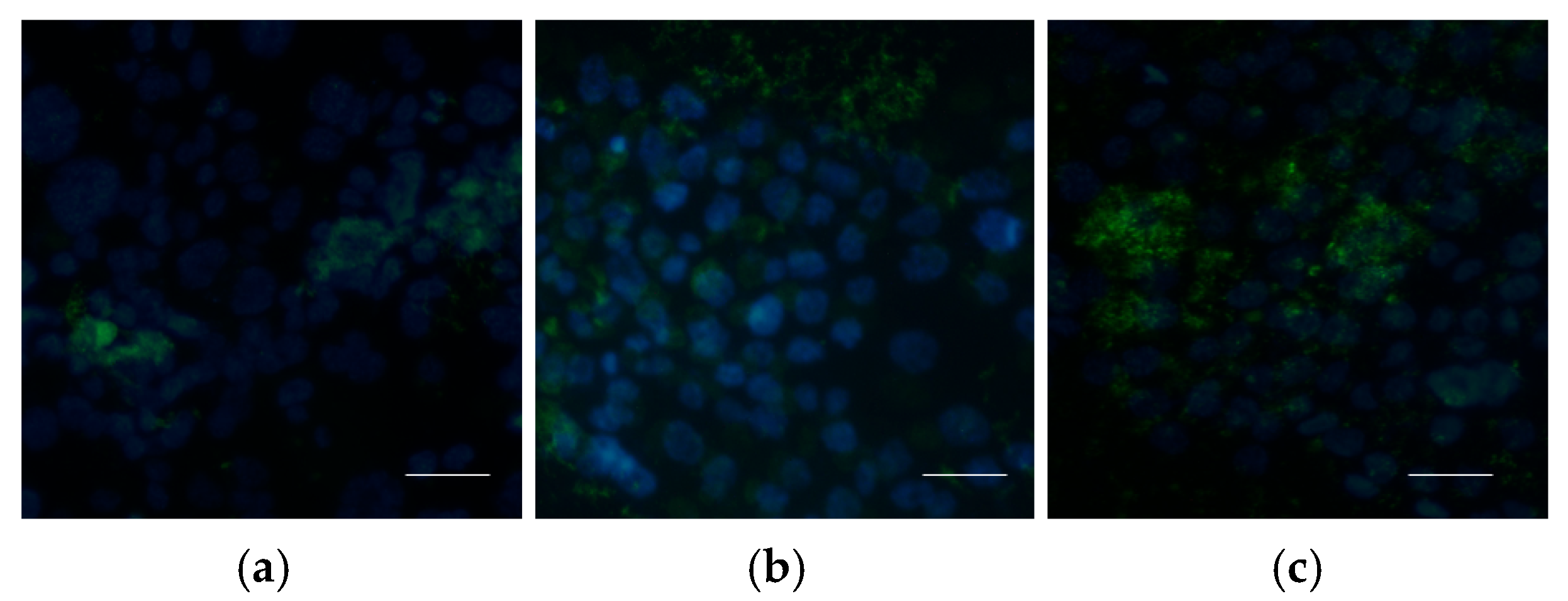
2.5.3. Uptake of Nanoparticles by Cells
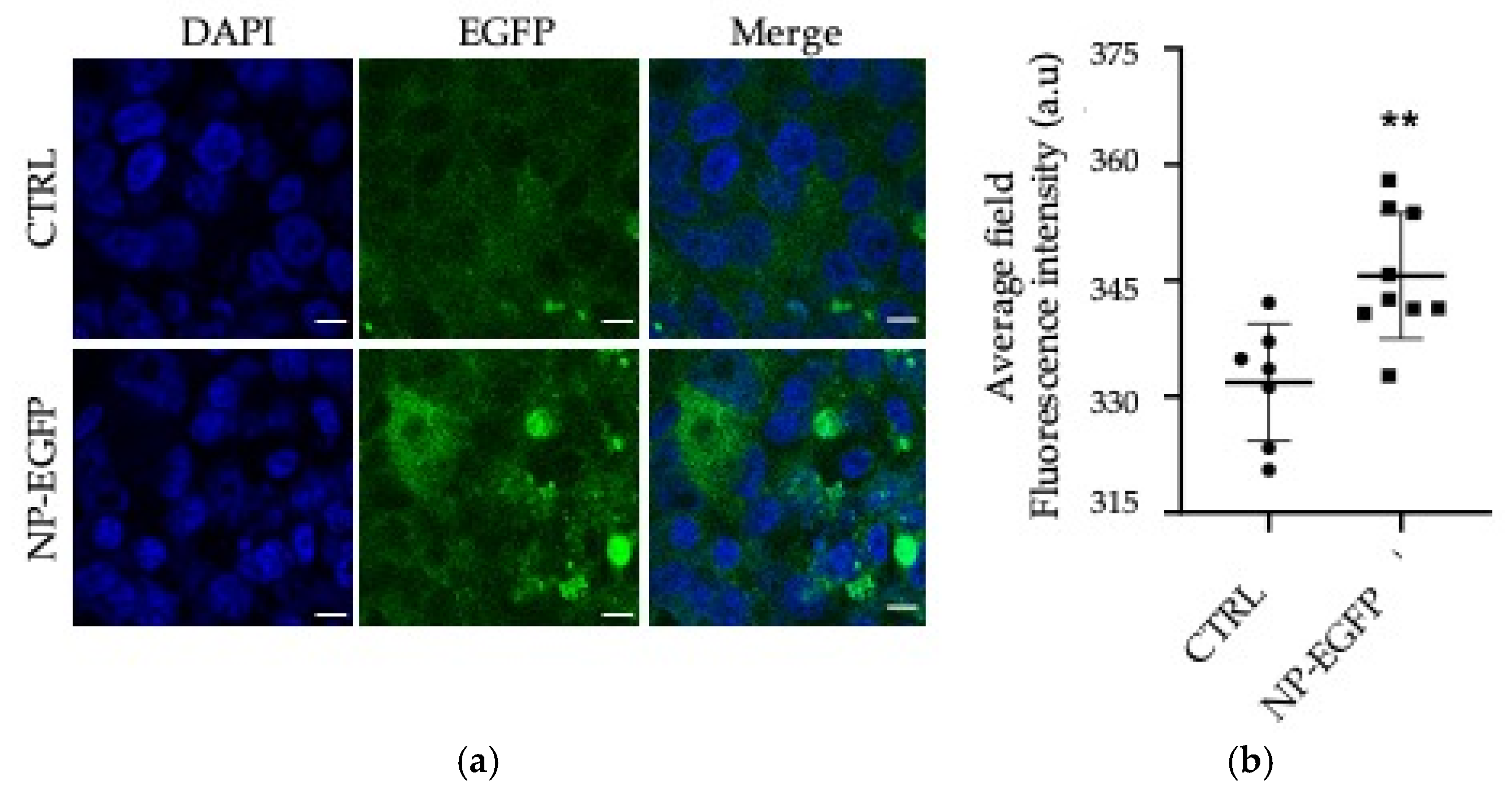
2.5.4. In Vitro Transfection Studies
2.5.5. Confocal Microscopy
2.6. Statistical Analysis
3. Results
3.1. NP Physical–Chemical Characterization
3.2. Biological Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Chitosan |
| TPP | Sodium tripolyphosphate |
| NPs | Nanoparticles |
| FITC | Fluorescein isothiocyanate |
| CS NPs | Reference chitosan blank nanoparticles |
| FITC-CS NPs | Fluorescent chitosan nanoparticles |
| bp | Base pairs |
| CS/DNA NPs | Chitosan–DNA nanoparticles |
| EE | Encapsulation efficiency |
| PFA | Paraformaldehyde |
| PBS | Phosphate-buffered saline |
| DMSO | Dimethyl sulfoxide |
| GFP | Green fluorescence protein |
| CELiDs | Closed-End Linear DNAs |
References
- Karahmet Sher, E.; Alebić, M.; Marković Boras, M.; Boškailo, E.; Karahmet Farhat, E.; Karahmet, A.; Pavlović, B.; Sher, F.; Lekić, L. Nanotechnology in medicine revolutionizing drug delivery for cancer and viral infection treatments. Int. J. Pharm. 2024, 660, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Palaniappan, T.; Wang, J.W.; Wacker, M.G. Revisiting nanomedicine design strategies for follow-on products: A model-informed approach to optimize performance. J. Control Release 2024, 376, 1251–1270. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Conniot, J.; Avita, L. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How nanomaterials are transforming drug delivery, bio-imaging, and diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Pan, X.; Veroniaina, H.; Su, N.; Sha, K.; Jiang, F.; Wu, Z.; Qi, X. Applications and developments of gene therapy drug delivery systems for genetic diseases. Asian J. Pharm. Sci. 2021, 16, 687–703. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Ramos Campos, E.V.; Rodriguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Kumara Swamy, M.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Valatabar, N.; Oroojalian, F.; Kazemzadeh, M.; Mokhtarzadeh, A.A.; Safaralizadeh, R.; Sahebkar, A. Recent advances in gene delivery nanoplatforms based on spherical nucleic acids. J. Nanobiotechnol. 2024, 22, 386. [Google Scholar] [CrossRef]
- Taghdiri, M.; Mussolino, C. Viral and Non-Viral Systems to Deliver Gene Therapeutics to Clinical Targets. Int. J. Mol. Sci. 2024, 25, 7333. [Google Scholar] [CrossRef]
