Abstract
Hematologic malignancies, including leukemia, lymphoma, and multiple myeloma, pose significant therapeutic challenges due to their heterogeneity and high relapse rates. Nanotechnology has emerged as a promising avenue for precision drug delivery in these malignancies, allowing for enhanced drug concentration at tumor sites and reducing systemic toxicity. Recent developments in nanocarriers—such as liposomes, polymeric nanoparticles, and inorganic nanoparticles—have enabled targeted approaches, utilizing molecular markers specific to malignant cells to increase therapeutic efficacy while minimizing adverse effects. Evidence from preclinical and clinical studies underscores the potential of nanotechnology to improve patient outcomes by facilitating controlled release, improved bioavailability, and reduced toxicity. However, translating these advancements into clinical practice requires further research to validate their safety and efficacy. This review provides a comprehensive analysis of the latest innovations in nanotechnology for targeted drug delivery in hematologic malignancies, addressing current achievements and future directions for integrating these approaches into Clinical Hemato-Oncology.
1. Introduction
Hematologic malignancies remain a significant global health challenge. Recent data underscore the global burden of these malignancies, with over one million new cases annually, contributing to a high morbidity and mortality rate [1,2,3]. These malignancies involve complex molecular pathways, often necessitating aggressive therapeutic interventions such as chemotherapy, radiation, and stem cell transplants. However, despite advancements in treatment, these conventional therapies are often limited by non-specific mechanisms of action, leading to significant off-target effects, toxicity, and sometimes therapy resistance [4,5,6,7].
The need for targeted therapeutic strategies in hematologic malignancies has driven a growing interest in precision medicine. Unlike traditional therapies, precision approaches allow for highly specific targeting of malignant cells while minimizing damage to healthy tissue, thus improving patient outcomes and quality of life [8,9,10]. The challenges of achieving precision are amplified in hematologic malignancies, where malignant cells circulate systemically, making selective targeting essential yet challenging [11]. Here, the role of nanotechnology is particularly promising, offering innovative ways to deliver drugs with high specificity, minimal toxicity, and enhanced therapeutic efficacy. This review aims to bridge the gap between theoretical advancements and clinical translation of nanotechnology for precision drug delivery in hematologic malignancies. By consolidating recent findings and emphasizing the clinical potential and limitations, this work highlights emerging strategies that offer unique advantages over existing reviews and specifically addresses the integration of nanotechnology with hematologic malignancies’ systemic characteristics, focusing on how targeted approaches overcome challenges associated with conventional therapies. Additionally, we provide insights into stimuli-responsive systems and combination therapies, offering a more focused outlook on clinical translation.
1.1. Precision Drug Delivery: Transforming Hematological Malignancies
Precision drug delivery aims to maximize therapeutic efficacy while reducing systemic toxicity by selectively targeting disease sites. In the context of hematologic malignancies, precision drug delivery has the potential to circumvent the issues associated with conventional therapies. By targeting drugs specifically to malignant cells, precision delivery minimizes collateral damage to healthy cells, particularly relevant for hematological malignancies that disseminate widely [12,13]. Nanotechnology plays a pivotal role in enhancing precision, enabling the design of sophisticated drug delivery systems capable of navigating the circulatory system to reach specific malignant cells [14,15,16].
Nanocarriers such as liposomes, polymeric micelles, dendrimers, and metal–organic frameworks allow for improved control over drug release, solubility, and bioavailability [17,18,19]. For instance, liposomal formulations of chemotherapeutic agents have demonstrated improved pharmacokinetics and reduced systemic toxicity in various types of hematologic malignancies, such as in pegylated liposomal doxorubicin for multiple myeloma [20,21,22,23,24]. Nanocarriers can also be functionalized with ligands that specifically recognize receptors on malignant cells, facilitating active targeting and further improving the precision of drug delivery [25,26]. Figure 1 highlights the four major categories of nanotherapeutic strategies for hematological malignancies, including chemonanotherapy, immunotherapy, cell-derived biomimetic nanoparticles, and targeted delivery systems.
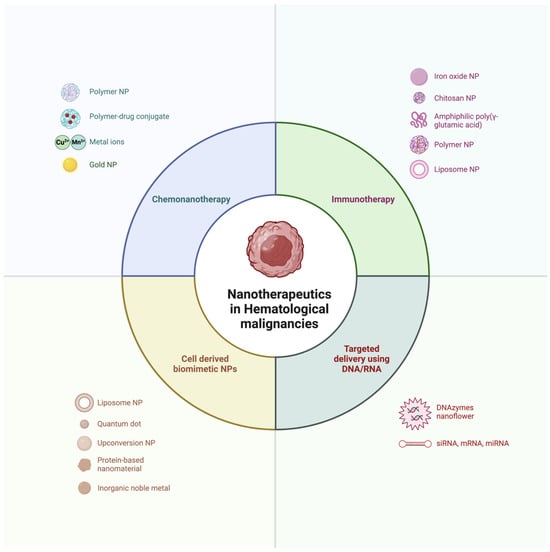
Figure 1.
Nanotherapeutic strategies in hematological malignancies, including categories such as chemonanotherapy and immunotherapy. (Created in BioRender. Mahmoud, A. (2025)).
The enhanced permeability and retention (EPR) effect is another advantage of nanocarriers, allowing them to accumulate selectively in tumor tissues due to the abnormal vasculature of tumors [27]. While this effect is often utilized in solid tumors, studies indicate that it can also be beneficial in treating hematologic malignancies by enabling targeted delivery within the bone marrow and lymphoid tissues where malignant cells proliferate [28,29]. Furthermore, recent developments in stimuli-responsive nanocarriers enable drug release in response to specific triggers in the tumor microenvironment, such as pH, redox potential, or enzymatic activity, further enhancing the precision of nanotechnology-based delivery systems [16,30,31,32,33].
1.2. Nanotechnology-Driven Advancements in Hematologic Malignancioes Treatment
Recent research in nanotechnology has expanded beyond passive targeting strategies to develop “smart” nanocarriers that release therapeutic agents in response to the unique microenvironment of hematologic malignancies [33,34]. For example, pH-sensitive nanocarriers can release drugs in the acidic conditions characteristic of the tumor microenvironment, providing a controlled release mechanism that limits systemic exposure and reduces toxicity [23,35]. In addition, enzyme-sensitive nanocarriers exploit the high expression of specific enzymes in certain cancers, such as matrix metalloproteinases in aggressive leukemias, to trigger the release of their therapeutic payload directly at the disease site [36,37].
One of the most exciting innovations in nanotechnology for hematologic malignancies is the development of multi-functional nanocarriers capable of co-delivering multiple therapeutic agents. These co-delivery systems allow for synergistic treatment approaches, where chemotherapeutic drugs can be combined with molecular inhibitors to target both cell proliferation and specific resistance pathways [38,39]. For example, liposomal formulations co-loaded with doxorubicin and paclitaxel have shown promise in preclinical models of lymphoma [40,41], enhancing treatment efficacy while reducing the side effects commonly associated with high-dose chemotherapy [42,43] (Figure 2).
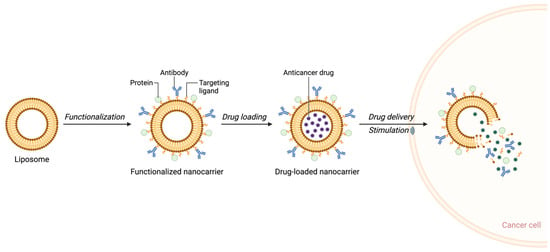
Figure 2.
Schematic representation of liposome-based nanocarrier functionalization and drug delivery to cancer cells. (Created in BioRender. Mahmoud, A. (2025)).
This review aims to comprehensively examine recent advancements in nanotechnology-based precision drug delivery for hematologic malignancies. Specifically, we focus on the mechanisms, design considerations, and therapeutic potential of various nanocarriers in targeting hematological malignancies. Additionally, this review discusses clinical applications and recent findings in leukemia, lymphoma, and multiple myeloma, highlighting the improvements in therapeutic outcomes associated with nanotechnology-enabled drug delivery. Challenges in translating these innovations to clinical practice, including scalability, regulatory approval, and cost considerations, will also be addressed.
Through this review, we seek to underscore the transformative potential of nanotechnology in hematological malignancies, offering a pathway toward more effective, personalized, and less toxic therapies that align with the broader goals of precision medicine. As illustrated in Figure 2, the drug delivery process begins with the functionalization of liposomes using bioactive molecules such as antibodies or surface proteins. This functionalization enhances the targeting of malignant cells, enabling precise drug delivery upon stimulation.
1.3. Current Landscape of Nanotechnology in Drug Delivery
Nanotechnology has significantly advanced the field of drug delivery, providing innovative approaches that address the inherent limitations of traditional pharmaceutical methods. Nanocarriers—nanoscale vehicles used to enhance the delivery of therapeutic agents—offer numerous advantages, including improved drug solubility [44,45,46], targeted drug delivery [47], controlled release [48,49], and reduced side effects [50,51], particularly in oncology [52,53,54]. By employing nanoscale engineering, these carriers can effectively improve the pharmacokinetics and bioavailability of drugs, making them a transformative tool in modern medicine [55,56,57,58].
1.4. Nanocarriers: Types and Mechanisms
Nanocarriers can be categorized into several types based on their composition and structure, including liposomes, polymeric nanoparticles, dendrimers, and metallic nanoparticles (Figure 3).
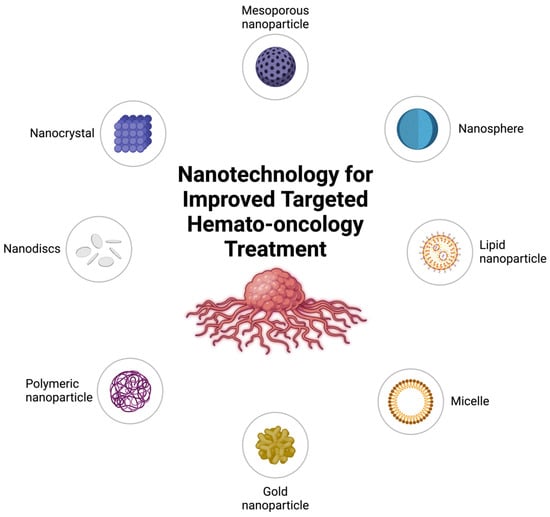
Figure 3.
Nanotechnology platforms for targeted hemato-oncology treatment. (Created in BioRender. Mahmoud, A. (2025)).
Liposomes are among the most well-established nanocarriers. These spherical vesicles are composed of one or more phospholipid bilayers, allowing them to encapsulate both hydrophilic and hydrophobic drugs. Liposomes enhance drug solubility, protect drugs from enzymatic degradation, and provide sustained release [55,57]. A notable example is Doxil®, a pegylated liposomal formulation of doxorubicin, which has demonstrated reduced toxicity and prolonged half-life compared to free doxorubicin [59,60,61].
Polymeric nanoparticles are constructed from biodegradable polymers, such as poly(lactic-co-glycolic acid) (PLGA). These nanoparticles are capable of encapsulating a variety of drugs and providing controlled, sustained release, thereby reducing dosing frequency and minimizing peak-related toxicities [62,63]. Polymeric nanoparticles are particularly effective in delivering chemotherapeutics, thereby enhancing efficacy while minimizing adverse effects on healthy tissues [64,65,66].
