AG1®, a Novel Synbiotic, Demonstrates Superior Mineral Bioaccessibility and Bioavailability Compared to a Tablet Multivitamin and Mineral Supplement Using an In Vitro Model of the Upper Gastrointestinal Tract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
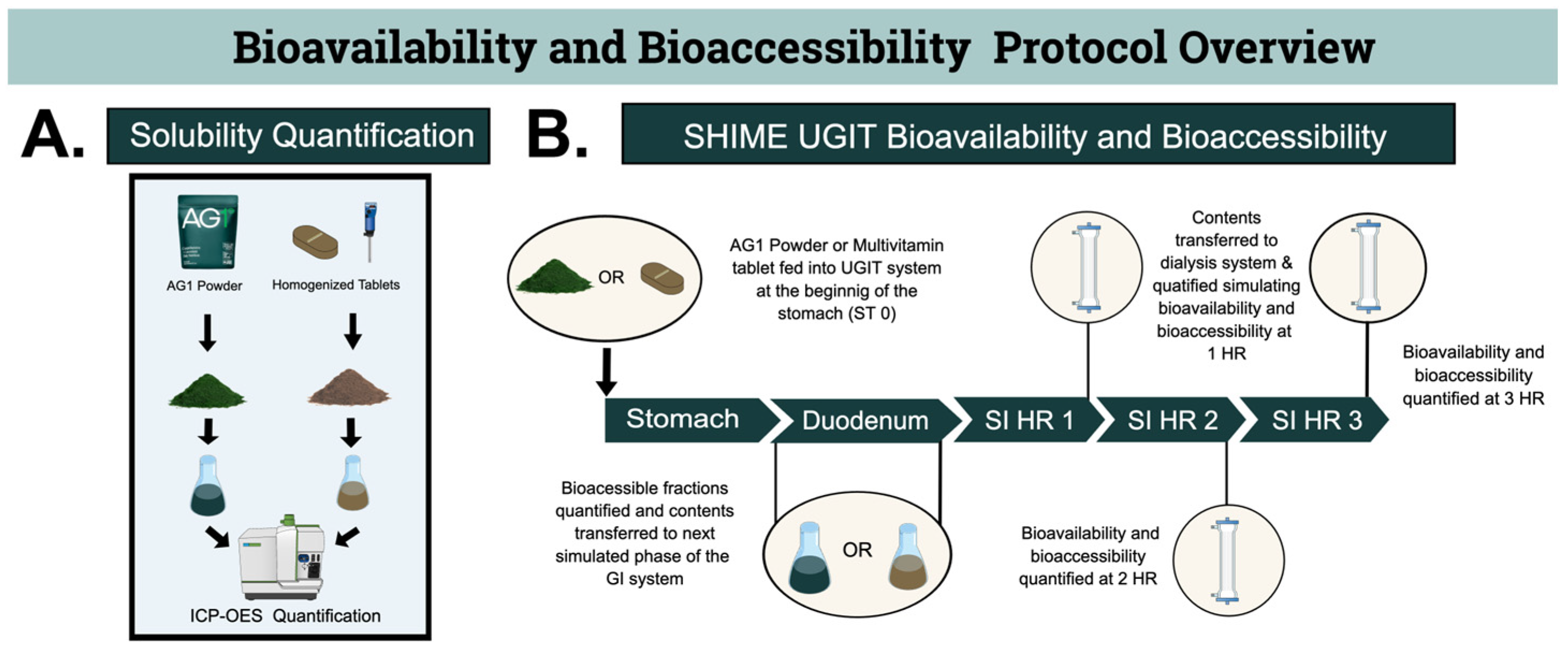
2.1.1. Solubility Quantification before the SHIME Model
2.1.2. SHIME UGIT Bioavailability and Bioaccessibility
2.2. Test Products
2.3. Determination of Initial Soluble Fraction, Subsequent Mineral Analysis, and Definitions
2.3.1. Determination of Absolute Initial Soluble Amounts and Fraction
2.3.2. Subsequent Mineral Analysis
