Wine Grapes Ripening: A Review on Climate Effect and Analytical Approach to Increase Wine Quality
Abstract
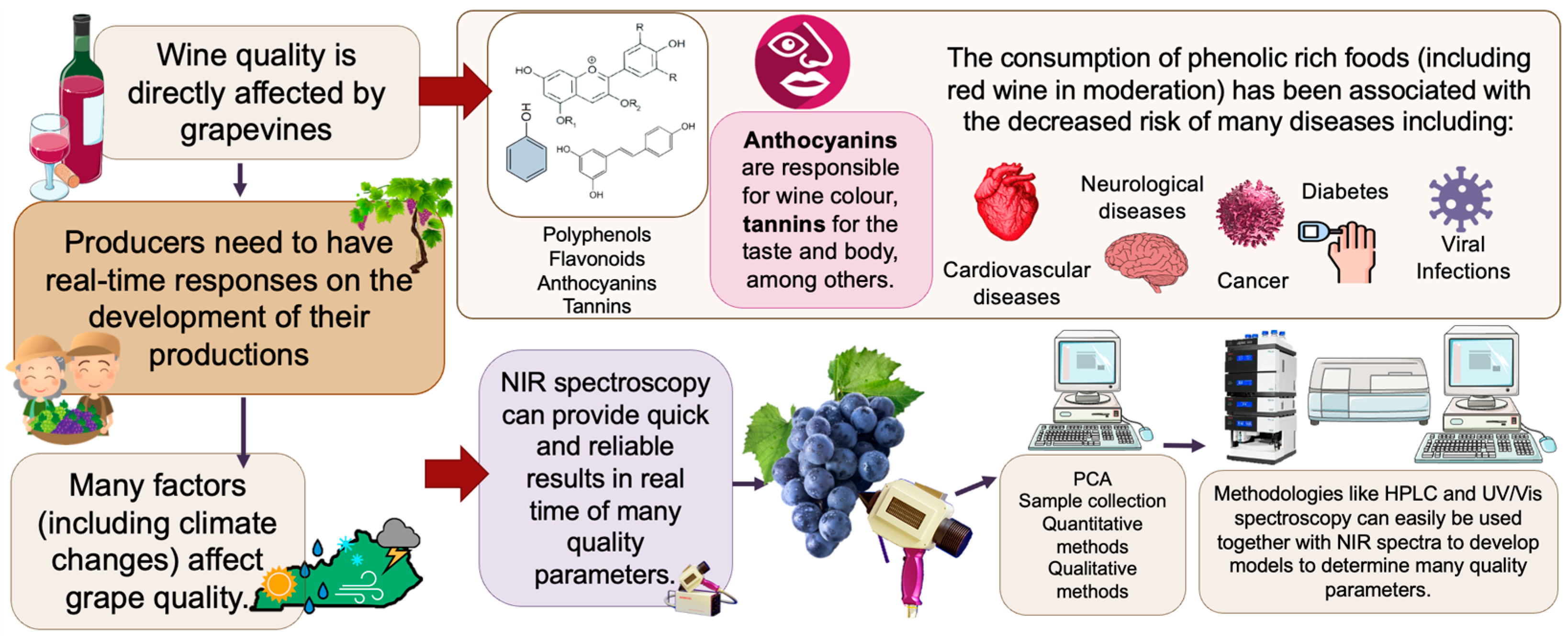
1. Introduction
2. Grape Production
3. Climate Influence on Grape Quality
3.1. The Impact of Temperature
3.2. The Impact of UV Radiation
3.3. The Influence of Water Availability
4. Grape Ripening
5. Plant Cell Wall Structure and Composition
6. Grape Phenolic Compounds
| Compound | Location | Function | References |
|---|---|---|---|
| Hydroxybenzoic and hydroxycinnamic acids | Seed; Skin; Pulp | Synthesis of key compounds in berry growth and development | [7,104] |
| Free radical scavengers; antimicrobial agents | [7] | ||
| Stilbenes | Seeds and skins | Berry growth and development | [7,105,106] |
| Protect the berry from biotic and abiotic stress | [7,105,106] | ||
| Flavonoids | Skins and seeds | Strong antioxidant capacity | [74,107] |
| Impact on wine organoleptic characteristics | [5,47] | ||
| Anthocyanins | Skins | Responsible for the colors red, blue, and purple in plant tissues | [108] |
| Important contributor to the sensory qualities | [109] |
7. Grape Quality Assessment Methods
7.1. Methods Used to Determine Phenolic Compounds in Grapes
7.2. Non-Destructive Methods for Grape Quality Assessment
7.3. Enzymatic Methods to Access Oxidative Stress in Grapes
8. Health Benefits of Red Wine Grape Consumption
9. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rouxinol, M.I.; Martins, M.R.; Salgueiro, V.; Costa, M.J.; Barroso, J.M.; Rato, A.E. Climate Effect on Morphological Traits and Polyphenolic Composition of Red Wine Grapes of Vitis vinifera. Beverages 2023, 9, 8. [Google Scholar] [CrossRef]
- Tomasi, D.; Gaiotti, F.; Jones, G.V. Organoleptic Characteristics of the Wines. In The Power of the Terroir: The Case Study of Prosecco Wine; Springer: Basel, Switzerland, 2013; pp. 149–166. ISBN 978-3-0348-0627-5. [Google Scholar]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and Colour Variability of Red Wines Made from Grapes Harvested at Different Ripeness Grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
- De Orduña, R.M. Climate Change Associated Effects on Grape and Wine Quality and Production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Merkytė, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. Grapes: Phenolic Composition, Antioxidant Characteristics and Health Benefits; Thomas, S., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017. [Google Scholar]
- Zarrouk, O.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Santos, R.R.; Pinheiro, C.; Costa, J.M.; Lopes, C.; Chaves, M.M. Impact of Irrigation Regime on Berry Development and Flavonoids Composition in Aragonez (Syn. Tempranillo) Grapevine. Agric. Water Manag. 2012, 114, 18–29. [Google Scholar] [CrossRef]
- Oancea, S.; Oprean, L. Anthocyanins, from Biosynthesis in Plants to Human Health Benefits. Food Technol. 2011, 15, 3–16. [Google Scholar]
- Gerós, H.; Chaves, M.M.; Delrot, S. The Biochemistry of the Grape Berry; Bentham Books: Sharjah, United Arab Emirates, 2012. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Determination of Phenolic Compounds of Grape Skins during Ripening by NIR Spectroscopy. LWT Food Sci. Technol. 2011, 44, 847–853. [Google Scholar] [CrossRef]