Dendrimers are highly branched, three-dimensional structures with numerous functional groups that allow for the attachment of multiple drugs. These carriers are well-suited for multifunctional applications, such as theranostics, where diagnostics and therapy are combined [67,68]. Metallic nanoparticles, particularly those made of gold and silver, have unique optical and thermal properties that are useful in drug delivery. Gold nanoparticles, for instance, can be used in combination with photothermal therapy to provide dual benefits for tumor treatment [69,70,71]. Figure 3 illustrates various nanotechnology platforms employed in hemato-oncological applications, such as lipid nanoparticles, polymeric micelles, gold nanoparticles, and nanodiscs.
The mechanisms by which nanocarriers deliver drugs often involve both passive and active targeting strategies. Passive targeting leverages the enhanced permeability and retention (EPR) effect, which enables nanoparticles to accumulate in tumor tissues due to the leaky vasculature and poor lymphatic drainage characteristic of tumors [72,73]. This approach is especially effective for solid tumors. Active targeting involves functionalizing nanocarriers with ligands such as antibodies, peptides, or small molecules that bind to receptors overexpressed on malignant cells, thereby increasing the specificity of drug delivery [74,75,76].
1.5. Advantages over Conventional Drug Delivery Methods
Nanocarriers offer several advantages over conventional drug delivery systems, particularly in terms of specificity, control, and therapeutic efficacy.
One major advantage is enhanced targeting specificity, which allows for the selective delivery of drugs to diseased tissues while sparing healthy cells. Traditional chemotherapy agents are distributed systemically, leading to significant side effects such as nausea, myelosuppression, and organ toxicity [77,78,79,80,81]. In contrast, nanocarriers can be engineered to release drugs specifically at the tumor site, thereby minimizing systemic exposure and reducing these adverse effects [82,83]. This specificity is especially critical in treating hematologic malignancies, where systemic circulation of malignant cells requires targeted approaches to minimize harm to normal cells [82,83,84].
Another key advantage is the controlled and sustained release of encapsulated drugs. Many anticancer drugs have narrow therapeutic windows, requiring precise dosing to maximize efficacy while minimizing toxicity. Nanocarriers can be designed to release drugs over an extended period, maintaining therapeutic concentrations and reducing the need for frequent dosing. For instance, PLGA-based nanoparticles have been used to deliver paclitaxel, extending the drug’s half-life and reducing toxicity compared to conventional formulations [85,86].
Nanocarriers also facilitate the co-delivery of multiple therapeutic agents, which is crucial in cancer treatment where combination therapy is often required. Co-delivery enables the simultaneous targeting of different pathways involved in tumor progression and resistance. For example, dendrimers and polymeric nanoparticles can encapsulate multiple agents within a single vehicle, delivering them in optimal ratios to the tumor site, enhancing their synergistic effects and overcoming multidrug resistance [87,88,89].
Furthermore, stimuli-responsive drug release is another advantage of nanocarrier-based systems. Nanocarriers can be designed to release their drug payload in response to specific environmental cues, such as pH changes, enzyme activity, or temperature variations. Tumor tissues often have an acidic microenvironment or overexpress specific enzymes, which can be exploited to achieve site-specific drug release. pH-sensitive liposomes, for instance, can release their encapsulated drugs in response to acidic conditions, thereby maximizing drug action at the target site while minimizing systemic exposure [74,90].
Finally, nanocarriers improve the pharmacokinetic properties of drugs, including solubility, stability, and circulation time [54]. Many anticancer drugs are hydrophobic and have poor solubility, limiting their clinical use [91]. Encapsulating these drugs in nanocarriers enhances their solubility and protects them from premature degradation or clearance. Surface modifications, such as pegylation, help nanocarriers evade immune detection, extending their circulation time and increasing the chances of reaching the target tissue [23,24]. This is particularly beneficial for treating hematologic malignancies, where widespread distribution is needed to target cancer cells in both the blood and bone marrow [90,92,93,94].
Nanotechnology-based drug delivery systems offer multiple advantages, enhancing the efficacy and safety of hematological malignancies treatments. Nanocarriers improve targeting specificity, allow for controlled drug release, facilitate the co-delivery of multiple agents, and enable stimuli-responsive drug release. These features are instrumental in modern hematoncology, contributing to better therapeutic outcomes and fewer side effects compared to traditional drug delivery methods. The subsequent sections will further explore the clinical applications and challenges associated with these technologies.
2. Applications in Hematologic Malignancies
Hematologic malignancies, which include leukemia, lymphoma, and multiple myeloma, present unique challenges for effective treatment due to their systemic nature and the involvement of the bone marrow and blood. Traditional chemotherapeutic approaches, while effective in some cases, often cause significant toxicity to healthy cells and have limited specificity for malignant cells. Nanocarriers provide a promising alternative by improving drug targeting, reducing toxicity, and enhancing the delivery of therapeutics to the affected areas [52].
2.1. Nanocarriers in Leukemia Treatment
Leukemia is characterized by the proliferation of malignant white blood cells, often leading to widespread involvement of the bone marrow and peripheral blood. Nanocarriers have been particularly effective in addressing the challenges associated with leukemia treatment. Liposomal formulations of chemotherapeutic agents such as cytarabine and daunorubicin have been developed to improve the delivery of these drugs to leukemic cells while minimizing systemic toxicity [55,95,96,97]. For instance, Vyxeos™ (a liposomal formulation of daunorubicin and cytarabine) has demonstrated improved overall survival in patients with newly diagnosed secondary acute myeloid leukemia (AML) compared to conventional chemotherapy [98].
Polymeric nanoparticles have also been explored for leukemia treatment. PLGA-based nanoparticles have been used to encapsulate drugs such as doxorubicin, allowing for controlled release and improved accumulation in leukemic cells [37]. Studies have shown that these nanoparticles can enhance the therapeutic efficacy of doxorubicin while reducing its cardiotoxicity [99], a major side effect associated with its use [100,101,102,103]. Furthermore, chitosan-based nanoparticles have been explored for the delivery of arsenic trioxide, which has demonstrated promising anti-leukemic activity in acute promyelocytic leukemia by improving drug solubility and reducing systemic toxicity [104,105,106,107].
2.2. Nanocarriers in Lymphoma
Lymphoma, which includes Hodgkin and non-Hodgkin lymphoma, affects the lymphatic system and can spread to other organs. Nanocarriers have been utilized to enhance the delivery of chemotherapeutic agents and monoclonal antibodies used in lymphoma treatment [108]. Liposomal formulations of doxorubicin, such as Doxil®, have been employed to treat relapsed or refractory lymphoma, demonstrating reduced cardiotoxicity and improved patient tolerance [57,109] (Figure 4).
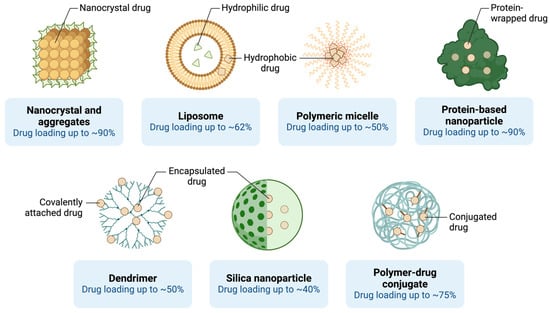
Figure 4.
Nanoparticle-based drug delivery platforms and their drug-loading efficiencies. (Created in BioRender. Mahmoud, A. (2025)).
Polymeric micelles have also shown potential in lymphoma therapy by encapsulating hydrophobic chemotherapeutic agents such as paclitaxel, allowing for improved solubility and targeted delivery to lymphoma cells [110,111,112]. These micelles have been conjugated with targeting ligands to improve specificity, thereby reducing the impact on healthy tissues and enhancing the therapeutic index of the treatment [113] (Figure 4).
Gold nanoparticles have also been investigated for their potential in lymphoma therapy. These nanoparticles can be conjugated with targeting ligands, such as antibodies against CD20—a surface marker overexpressed in B-cell lymphomas [41,114,115]. By specifically targeting lymphoma cells, gold nanoparticles can deliver chemotherapeutic agents directly to the tumor site, thereby enhancing efficacy and reducing off-target effects [116]. Additionally, gold nanoparticles have been explored for use in photothermal therapy, where they generate localized heat upon exposure to infrared light, selectively killing lymphoma cells [117]. Photothermal therapy, combined with traditional chemotherapy, has shown synergistic effects, providing an enhanced anti-tumor response and reducing the required dosage of chemotherapeutic agents [118,119,120,121,122,123]. Various nanoscale delivery platforms, including liposomes, polymeric micelles, and nanocrystals, are explored for their drug-loading capacities in lymphoma treatment (Figure 4).
2.3. Nanocarriers in Multiple Myeloma
Multiple myeloma is a hematologic malignancy characterized by the proliferation of malignant plasma cells within the bone marrow. Effective treatment of multiple myeloma is complicated by the involvement of the bone marrow microenvironment, which can protect malignant cells from conventional chemotherapeutic agents. Nanocarriers offer a means to overcome these challenges by enhancing drug penetration into the bone marrow and improving drug stability.
Bortezomib, a proteasome inhibitor commonly used in multiple myeloma treatment, has been encapsulated in liposomal formulations to enhance its delivery and reduce peripheral neuropathy, a common side effect [124]. Liposomal bortezomib has shown promising results in preclinical studies, with improved drug accumulation in the bone marrow and enhanced anti-myeloma activity [118,119,120,121,122,125]. Additionally, polymeric nanoparticles have been used to co-deliver bortezomib and dexamethasone, a corticosteroid that is often used in combination therapy for multiple myeloma [126]. This co-delivery approach has demonstrated synergistic effects, leading to enhanced apoptosis of myeloma cells and improved therapeutic outcomes [120,127,128].
Furthermore, dendrimer-based nanocarriers have been utilized for the delivery of thalidomide, another key drug used in multiple myeloma treatment [129]. Dendrimers allow for controlled drug release and improved targeting of the bone marrow, enhancing the drug’s efficacy while reducing its notorious side effects such as neurotoxicity [130].
2.4. Emerging Clinical Trials and Future Directions
Recent clinical trials have highlighted the potential of nanocarriers in the treatment of hematologic malignancies. For instance, a phase II clinical trial investigating the use of liposomal vincristine (Marqibo®) in patients with relapsed or refractory acute lymphoblastic leukemia (ALL) demonstrated significant clinical activity with an acceptable safety profile [131,132,133]. This suggests that liposomal formulations can improve the therapeutic index of chemotherapeutic agents, particularly in patients with limited treatment options.
Furthermore, targeted nanoparticles conjugated with monoclonal antibodies are being explored in clinical trials for various hematologic malignancies. Antibody-drug conjugates (ADCs), which consist of a cytotoxic drug linked to a monoclonal antibody, represent a promising approach for delivering potent chemotherapeutic agents directly to cancer cells. Nanoparticles can be used to improve the stability and delivery of these conjugates, thereby enhancing their efficacy and reducing systemic toxicity [134,135]. Examples include brentuximab vedotin, an ADC used for treating relapsed Hodgkin lymphoma, which has shown significant efficacy in clinical trials with reduced systemic side effects [136].
