Review of Partial Hybrids between Herbaceous Medicago sativa and Woody Medicago arborea and Their Potential Role in Alfalfa Improvement
Simple Summary
Abstract
1. Introduction
2. Phylogenetic Relationships between M. sativa subspp. and M. arborea
Molecular Phylogenies
3. Early Attempts to Hybridise M. sativa and M. arborea
4. Generation of Alborea and Its Characteristics
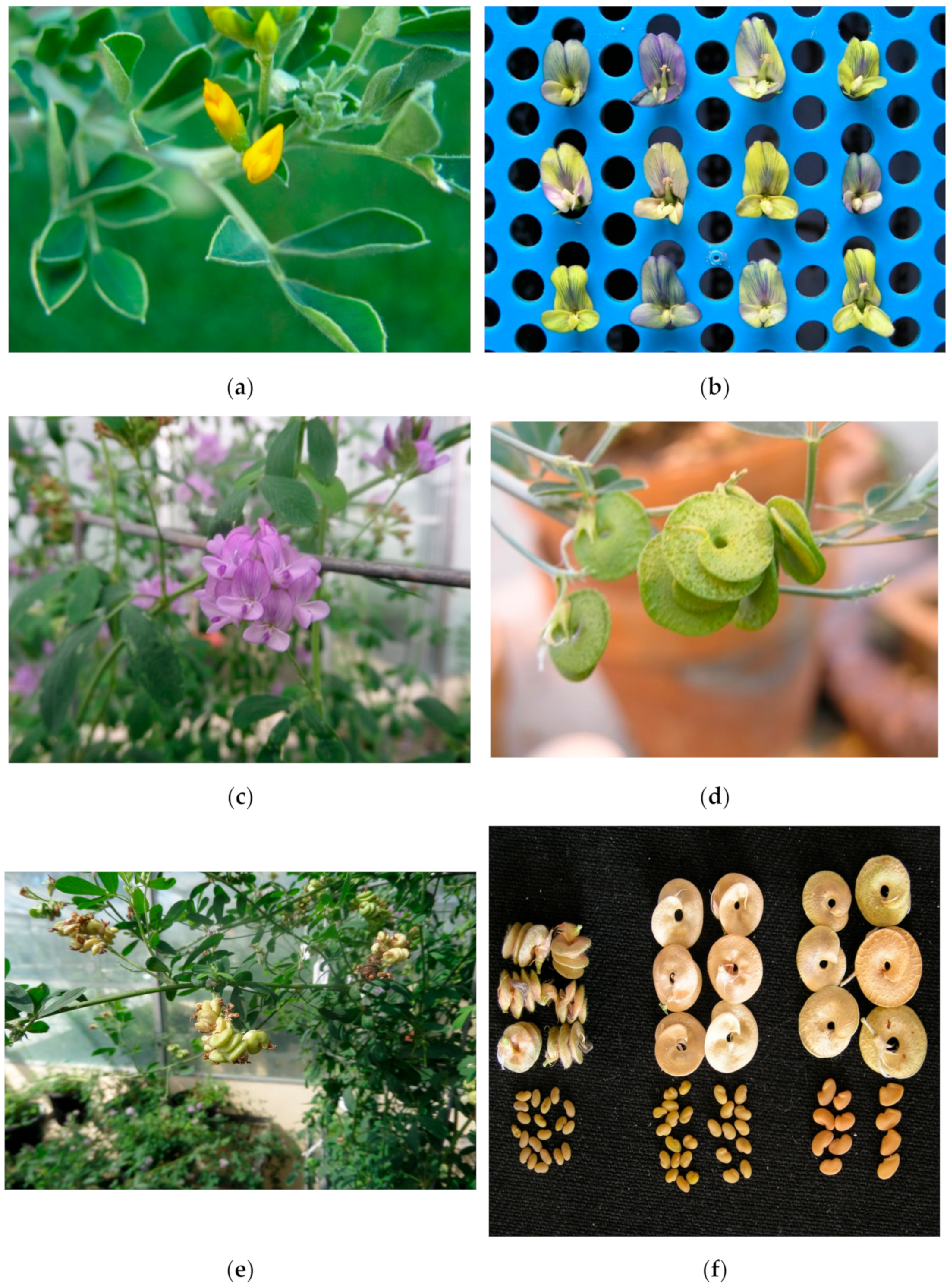
4.1. Morphological Characterization
4.2. Genomic Characterisation
5. Similarities between Alborea and Other Asymmetric Plant Hybrids
6. Importance of Reproductive Abnormalities in Generating Interspecific Hybrids
7. Use of Alborea in Alfalfa Breeding
8. The Future
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendry, G. Alfalfa in history. J. Am. Soc. Agron. 1923, 15, 171–174. [Google Scholar] [CrossRef]
- Michaud, R.; Lehman, W.; Rumbaugh, M. World distribution and development. In Alfalfa and Alfalfa Improvement; American Society of Agronomy: Madison, WI, USA, 1988; Volume 29, pp. 26–28. [Google Scholar]
- Small, E. Alfalfa and Relatives: Evolution and Classification of Medicago; NRC Research Press: Ottawa, ON, Canada, 2011; p. 727. [Google Scholar]
- Stanford, E. Tetrasomic inheritance in alfalfa. Agronomy 1951, 43, 222–225. [Google Scholar] [CrossRef]
- Tysdal, H.; Kiesselbach, T.; Westover, H. Alfalfa breeding. Nebraska Agric. Expt. Station Res Bull. 1942 Number 124. Available online: https://digitalcommons.unl.edu/ardhistrb/219/ (accessed on 30 June 2023).
