Umckalin Promotes Melanogenesis in B16F10 Cells Through the Activation of Wnt/β-Catenin and MAPK Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Measurement of Cell Viability
2.4. Melanin Content Assay
2.5. Tyrosinase Activity Assay
2.6. Western Blotting Assay
2.7. Statistical Analysis
3. Results and Discussion
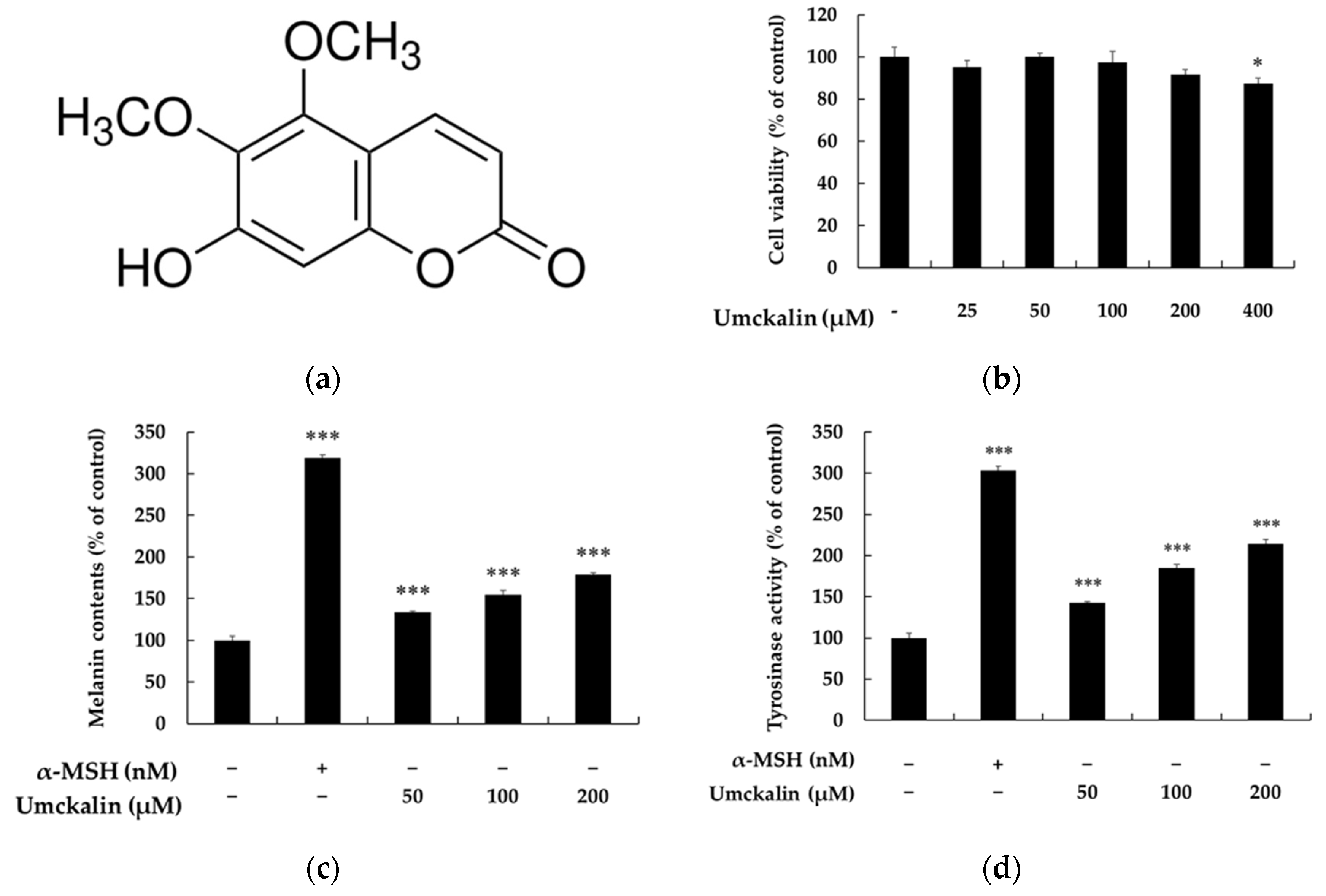
3.1. Umckalin Promotes Melanin Synthesis in B16F10 Cells
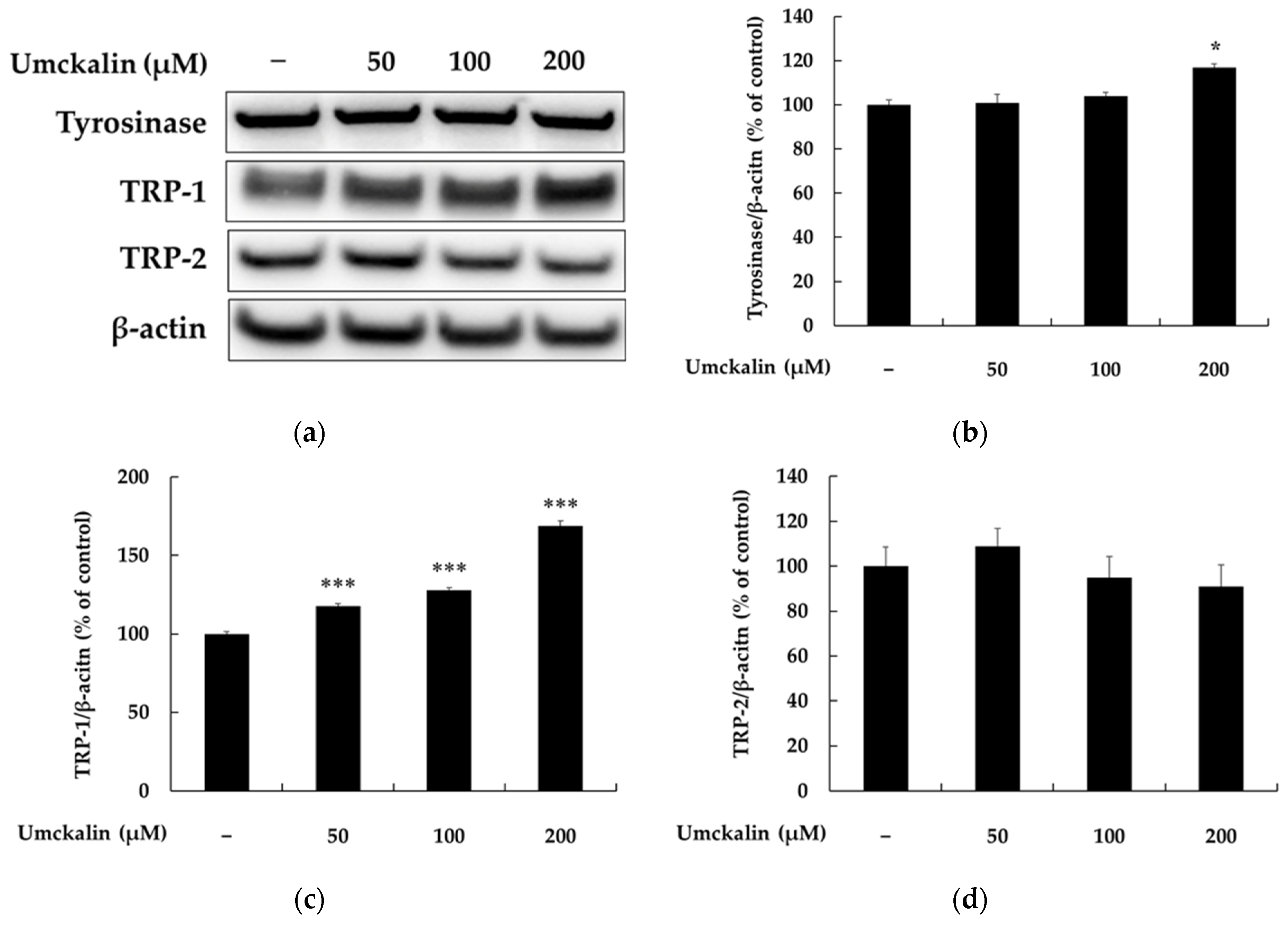
3.2. Umckalin Promotes Melanin Synthesis by Inducing TRP-1 Expression via the MITF Signaling Pathway
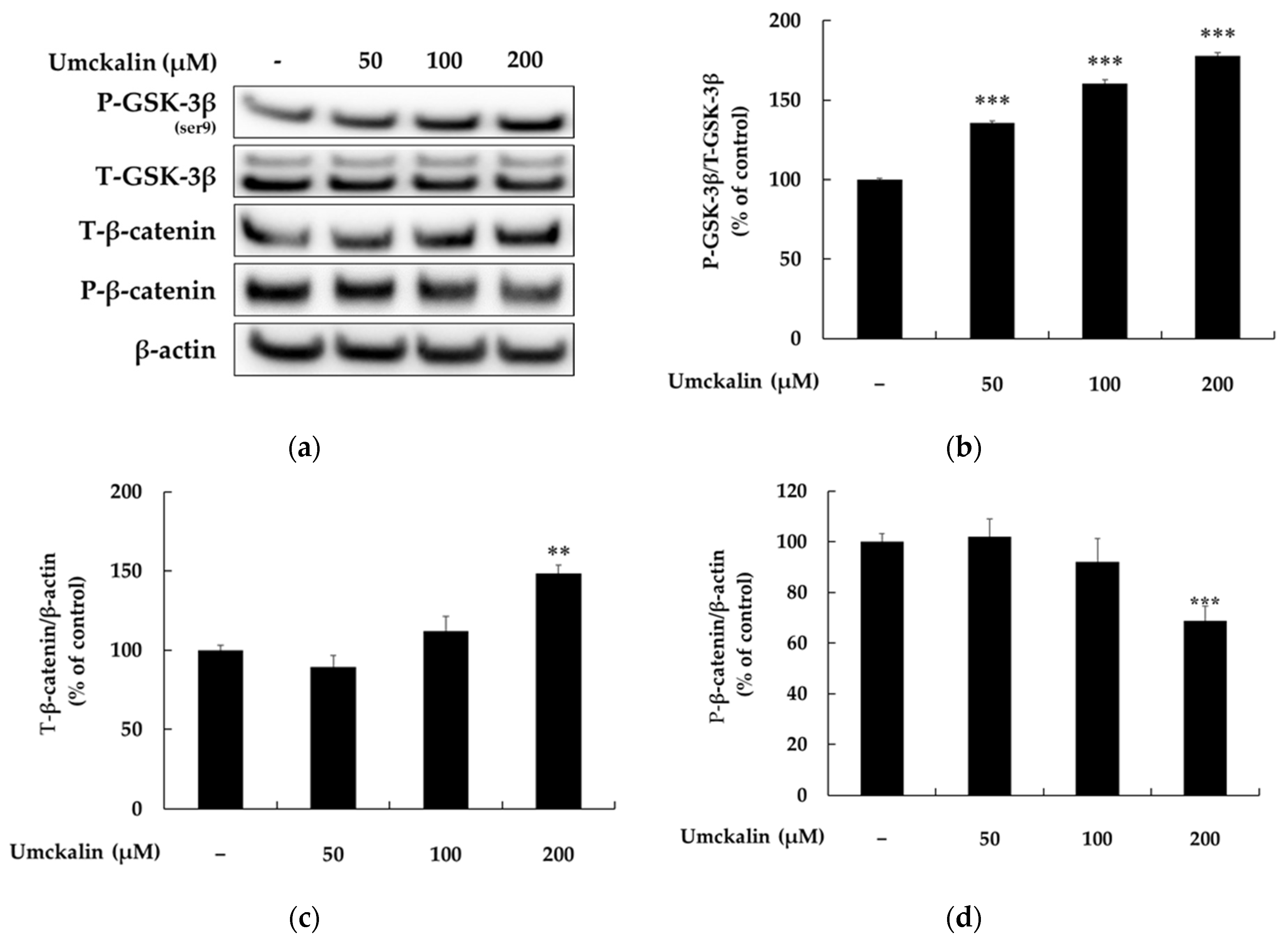
3.3. Umckalin Promotes Melanin Synthesis via GSK-3β/β-Catenin Signaling Pathway
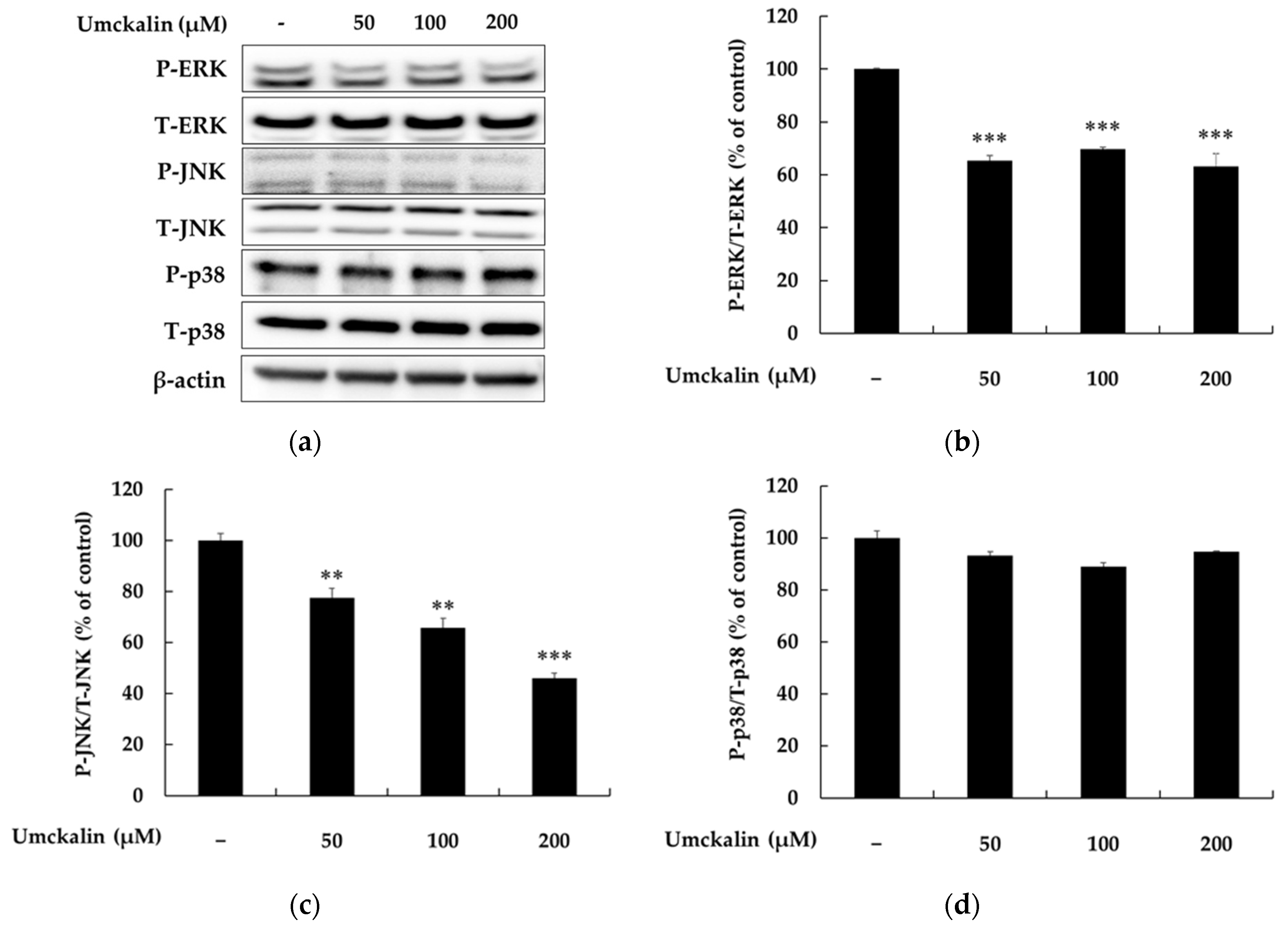
3.4. Umckalin Promotes Melanin Synthesis via MAPK Signaling Pathway
3.5. Umckalin Promotes Melanin Synthesis Independently of the PI3K/AKT Signaling Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postep. Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Su, H.; Yang, F.; Lu, K.; Ma, J.; Huo, G.; Li, S.; Li, J. Carnosic acid ameliorates postinflammatory hyperpigmentation by inhibiting inflammatory reaction and melanin deposition. Biomed. Pharmacother. 2024, 180, 117522. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Cardinali, G.; Picardo, M.; Bastonini, E. Shining Light on Autophagy in Skin Pigmentation and Pigmentary Disorders. Cells 2022, 11, 2999. [Google Scholar] [CrossRef]