The future of nanocarrier-based therapies in hematologic malignancies lies in the development of personalized nanomedicines that can be tailored to the specific genetic and molecular characteristics of each patient’s cancer. Advances in nanotechnology, combined with insights from genomics and precision medicine, are expected to lead to more effective and less toxic treatment options for patients with hematologic malignancies. Artificial intelligence and machine learning are increasingly being applied to optimize nanomedicine design, accelerating the development of next-generation, patient-specific therapies and release profiles, thereby paving the way for next-generation nanomedicines [137,138].
3. Mechanisms of Precision Delivery via Nanotechnology
Nanotechnology-based drug delivery systems rely on precise mechanisms to achieve targeted therapeutic outcomes in cancer treatment. These mechanisms can be broadly categorized into passive targeting and active targeting, each with distinct strategies for directing drug-laden nanocarriers to malignant tissues. In addition to these targeting methods, stimuli-responsive nanocarriers represent an emerging approach that utilizes environmental cues to achieve site-specific drug release. Together, these mechanisms help optimize the efficacy and safety of treatment in hematologic malignancies, where conventional chemotherapies often face limitations in selectivity and systemic toxicity.
3.1. Controlled Drug Release and Passive Targeting Mechanisms
Passive targeting relies on the enhanced permeability and retention (EPR) effect, which is characteristic of tumor tissues. Tumors tend to have leaky vasculature due to the rapid and abnormal growth of blood vessels, coupled with poor lymphatic drainage. This phenomenon allows nanoscale drug carriers to accumulate preferentially at tumor sites. Nanocarriers typically range from 10 to 200 nanometers, making them ideally suited to pass through these leaky vessels and reach the tumor microenvironment [67] (Figure 5).
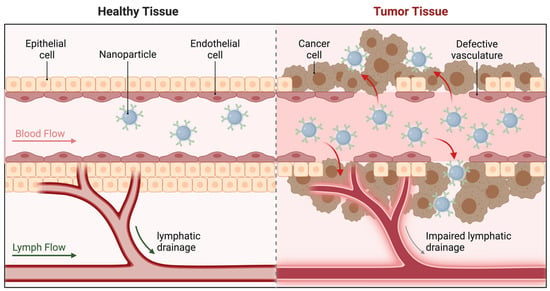
Figure 5.
Passive targeting of nanoparticles Via the enhanced permeability and retention (EPR) effect. (Created in BioRender. Mahmoud, A. (2025)).
In hematologic malignancies, passive targeting has been utilized to direct chemotherapeutic agents to the bone marrow, which is often affected by lymphomas and multiple myeloma. Liposomal formulations of drugs, such as Doxil®, leverage passive targeting to enhance drug accumulation in malignant tissues while reducing distribution to healthy cells, thereby minimizing side effects [40,57,74]. These nanocarriers typically enter cells through endocytosis mediated by lipid–protein interactions. After internalization, the liposomes are trafficked to acidic endosomes, where the low pH destabilizes the lipid bilayer, triggering drug release into the cytoplasm [53]. For instance, pegylated liposomes, which are coated with polyethylene glycol (PEG), evade immune recognition and prolong circulation times, further enhancing the opportunity for passive targeting [69]. As illustrated in Figure 5, passive targeting mechanisms exploit the EPR effect, which enables nanoparticles to accumulate selectively in tumor tissues due to their abnormal vasculature.
Passive targeting has also been leveraged to enhance the delivery of drugs in the treatment of lymphomas, where the abnormal vasculature of tumor masses provides an opportunity for preferential accumulation of nanocarriers [139]. This approach is particularly important in treating bulky lymphomas, where direct drug penetration is otherwise limited [41,140,141]. Despite the advantages of passive targeting, its reliance on the unique properties of the tumor vasculature can result in variable efficiency, as not all tumors exhibit equally leaky vasculature. In hematologic malignancies, where malignant cells circulate in the blood, passive targeting must be complemented by other mechanisms to ensure adequate drug delivery to the affected areas. For example, this approach has limited utility in leukemias, where malignant cells are systemically distributed. Although passive targeting offers some degree of selectivity, it lacks the specificity achieved through active targeting strategies.
3.2. Active Targeting Mechanisms
Active targeting involves the functionalization of nanocarriers with ligands that bind specifically to receptors overexpressed on cancer cells [142]. These ligands can be antibodies, peptides, aptamers, or small molecules that recognize and bind to unique surface markers of malignant cells. By enhancing the specificity of drug delivery, active targeting aims to further improve the therapeutic index of nanomedicines [23] (Figure 6).
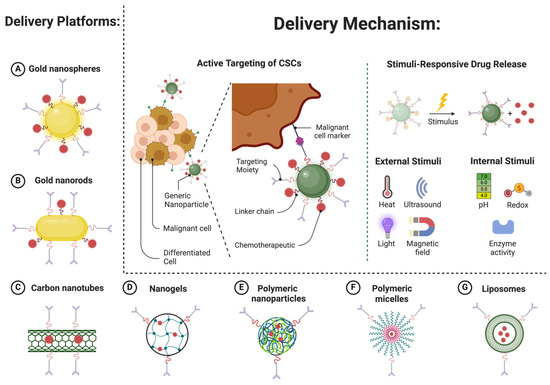
Figure 6.
Active targeting strategies using nanoparticle-based platforms for hematologic malignancies. (Created in BioRender. Mahmoud, A. (2025)).
In hematologic malignancies, specific surface markers such as CD19, CD20, and CD38 are overexpressed in various subtypes of leukemia, lymphoma, and multiple myeloma [143,144,145]. Nanocarriers conjugated with antibodies against these markers have demonstrated improved targeting efficiency. For example, gold nanoparticles conjugated with anti-CD20 antibodies have been shown to selectively target B-cell lymphoma cells, delivering cytotoxic agents directly to the site of action [116]. This targeted delivery reduces off-target toxicity and enhances the therapeutic outcome.
Another promising approach is the use of aptamer-functionalized nanoparticles. Aptamers are short, single-stranded nucleic acids that can bind to specific cellular targets with high affinity [146]. Aptamer-conjugated nanoparticles have been used to target CD30-positive Hodgkin lymphoma cells, achieving significant tumor reduction in preclinical models [147,148]. These targeted therapies show great promise in improving the precision and efficacy of treatment for hematologic malignancies. Figure 6 highlights active targeting mechanisms employed by nanoparticle platforms, emphasizing tumor-specific ligand functionalization and stimuli-responsive drug release.
Dual-targeting strategies are also being explored, where nanocarriers are functionalized with two or more targeting ligands to improve specificity and reduce the likelihood of drug resistance [17,149,150]. Such dual-targeting approaches are especially valuable in hematologic malignancies, in which tumor heterogeneity can limit the efficacy of single-targeted therapies.
3.3. Stimuli-Responsive Nanocarriers
Stimuli-responsive nanocarriers represent an advanced strategy for achieving site-specific drug release by responding to unique environmental cues present within the tumor microenvironment. These stimuli can be internal (e.g., pH, enzyme activity, or redox potential) or external (e.g., temperature, magnetic field, or light). The ability of these nanocarriers to release their payload in response to specific stimuli provides a higher level of control, allowing drugs to be activated only at the intended site of action, thereby minimizing systemic side effects [85,151,152].
In hematologic malignancies, the acidic microenvironment of the bone marrow and the overexpression of specific enzymes have been exploited to achieve targeted drug release. pH-responsive nanocarriers, such as those composed of polyhistidine or other pH-sensitive polymers, are designed to degrade or change conformation in response to the acidic pH of tumor tissues, thereby releasing their drug payload [153,154]. These nanoparticles are internalized via receptor-mediated endocytosis and accumulate in the acidic bone marrow microenvironment. Upon exposure to the low pH environment, the polymer matrix degrades, allowing the release of encapsulated agents such as bortezomib [119]. In multiple myeloma, pH-sensitive liposomes encapsulating bortezomib have demonstrated improved efficacy by selectively releasing the drug in the acidic bone marrow niche and drug bioavailability while minimizing peripheral neuropathy, a common side effect of bortezomib therapy [118,129].
Enzyme-responsive nanocarriers are designed to degrade in the presence of enzymes overexpressed by cancer cells or within the tumor microenvironment. For example, matrix metalloproteinases (MMPs) are often overexpressed in lymphoma and leukemia. Nanocarriers containing MMP-cleavable linkers release their therapeutic payload specifically in the vicinity of malignant cells, thereby enhancing drug delivery while sparing normal tissues [155]. Enzyme-responsive nanoparticles have been tested in preclinical models of acute myeloid leukemia (AML), showing promising results in terms of targeted drug release and reduced off-target toxicity [156].
Another innovative approach involves the use of redox-responsive nanocarriers, which release drugs in response to the high levels of glutathione found within cancer cells. These nanocarriers are stabilized by disulfide bonds that are cleaved in the reductive environment of cancer cells, resulting in the rapid release of the encapsulated drug. This mechanism has been used to enhance the delivery of doxorubicin to leukemia cells, showing significant tumor inhibition in preclinical studies [157,158].
External stimuli, such as magnetic fields and light, have also been explored for precision drug delivery. Magnetic nanoparticles, when subjected to an external magnetic field, can be directed to specific areas of the body, such as the bone marrow, which is often the site of hematologic malignancies. Once localized, these nanoparticles can release their therapeutic payload in response to heat generated by the magnetic field, providing a synergistic treatment effect through hyperthermia [159,160]. Magnetic hyperthermia combined with chemotherapeutic agents has been shown to enhance cell death in lymphoma models, demonstrating the potential of this dual-therapy approach [161].
Light-responsive nanocarriers, such as gold nanoshells, can be triggered by near-infrared light to release their drug payload. This approach has been tested in lymphoma models, where light irradiation led to the selective release of chemotherapeutic agents at the tumor site, reducing systemic toxicity and improving therapeutic efficacy [117]. Researchers are also exploring photoimmunotherapy, in which light-activated nanoparticles are used in combination with immune checkpoint inhibitors to boost the immune response against lymphoma cells [162,163,164].
The mechanisms of precision drug delivery via nanotechnology—including passive targeting, active targeting, and stimuli-responsive release—are transforming the treatment landscape for hematologic malignancies. These strategies enhance specificity and minimize systemic toxicity, thereby significantly improving patient outcomes. The upcoming sections will delve further into the clinical applications, ongoing trials, and future directions for nanotechnology-based treatments in leukemia, lymphoma, and multiple myeloma. By enhancing specificity and minimizing systemic toxicity, these strategies hold the potential to significantly improve patient outcomes. The development of multifunctional nanocarriers that combine multiple targeting strategies further advances the goal of achieving precise, personalized therapy for patients with leukemia, lymphoma, and multiple myeloma. Subsequent sections will explore clinical applications and challenges, as well as recent advances in nanomedicine for treating these malignancies.
3.4. Clinical Advances and Challenges in Nanotechnology for Hematologic Malignancies
The integration of nanotechnology in the clinical treatment of hematologic malignancies has seen significant advancements, marked by numerous clinical trials and real-world applications. While nanocarrier-based drug delivery systems offer substantial potential in terms of targeted drug delivery and improved therapeutic efficacy, they are also associated with challenges related to safety, toxicity, scalability, and regulatory compliance. Addressing these challenges is crucial for ensuring the successful translation of nanomedicine from bench to bedside. To provide a comprehensive overview of current nanomedicine strategies and their therapeutic applications in hematologic malignancies, we summarize key studies in Table 1. This table highlights the diversity of nanocarriers, their target malignancies, therapeutic agents, and the associated clinical or preclinical findings.