- Genisheva, Z.; Quintelas, C.; Mesquita, D.P.; Ferreira, E.C.; Oliveira, J.M.; Amaral, A.L. New PLS Analysis Approach to Wine Volatile Compounds Characterization by near Infrared Spectroscopy (NIR). Food Chem. 2018, 246, 172–178. [Google Scholar] [CrossRef]
- Rouxinol, M.I.; Martins, M.R.; Murta, G.C.; Mota Barroso, J.; Rato, A.E. Quality Assessment of Red Wine Grapes through NIR Spectroscopy. Agronomy 2022, 12, 637. [Google Scholar] [CrossRef]
- Mark, L.; Nikfardjam, M.S.P.; Avar, P.; Ohmacht, R. A Validated HPLC Method for the Quantitative Analysis of Trans-Resveratrol and Trans-Piceid in Hungarian Wines. J. Chromatogr. Sci. 2005, 43, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; De Rosso, M.; Bavaresco, L. Study of Grape Polyphenols by Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC/QTOF) and Suspect Screening Analysis. J. Anal. Methods Chem. 2015, 2015, 350259. [Google Scholar] [CrossRef] [PubMed]
- Alberts, P.; Stander, M.A.; De Villiers, A. Advanced Ultra High Pressure Liquid Chromatography-Tandem Mass Spectrometric Methods for the Screening of Red Wine Anthocyanins and Derived Pigments. J. Chromatogr. A 2012, 1235, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Boulet, J.-C.; Ducasse, M.-A.; Cheynier, V. Ultraviolet Spectroscopy Study of Phenolic Substances and Other Major Compounds in Red Wines: Relationship between Astringency and the Concentration of Phenolic Substances: UV Spectroscopy of Red Wine Components. Aust. J. Grape Wine Res. 2017, 23, 193–199. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and Grape Polyphenols—A Chemical Perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Barnes, J.S. Analytical Characterization of Anthocyanins from Natural Products by Reverse-Phase Liquid-Chromatography-Photodiode Array-Electrospray Ionization-Ion Trap-Time of Flight Mass Spectrometry. Master’s Thesis, University of Texas, Arlington, TX, USA, 2010. [Google Scholar]
- Flamini, R.; Traldi, P. Mass Spectrometry in Grape and Wine Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-55292-6. [Google Scholar]
- Kalivas, J.H. Multivariate Calibration, an Overview. Anal. Lett. 2005, 38, 2259–2279. [Google Scholar] [CrossRef]
- Carlini, P.; Massantini, R.; Mencarelli, F. Vis-NIR Measurement of Soluble Solids in Cherry and Apricot by PLS Regression and Wavelength Selection. J. Agric. Food Chem. 2000, 48, 5236–5242. [Google Scholar] [CrossRef]
- Joslyn, M.A. Methods in Food Analysis: Physical, Chemical, and Instrumental Methods of Analysis, 2nd ed.; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Higgins, L.M.; Llanos, E. A Healthy Indulgence? Wine Consumers and the Health Benefits of Wine. Wine Econ. Policy 2015, 4, 3–11. [Google Scholar] [CrossRef]
- Nowshehri, J.A.; Bhat, Z.A.; Shah, M.Y. Blessings in Disguise: Bio-Functional Benefits of Grape Seed Extracts. Food Res. Int. 2015, 77, 333–348. [Google Scholar] [CrossRef]