- Holland, J.; Bingham, E. Genetic improvement for yield and fertility of alfalfa cultivars representing different eras of breeding. Crop Sci. 1994, 34, 953–957. [Google Scholar] [CrossRef]
- Lamb, J.; Shaeffer, C.; Rhodes, L.; Sule, R.; Undersander, D.; Brummer, E. Five decades of alfalfa cultivar improvement: Impact on forage yield, persistence, and nutritive value. Crop Sci. 2006, 46, 902–909. [Google Scholar] [CrossRef]
- Lowe, K.; Bowdler, T.; Casey, N.; Pepper, P. Evaluating temperate species in the subtropics. 3. Irrigated lucerne. Trop. Grassl. 2009, 44, 1–23. [Google Scholar]
- Sakiroglu, M.; Llhan, D. Medicago sativa species complex: Revisiting the century old problem in the light of molecular tools. Crop Sci. 2020, 61, 827–838. [Google Scholar] [CrossRef]
- Bingham, E.; Groose, R.; Woodfield, D.; Kidwell, K. Complementary gene interactions in alfalfa are greater in autotetraploids than in diploids. Crop Sci. 1994, 34, 823–829. [Google Scholar] [CrossRef]
- Nenz, E.; Pupilli, F.; Damiani, F.; Arcioni, S. Somatic hybrid plants between the forage legumes Medicago sativa L. and Medicago arborea L. Theor. Appl. Genet. 1994, 93, 183–189. [Google Scholar] [CrossRef]
- Urban, I. Prodromus einer Monographie der Gattung. Medicago L.; Kommissions-Verlag von R. Gaertner: Brandenb, Germany, 1873; Volume 15, pp. 1–85. [Google Scholar]
- Small, E.; Jomphe, M. A synopsis of the genus Medicago (Leguminosae). Can. J. Bot. 1988, 67, 3260–3294. [Google Scholar] [CrossRef]
- Steele, K.; Ickert-Bond, S.; Zarre, S.; Wojciechowski, M. Phylogeny and character evolution in Medicago (Leguminosae): Evidence from analyses of plastid TRN/MatK and nuclear GA 30x1 sequences. Am. J. Bot. 2010, 97, 1142–1155. [Google Scholar] [CrossRef]
- Fridriksson, S.; Bolton, J. Development of the embryo of Medicago sativa L. after normal fertilisation and after pollination by other species of Medicago. Can. J. Bot. 1963, 41, 23–33. [Google Scholar] [CrossRef]
- Lesins, K.; Lesins, I. Genus Medicago (Leguminosae), a Taxonomic Study; Dr W Jurk: The Hague, The Netherlands, 1979. [Google Scholar]
- McCoy, T.; Smith, L. Interspecific hybrids of perennial Medicago species using ovule-embryo culture. Theor. Appl. Genet. 1986, 71, 772–783. [Google Scholar] [CrossRef] [PubMed]
- McCoy, T.; Quarisa, G. Allotetraploid behaviour of hybrids of Medicago sativa L. and Medicago papillosa. Bioss Genome 1989, 32, 6–11. [Google Scholar] [CrossRef]
- Mizukami, Y.; Kato, M.; Takamizo, T.; Kanbe, M.; Inami, S.; Hattori, K. Interspecific hybrids between Medicago sativa L. and annual Medicago containing Alfalfa weevil resistance. Plant Cell Tissue Organ Cult. 2006, 84, 80–89. [Google Scholar] [CrossRef]
- McCoy, T.; Echt, C. Potential of trispecies bridge crosses and random amplified polymorphic DNA markers for introgression of Medicago daghestanica and M. pironae germplasm into alfalfa (M. sativa). Genome 1993, 36, 594–601. [Google Scholar] [CrossRef]
- Bingham, E.; Armour, D.; Irwin, J. The hybridization barrier between herbaceous Medicago sativa and woody M. arborea is weakened by selection of seed parents. Plants 2013, 2, 343–353. [Google Scholar] [CrossRef]
- Bingham, E.; Irwin, J. “Alborea”: A new cultigen developed from hybrids of alfalfa X M. arborea. Medicago Genetic Reports. 2014. Available online: www.medicago-reports.org (accessed on 30 June 2023).