2.3.3. Definitions
2.4. Test Gastrointestinal Tract System
2.4.1. Gastric Phase
2.4.2. Small Intestine Phase
2.5. Statistics
3. Results
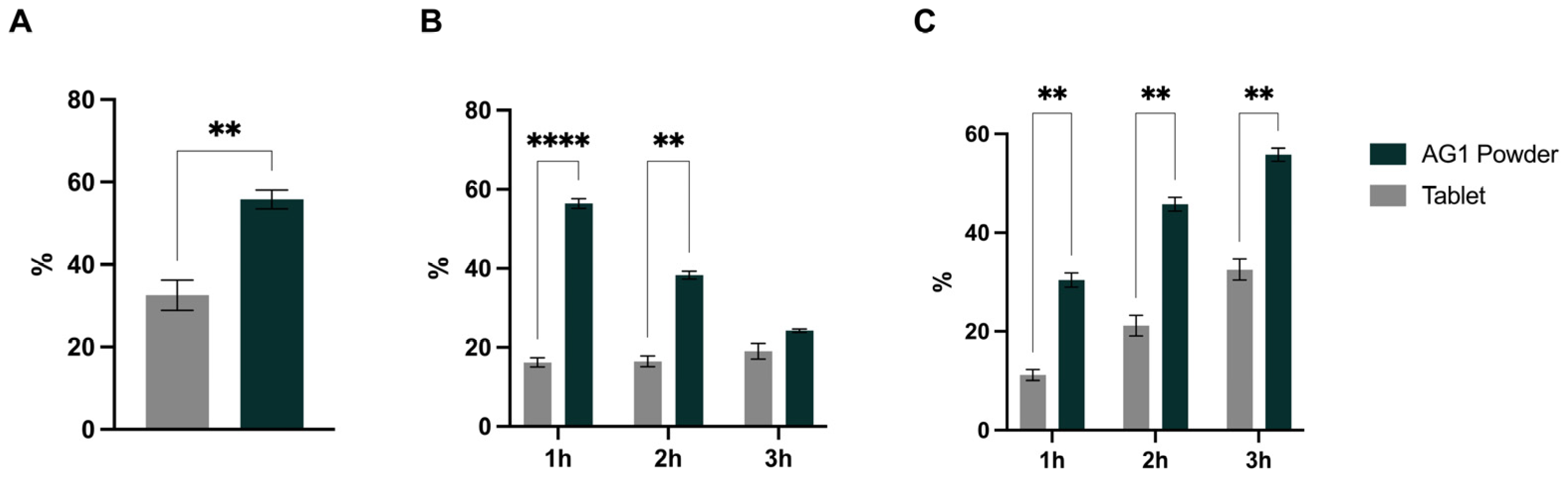
3.1. Mineral Amounts Present at Stomach and Duodenum End
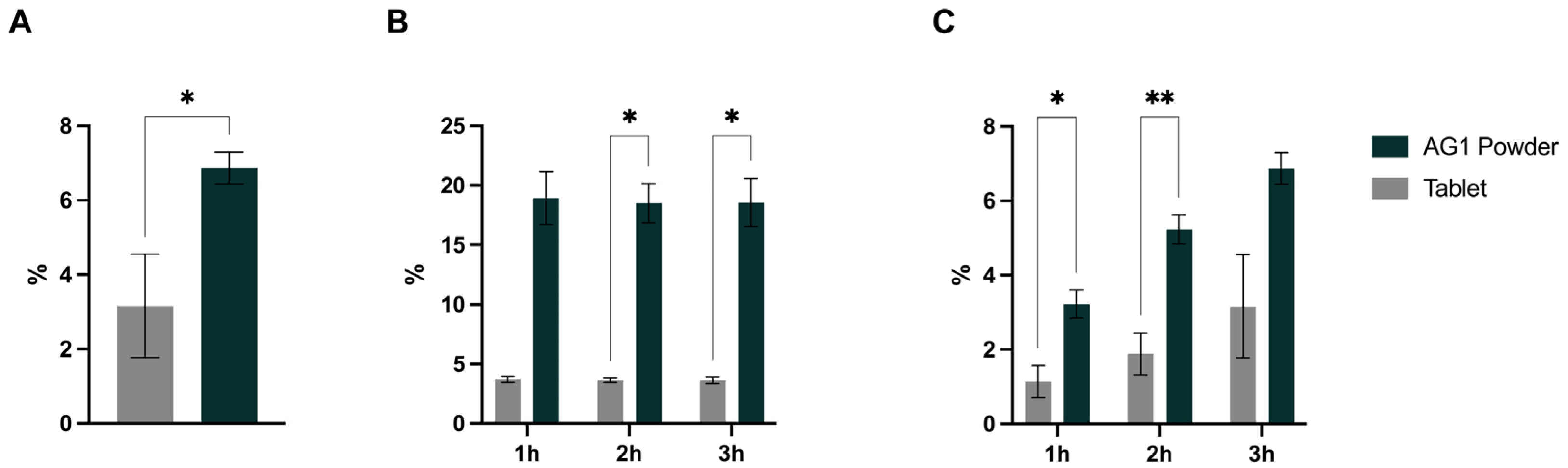
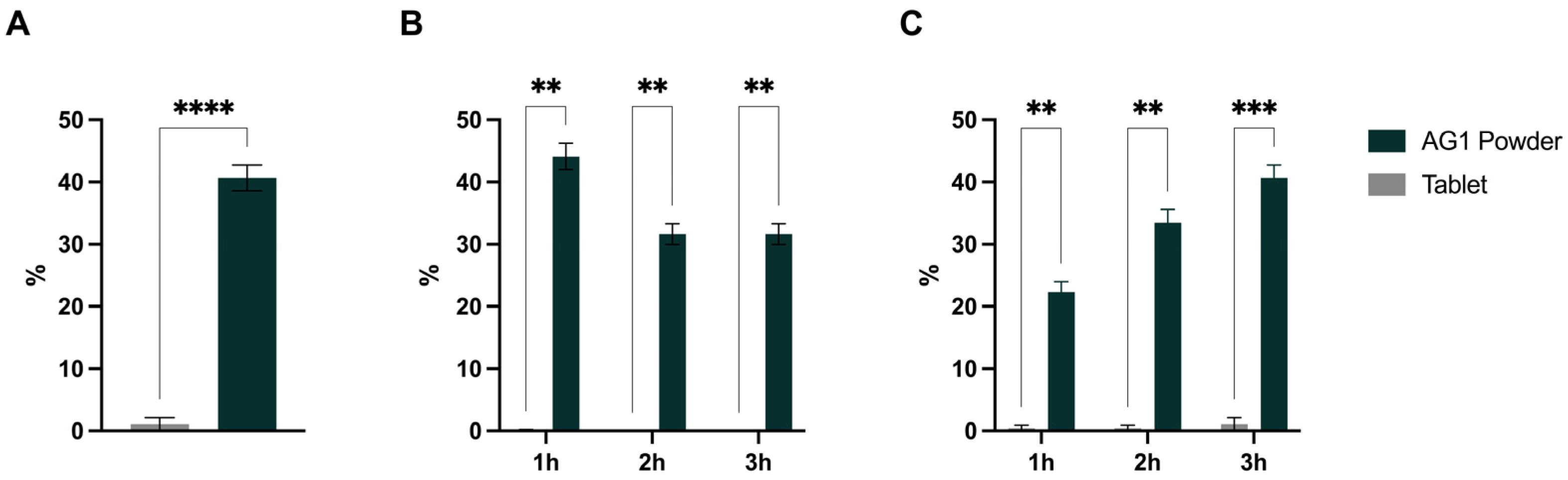
3.2. Bioaccessibility, Bioavailability, and Cmax
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US Burden of Disease Collaborators; Mokdad, A.H.; Ballestros, K.; Echko, M.; Glenn, S.; Olsen, H.E.; Mullany, E.; Lee, A.; Khan, A.R.; Ahmadi, A.; et al. The State of US Health, 1990–2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA 2018, 319, 1444–1472. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Pannucci, T.E.; Lerman, J.L.; Herrick, K.A.; Zimmer, M.; Meyers Mathieu, K.; Stoody, E.E.; Reedy, J. Healthy Eating Index-2020: Review and Update Process to Reflect the Dietary Guidelines for Americans, 2020–2025. J. Acad. Nutr. Diet. 2023, 123, 1280–1288. [Google Scholar] [CrossRef]
- USDA, Agricultural Research Service. Usual Nutrient Intake from Food and Beverages, by Gender and Age, What We Eat in America, NHANES 2017–March 2020 Prepandemic. 2023. Available online: http://www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 10 September 2023).