Table 1.
Key studies on nanocarrier-based therapies for hematologic malignancies.
3.5. Clinical Trials and Real-World Applications
Nanotechnology-based therapeutics have advanced from preclinical studies to clinical trials, showing promising results in safety and efficacy. Liposomal formulations, such as Doxil®, a pegylated liposomal doxorubicin, have been used successfully in the treatment of multiple hematologic malignancies, including relapsed/refractory multiple myeloma and non-Hodgkin lymphoma. Doxil® has demonstrated reduced cardiotoxicity compared to conventional doxorubicin, making it a safer alternative for patients who require anthracycline-based chemotherapy [53].
In acute myeloid leukemia (AML), the Phase III study evaluating Vyxeos™ (CPX-351), a liposomal formulation combining daunorubicin and cytarabine, has shown superior efficacy compared to the standard 7 + 3 regimen in elderly patients with newly diagnosed high-risk AML. The phase III clinical trial demonstrated a significant improvement in median overall survival (9.6 months vs. 5.9 months) compared to the traditional 7 + 3 regimen, leading to FDA approval in 2017 for specific AML subtypes [98]. Vyxeos™ represents a notable example of how nanotechnology can optimize drug ratios and delivery kinetics to improve therapeutic outcomes.
Another significant development is the use of nanoparticle albumin-bound paclitaxel (Abraxane®). Although initially developed for solid tumors, Abraxane® has shown potential in treating hematologic cancers due to its enhanced tumor delivery properties, facilitated by albumin’s natural affinity to tumor cells [182,183,184]. Moreover, the application of gold nanoparticles conjugated with antibodies targeting CD20 in B-cell lymphomas has shown promise in preclinical studies. These nanoparticle-antibody conjugates have demonstrated improved binding affinity and specificity, thereby enhancing the efficacy of rituximab, a monoclonal antibody therapy, in clinical trials [185,186].
Nanomedicines, such as Marqibo® (vincristine sulfate liposome injection), have been employed for the treatment of relapsed acute lymphoblastic leukemia (ALL). Marqibo® has shown prolonged drug exposure and improved efficacy in relapsed acute ALL in a Phase II clinical trial, which leverages liposomal encapsulation to enhance the pharmacokinetics of vincristine, resulting in prolonged drug circulation and increased efficacy [131,132,187,188]. Such developments underscore the growing role of nanotechnology in enhancing the therapeutic index of established chemotherapeutic agents, thereby providing better options for patients with limited treatment alternatives.
3.6. Safety, Toxicity, and Efficacy Concerns
While nanocarriers offer significant advantages for targeted drug delivery, their safety profile requires thorough evaluation. The toxicity of nanomaterials is influenced by factors such as size, shape, surface charge, and the materials used in their synthesis. Metallic nanoparticles, including gold and silver, have been associated with potential toxicity, including oxidative stress and inflammation, which can lead to organ damage [189,190]. The small size of nanoparticles allows them to cross biological barriers, potentially resulting in unintended interactions with healthy tissues. Clinical studies have shown that pegylated liposomal formulations, such as Doxil®, tend to exhibit reduced toxicity compared to free drugs, as the PEG coating helps evade immune detection and minimizes off-target effects. However, hypersensitivity reactions, such as complement activation-related pseudoallergy (CARPA), remain a concern with liposomal drugs [191].
The efficacy of nanocarrier-based treatments hinges on their ability to achieve specific targeting while maintaining adequate therapeutic concentrations at the tumor site. In hematologic malignancies, where malignant cells are often widely distributed, effective targeting can be challenging. Despite these hurdles, nanocarriers have demonstrated improved therapeutic efficacy by enhancing drug accumulation in the bone marrow and lymphoid tissues [57]. For instance, in the treatment of lymphoma, gold nanoparticle-conjugated monoclonal antibodies have shown increased tumor penetration and enhanced therapeutic effects when used in combination with chemotherapeutic agents [185].
3.7. Scalability, Manufacturing, and Regulatory Challenges
The scalability of nanomedicine production remains a significant challenge, as nanoparticle synthesis must be highly reproducible to ensure consistent drug loading, particle size, and surface characteristics—all critical factors for maintaining safety and efficacy. The production of nanocarriers, especially those with complex surface modifications for active targeting, requires precise control and adherence to high-quality manufacturing practices that can be difficult to achieve on a large scale [192]. Variability in the production process can lead to batch-to-batch inconsistencies, potentially affecting the therapeutic outcomes of nanocarrier-based drugs.
The cost of manufacturing nanomedicines is significantly higher than that of traditional small-molecule drugs. This is partly due to the sophisticated equipment, materials, and quality control processes required to produce nanoparticles with the necessary precision. Addressing these manufacturing and cost challenges is crucial for making nanomedicines more accessible to a broader patient population [193,194,195].
Regulatory approval of nanomedicines presents unique challenges due to the complexity of these therapeutic platforms. Regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have developed guidelines to address the specific characteristics of nanomedicines, including their pharmacokinetics, biodistribution, and toxicity. However, the lack of standardized testing protocols and the need for comprehensive safety assessments have slowed the approval process for many nanocarrier-based therapeutics [196,197]. Additionally, concerns about the long-term safety of nanomedicines, particularly the potential accumulation of nanoparticles in organs such as the liver and spleen, necessitate extensive preclinical and clinical evaluation [198,199]. Collaboration among regulatory bodies, academic researchers, and industry stakeholders will be essential to establish clear guidelines and accelerate the approval of nanomedicines.
3.8. Future Directions and Prospects in Nanotechnology for Hematologic Malignancies
The field of nanotechnology continues to evolve, and its application in the treatment of hematologic malignancies has opened new frontiers in personalized medicine, improved treatment efficacy, and reduction in adverse effects. However, several research gaps and challenges remain to be addressed in order to fully realize the potential clinical impact of nanotechnology.
The future of nanotechnology-based therapies for hematologic malignancies lies in personalized approaches and multifunctional nanocarriers. For example, combination therapies, in which nanocarriers deliver multiple therapeutic agents or are used in conjunction with other treatment modalities such as immunotherapy [200]. Nanocarriers designed to co-deliver chemotherapeutic drugs and immune modulators could potentially overcome resistance mechanisms and enhance antitumor responses [201]. Resistance to free drugs often arises due to mechanisms such as efflux pump overexpression (e.g., P-glycoprotein), metabolic enzyme activation, or alterations in drug target binding sites. Nanoparticles can help overcome these challenges in several ways. First, by encapsulating the drug, nanocarriers can evade efflux pumps, ensuring higher intracellular drug concentrations [91]. Second, sustained drug release from nanoparticles maintains therapeutic levels over longer periods, reducing the likelihood of resistance associated with subtherapeutic dosing [86]. Finally, functionalized nanoparticles can deliver drug combinations that target multiple pathways simultaneously, thereby reducing the emergence of resistant cancer cell clones [39].
Another area of interest is the development of biodegradable nanocarriers that can be safely metabolized and excreted by the body, reducing the risk of long-term toxicity. The use of natural biomaterials, such as lipids, proteins, and polysaccharides, in the synthesis of nanocarriers has shown promise in improving biocompatibility and safety profiles [202,203]. Furthermore, advancements in manufacturing processes, such as the adoption of continuous manufacturing, could enhance the scalability of nanomedicines, making these therapies more widely available and affordable.
3.9. Emerging Trends and Personalized Medicine
One of the most promising areas of development is the use of nanotechnology in personalized medicine, where treatments are tailored to the individual genetic and molecular profiles of each patient’s cancer. Nanocarriers can be customized to deliver drugs directly to the specific biomarkers expressed by malignant cells, significantly enhancing specificity and minimizing off-target effects. For instance, the use of aptamer-conjugated nanoparticles that specifically target CD19 or CD20 markers has shown promise in B-cell lymphomas, providing a more precise therapeutic approach [204,205,206].
Another emerging trend is the use of multifunctional nanocarriers capable of combining diagnostic and therapeutic capabilities, often referred to as “theranostics.” These nanocarriers are engineered to provide real-time monitoring of drug delivery and treatment response while simultaneously delivering therapeutic agents. For example, magnetic nanoparticles conjugated with chemotherapeutic agents can be used to monitor tumor regression through imaging techniques such as MRI, while also delivering the treatment directly to malignant cells [207]. This dual functionality holds significant promise for improving treatment outcomes in hematologic cancers, as it allows clinicians to tailor treatment regimens based on real-time data.
3.10. Research Gaps and Challenges
Despite the progress made in nanotechnology-based therapies, several research gaps need to be addressed to advance their clinical application in hematologic malignancies. One of the primary challenges is the heterogeneity of hematologic malignancies, which limits the effectiveness of targeted therapies. For example, the expression of specific surface markers, such as CD19 or CD20, can vary significantly among patients or even within subpopulations of malignant cells, leading to suboptimal therapeutic responses [208,209]. To overcome this, future research must focus on developing adaptive nanocarriers capable of targeting multiple markers or dynamically adjusting their targeting mechanisms based on the tumor environment.
Another critical area requiring further exploration is the tumor microenvironment in hematologic malignancies, such as the bone marrow niche in multiple myeloma or the lymphatic system in lymphomas. These environments play a significant role in promoting tumor growth and resistance to therapy. Nanoparticles designed to modulate the tumor microenvironment—for instance, by delivering agents that disrupt cell signaling pathways or alter immune cell behavior—could help enhance the efficacy of existing treatments and prevent relapse [210]. Understanding how nanoparticles interact with the tumor microenvironment will be crucial in developing more effective therapies.
3.11. Potential Clinical Impact and Future Directions
Emerging treatment modalities include immunotherapy and gene editing. Recent studies demonstrate the potential of nanoparticles to deliver immune checkpoint inhibitors, including anti-PD-1 and anti-CTLA-4 antibodies, resulting in enhanced immune responses in preclinical models of hematologic cancers [195,196]. In addition, recent studies have explored the use of CRISPR-Cas9 gene editing delivered via lipid nanoparticles to correct genetic mutations that drive cancer progression, offering new hope for patients with limited treatment options [211,212,213].
The integration of artificial intelligence (AI) and machine learning in nanomedicine design can be used to predict optimal drug loading, release profiles, and targeting ligands for nanoparticles, thereby enhancing their precision and effectiveness [214]. This computational approach could accelerate the development of next-generation nanomedicines that are tailored to individual patients’ needs, ultimately improving clinical outcomes.
Combination therapies involving nanocarriers are another avenue for enhancing treatment efficacy. For example, combining chemotherapy-loaded nanoparticles with CAR T-cell therapy has demonstrated enhanced tumor cell killing in preclinical models by promoting synergistic effects between the chemotherapeutic agents and immune cell activation [215,216]. Such combinations have the potential to overcome resistance mechanisms that have traditionally limited the success of monotherapies in hematologic malignancies.
Finally, addressing the regulatory and scalability challenges associated with nanomedicine production will be crucial for ensuring that these innovative treatments reach patients. Regulatory bodies need to establish standardized protocols for evaluating the safety and efficacy of nanomedicines, including their pharmacokinetics and long-term toxicity. Furthermore, improvements in manufacturing processes, such as continuous production techniques, will help lower production costs and ensure consistent quality, thereby making these advanced therapies more accessible to a wider patient population [217,218,219].