- Patil, S.; Gao, Y.G.; Lin, X.; Li, Y.; Dang, K.; Tian, Y.; Zhang, W.J.; Jiang, S.F.; Qadir, A.; Qian, A.R. The Development of Functional Non-Viral Vectors for Gene Delivery. Int. J. Mol. Sci. 2019, 20, 5491. [Google Scholar] [CrossRef] [PubMed]
- Baylot, V.; Le, T.K.; Taïeb, D.; Rocchi, P.; Colleaux, L. Between hope and reality: Treatment of genetic diseases through nucleic acid-based drugs. Commun. Biol. 2024, 7, 489. [Google Scholar] [CrossRef]
- Migone, C.; Mattii, L.; Giannasi, M.; Moscato, S.; Cesari, A.; Zambito, Y.; Piras, A.M. Nanoparticles Based on Quaternary Ammonium Chitosan-methyl--cyclodextrin Conjugate for the Neuropeptide Dalargin Delivery to the Central Nervous System: An In Vitro Study. Pharmaceutics 2021, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Haider, A.; Khan, S.; Iqbal, D.N.; Shrahili, M.; Haider, S.; Mohammad, K.; Mohammad, A.; Rizwan, M.; Kanwal, Q.; Mustafa, G. Advances in chitosan-based drug delivery systems: A comprehensive review for therapeutic applications. Eur. Polym. J. 2024, 210, 1–23. [Google Scholar] [CrossRef]
- Chuan, D.; Jin, T.; Fan, R.; Zhou, L.; Guo, G. Chitosan for gene delivery: Methods for improvement and applications. Adv. Colloid. Interface Sci. 2019, 268, 25–38. [Google Scholar] [CrossRef]
- Genedy, H.H.; Delair, T.; Montembault, A. Chitosan Based MicroRNA Nanocarriers. Pharmaceuticals 2022, 15, 1036. [Google Scholar] [CrossRef]
- Antoniou, V.; Mourelatou, E.A.; Galatou, E.; Avgoustakis, K.; Hatziantoniou, S. Gene Therapy with Chitosan Nanoparticles: Modern Formulation Strategies for Enhancing Cancer Cell Transfection. Pharmaceutics 2024, 16, 868. [Google Scholar] [CrossRef]
- Karayianni, M.; Sentoukas, T.; Skandalis, A.; Pippa, N.; Pispas, S. Chitosan-Based Nanoparticles for Nucleic Acid Delivery: Technological Aspects, Applications, and Future Perspectives. Pharmaceutics 2023, 15, 1849. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Lim, S.; Yocum, R.R.; Silver, P.A.; Way, J.C. High spontaneous integration rates of end-modified linear DNAs upon mammalian cell transfection. Sci. Rep. 2023, 26, 6835. [Google Scholar] [CrossRef] [PubMed]