- Karkoszka, M.; Rok, J.; Wrześniok, D. Melanin Biopolymers in Pharmacology and Medicine-Skin Pigmentation Disorders, Implications for Drug Action, Adverse Effects and Therapy. Pharmaceuticals 2024, 17, 521. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Cavaco-Paulo, A.; Matamá, T. A Comprehensive Review of Mammalian Pigmentation: Paving the Way for Innovative Hair Colour-Changing Cosmetics. Biology 2023, 12, 290. [Google Scholar] [CrossRef]
- Costa, E.F.; Magalhães, W.V.; Di Stasi, L.C. Recent Advances in Herbal-Derived Products with Skin Anti-Aging Properties and Cosmetic Applications. Molecules 2022, 27, 7518. [Google Scholar] [CrossRef] [PubMed]
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Wan, P.; Hu, Y.; He, L. Regulation of melanocyte pivotal transcription factor MITF by some other transcription factors. Mol. Cell. Biochem. 2011, 354, 241–246. [Google Scholar] [CrossRef]
- Fu, C.; Chen, J.; Lu, J.; Yi, L.; Tong, X.; Kang, L.; Pei, S.; Ouyang, Y.; Jiang, L.; Ding, Y.; et al. Roles of inflammation factors in melanogenesis (Review). Mol. Med. Rep. 2020, 21, 1421–1430. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Stelmach, J.; Zajdel, K.; Kucharska, E.; Zajdel, R. Plants as Modulators of Melanogenesis: Role of Extracts, Pure Compounds and Patented Compositions in Therapy of Pigmentation Disorders. Int. J. Mol. Sci. 2022, 23, 14787. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. Skin Pigmentation Types, Causes and Treatment-A Review. Molecules 2023, 28, 4839. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, Z.; Golkar, P.; Mirjalili, M.H. Production enhancement of medicinally active coumarin and phenolic compounds in hairy root cultures of Pelargonium sidoides: The effect of elicitation and sucrose. J. Plant Growth Reg. 2021, 40, 628–641. [Google Scholar] [CrossRef]