4. Conclusions
The advancements in nanotechnology for the treatment of hematologic malignancies hold significant promise in transforming clinical outcomes. Nanocarrier-based therapies, including liposomal formulations, gold nanoparticles, and multifunctional nanocarriers, have shown substantial improvements in targeted drug delivery, minimizing off-target toxicity, and enhancing treatment efficacy. The application of personalized nanomedicine has the potential to tailor therapies to individual patient profiles, thereby improving response rates and minimizing adverse effects. Among the various nanocarriers examined, liposomal formulations and polymeric nanoparticles have shown the most promising potential in clinical applications. Liposomes, particularly pegylated formulations such as Doxil®, have demonstrated improved bioavailability and reduced toxicity in hematologic malignancies. Polymeric nanoparticles, including PLGA-based systems, have excelled in delivering chemotherapeutic agents with controlled release and enhanced tumor accumulation. Additionally, the integration of theranostic nanocarriers has enabled simultaneous diagnosis and therapy, providing real-time monitoring that can significantly enhance treatment outcomes.
In terms of disease-specific applications, nanomedicine has made the most revolutionary impact in the treatment of AML and multiple myeloma. Liposomal formulations such as CPX-351 have demonstrated superior outcomes compared to conventional chemotherapy and maintain a fixed molar ratio that prevents resistance mechanisms often observed with free drug administration in AML. In multiple myeloma, nanoparticle systems have successfully reduced peripheral neuropathy while improving drug penetration in the bone marrow microenvironment.
Despite the numerous advancements, several research gaps and challenges remain. The heterogeneity of hematologic malignancies presents an obstacle to effective targeting, requiring adaptive strategies to improve precision. Addressing the tumor microenvironment and its role in drug resistance also remains a priority. Research efforts should continue to explore combination therapies involving nanocarriers, including their use alongside immunotherapy or gene-editing technologies like CRISPR-Cas9.
Regulatory and scalability challenges must be addressed to ensure that these novel therapies reach a broader patient population. Developing standardized protocols for evaluating nanomedicines and improving manufacturing processes are crucial steps in achieving this goal. Future research should focus on refining the production of biodegradable and adaptive nanocarriers that can better navigate the complex tumor microenvironment and effectively target multiple disease markers.
Comparing nanocarrier-based systems reveals that functionalized nanoparticles with active targeting ligands offer superior efficacy compared to passive targeting systems. However, challenges related to cost, scalability, and regulatory compliance remain significant hurdles to clinical translation. Continued research on multifunctional and biodegradable nanocarriers is essential for overcoming these barriers.
Future advancements in nanotechnology for hematologic malignancies will likely be driven by personalized medicine approaches and combination therapies. The integration of artificial intelligence and machine learning to optimize nanocarrier design and delivery is expected to accelerate clinical translation, offering more effective and less toxic treatment options for patients.
In conclusion, the potential impact of nanotechnology on clinical practice in the treatment of hematologic malignancies is profound. By improving the specificity, safety, and efficacy of cancer therapies, nanomedicine offers a promising path toward more personalized and effective treatments. As the field continues to evolve, collaborative efforts among researchers, clinicians, and regulatory bodies will be vital in overcoming existing challenges and realizing the full clinical potential of nanotechnology in oncology.
Author Contributions
A.M.M.: Conceptualization, Methodology, Investigation, Writing—Original Draft Preparation, Writing—Review and Editing, Supervision, Project Administration. C.D.: Conceptualization, Validation, Resources, Writing—Review and Editing, Visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by Department of Excellence—DIMET, Università del Piemonte Orientale, Novara, Italy; and AIL Novara VCO ODV, Italy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Malard, F.; Neri, P.; Bahlis, N.J.; Terpos, E.; Moukalled, N.; Hungria, V.T.M.; Manier, S.; Mohty, M. Multiple myeloma. Nat. Rev. Dis. Primers 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Nogami, A.; Sasaki, K. Therapeutic Advances in Immunotherapies for Hematological Malignancies. Int. J. Mol. Sci. 2022, 23, 11526. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y. Novel treatment strategies for hematological malignancies in the immunotherapy era. Int. J. Hematol. 2024, 120, 3–5. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Tang, L.; Huang, Z.; Mei, H.; Hu, Y. Immunotherapy in hematologic malignancies: Achievements, challenges and future prospects. Signal Transduct. Target. Ther. 2023, 8, 306. [Google Scholar] [CrossRef]
- Lica, J.J.; Pradhan, B.; Safi, K.; Jakóbkiewicz-Banecka, J.; Hellmann, A. Promising Therapeutic Strategies for Hematologic Malignancies: Innovations and Potential. Molecules 2024, 29, 4280. [Google Scholar] [CrossRef]
- Garg, A.; Nair, K.; Chavan, S.; Mukundan, M.; Kumar, P. Quality of life in adult patients with hematological malignancy- treading a road less travelled. Indian J. Hematol. Blood Transfus. 2024. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Megías-Vericat, J.E.; Martínez-Cuadrón, D.; Solana-Altabella, A.; Montesinos, P. Precision medicine in acute myeloid leukemia: Where are we now and what does the future hold? Expert. Rev. Hematol. 2020, 13, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H. Precision medicine in acute lymphoblastic leukemia. Front. Med. 2020, 14, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Gioacchino, M.D.; Tonacci, A.; Petrarca, C.; Gangemi, S. Nanomedicine for Immunotherapy Targeting Hematological Malignancies: Current Approaches and Perspective. Nanomaterials 2021, 11, 2792. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef]
- Amin, M.; Seynhaeve, A.L.B.; Sharifi, M.; Falahati, M.; Ten Hagen, T.L.M. Liposomal Drug Delivery Systems for Cancer Therapy: The Rotterdam Experience. Pharmaceutics 2022, 14, 2165. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Chen, J.; Hu, S.; Sun, M.; Shi, J.; Zhang, H.; Yu, H.; Yang, Z. Recent advances and clinical translation of liposomal delivery systems in cancer therapy. Eur. J. Pharm. Sci. 2024, 193, 106688. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Subhan, M.A.; Parveen, F.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P. Approaches to Improve EPR-Based Drug Delivery for Cancer Therapy and Diagnosis. J. Pers. Med. 2023, 13, 389. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Khawar, I.A.; Kim, J.H.; Kuh, H.J. Improving drug delivery to solid tumors: Priming the tumor microenvironment. J. Control Release 2015, 201, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ruchika; Dhritlahre, R.K.; Saneja, A. Recent advances in dual-ligand targeted nanocarriers for cancer therapy. Drug Discov. Today 2022, 27, 2288–2299. [Google Scholar] [CrossRef]
- Yan, S.; Na, J.; Liu, X.; Wu, P. Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy. Pharmaceutics 2024, 16, 248. [Google Scholar] [CrossRef]
- Sharifi, M.; Cho, W.C.; Ansariesfahani, A.; Tarharoudi, R.; Malekisarvar, H.; Sari, S.; Bloukh, S.H.; Edis, Z.; Amin, M.; Gleghorn, J.P.; et al. An Updated Review on EPR-Based Solid Tumor Targeting Nanocarriers for Cancer Treatment. Cancers 2022, 14, 2868. [Google Scholar] [CrossRef]
- Chen, H.-J.; Cheng, Y.-A.; Chen, Y.-T.; Li, C.-C.; Huang, B.-C.; Hong, S.-T.; Chen, I.J.; Ho, K.-W.; Chen, C.-Y.; Chen, F.-M.; et al. Targeting and internalizing PEGylated nanodrugs to enhance the therapeutic efficacy of hematologic malignancies by anti-PEG bispecific antibody (mPEG × CD20). Cancer Nanotechnol. 2023, 14, 78. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Targeted drug delivery in cancer therapy. Technol. Cancer Res. Treat. 2005, 4, 363–374. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Nteli, P.; Bajwa, D.E.; Politakis, D.; Michalopoulos, C.; Kefala-Narin, A.; Efstathopoulos, E.P.; Gazouli, M. Nanomedicine approaches for treatment of hematologic and oncologic malignancies. World J. Clin. Oncol. 2022, 13, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Qian, S.; Zheng, C.; Wu, Y.; Huang, H.; Wu, G.; Zhang, J. Targeted therapy for leukemia based on nanomaterials. Heliyon 2024, 10, e34951. [Google Scholar] [CrossRef]
- Salama, M.M.; Aborehab, N.M.; El Mahdy, N.M.; Zayed, A.; Ezzat, S.M. Nanotechnology in leukemia: Diagnosis, efficient-targeted drug delivery, and clinical trials. Eur. J. Med. Res. 2023, 28, 566. [Google Scholar] [CrossRef]
- Fumoto, S.; Nishida, K. Co-delivery Systems of Multiple Drugs Using Nanotechnology for Future Cancer Therapy. Chem. Pharm. Bull. 2020, 68, 603–612. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, P.; Chen, Y.; Sun, J.; Kong, F. Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. Int. J. Mol. Med. 2014, 34, 191–196. [Google Scholar] [CrossRef]
- Aloss, K.; Hamar, P. Recent Preclinical and Clinical Progress in Liposomal Doxorubicin. Pharmaceutics 2023, 15, 893. [Google Scholar] [CrossRef]
- Vinhas, R.; Mendes, R.; Fernandes, A.R.; Baptista, P.V. Nanoparticles-Emerging Potential for Managing Leukemia and Lymphoma. Front. Bioeng. Biotechnol. 2017, 5, 79. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, K.; Zhang, J.; Duan, X.; Sun, Q.; Men, K. Multifunctional nanoparticle for cancer therapy. MedComm 2023, 4, e187. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.; Bazad, N.; Mukherjee, R.; Himanshu; Gunjan; Leal, E.; Ahmad, S.; Kaur, K.; Raj, V.S.; Chang, C.-M.; et al. Enhanced drug delivery with nanocarriers: A comprehensive review of recent advances in breast cancer detection and treatment. Discov. Nano 2024, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef]