- Dickinson, A.; Blatman, J.; El-Dash, N.; Franco, J.C. Consumer Usage and Reasons for Using Dietary Supplements: Report of a Series of Surveys. J. Am. Coll. Nutr. 2014, 33, 176–182. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; U.S. Department of Health and Human Service. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2020. Available online: https://www.DietaryGuidelines.gov (accessed on 10 September 2023).
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Data Brief 399: Dietary Supplement Use among Adults: United States, 2017–2018; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [Google Scholar]
- Fact.MR. Powder Dietary Supplements Market by Ingredient (Vitamins, Botanicals, Proteins & Amino Acids, Fibers & Specialty Carbohydrates, Omega Fatty Acids), by Application (Bone & Joint Health, Immunity, Cardiac Health), by End User, by Region—Global Insights 2022–2032. 2022. Available online: https://www.factmr.com/report/48/powder-dietary-supplements-market (accessed on 10 September 2023).
- Wang, H.; Bua, P.; Capodice, J. A Comparative Study of Calcium Absorption Following a Single Serving Administration of Calcium Carbonate Powder versus Calcium Citrate Tablets in Healthy Premenopausal Women. Food Nutr. Res. 2014, 58. [Google Scholar] [CrossRef] [PubMed]
- Löbenberg, R.; Steinke, W. Investigation of Vitamin and Mineral Tablets and Capsules on the Canadian Market. J. Pharm. Pharm. Sci. 2006, 9, 40–49. [Google Scholar] [PubMed]
- Thakker, K.M.; Sitren, H.S.; Gregory, J.F.; Schmidt, G.L.; Baumgartner, T.G. Dosage Form and Formulation Effects on the Bioavailability of Vitamin E, Riboflavin, and Vitamin B-6 from Multivitamin Preparations. Am. J. Clin. Nutr. 1987, 45, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Oner, L.; Arcasoy, A.; Kas, H.S.; Hincal, A.A. Studies on Zinc Sulphate Microcapsules: (III) in Vivo Evaluation. Eur. J. Drug Metab. Pharmacokinet. 1989, 14, 107–110. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Wolkoff, B.I. Correlation between the Disintegration Time and the Bioavailability of Vitamin C Tablets. Pharm. Res. 1993, 10, 239–242. [Google Scholar] [CrossRef]
- Sakurada, T.; Oishi, D.; Shibagaki, Y.; Yasuda, T.; Kimura, K. Efficacy of Oral Powder Compared with Chewable Tablets for Lanthanum Carbonate Administration in Hemodialysis Patients. Hemodial. Int. 2013, 17, S2–S6. [Google Scholar] [CrossRef]
- Bende, G.; Biswal, S.; Bhad, P.; Chen, Y.; Salunke, A.; Winter, S.; Wagner, R.; Sunkara, G. Relative Bioavailability of Diclofenac Potassium from Softgel Capsule versus Powder for Oral Solution and Immediate-Release Tablet Formulation. Clin. Pharmacol. Drug Dev. 2016, 5, 76–82. [Google Scholar] [CrossRef]
- Navarro, M.; Wood, R.J. Plasma Changes in Micronutrients Following a Multivitamin and Mineral Supplement in Healthy Adults. J. Am. Coll. Nutr. 2003, 22, 124–132. [Google Scholar] [CrossRef]
- Blancquaert, L.; Vervaet, C.; Derave, W. Predicting and Testing Bioavailability of Magnesium Supplements. Nutrients 2019, 11, 1663. [Google Scholar] [CrossRef]
- AG1 Supplement Facts. Available online: https://drinkag1.com/ingredients/en (accessed on 24 October 2023).