- Alossaimi, M.A.; Alzeer, M.A.; Abdel Bar, F.M.; ElNaggar, M.H. Pelargonium sidoides Root Extract: Simultaneous HPLC Separation, Determination, and Validation of Selected Biomolecules and Evaluation of SARS-CoV-2 Inhibitory Activity. Pharmaceuticals 2022, 15, 1184. [Google Scholar] [CrossRef]
- Reina, B.D.; Malheiros, S.S.; Vieira, S.M.; Ferreira de Andrade, P.; Dovigo, L.N. Unlocking the therapeutic potential of Pelargonium sidoides natural extract: A scoping review. Heliyon 2024, 10, e40554. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hyun, C.G. Mechanistic Insights into the Stimulatory Effect of Melanogenesis of 4-Methylcoumarin Derivatives in B16F10 Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 12421. [Google Scholar] [CrossRef]
- Cheng, M.C.; Lee, T.H.; Chu, Y.T.; Syu, L.L.; Hsu, S.J.; Cheng, C.H.; Wu, J.; Lee, C.K. Melanogenesis Inhibitors from the Rhizoma of Ligusticum Sinense in B16-F10 Melanoma Cells In Vitro and Zebrafish In Vivo. Int. J. Mol. Sci. 2018, 19, 3994. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Hyun, C.G. Anti-Inflammatory Potential of Umckalin Through the Inhibition of iNOS, COX-2, Pro-Inflammatory Cytokines, and MAPK Signaling in LPS-Stimulated RAW 264.7 Cells. Future Pharmacol. 2025, 5, 6. [Google Scholar] [CrossRef]