- Bingham, E.; Irwin, J. The hybridization barrier between herbaceous Medicago sativa and woody M. arborea is weakened by reproductive abnormalities in M. sativa seed parents. Plants 2023, 12, 962. [Google Scholar] [CrossRef]
- Irwin, J.; Sewell, J.; Woodfield, D.; Bingham, E. Restructuring lucerne (Medicago sativa) through introgression of the Medicago arborea genome. Agric. Sci. 2016, 28, 40–47. [Google Scholar]
- Humphries, A.; Ovalle, C.; Hughes, S.; del Pozo, A.; Inostroza, L.; Barahona, V.; Yu, L.; Yerzhanova, S.; Rowe, T.; Hill, J.; et al. Characterization, preliminary evaluation and pre-breeding of diverse alfalfa crop wild relatives originating from drought-stressed environments. Crop Sci. 2020, 61, 69–88. [Google Scholar] [CrossRef]
- Inostroza, L.; Espinoza, S.; Barahona, V.; Gerding, M.; Humphries, A.; del Pozo, A.; Ovalle, C. Phenotypic diversity and productivity of Medicago sativa subspecies from drought-prone environments in Mediterranean type climates. Plants 2021, 10, 862. [Google Scholar] [CrossRef]
- Armour, D.; Mackie, J.; Musial, J.; Irwin, J. Transfer of anthracnose resistance and pod coiling traits from Medicago arborea to Medicago sativa by sexual reproduction. Theor. Appl. Genet. 2008, 117, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bingham, E. Medicago arborea Project at the University of Wisconsin. Medicago Genetic Reports 5, 1–7 and Several Reports in Volums 7, 9, 10 and 11 and Subsequent Years. Available online: www.medicago-reports.org (accessed on 1 July 2023).
- Julier, B.; Fajoulot, S.; Barre, P.; Cardinet, G.; Santoni, S.; Huguet, T.; Huyghe, C. Construction of two genetic linkage maps in cultivated tetraploid alfalfa (Medicago sativa) using microsatellite and AFLP markers. BMC Plant Biol. 2003, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.; Kim, D.; Choi, H.; Gish, J.; Debelle, F.; Mudge, J.; Denny, R.; Endre, G.; Saurat, O.; Dudez, A.-M.; et al. Distribution of microsatellites in the genome of Medicago truncatula: A resource of genetic markers that integrate genetic and physical maps. Genetics 2006, 172, 2541–2555. [Google Scholar] [CrossRef]
- Mackie, J.; Musial, J.; Armour, D.; Phan, H.; Elwood, S.; Aitken, K.; Irwin, J. Identification of QTL for reaction to three races of Colletotrichum trifolii and further analysis of inheritance of resistance in autotetraploid lucerne. Theor. Appl. Genet. 2007, 114, 1417–1426. [Google Scholar] [CrossRef]
- Irwin, J.; Armour, D.; Pepper, P.; Lowe, K. Heterosis in lucerne testcrosses with Medicago arborea introgressions and Omani landraces and their performance in synthetics. Crop Pasture Sci. 2010, 61, 450–463. [Google Scholar] [CrossRef]
- De Wet, J.; Newell, C.; Brink, D. Counterfeit hybrids between Tripsacum and Zea (Gramineae). Am. J. Bot. 1984, 71, 245–251. [Google Scholar] [CrossRef]
- Bingham, E.; Irwin, J. Evidence that 2n eggs explain partial hybrids between Medicago sativa and Medicago arborea. Plants 2022, 11, 1380. [Google Scholar] [CrossRef]
- Allard, R. Principles of Plant Breeding; John Wiley and Sons: New York, NY, USA, 1960; p. 485. [Google Scholar]
- Doebly, J. The genetics of maize evolution. Annu. Rev. Genet. 2004, 38, 37–59. [Google Scholar] [CrossRef]