- Travis, J.; Lattimore, L.G.; Harvey, M.; Frey, T. NSF International’s Role in the Dietary Supplements and Nutraceuticals Industries. In Nutraceutical and Functional Food Regulations in the United States and around the World; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–158. [Google Scholar]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Development of a 5-Step Multi-Chamber Reactor as a Simulation of the Human Intestinal Microbial Ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.; Rigby, N. InfoGest Consensus Method. In The Impact of Food Bioactives on Health; Springer International Publishing: Cham, Switzerland, 2015; pp. 13–22. [Google Scholar]
- Riethorst, D.; Mols, R.; Duchateau, G.; Tack, J.; Brouwers, J.; Augustijns, P. Characterization of Human Duodenal Fluids in Fasted and Fed State Conditions. J. Pharm. Sci. 2016, 105, 673–681. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Erdogan, A.; Rao, S.S.C. How to Assess Regional and Whole Gut Transit Time with Wireless Motility Capsule. J. Neurogastroenterol. Motil. 2014, 20, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Barker, J.; ElShaer, A. Pharmaceutical Excipients and Drug Metabolism: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 8224. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bhatia, D.; Dave, V.; Sutariya, V.; Varghese Gupta, S. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Molecules 2018, 23, 1719. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Wang, J.; Shi, G.; Chen, L. Influence of the Formula on the Properties of a Fast Dispersible Fruit Tablet Made from Mango, Chlorella, and Cactus Powder. Food Sci. Nutr. 2020, 8, 479–488. [Google Scholar] [CrossRef]
- CK-12 Foundation. 16.2: Rate of Dissolution. In Introductory, Conceptual, and GOB Chemistry; LibreTexts: Davis, CA, USA, 2023. [Google Scholar]
- Bergstrom, G. 2.4: Water Chemistry. In Cell and Molecular Biology (Bergstrom); LibreTexts: Davis, CA, USA, 2023. [Google Scholar]
- Molavi, F.; Hamishehkar, H.; Nokhodchi, A. Impact of Tablet Shape on Drug Dissolution Rate Through Immediate Released Tablets. Adv. Pharm. Bull. 2020, 10, 656–661. [Google Scholar] [CrossRef]
- Babu, V.R.; Areefulla, S.H.; Mallikarjun, V. Solubility and Dissolution Enhancement: An Overview. J. Pharm. Res. 2010, 3, 141–145. [Google Scholar]
- Yassin, S.; Goodwin, D.J.; Anderson, A.; Sibik, J.; Ian Wilson, D.; Gladden, L.F.; Axel Zeitler, J. The Disintegration Process in Microcrystalline Cellulose Based Tablets, Part 1: Influence of Temperature, Porosity and Superdisintegrants. J. Pharm. Sci. 2015, 104, 3440–3450. [Google Scholar] [CrossRef]
- Han, S.; Hong, J.; Luo, Q.; Xu, H.; Tan, H.; Wang, Q.; Tao, J.; Zhou, Y.; Peng, L.; He, Y.; et al. Hygroscopicity of Organic Compounds as a Function of Organic Functionality, Water Solubility, Molecular Weight and Oxidation Level. Atmos. Chem. Phys. Discuss. 2022, 22, 2004–3985. [Google Scholar] [CrossRef]
- Maclean, N.; Khadra, I.; Mann, J.; Williams, H.; Abbott, A.; Mead, H.; Markl, D. Investigating the Role of Excipients on the Physical Stability of Directly Compressed Tablets. Int. J. Pharm. X 2022, 4, 100106. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Wang, L.H.; Gordon, M.S.; Chowhan, Z.T. Effect of Formulation Solubility and Hygroscopicity on Disintegrant Efficiency in Tablets Prepared by Wet Granulation, in Terms of Dissolution. J. Pharm. Sci. 1991, 80, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Ekmekciyan, N.; Tuglu, T.; El-Saleh, F.; Muehlenfeld, C.; Stoyanov, E.; Quodbach, J. Competing for Water: A New Approach to Understand Disintegrant Performance. Int. J. Pharm. 