- Creasy, G.L.; Creasy, L.L. Crop Production Science in Horticulture: Grapes; Creasy, G.L., Creasy, L.L., Eds.; CABI Publishing: London, UK, 2009; ISBN 9781845933999. [Google Scholar]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. Switz. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Williams, P.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M.; Doco, T. Polysaccharide Composition of Monastrell Red Wines from Four Different Spanish Terroirs: Effect of Wine-Making Techniques. J. Agric. Food Chem. 2013, 61, 2538–2547. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.T.; Lehmann, J.; Dias, J.E.E.; Böhm, J. Atlas Das Castas Da Península Ibérica: História, Terroir, Ampelografia; Dinalivros: Lisboa, Portugal, 2011; ISBN 9789725765913. [Google Scholar]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.; Gerós, H. Berry Phenolics of Grapevine under Challenging Environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. 2019 Statistical Report on World Vitiviniculture; International Organisation of Vine and Wine: Dijon, France, 2019. [Google Scholar]
- International Organization of Vine and Wine. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2017; ISBN 979-10-91799-64-5. [Google Scholar]
- FAO FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 7 July 2020).
- Ponti, L.; Gutierrez, A.P.; Boggia, A.; Neteler, M. Analysis of Grape Production in the Face of Climate Change. Climate 2018, 6, 20. [Google Scholar] [CrossRef]
- OIV. Distribution of the World’s Grapevine Varieties; International Organization of Vine and Wine: Dijon, France, 2017; ISBN 9791091799898. [Google Scholar]
- CVRA. Castas. Available online: https://www.vinhosdoalentejo.pt/pt/vinhos/castas/ (accessed on 23 January 2023).
- Häusler, M. Assessment of Vegetation Parameters in Olive Trees in the Region of Alentejo Assessment of Vegetation Parameters in Olive Trees in the Region of Alentejo a Comparison of Direct and Indirect Methods; Universidade Técnica de Lisboa: Lisboa, Portugal, 2011. [Google Scholar]
- Kolyva, F.; Rhizopoulou, S.; Meletiou-Christou, M.-S.; Stratakis, E. Physiological Characteristics of Expanding and Expanded Leaves of Vitis vinifera L. Cv. Assyrtiko in Climate Change Conditions. Biol. Life Sci. Forum 2020, 4, 55. [Google Scholar] [CrossRef]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Romero, E.; Gómez-Alonso, S.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of Water Stress on the Phenolic Compounds of ‘Merlot’ Grapes in a Semi-Arid Mediterranean Climate. Horticulturae 2021, 7, 161. [Google Scholar] [CrossRef]
- Tomaz, A.; Martinez, J.M.C.; Pacheco, C.A. Yield and Quality Responses of “Aragonez” Grapevines under Deficit Irrigation and Different Soil Management Practices in a Mediterranean Climate. Cienc. Tec. Vitivinic. 2015, 30, 9–20. [Google Scholar] [CrossRef]
- Costa, E.; Cosme, F.; Jordão, A.M.; Mendes-Faia, A. Anthocyanin Profile and Antioxidant Activity from 24 Grape Varieties Cultivated in Two Portuguese Wine Regions. OENO One 2014, 48, 51. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Martínez-Carballo, E.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Pattern Recognition of Three Vitis vinifera L. Red Grapes Varieties Based on Anthocyanin and Flavonol Profiles, with Correlations between Their Biosynthesis Pathways. Food Chem. 2012, 130, 9–19. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Influência Da Rega Na Composição Fenólica Das Uvas Tintas Da Casta Touriga Francesa (Vitis vinifera L.). Cienc. Tecnol. Aliment. 1998, 2, 60–73. [Google Scholar] [CrossRef]
- Sugiura, T.; Sato, A.; Shiraishi, M.; Amamiya, H.; Ohno, H.; Takayama, N.; Miyata, N.; Sakaue, T.; Konno, S. Prediction of Acid Concentration in Wine and Table Grape Berries from Air Temperature. Hortic. J. 2020, 89, 208–215. [Google Scholar] [CrossRef]