- Naghib, S.M.; Ahmadi, B.; Mikaeeli Kangarshahi, B.; Mozafari, M.R. Chitosan-based smart stimuli-responsive nanoparticles for gene delivery and gene therapy: Recent progresses on cancer therapy. Int. J. Biol. Macromol. 2024, 278, 134542. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.M.; Maluf, D.F.; Pontarolo, R.; Saul, C.K.; Almouazen, E.; Chevalier, Y. Negatively charged chitosan nanoparticles prepared by ionotropic gelation for encapsulation of positively charged proteins. Int. J. Pharm. 2023, 642, 123164. [Google Scholar] [CrossRef] [PubMed]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwy, J. Polyethylenimine (PEI) in gene therapy: Current status and clinical applications. J. Control Release 2023, 362, 667–691. [Google Scholar] [CrossRef]
- Souto, E.B.; Blanco-Llamero, C.; Krambeck, K.; Kiran, N.S.; Yashaswini, C.; Postwala, H.; Severino, P.; Priefer, R.; Prajapati, B.G.; Maheshwari, R. Regulatory insights into nanomedicine and gene vaccine innovation: Safety assessment, challenges, and regulatory perspectives. Acta Biomater. 2024, 180, 1–17. [Google Scholar] [CrossRef]
- Tasset, A.; Bellamkonda, A.; Wang, W.; Pyatnitskiy, I.; Ward, D.; Peppas, N.; Wang, H. Overcoming barriers in non-viral gene delivery for neurological applications. Nanoscale 2022, 10, 3698–3719. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef]
- Tran, T.-T.; Amalina, N.; Cheow, W.S.; Hadinoto, K. Effects of storage on the stability and aerosolization efficiency of dry powder inhaler formulation of plasmid DNA-Chitosan nanoparticles. J. Drug Deliv. Technol. 2020, 59, 101866. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; van Meijgaarden, K.E.; Franken, K.L.M.C.; Junginger, H.E.; Borchard, G.; Ottenhoff, T.H.M.; Geluk, A. Pulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A*0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine 2004, 22, 1609–1615. [Google Scholar] [CrossRef]
- Ma, W.; Zhan, Y.; Zhang, Y.; Mao, C.; Xie, X.; Lin, Y. The biological applications of DNA nanomaterials: Current challenges and future directions. Signal Transduct. Target. Ther. 2021, 6, 351. [Google Scholar] [CrossRef]
- Kolonko, A.K.; Bangel-Ruland, N.; Goycoolea, F.M.; Weber, W.M. Chitosan Nanocomplexes for the Delivery of ENaC Antisense Oligonucleotides to Airway Epithelial Cells. Biomolecules 2020, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Plasschaert, L.W.; MacDonald, K.D.; Moffit, J.S. Current landscape of cystic fibrosis gene therapy. Front. Pharmacol. 2024, 15, 1476331. [Google Scholar] [CrossRef]
- Zambito, Y.; Felice, F.; Fabiano, A.; Di Stefano, R.; Di Colo, G. Mucoadhesive nanoparticles made of thiolated quaternary chitosan crosslinked with hyaluronan. Carbohydr. Polym. 2013, 92, 33–39. [Google Scholar] [CrossRef]