- Goelzer Neto, C.F.; do Nascimento, P.; da Silveira, V.C.; de Mattos, A.B.N.; Bertol, C.D. Natural sources of melanogenic inhibitors: A systematic review. Int. J. Cosmet. Sci. 2022, 44, 143–153. [Google Scholar] [CrossRef]
- Zhou, S.; Zeng, H.; Huang, J.; Lei, L.; Tong, X.; Li, S.; Zhou, Y.; Guo, H.; Khan, M.; Luo, L.; et al. Epigenetic regulation of melanogenesis. Ageing Res. Rev. 2021, 69, 101349. [Google Scholar] [CrossRef] [PubMed]
- Gelmi, M.C.; Houtzagers, L.E.; Strub, T.; Krossa, I.; Jager, M.J. MITF in Normal Melanocytes, Cutaneous and Uveal Melanoma: A Delicate Balance. Int. J. Mol. Sci. 2022, 23, 6001. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, J.H.; Youn, K.; Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef] [PubMed]
- Drira, R.; Sakamoto, K. Sakuranetin Induces Melanogenesis in B16BL6 Melanoma Cells through Inhibition of ERK and PI3K/AKT Signaling Pathways. Phytother. Res. 2016, 30, 997–1002. [Google Scholar] [CrossRef]
- Kim, J.G.; Mahmud, S.; Min, J.K.; Lee, Y.B.; Kim, H.; Kang, D.C.; Park, H.S.; Seong, J.; Park, J.B. RhoA GTPase phosphorylated at tyrosine 42 by src kinase binds to β-catenin and contributes transcriptional regulation of vimentin upon Wnt3A. Redox Biol. 2021, 40, 101842. [Google Scholar] [CrossRef]
- Trejo-Solis, C.; Escamilla-Ramirez, A.; Jimenez-Farfan, D.; Castillo-Rodriguez, R.A.; Flores-Najera, A.; Cruz-Salgado, A. Crosstalk of the Wnt/β-Catenin Signaling Pathway in the Induction of Apoptosis on Cancer Cells. Pharmaceuticals 2021, 14, 871. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L. The potential role of ubiquitination and deubiquitination in melanogenesis. Exp. Dermatol. 2023, 32, 2062–2071. [Google Scholar] [CrossRef]