2018, 548, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Baye, K.; Guyot, J.-P.; Mouquet-Rivier, C. The Unresolved Role of Dietary Fibers on Mineral Absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Botek, M.; McKune, A.J.; Sergi, D.; Georgousopoulou, E.; Mellor, D.D.; Naumovski, N. The Effects of Dietary Polyphenols on Circulating Cardiovascular Disease Biomarkers and Iron Status: A Systematic Review. Nutr. Metab. Insights 2019, 12, 1178638819882739. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut Microbiome-Micronutrient Interaction: The Key to Controlling the Bioavailability of Minerals and Vitamins? Biofactors 2022, 48, 307–314. [Google Scholar] [CrossRef]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef]

| AG1 Powder | Tablet | p-Value | |
|---|---|---|---|
| % soluble 1 | |||
| Magnesium | 79.37 ± 0.44 | 83.03 ± 1.20 | 0.0236 |
| Potassium | 100.4 ± 11.30 | 94.34 ± 1.84 | 0.4489 |
| Calcium | 93.32 ± 3.77 * | 36.06 ± 6.96 | 0.0010 |
| Zinc | 84.30 ± 0.39 * | 56.25 ± 2.65 | 0.0025 |
| AG1 Powder | Tablet 2 | p-Value | |
|---|---|---|---|
| % Bioaccessible at stomach end 1 | |||
| Magnesium | 102.2 ± 1.57 * | 9.3 ± 6.48 | 0.0010 |
| Potassium | 106.5 ± 18.8 * | 24.1 ± 15.9 | 0.0048 |
| Calcium | 100.1 ± 6.51 * | 0.7 ± 0.40 | 0.0014 |
| Zinc | 106.5 ± 3.91 * | 5.1 ± 3.57 | <0.0001 |
| % Bioaccessible at duodenum end 1 | |||
| Magnesium | 94.4 ± 2.57 * | 12.7 ± 7.61 | 0.0012 |
| Potassium | 108.2 ± 18.3 * | 35.2 ± 19.66 | 0.0094 |
| Calcium | 73.9 ± 0.11 * | 0.7 ± 0.62 | <0.0001 |
| Zinc | 25.9 ± 4.59 * | 3.7 ± 2.32 | 0.0052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapp, P.A.; Townsend, J.R.; Kirby, T.O.; Govaert, M.; Duysburgh, C.; Marzorati, M.; Marshall, T.M.; Esposito, R. AG1®, a Novel Synbiotic, Demonstrates Superior Mineral Bioaccessibility and Bioavailability Compared to a Tablet Multivitamin and Mineral Supplement Using an In Vitro Model of the Upper Gastrointestinal Tract. Appl. Biosci. 2023, 2, 656-667. https://doi.org/10.3390/applbiosci2040041
Sapp PA, Townsend JR, Kirby TO, Govaert M, Duysburgh C, Marzorati M, Marshall TM, Esposito R. AG1®, a Novel Synbiotic, Demonstrates Superior Mineral Bioaccessibility and Bioavailability Compared to a Tablet Multivitamin and Mineral Supplement Using an In Vitro Model of the Upper Gastrointestinal Tract. Applied Biosciences. 2023; 2(4):656-667. https://doi.org/10.3390/applbiosci2040041
Chicago/Turabian StyleSapp, Philip A., Jeremy R. Townsend, Trevor O. Kirby, Marlies Govaert, Cindy Duysburgh, Massimo Marzorati, Tess M. Marshall, and Ralph Esposito. 2023. "AG1®, a Novel Synbiotic, Demonstrates Superior Mineral Bioaccessibility and Bioavailability Compared to a Tablet Multivitamin and Mineral Supplement Using an In Vitro Model of the Upper Gastrointestinal Tract" Applied Biosciences 2, no. 4: 656-667. https://doi.org/10.3390/applbiosci2040041
APA StyleSapp, P. A., Townsend, J. R., Kirby, T. O., Govaert, M., Duysburgh, C., Marzorati, M., Marshall, T. M., & Esposito, R. (2023). AG1®, a Novel Synbiotic, Demonstrates Superior Mineral Bioaccessibility and Bioavailability Compared to a Tablet Multivitamin and Mineral Supplement Using an In Vitro Model of the Upper Gastrointestinal Tract. Applied Biosciences, 2(4), 656-667. https://doi.org/10.3390/applbiosci2040041