- Crintea, A.; Dutu, A.G.; Sovrea, A.; Constantin, A.-M.; Samasca, G.; Masalar, A.L.; Ifju, B.; Linga, E.; Neamti, L.; Tranca, R.A.; et al. Nanocarriers for Drug Delivery: An Overview with Emphasis on Vitamin D and K Transportation. Nanomaterials 2022, 12, 1376. [Google Scholar] [CrossRef]
- Cong, X.; Zhang, Z.; Li, H.; Yang, Y.-G.; Zhang, Y.; Sun, T. Nanocarriers for targeted drug delivery in the vascular system: Focus on endothelium. J. Nanobiotechnol. 2024, 22, 620. [Google Scholar] [CrossRef]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef]
- Kapalatiya, H.; Madav, Y.; Tambe, V.S.; Wairkar, S. Enzyme-responsive smart nanocarriers for targeted chemotherapy: An overview. Drug Deliv. Transl. Res. 2022, 12, 1293–1305. [Google Scholar] [CrossRef]
- Raikwar, S.; Vyas, S.; Sharma, R.; Mody, N.; Dubey, S.; Vyas, S.P. Nanocarrier-Based Combination Chemotherapy for Resistant Tumor: Development, Characterization, and Ex Vivo Cytotoxicity Assessment. AAPS PharmSciTech 2018, 19, 3839–3849. [Google Scholar] [CrossRef]
- Kumar, A.; Lunawat, A.K.; Kumar, A.; Sharma, T.; Islam, M.M.; Kahlon, M.S.; Mukherjee, D.; Narang, R.K.; Raikwar, S. Recent Trends in Nanocarrier-Based Drug Delivery System for Prostate Cancer. AAPS PharmSciTech 2024, 25, 55. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Fobian, S.-F.; Gurrieri, E.; Amin, M.; D’Agostino, V.G.; Falahati, M.; Zalba, S.; Debets, R.; Garrido, M.J.; Saeed, M.; et al. Lipid-based nanosystems: The next generation of cancer immune therapy. J. Hematol. Oncol. 2024, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Kaushik, A.; Khatib, Z.M.; Nair, M.; McGoron, A.J. Recalcitrant Issues and New Frontiers in Nano-Pharmacology. Front. Pharmacol. 2019, 10, 1369. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Norouzi, M.; Amerian, M.; Amerian, M.; Atyabi, F. Clinical applications of nanomedicine in cancer therapy. Drug Discov. Today 2020, 25, 107–125. [Google Scholar] [CrossRef]
- Prajapati, S.; Maurya, S.; Das, M.; Tilak, V.; Verma, K.; Dhakar, R.C. Dendrimers in Drug Delivery, Diagnosis and Therapy: Basics and Potential Applications. J. Drug Deliv. Ther. 2016, 6, 67–92. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhatt, G.; Kothiyal, P. Gold Nanoparticles synthesis, properties, and forthcoming applications: A review. Indian J. Pharm. Biol. Res. 2015, 3. [Google Scholar] [CrossRef]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications—A Review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef]
- Pisitsak, P.; Chamchoy, K.; Chinprateep, V.; Khobthong, W.; Chitichotpanya, P.; Ummartyotin, S. Synthesis of Gold Nanoparticles Using Tannin-Rich Extract and Coating onto Cotton Textiles for Catalytic Degradation of Congo Red. J. Nanotechnol. 2021, 2021, 6380283. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Blanco, M.D.; Teijón, C.; Olmo, R.M.; Teijón, J.M. Targeted Nanoparticles for Cancer Therapy. In Recent Advances in Novel Drug Carrier Systems; Ali Demir, S., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 9. [Google Scholar]
- Bhirde, A.A.; Chikkaveeraiah, B.V.; Srivatsan, A.; Niu, G.; Jin, A.J.; Kapoor, A.; Wang, Z.; Patel, S.; Patel, V.; Gorbach, A.M.; et al. Targeted therapeutic nanotubes influence the viscoelasticity of cancer cells to overcome drug resistance. ACS Nano 2014, 8, 4177–4189. [Google Scholar] [CrossRef]
- Aborode, A.T.; Oluwajoba, A.S.; Ibrahim, A.M.; Ahmad, S.; Mehta, A.; Osayawe, O.J.-K.; Oyebode, D.; Akinsola, O.; Osinuga, A.; Onifade, I.A.; et al. Nanomedicine in cancer therapy: Advancing precision treatments. Adv. Biomark. Sci. Technol. 2024, 6, 105–119. [Google Scholar] [CrossRef]
- Wu, X.; Xin, Y.; Zhang, H.; Quan, L.; Ao, Q. Biopolymer-Based Nanomedicine for Cancer Therapy: Opportunities and Challenges. Int. J. Nanomed. 2024, 19, 7415–7471. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S. Nanomedicine Magic Bullet for Human Cancer; IGI Global: Hershey, PA, USA, 2017. [Google Scholar] [CrossRef]
- Minru, G.; Venkatraman, K.; Venkatraman, S. The magic bullet as cancer therapeutic- has nanotechnology failed to find its mark? Prog. Biomed. Eng. 2020, 2, 042004. [Google Scholar] [CrossRef]
- Hani, U.; Gowda, B.H.J.; Haider, N.; Ramesh, K.; Paul, K.; Ashique, S.; Ahmed, M.G.; Narayana, S.; Mohanto, S.; Kesharwani, P. Nanoparticle-Based Approaches for Treatment of Hematological Malignancies: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 233. [Google Scholar] [CrossRef]
- Tiwari, H.; Rai, N.; Singh, S.; Gupta, P.; Verma, A.; Singh, A.K.; Kajal; Salvi, P.; Singh, S.K.; Gautam, V. Recent Advances in Nanomaterials-Based Targeted Drug Delivery for Preclinical Cancer Diagnosis and Therapeutics. Bioengineering 2023, 10, 760. [Google Scholar] [CrossRef]
- Samir, A.; Elgamal, B.M.; Gabr, H.; Sabaawy, H.E. Nanotechnology applications in hematological malignancies (Review). Oncol. Rep. 2015, 34, 1097–1105. [Google Scholar] [CrossRef]
- Xiao, X.; Teng, F.; Shi, C.; Chen, J.; Wu, S.; Wang, B.; Meng, X.; Essiet Imeh, A.; Li, W. Polymeric nanoparticles-Promising carriers for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1024143. [Google Scholar] [CrossRef]
- Yousefi Rizi, H.A.; Hoon Shin, D.; Yousefi Rizi, S. Polymeric Nanoparticles in Cancer Chemotherapy: A Narrative Review. Iran. J. Public Health 2022, 51, 226–239. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Dehaghi, H.M.; Ghaemi, A.; Maleki, H.; Yazdian, F.; Rahdar, A.; Pandey, S. Polymeric nanoparticles as delivery vehicles for targeted delivery of chemotherapy drug fludarabine to treat hematological cancers. Inorg. Chem. Commun. 2024, 167, 112819. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Han, Y.; Jiang, L.; Lu, S.; Wang, B.; Qian, W.; Zhu, M.; Huang, H.; Qian, P. Development and application of nanomaterials, nanotechnology and nanomedicine for treating hematological malignancies. J. Hematol. Oncol. 2023, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Alwattar, J.K.; Mneimneh, A.T.; Abla, K.K.; Mehanna, M.M.; Allam, A.N. Smart Stimuli-Responsive Liposomal Nanohybrid Systems: A Critical Review of Theranostic Behavior in Cancer. Pharmaceutics 2021, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K.; et al. Stimuli-responsive liposomal nanoformulations in cancer therapy: Pre-clinical & clinical approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar] [CrossRef]
- Lee, S.-M.; Nguyen, S.T. Smart Nanoscale Drug Delivery Platforms from Stimuli-Responsive Polymers and Liposomes. Macromolecules 2013, 46, 9169–9180. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef]
- Xu, M.; Han, X.; Xiong, H.; Gao, Y.; Xu, B.; Zhu, G.; Li, J. Cancer Nanomedicine: Emerging Strategies and Therapeutic Potentials. Molecules 2023, 28, 5145. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.J.; Lancet, J.E.; Kolitz, J.E.; Ritchie, E.K.; Roboz, G.J.; List, A.F.; Allen, S.L.; Asatiani, E.; Mayer, L.D.; Swenson, C.; et al. First-in-man study of CPX-351: A liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Tsukagoshi, S.; Sakurai, Y. Enhancement of the cancer chemotherapeutic effect of cytosine arabinoside entrapped in liposomes on mouse leukemia L-1210. Gan 1975, 66, 719–720. [Google Scholar] [PubMed]
- Mayhew, E.; Papahadjopoulos, D.; Rustum, Y.M.; Dave, C. Inhibition of tumor cell growth in vitro and in vivo by 1-beta-D-arabinofuranosylcytosine entrapped within phospholipid vesicles. Cancer Res. 1976, 36, 4406–4411. [Google Scholar]
- Lancet, J.E.; Uy, G.L.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; Bixby, D.L.; et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021, 8, e481–e491. [Google Scholar] [CrossRef]
- Drinković, N.; Beus, M.; Barbir, R.; Debeljak, Ž.; Tariba Lovaković, B.; Kalčec, N.; Ćurlin, M.; Bekavac, A.; Gorup, D.; Mamić, I.; et al. Novel PLGA-based nanoformulation decreases doxorubicin-induced cardiotoxicity. Nanoscale 2024, 16, 9412–9425. [Google Scholar] [CrossRef]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Biodegradable Nanoparticles for Drug Delivery and Targeting. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Cham, Switzerland, 2019; pp. 167–181. [Google Scholar] [CrossRef]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA-based nanoparticles for the treatment of cancer: Current strategies and perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Sönksen, M.; Kerl, K.; Bunzen, H. Current status and future prospects of nanomedicine for arsenic trioxide delivery to solid tumors. Med. Res. Rev. 2022, 42, 374–398. [Google Scholar] [CrossRef]
- Houshmand, M.; Garello, F.; Circosta, P.; Stefania, R.; Aime, S.; Saglio, G.; Giachino, C. Nanocarriers as Magic Bullets in the Treatment of Leukemia. Nanomaterials 2020, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Alimoghaddam, K. A review of arsenic trioxide and acute promyelocytic leukemia. Int. J. Hematol. Oncol. Stem Cell Res. 2014, 8, 44–54. [Google Scholar] [PubMed]
- Jiang, Y.; Shen, X.; Zhi, F.; Wen, Z.; Gao, Y.; Xu, J.; Yang, B.; Bai, Y. An overview of arsenic trioxide-involved combined treatment algorithms for leukemia: Basic concepts and clinical implications. Cell Death Discov. 2023, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; da Silva, P.B.; Eloy, J.O.; Chorilli, M. Nanocarriers for the Diagnosis and Treatment of Cancer. In Nanocarriers for Drug Delivery: Concepts and Applications; Eloy, J.O., Abriata, J.P., Marchetti, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 223–252. [Google Scholar] [CrossRef]
- Acuña Cruz, E.; Cannata Ortiz, J.; García-Noblejas, A.; Alegre, A.; Arranz Sáez, R. Liposomal Doxorubicin in Aggressive B Cell Lymphoma Has Similar Efficacy to the Conventional Formulation: Results from a Retrospective Cohort Study. Blood 2015, 126, 5106. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009, 100, 572–579. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric Micelles: Nanocarriers for Cancer-Targeted Drug Delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric micelles and cancer therapy: An ingenious multimodal tumor-targeted drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 135–163. [Google Scholar] [CrossRef]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.F.; Zhou, X. Lipid-based nanoparticles as drug delivery carriers for cancer therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef]