- Di Colo, G.; Zambito, Y.; Zaino, C.; Sansò, M. Selected polysaccharides at comparison for their mucoadhesiveness and effect on precorneal residence of different drugs in the rabbit model. Drug Dev. Ind. Pharm. 2009, 35, 941–949. [Google Scholar] [CrossRef]
- Lv, Y.; He, H.; Qi, J.; Lu, Y.; Zhao, W.; Dong, X.; Wu, W. Visual validation of the measurement of entrapment efficiency of drugnanocarriers. Int. J. Pharm. 2018, 547, 395–403. [Google Scholar] [CrossRef]
- Piccarducci, R.; Germelli, L.; Falleni, A.; Luisotti, L.; Masciulli, B.; Signore, G.; Migone, C.; Fabiano, A.; Bizzarri, R.; Piras, A.M.; et al. GFP Farnesylation as a Suitable Strategy for Selectively Tagging Exosomes. ACS Appl. Bio Mater. 2024, 7, 8305–8318. [Google Scholar] [CrossRef]
- Santos-Carballal, B.; Fernández Fernández, E.; Goycoolea, F.M. Chitosan in Non-Viral Gene Delivery: Role of Structure, Characterization Methods, and Insights in Cancer and Rare Diseases Therapies. Polymers 2018, 10, 444. [Google Scholar] [CrossRef]
- Sato, T.; Ishii, T.; Okahata, Y. In vitro gene delivery mediated by chitosan: Effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials 2001, 22, 2075–2080. [Google Scholar] [CrossRef]
- Lin, G.; Huang, J.; Zhang, M.; Chen, S.; Zhang, M. Chitosan-Crosslinked Low Molecular Weight PEI-Conjugated Iron Oxide Nanoparticle for Safe and Effective DNA Delivery to Breast Cancer Cells. Nanomaterials 2022, 12, 584. [Google Scholar] [CrossRef]
- Lebre, F.; Borchard, G.; Faneca, H.; Pedroso de Lima, M.; Borges, O. Intranasal administration of novel chitosan nanoparticle/DNA complexes induces antibody response to hepatitis B surface antigen in mice. Mol. Pharm. 2016, 13, 472–482. [Google Scholar] [CrossRef]
- Lavertu, M.; Methot, S.; Tran-Khanh, N.; Buschmann, M.D. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials 2006, 27, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.; Sleiman, H. DNA nanotechnology. Nat. Rev. Mater. 2018, 3, 17068. [Google Scholar] [CrossRef]
- Xu, F.; Xia, Q.; Wang, P. Rationally Designed DNA Nanostructures for Drug Delivery. Front. Chem. 2020, 8, 751. [Google Scholar] [CrossRef]
- Keller, A.; Linko, V. Challenges and Perspectives of DNA Nanostructures in Biomedicine. Angew. Chem. Int. 2020, 59, 15818–15833. [Google Scholar] [CrossRef]