- Li, Z.; Heneen, W. Production and cytogenetics of intergeneric hybrids between the three cultivated Brassica diploids and Orychophragmus violaceus. Theor. Appl. Genet. 1999, 99, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Sun, J.; Ge, X.; Li, Z. Production and genetic analysis of partial hybrids from intertribal sexual crosses between Brassica napus and Isatis indigotica and progenies. Genome 2010, 53, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Bingham, E. Abnormal meiosis in alfalfa, Medicago sativa; Cytology of 2N egg and 4N pollen formation. Can. J. Genet. Cytol. 1983, 25, 107–112. [Google Scholar] [CrossRef]
- Brownfield, L.; Kohler, C. Unreduced gamete formation in plants: Mechanisms and prospects. J. Exp. Bot. 2010, 62, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Seguin-Swartz, G.; Somers, D. Cytogenetic and molecular characterization of intergeneric hybrids between Brassica napus and Orychophragmus violaceus. Genome 2002, 45, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Baraccia, G.; Albertini, D.; Rosellini, S.; Tavoletti, S.; Veronesi, F. Inheritance and mapping of 2n-egg production in diploid alfalfa. Genome 2000, 43, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Camadro, E.; Carputo, D.; Peloquin, S. Substitutes for genome differentiation in tuber-bearing Solanum; interspecific pollen-pistil incompatibility, nuclear-cytoplasmic male sterility, and endosperm. Theor. Appl. Genet. 2004, 109, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Boyes, J.; Thompson, W. The development of the endosperm and embryo in reciprocal crosses in cereals. J. Genet. Breed. 1937, 34, 203–227. [Google Scholar] [CrossRef]
- Valentine, D. Studies in British primulas. V. The inheritance of seed compatibility. New Phytol. 1956, 55, 305–318. [Google Scholar]
- Carputo, D.; Monti, L.; Werner, J.; Frusciante, L. Uses and usefulness of the endosperm balance number. Theor. Appl. Genet. 1999, 98, 478–484. [Google Scholar] [CrossRef]
- Barnes, D.; Bingham, E.; Murphy, R.; Hunt, O.; Beard, D.; Skrdla, W.; Teuber, L. Alfalfa Germplasm in the United States: Genetic Vulnerability, Use, Improvement and Maintenance; Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 1977. [Google Scholar]
- Kidwell, K.; Austin, D.; Osborn, T. RFLP evaluation of nine Medicago accessions representing the original germplasm sources for North American alfalfa cultivars. Crop Sci. 1994, 34, 230–236. [Google Scholar] [CrossRef]
- Shen, C.; Du, H.; Chen, Z.; Lu, H.; Zhu, F.; Chen, H.; Meng, X.; Liu, Q.; Liu, P.; Zheng, L.; et al. The chromosome- level genome sequence of the autotetraploid alfalfa and resequencing of core germplasms provide genomic resources for alfalfa research. Mol. Plant 2020, 13, 1250–1261. [Google Scholar] [CrossRef]
- Elgin, J.; Ostazeski, S. Evaluation of selected alfalfa cultivars and related Medicago species for resistance to race-1 and race-2 anthracnose. Crop Sci. 1982, 22, 39–42. [Google Scholar] [CrossRef]
- Tani, E.; Sarri, E.; Goufa, M.; Asimakopoulou, G.; Psychogiou, M.; Bingham, E.; Skaricis, G.; Abraham, E. Sedling growth and transcriptional responses to salt shock and stress in Medicago sativa L., Medicago arborea L., and their hybrid (Alborea). Agronomy 2018, 8, 231. [Google Scholar] [CrossRef]
- Bingham, E.; Armour, D.; Irwin, J.; Jayaraman, D.; Ane, J. Weakening the Hybridization Barrier between Herbaceous Medicago sativa and Woody M. arborea by Genetic Selection and Bridge Crossing. Available online: http://www.medicago-reports.org/volumes09.php (accessed on 6 June 2023).