- Chowdhury, M.A.R.; Haq, M.M.; Lee, J.H.; Jeong, S. Multi-faceted regulation of CREB family transcription factors. Front. Mol. Neurosci. 2024, 17, 1408949. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhao, B.; Liu, Y.; Wang, R.; Yang, Y.; Yang, L.; Dong, C. MITF-M regulates melanogenesis in mouse melanocytes. J. Dermatol. Sci. 2018, 90, 253–262. [Google Scholar] [CrossRef]
- Bellei, B.; Maresca, V.; Flori, E.; Pitisci, A.; Larue, L.; Picardo, M. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J. Biol. Chem. 2010, 285, 7288–7299. [Google Scholar] [CrossRef]
- Fu, T.; Chai, B.; Shi, Y.; Dang, Y.; Ye, X. Fargesin inhibits melanin synthesis in murine malignant and immortalized melanocytes by regulating PKA/CREB and P38/MAPK signaling pathways. J. Dermatol. Sci. 2019, 94, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Hu, N.; Wang, H. Petanin Potentiated JNK Phosphorylation to Negatively Regulate the ERK/CREB/MITF Signaling Pathway for Anti-Melanogenesis in Zebrafish. Int. J. Mol. Sci. 2024, 25, 5939. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hong, A.R.; Kim, Y.H.; Yoo, H.; Kang, S.W.; Chang, S.E.; Song, Y. JNK suppresses melanogenesis by interfering with CREB-regulated transcription coactivator 3-dependent MITF expression. Theranostics 2020, 10, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Mosca, S.; Cardinali, G.; Flori, E.; Briganti, S.; Bottillo, I.; Mileo, A.M.; Maresca, V. The PI3K pathway induced by αMSH exerts a negative feedback on melanogenesis and contributes to the release of pigment. Pigment Cell Melanoma Res. 2021, 34, 72–88. [Google Scholar] [CrossRef]