- Adamo, F.M.; De Falco, F.; Dorillo, E.; Sorcini, D.; Stella, A.; Esposito, A.; Arcaleni, R.; Rosati, E.; Sportoletti, P. Nanotechnology Advances in the Detection and Treatment of Lymphoid Malignancies. Int. J. Mol. Sci. 2024, 25, 9253. [Google Scholar] [CrossRef]
- Minai, L.; Yeheskely-Hayon, D.; Yelin, D. High levels of reactive oxygen species in gold nanoparticle-targeted cancer cells following femtosecond pulse irradiation. Sci. Rep. 2013, 3, 2146. [Google Scholar] [CrossRef]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kuo, J.C.; Huang, Y.; Hu, Y.; Deng, L.; Yung, B.C.; Zhao, X.; Zhang, Z.; Pan, J.; Ma, Y.; et al. Optimized Liposomal Delivery of Bortezomib for Advancing Treatment of Multiple Myeloma. Pharmaceutics 2023, 15, 2674. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.D.; Stefanick, J.F.; Schroeder, V.A.; Suckow, M.A.; Kiziltepe, T.; Bilgicer, B. Liposomal Bortezomib Nanoparticles via Boronic Ester Prodrug Formulation for Improved Therapeutic Efficacy in Vivo. J. Med. Chem. 2014, 57, 5282–5292. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, R.; Jiang, X.; Li, Z.; Zhang, B. Progress on the Application of Bortezomib and Bortezomib-Based Nanoformulations. Biomolecules 2021, 12, 51. [Google Scholar] [CrossRef]
- Federico, C.; Alhallak, K.; Sun, J.; Duncan, K.; Azab, F.; Sudlow, G.P.; de la Puente, P.; Muz, B.; Kapoor, V.; Zhang, L.; et al. Tumor microenvironment-targeted nanoparticles loaded with bortezomib and ROCK inhibitor improve efficacy in multiple myeloma. Nat. Commun. 2020, 11, 6037. [Google Scholar] [CrossRef]
- Cholujova, D.; Koklesova, L.; Lukacova Bujnakova, Z.; Dutkova, E.; Valuskova, Z.; Beblava, P.; Matisova, A.; Sedlak, J.; Jakubikova, J. In vitro and ex vivo anti-myeloma effects of nanocomposite As4S4/ZnS/Fe3O4. Sci. Rep. 2022, 12, 17961. [Google Scholar] [CrossRef]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Iconomou, G.; Kalofonos, H.P. Bortezomib-induced peripheral neuropathy in multiple myeloma: A comprehensive review of the literature. Blood 2008, 112, 1593–1599. [Google Scholar] [CrossRef]
- Boccadoro, M.; Morgan, G.; Cavenagh, J. Preclinical evaluation of the proteasome inhibitor bortezomib in cancer therapy. Cancer Cell Int. 2005, 5, 18. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, H.; Liu, H.; Ding, K.; Song, J.; Zhang, J.; Ding, D.; Fu, R. Advancements in drugs restructured with nanomedicines for multiple myeloma treatment. Sci. China Mater. 2024, 67, 3780–3795. [Google Scholar] [CrossRef]
- Zheleznyak, A.; Shokeen, M.; Achilefu, S. Nanotherapeutics for multiple myeloma. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2018, 10, e1526. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Xiong, S.; Liu, J.; Sun, S. Targeted Delivery Strategies for Multiple Myeloma and Their Adverse Drug Reactions. Pharmaceuticals 2024, 17, 832. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, Y.; Zhu, L.; You, L.; Tong, H.; Meng, H.; Sheng, J.; Jin, J. Harnessing Nanotechnology: Emerging Strategies for Multiple Myeloma Therapy. Biomolecules 2024, 14, 83. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimer nanotechnology for enhanced formulation and controlled delivery of resveratrol. Ann. N. Y. Acad. Sci. 2015, 1348, 134–140. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Silverman, J.A.; Reynolds, L.; Deitcher, S.R. Pharmacokinetics and pharmacodynamics of vincristine sulfate liposome injection (VSLI) in adults with acute lymphoblastic leukemia. J. Clin. Pharmacol. 2013, 53, 1139–1145. [Google Scholar] [CrossRef]
- Sasaki, K.; Jabbour, E.J.; Khouri, M.; Thomas, D.A.; Garcia-Manero, G.; Ravandi, F.; Borthakur, G.; Short, N.J.; Issa, G.C.; Kadia, T.; et al. Phase II Study of Hyper-Cmad with Liposomal Vincristine (Marqibo) for Patients with Newly Diagnosed Acute Lymphoblastic Leukemia (ALL). Blood 2017, 130, 2554. [Google Scholar] [CrossRef]
- Goli, N.; Bolla, P.K.; Talla, V. Antibody-drug conjugates (ADCs): Potent biopharmaceuticals to target solid and hematological cancers- an overview. J. Drug Deliv. Sci. Technol. 2018, 48, 106–117. [Google Scholar] [CrossRef]
- Gébleux, R.; Casi, G. Antibody-drug conjugates: Current status and future perspectives. Pharmacol. Ther. 2016, 167, 48–59. [Google Scholar] [CrossRef]
- Younes, A.; Bartlett, N.L.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.L.; Forero-Torres, A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef] [PubMed]
- Hristova-Panusheva, K.; Xenodochidis, C.; Georgieva, M.; Krasteva, N. Nanoparticle-Mediated Drug Delivery Systems for Precision Targeting in Oncology. Pharmaceuticals 2024, 17, 677. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Passive and Active Drug Targeting: Drug Delivery to Tumors as an Example. In Drug Delivery; Schäfer-Korting, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–53. [Google Scholar] [CrossRef]
- Etrych, T.; Braunova, A.; Zogala, D.; Lambert, L.; Renesova, N.; Klener, P. Targeted Drug Delivery and Theranostic Strategies in Malignant Lymphomas. Cancers 2022, 14, 626. [Google Scholar] [CrossRef]
- Jiang, Y.; Lin, W.; Zhu, L. Targeted Drug Delivery for the Treatment of Blood Cancers. Molecules 2022, 27, 1310. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Das, T.; Nandy, S.; Sahib, S.; Preetam, S.; Gopalakrishnan, A.V.; Dey, A. Ligand-based active targeting strategies for cancer theranostics. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3417–3441. [Google Scholar] [CrossRef]
- Wang, K.; Wei, G.; Liu, D. CD19: A biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 2012, 1, 36. [Google Scholar] [CrossRef]
- Horna, P.; Nowakowski, G.; Endell, J.; Boxhammer, R. Comparative Assessment of Surface CD19 and CD20 Expression on B-Cell Lymphomas from Clinical Biopsies: Implications for Targeted Therapies. Blood 2019, 134, 5345. [Google Scholar] [CrossRef]
- Morandi, F.; Horenstein, A.L.; Costa, F.; Giuliani, N.; Pistoia, V.; Malavasi, F. CD38: A Target for Immunotherapeutic Approaches in Multiple Myeloma. Front. Immunol. 2018, 9, 2722. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide aptamers: New tools for targeted cancer therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, X. Aptamer-based targeted therapy. Adv. Drug Deliv. Rev. 2018, 134, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, F.; Ahmari, A.; Shabani, S.; Sadeghi, B.; Fahimirad, S.; Fattahi, F. Aptamers as an approach to targeted cancer therapy. Cancer Cell Int. 2024, 24, 108. [Google Scholar] [CrossRef] [PubMed]
- Agiba, A.M.; Arreola-Ramírez, J.L.; Carbajal, V.; Segura-Medina, P. Light-Responsive and Dual-Targeting Liposomes: From Mechanisms to Targeting Strategies. Molecules 2024, 29, 636. [Google Scholar] [CrossRef]
- McCallion, C.; Peters, A.D.; Booth, A.; Rees-Unwin, K.; Adams, J.; Rahi, R.; Pluen, A.; Hutchinson, C.V.; Webb, S.J.; Burthem, J. Dual-action CXCR4-targeting liposomes in leukemia: Function blocking and drug delivery. Blood Adv. 2019, 3, 2069–2081. [Google Scholar] [CrossRef]
- Murugan, B.; Sagadevan, S.; Fatimah, I.; Oh, W.-C.; Motalib Hossain, M.A.; Johan, M.R. Smart stimuli-responsive nanocarriers for the cancer therapy—Nanomedicine. Nanotechnol. Rev. 2021, 10, 933–953. [Google Scholar] [CrossRef]
- Zhang, Q.; Kuang, G.; Li, W.; Wang, J.; Ren, H.; Zhao, Y. Stimuli-Responsive Gene Delivery Nanocarriers for Cancer Therapy. Nanomicro Lett. 2023, 15, 44. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2021, 18, 205–227. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Sullivan, H.L.; Liang, Y.; Worthington, K.; Luo, C.; Gianneschi, N.C.; Christman, K.L. Enzyme-Responsive Nanoparticles for the Targeted Delivery of an MMP Inhibitor to Acute Myocardial Infarction. Biomacromolecules 2023, 24, 4695–4704. [Google Scholar] [CrossRef]
- Abed, H.F.; Abuwatfa, W.H.; Husseini, G.A. Redox-Responsive Drug Delivery Systems: A Chemical Perspective. Nanomaterials 2022, 12, 3183. [Google Scholar] [CrossRef]
- Meng, X.; Shen, Y.; Zhao, H.; Lu, X.; Wang, Z.; Zhao, Y. Redox-manipulating nanocarriers for anticancer drug delivery: A systematic review. J. Nanobiotechnol. 2024, 22, 587. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Roland, F. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Fatima, H.; Charinpanitkul, T.; Kim, K.S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhou, F.; Doughty, A.; Hoover, A.R.; Nordquist, R.E.; Chen, W.R. Nanotechnology-based photoimmunological therapies for cancer. Cancer Lett. 2019, 442, 429–438. [Google Scholar] [CrossRef]
- Zhou, F.; Hasan, T.; Frochot, C.; Chen, W.R. Nanotechnology, photonics, and immunotherapy for cancer diagnostics and therapeutics. Nanophotonics 2021, 10, 2969–2971. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Jin, B.; Sun, T.; Sun, K.; Wang, S.; Fan, Z. Advances in smart nanotechnology-supported photodynamic therapy for cancer. Cell Death Discov. 2024, 10, 466. [Google Scholar] [CrossRef]
- Shakil, M.S.; Niloy, M.S.; Mahmud, K.M.; Kamal, M.A.; Islam, M.A. Theranostic Potentials of Gold Nanomaterials in Hematological Malignancies. Cancers 2022, 14, 3047. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, J.; Agola, J.; Croissant, J.; Wang, Z.; Shang, J.; Coker, E.; Motevalli, B.; Zimpel, A.; Wuttke, S.; et al. Metal-Organic Framework Nanoparticle-Assisted Cryopreservation of Red Blood Cells. J. Am. Chem. Soc. 2019, 141, 7789–7796. [Google Scholar] [CrossRef]
- Wang, R.; Gao, J.; Vijayalakshmi, M.; Tang, H.; Chen, K.; Reddy, C.V.; Kakarla, R.R.; Anjana, P.M.; Rezakazemi, M.; Cheolho, B.; et al. Metal–organic frameworks and their composites: Design, synthesis, properties, and energy storage applications. Chem. Eng. J. 2024, 496, 154294. [Google Scholar] [CrossRef]