- Annicchiarico, P.; Barrett, B.; Brummer, E.; Julier, B.; Marshall, A. Achievements and challenges in improving temperate perennial forage legumes. Front. Plant Sci. 2015, 34, 327–380. [Google Scholar] [CrossRef]
- Brummer, E. Capturing heterosis in forage crop cultivar development. Crop Sci. 1999, 39, 943–954. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Pecetti, L. Exploiting heterosis of semi-hybrids and heterogeneity of cultivar mixtures to enhance alfalfa crop performance. Field Crop Res. 2022, 283, 108522. [Google Scholar] [CrossRef]
- Kang, Y.; Seminario, A.; Udvardi, M.; Annicchiarico, P. Physiological and biochemical adaptive traits support the specific breeding of alfalfa (Medicago sativa) for severely drought-stressed or moisture-stressed environments. J. Agron. Crop Sci. 2022, 209, 132–143. [Google Scholar] [CrossRef]
- Del Pozo, A.; Espinoza, S.; Barahona, V.; Inostroza, L.; Gerding, M.; Humphries, A.; Lobos, G.; Cares, J.; Ovalle, C. Ariel and ground-based phenotyping of an alfalfa diversity panel to assess adaptation to a prolonged drought period in a Mediterranean environment of central Chile. Eur. J. Agron. 2023, 144, 126751. [Google Scholar] [CrossRef]
| Trait | Observations |
|---|---|
| Observed in greenhouses and fields | |
| Flower colour | Yellow flowers per se and variegated flowers |
| Indeterminate growth | Plants grow up to 4 m in height |
| Minimal crown | Observed in ca. 20% of plants |
| Large leaves | Leaves larger than in both parents in some plants |
| M. arborea pod shape and size | 1- to 1.5-coil flat pods ca. 1 cm in diameter observed in ca. 20% of plants |
| Large seeds | Seeds twice the size of alfalfa and half the size of M. arborea |
| Short racemes | 5–6 florets versus 15–25 in alfalfa |
| Fewer seeds per pod | 0–50% of alfalfa, although 8–9 per pod observed in one plant |
| Pollen quantity | 0–25% of alfalfa |
| Autogamy | Full seed set not observed; 10–25% of cross-fertility level |
| Crossability | Low frequency of Alborea plants that do not cross well with alfalfa but cross with Alborea |
| Observed in fields | |
| Lodging resistance | About 25% of Alborea plants resist lodging after rain and wind |
| Frost resistance | Low frequency of Alborea plants (ca. 5–10%) stay green down to −8 °C |
| Solid stem | About 5% of Alborea plants have a solid stem above the base like M. arborea; alfalfa has hollow stems |
| Heterosis in crosses | About 25% have heterosis for vigour in crosses with alfalfa |
| Branching roots | Some plants show absence of a tap root like M. arborea |
| Winter activity | Around 65% of plants with a group 9 dormancy level |
| Late flowering | May take some plants 2 years to flower, as for M. arborea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irwin, J.; Bingham, E. Review of Partial Hybrids between Herbaceous Medicago sativa and Woody Medicago arborea and Their Potential Role in Alfalfa Improvement. Appl. Biosci. 2023, 2, 373-383. https://doi.org/10.3390/applbiosci2030024
Irwin J, Bingham E. Review of Partial Hybrids between Herbaceous Medicago sativa and Woody Medicago arborea and Their Potential Role in Alfalfa Improvement. Applied Biosciences. 2023; 2(3):373-383. https://doi.org/10.3390/applbiosci2030024
Chicago/Turabian StyleIrwin, John, and Edwin Bingham. 2023. "Review of Partial Hybrids between Herbaceous Medicago sativa and Woody Medicago arborea and Their Potential Role in Alfalfa Improvement" Applied Biosciences 2, no. 3: 373-383. https://doi.org/10.3390/applbiosci2030024
APA StyleIrwin, J., & Bingham, E. (2023). Review of Partial Hybrids between Herbaceous Medicago sativa and Woody Medicago arborea and Their Potential Role in Alfalfa Improvement. Applied Biosciences, 2(3), 373-383. https://doi.org/10.3390/applbiosci2030024