- Zhou, S.; Sakamoto, K. Pyruvic acid/ethyl pyruvate inhibits melanogenesis in B16F10 melanoma cells through PI3K/AKT, GSK3β, and ROS-ERK signaling pathways. Genes Cells 2019, 24, 60–69. [Google Scholar] [CrossRef]
- Hermida, M.A.; Dinesh Kumar, J.; Leslie, N.R. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv. Biol. Regul. 2017, 65, 5–15. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.-Y.; Hyun, C.-G. Umckalin Promotes Melanogenesis in B16F10 Cells Through the Activation of Wnt/β-Catenin and MAPK Signaling Pathways. Appl. Biosci. 2025, 4, 20. https://doi.org/10.3390/applbiosci4020020
Oh S-Y, Hyun C-G. Umckalin Promotes Melanogenesis in B16F10 Cells Through the Activation of Wnt/β-Catenin and MAPK Signaling Pathways. Applied Biosciences. 2025; 4(2):20. https://doi.org/10.3390/applbiosci4020020
Chicago/Turabian StyleOh, So-Yeon, and Chang-Gu Hyun. 2025. "Umckalin Promotes Melanogenesis in B16F10 Cells Through the Activation of Wnt/β-Catenin and MAPK Signaling Pathways" Applied Biosciences 4, no. 2: 20. https://doi.org/10.3390/applbiosci4020020
APA StyleOh, S.-Y., & Hyun, C.-G. (2025). Umckalin Promotes Melanogenesis in B16F10 Cells Through the Activation of Wnt/β-Catenin and MAPK Signaling Pathways. Applied Biosciences, 4(2), 20. https://doi.org/10.3390/applbiosci4020020