- Vavassori, V.; Ferrari, S.; Beretta, S.; Asperti, C.; Albano, L.; Annoni, A.; Gaddoni, C.; Varesi, A.; Soldi, M.; Cuomo, A.; et al. Lipid nanoparticles allow efficient and harmless ex vivo gene editing of human hematopoietic cells. Blood 2023, 142, 812–826. [Google Scholar] [CrossRef]
- Su, K.; Shi, L.; Sheng, T.; Yan, X.; Lin, L.; Meng, C.; Wu, S.; Chen, Y.; Zhang, Y.; Wang, C.; et al. Reformulating lipid nanoparticles for organ-targeted mRNA accumulation and translation. Nat. Commun. 2024, 15, 5659. [Google Scholar] [CrossRef]
- Djayanti, K.; Maharjan, P.; Cho, K.H.; Jeong, S.; Kim, M.S.; Shin, M.C.; Min, K.A. Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. Int. J. Mol. Sci. 2023, 24, 6349. [Google Scholar] [CrossRef]
- Houshmand, F.; Schofield, J.; Moafi, Z. Electronic and structural properties of functionalized Silica nanoparticles: DFT and SCC DFTB calculation. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Rezigue, M.; Alsharedeh, R.H.; Obeid, M.A.; Mishra, V.; Serrano-Aroca, Á.; El-Tanani, M.; Tambuwala, M.M. Protein-based nanomaterials: A new tool for targeted drug delivery. Ther. Deliv. 2022, 13, 321–338. [Google Scholar] [CrossRef]
- Kaltbeitzel, J.; Wich, P.R. Protein-based Nanoparticles: From Drug Delivery to Imaging, Nanocatalysis and Protein Therapy. Angew. Chem. Int. Ed. 2023, 62, e202216097. [Google Scholar] [CrossRef]
- Thakare, V.S.; Das, M.; Jain, A.K.; Patil, S.; Jain, S. Carbon nanotubes in cancer theragnosis. Nanomedicine 2010, 5, 1277–1301. [Google Scholar] [CrossRef]
- Huang, J.Y.; Chen, S.; Wang, Z.Q.; Kempa, K.; Wang, Y.M.; Jo, S.H.; Chen, G.; Dresselhaus, M.S.; Ren, Z.F. Superplastic carbon nanotubes. Nature 2006, 439, 281. [Google Scholar] [CrossRef]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef]
- Ahmed, M.; Douek, M. The role of magnetic nanoparticles in the localization and treatment of breast cancer. Biomed. Res. Int. 2013, 2013, 281230. [Google Scholar] [CrossRef]
- Patel, A.; Patel, K.; Patel, V.; Rajput, M.S.; Patel, R.; Rajput, A. Nanocrystals: An emerging paradigm for cancer therapeutics. Future J. Pharm. Sci. 2024, 10, 4. [Google Scholar] [CrossRef]
- Straus, D.J.; Długosz-Danecka, M.; Connors, J.M.; Alekseev, S.; Illés, Á.; Picardi, M.; Lech-Maranda, E.; Feldman, T.; Smolewski, P.; Savage, K.J.; et al. Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON-1): 5-year update of an international, open-label, randomised, phase 3 trial. Lancet Haematol. 2021, 8, e410–e421. [Google Scholar] [CrossRef]
- Borchmann, P.; Ferdinandus, J.; Schneider, G.; Moccia, A.; Greil, R.; Hertzberg, M.; Schaub, V.; Hüttmann, A.; Keil, F.; Dierlamm, J.; et al. Assessing the efficacy and tolerability of PET-guided BrECADD versus eBEACOPP in advanced-stage, classical Hodgkin lymphoma (HD21): A randomised, multicentre, parallel, open-label, phase 3 trial. Lancet 2024, 404, 341–352. [Google Scholar] [CrossRef]
- Cucinotto, I.; Fiorillo, L.; Gualtieri, S.; Arbitrio, M.; Ciliberto, D.; Staropoli, N.; Grimaldi, A.; Luce, A.; Tassone, P.; Caraglia, M.; et al. Nanoparticle albumin bound Paclitaxel in the treatment of human cancer: Nanodelivery reaches prime-time? J. Drug Deliv. 2013, 2013, 905091. [Google Scholar] [CrossRef]
- Giordano, G.; Pancione, M.; Olivieri, N.; Parcesepe, P.; Velocci, M.; Di Raimo, T.; Coppola, L.; Toffoli, G.; D’Andrea, M.R. Nano albumin bound-paclitaxel in pancreatic cancer: Current evidences and future directions. World J. Gastroenterol. 2017, 23, 5875–5886. [Google Scholar] [CrossRef]
- Hassan, M.S.; Awasthi, N.; Ponna, S.; von Holzen, U. Nab-Paclitaxel in the Treatment of Gastrointestinal Cancers—Improvements in Clinical Efficacy and Safety. Biomedicines 2023, 11, 2000. [Google Scholar] [CrossRef]
- Rosi, N.L.; Giljohann, D.A.; Thaxton, C.S.; Lytton-Jean, A.K.R.; Han, M.S.; Mirkin, C.A. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science 2006, 312, 1027–1030. [Google Scholar] [CrossRef]
- Bavelaar, B.M.; Song, L.; Jackson, M.R.; Able, S.; Tietz, O.; Skaripa-Koukelli, I.; Waghorn, P.A.; Gill, M.R.; Carlisle, R.C.; Tarsounas, M.; et al. Oligonucleotide-Functionalized Gold Nanoparticles for Synchronous Telomerase Inhibition, Radiosensitization, and Delivery of Theranostic Radionuclides. Mol. Pharm. 2021, 18, 3820–3831. [Google Scholar] [CrossRef]
- Kaplan, L.D.; Deitcher, S.R.; Silverman, J.A.; Morgan, G. Phase II Study of Vincristine Sulfate Liposome Injection (Marqibo) and Rituximab for Patients With Relapsed and Refractory Diffuse Large B-Cell Lymphoma or Mantle Cell Lymphoma in Need of Palliative Therapy. Clin. Lymphoma Myeloma Leuk. 2014, 14, 37–42. [Google Scholar] [CrossRef]
- Silverman, J.A.; Aulitzky, W.E.; Lister, J.; Maness, L.; Schiller, G.J.; Seiter, K.; Smith, S.; Stock, W.; Yehuda, D.B.; Deitcher, S.R. Marqibo® (vincristine sulfate liposomes injection; VSLI) Optimizes the Dosing, Delivery, and Pharmacokinetic (PK) Profile of Vincristine Sulfate (VCR) In Adults with Relapsed and Refractory Acute Lymphoblastic Leukemia (ALL). Blood 2010, 116, 2142. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef]
- Szebeni, J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef]
- Hertig, J.B.; Shah, V.P.; Flühmann, B.; Mühlebach, S.; Stemer, G.; Surugue, J.; Moss, R.; Di Francesco, T. Tackling the challenges of nanomedicines: Are we ready? Am. J. Health Syst. Pharm. 2021, 78, 1047–1056. [Google Scholar] [CrossRef]
- Mühlebach, S. Regulatory challenges of nanomedicines and their follow-on versions: A generic or similar approach? Adv. Drug Deliv. Rev. 2018, 131, 122–131. [Google Scholar] [CrossRef]
- Md Anwar Nawaz, R.; Darul Raiyaan, G.I.; Sivakumar, K.; Arunachalam, K.D. Nanomedicine Regulation and Future Prospects. In Advances in Novel Formulations for Drug Delivery; Scrivener Publishing: Beverly, MA, USA, 2023; pp. 67–80. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Yu, J.; Dan, N.; Eslami, S.M.; Lu, X. State of the Art of Silica Nanoparticles: An Overview on Biodistribution and Preclinical Toxicity Studies. AAPS J. 2024, 26, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical translation of gold nanoparticles. Drug Deliv. Transl. Res. 2023, 13, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Y.; Zhao, D.; Zhao, W.; Wu, M.; Zhang, W.; Cui, Y.; Zhang, P.; Zhang, Z. Nanocarriers for intracellular co-delivery of proteins and small-molecule drugs for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 994655. [Google Scholar] [CrossRef] [PubMed]
- Rana, I.; Oh, J.; Baig, J.; Moon, J.H.; Son, S.; Nam, J. Nanocarriers for cancer nano-immunotherapy. Drug Deliv. Transl. Res. 2023, 13, 1936–1954. [Google Scholar] [CrossRef]
- Fadilah, N.I.; Isa, I.L.; Zaman, W.S.; Tabata, Y.; Fauzi, M.B. The Effect of Nanoparticle-Incorporated Natural-Based Biomaterials towards Cells on Activated Pathways: A Systematic Review. Polymers 2022, 14, 476. [Google Scholar] [CrossRef]
- Saikia, N. Inorganic-Based Nanoparticles and Biomaterials as Biocompatible Scaffolds for Regenerative Medicine and Tissue Engineering: Current Advances and Trends of Development. Inorganics 2024, 12, 292. [Google Scholar] [CrossRef]
- Wei, Z.; Zhou, Y.; Wang, R.; Wang, J.; Chen, Z. Aptamers as Smart Ligands for Targeted Drug Delivery in Cancer Therapy. Pharmaceutics 2022, 14, 2561. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, C.; Wang, Y.; Fang, X.; Zhang, X.; Chen, Z.; Tan, W. Aptamer-conjugated nanomaterials for specific cancer cell recognition and targeted cancer therapy. NPG Asia Mater. 2014, 6, e95. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamer-Functionalized Nanoparticles in Targeted Delivery and Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9123. [Google Scholar] [CrossRef]
- Tiwari, R.; Tiwari, G.; Parashar, P. Theranostics Applications of Functionalized Magnetic Nanoparticles. In Multifunctional And Targeted Theranostic Nanomedicines: Formulation, Design And Applications; Jain, K., Jain, N.K., Eds.; Springer Nature: Singapore, 2023; pp. 361–382. [Google Scholar] [CrossRef]
- Sermer, D.; Elavalakanar, P.; Abramson, J.S.; Palomba, M.L.; Salles, G.; Arnason, J. Targeting CD19 for diffuse large B cell lymphoma in the era of CARs: Other modes of transportation. Blood Rev. 2023, 57, 101002. [Google Scholar] [CrossRef]
- Marvin-Peek, J.; Savani, B.N.; Olalekan, O.O.; Dholaria, B. Challenges and Advances in Chimeric Antigen Receptor Therapy for Acute Myeloid Leukemia. Cancers 2022, 14, 497. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chu, B.; Wei, X.; Chen, Y.; Yang, Y.; Hu, D.; Huang, J.; Wang, F.; Chen, M.; Zheng, Y.; et al. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Multiple Myeloma Based on Bone Marrow Homing. Adv. Mater. 2022, 34, e2107883. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, P.; Yu, S.Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Hatit, M.Z.C.; Zhao, K.; Loughrey, D.; Lokugamage, M.P.; Peck, H.E.; Cid, A.D.; Muralidharan, A.; Kim, Y.; Santangelo, P.J.; et al. Piperazine-derived lipid nanoparticles deliver mRNA to immune cells in vivo. Nat. Commun. 2022, 13, 4766. [Google Scholar] [CrossRef]
- Duan, L.; Ouyang, K.; Xu, X.; Xu, L.; Wen, C.; Zhou, X.; Qin, Z.; Xu, Z.; Sun, W.; Liang, Y. Nanoparticle Delivery of CRISPR/Cas9 for Genome Editing. Front. Genet. 2021, 12, 673286. [Google Scholar] [CrossRef]
- Das, K.P.; J, C. Nanoparticles and convergence of artificial intelligence for targeted drug delivery for cancer therapy: Current progress and challenges. Front. Med. Technol. 2022, 4, 1067144. [Google Scholar] [CrossRef]
- Rodríguez-Criado, J.; Quiñonero, F.; Prados, J.; Melguizo, C. Nanoparticles Combining Gene Therapy and Chemotherapy as a Treatment for Gastrointestinal Tumors: A Systematic Review. Appl. Sci. 2024, 14, 7872. [Google Scholar] [CrossRef]
- Wang, A.X.; Ong, X.J.; D’Souza, C.; Neeson, P.J.; Zhu, J.J. Combining chemotherapy with CAR-T cell therapy in treating solid tumors. Front. Immunol. 2023, 14, 1140541. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; De la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef]
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.C.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).