- Azeez, Y.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Dey, G.R.; McCormick, C.R.; Soliman, S.S.; Darling, A.J.; Schaak, R.E. Chemical Insights into the Formation of Colloidal High Entropy Alloy Nanoparticles. ACS Nano 2023, 17, 5943–5955. [Google Scholar] [CrossRef]
- Fernández-Paz, E.; Feijoo-Siota, L.; Gaspar, M.M.; Csaba, N.; Remuñán-López, C. Microencapsulated Chitosan-Based Nanocap- sules: A New Platform for Pulmonary Gene Delivery. Pharmaceutics 2021, 13, 1377. [Google Scholar] [CrossRef]
- Khan, I.N.; Navaid, S.; Waqar, W.; Hussein, D.; Ullah, N.; Khan, M.U.A.; Hussain, Z.; Javed, A. Chitosan-Based Polymeric Nanoparticles as an Efficient Gene Delivery System to Cross Blood Brain Barrier: In Vitro and In Vivo Evaluations. Pharmaceuticals 2024, 17, 169. [Google Scholar] [CrossRef]
- Scarpin, D.; Nerva, L.; Chitarra, W.; Moffa, L.; D’Este, F.; Vuerich, M.; Filippi, A.; Braidot, E.; Petrussa, E. Characterisation and functionalisation of chitosan nanoparticles as carriers for double-stranded RNA (dsRNA) molecules towards sustainable crop protection. Biosci Rep. 2023, 43, BSR20230817. [Google Scholar] [CrossRef]
- Kolonko, A.K.; Efing, J.; González-Espinosa, Y.; Bangel-Ruland, N.; van Driessche, W.; Goycoolea, F.M.; Weber, W.M. Capsaicin-Loaded Chitosan Nanocapsules for wtCFTR-mRNA Delivery to a Cystic Fibrosis Cell Line. Biomedicines 2020, 8, 364. [Google Scholar] [CrossRef]
- Fernández Fernández, E.; Santos-Carballal, B.; Weber, W.M.; Goycoolea, F.M. Chitosan as a non-viral co-transfection system in a cystic fibrosis cell line. Int. J. Pharm. 2016, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Bentley, I.; Ghadiali, S.N.; Englert, J.A. Pulmonary drug delivery for acute respiratory distress syndrome. Pulm. Pharmacol. Ther. 2023, 79, 102196. [Google Scholar] [CrossRef] [PubMed]
- Zacaron, T.M.; Silva, M.L.S.e.; Costa, M.P.; Silva, D.M.e.; Silva, A.C.; Apolônio, A.C.M.; Fabri, R.L.; Pittella, F.; Rocha, H.V.A.; Tavares, G.D. Advancements in Chitosan-Based Nanoparticles for Pulmonary Drug Delivery. Polymers 2023, 15, 3849. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.J.; Muchitsch, V.E.; Gausterer, J.C.; Schwagerus, E.; Huwer, H.; Daum, N.; Lehr, C.M.; Ehrhardt, C. The cell line NCl-H441 is a useful in vitro model for transport studies of human distal lung epithelial barrier. Mol. Pharm. 2014, 3, 995–1006. [Google Scholar] [CrossRef]

| Sample Name | DNA Construct | |
|---|---|---|
| Base Pair (bp) | Solution Concentration (mg/mL) | |
| CS NP | - | - |
| FITC-CS NP | - | - |
| CS/DNA(41)a NP | 41 | 0.1 |
| CS/DNA(41)b NP | 41 | 0.4 |
| CS/DNA(720)a NP | 720 | 0.1 |
| CS/DNA(720)b NP | 720 | 0.4 |
| CS/DNA GFP(1000)a NP | 1000 | 0.1 |
| CS/DNA GFP (1000)b NP | 1000 | 0.4 |
| CS/DNA(5000)a NP | 5000 | 0.1 |
| CS/DNA(5000)b NP | 5000 | 0.4 |
| Sample Name | NP Size (nm) | PDI | EE (%) | Zeta Potential (z) |
|---|---|---|---|---|
| CS NP | 150.2 ± 3.6 | 0.289 ± 0.018 | / | +21 ± 2.17 |
| FITC-CS NP | 182.4 ± 2.4 | 0.301 ± 0.032 | / | +20.0 ± 1.90 |
| CS/DNA(41)a NP | 323.3 ± 8.3 | 0.447 ± 0.016 | 20 | +21.0 ± 2.17 |
| CS/DNA(41)b NP | 442.6 ± 2.6 | 0.348 ± 0.060 | 78 | +21.0 ± 2.31 |
| CS/DNA(720)a NP | 500.4 ± 1.8 | 0.429 ± 0.043 | 38 | +20.0 ± 3.90 |
| CS/DNA(720)b NP | 340.4 ± 17.5 | 0.298 ± 0.062 | 58 | +22.0 ± 1.78 |
| CS/DNA-GFP(1000)a NP | 720.1 ± 8.7 | 0.775 ± 0.720 | 40 | +23.90 ± 1.56 |
| CS/DNA-GFP(1000)b NP | 290.7 ± 2.3 | 0.190 ± 0.032 | 86 | +25.50 ± 2.09 |
| CS/DNA(5000)a NP | 374.4 ± 24.2 | 0.445 ± 0.011 | 43 | +23.0 ± 2.11 |
| CS/DNA(5000)b NP | 308.8 ± 1.2 | 0.219 ± 0.042 | 80 | +28.0 ± 2.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migone, C.; Fabiano, A.; Zambito, Y.; Piccarducci, R.; Marchetti, L.; Giacomelli, C.; Martini, C.; Piras, A.M. Linear DNA–Chitosan Nanoparticles: Formulation Challenges and Transfection Efficiency in Lung Cell Line. Appl. Biosci. 2025, 4, 29. https://doi.org/10.3390/applbiosci4020029
Migone C, Fabiano A, Zambito Y, Piccarducci R, Marchetti L, Giacomelli C, Martini C, Piras AM. Linear DNA–Chitosan Nanoparticles: Formulation Challenges and Transfection Efficiency in Lung Cell Line. Applied Biosciences. 2025; 4(2):29. https://doi.org/10.3390/applbiosci4020029
Chicago/Turabian StyleMigone, Chiara, Angela Fabiano, Ylenia Zambito, Rebecca Piccarducci, Laura Marchetti, Chiara Giacomelli, Claudia Martini, and Anna Maria Piras. 2025. "Linear DNA–Chitosan Nanoparticles: Formulation Challenges and Transfection Efficiency in Lung Cell Line" Applied Biosciences 4, no. 2: 29. https://doi.org/10.3390/applbiosci4020029
APA StyleMigone, C., Fabiano, A., Zambito, Y., Piccarducci, R., Marchetti, L., Giacomelli, C., Martini, C., & Piras, A. M. (2025). Linear DNA–Chitosan Nanoparticles: Formulation Challenges and Transfection Efficiency in Lung Cell Line. Applied Biosciences, 4(2), 29. https://doi.org/10.3390/applbiosci4020029