- Liang, N.N.; Zhu, B.Q.; Han, S.; Wang, J.H.; Pan, Q.H.; Reeves, M.J.; Duan, C.Q.; He, F. Regional Characteristics of Anthocyanin and Flavonol Compounds from Grapes of Four Vitis vinifera Varieties in Five Wine Regions of China. Food Res. Int. 2014, 64, 264–274. [Google Scholar] [CrossRef]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Grape Flavonoid Evolution and Composition Under Altered Light and Temperature Conditions in Cabernet Sauvignon (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1062. [Google Scholar] [CrossRef]
- Barnuud, N.N.; Zerihun, A.; Gibberd, M.; Bates, B. Berry Composition and Climate: Responses and Empirical Models. Int. J. Biometeorol. 2014, 58, 1207–1223. [Google Scholar] [CrossRef]
- Marchica, A.; Cotrozzi, L.; Detti, R.; Lorenzini, G.; Pellegrini, E.; Petersen, M.; Nali, C. The Biosynthesis of Phenolic Compounds Is an Integrated Defence Mechanism to Prevent Ozone Injury in Salvia Officinalis. Antioxidants 2020, 9, 1274. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of Anthocyanin-Based Aqueous Extracts of Andean Purple Corn and Red-Fleshed Sweet Potato Compared to Synthetic and Natural Colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Laleh, G.H.; Frydoonfar, H.; Heidary, R.; Jameei, R.; Zare, S. The Effect of Light, Temperature, PH and Species on Stability of Anthocyanin Pigments in Four Berberis Species. Pak. J. Nutr. 2006, 5, 90–92. [Google Scholar] [CrossRef]
- Niculescu, V.-C.; Paun, N.; Ionete, R.-E. The Evolution of Polyphenols from Grapes to Wines. In Grapes and Wines; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-Vilaplana, A.; Teixeira, A.; Collado-González, J.; Moreno, D.A.; Gil-Izquierdo, A.; Rosa, E.; Domínguez-Perles, R. Evaluation of Grape (Vitis vinifera L.) Stems from Portuguese Varieties as a Resource of (Poly)Phenolic Compounds: A Comparative Study. Food Res. Int. 2014, 65, 375–384. [Google Scholar] [CrossRef]
- Torres, N.; Goicoechea, N.; Morales, F.; Antolín, M.C. Berry Quality and Antioxidant Properties in Vitis vinifera Cv. Tempranillo as Affected by Clonal Variability, Mycorrhizal Inoculation and Temperature. Crop Pasture Sci. 2016, 67, 961–977. [Google Scholar] [CrossRef]
- Rodríguez Montealegre, R.; Romero Peces, R.; Chacón Vozmediano, J.L.; Martínez Gascueña, J.; García Romero, E. Phenolic Compounds in Skins and Seeds of Ten Grape Vitis vinifera Varieties Grown in a Warm Climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Vilas Boas, A.C.; Henrique, P.d.C.; Lima, L.C.d.O.; Decarlos Neto, A. Antioxidant Activity, Anthocyanins and Organic Acids Content of Grape Juices Produced in Southwest of Minas Gerais, Brazil. Ciênc. Agrotecnol. 2014, 38, 480–486. [Google Scholar] [CrossRef]
- Buglass, A.J.; Garnham, S.C. A Novel Method for the Determination of Lactic Acid. Comparison of Lactic Acid Content of English and North European Wines. Am. J. Enol. Vitic. 1991, 42, 63–66. [Google Scholar] [CrossRef]
- Costa, C.; Graça, A.; Fontes, N.; Teixeira, M.; Gerós, H.; Santos, J.A. The Interplay between Atmospheric Conditions and Grape Berry Quality Parameters in Portugal. Appl. Sci. Switz. 2020, 10, 4943. [Google Scholar] [CrossRef]
- Pirata, M.S. Estudo Do Stress Hídrico Da Vinha-Castas Aragonês e Trincadeira. Ph.D. Thesis, Universidade de Évora, Évora, Portugal, 2018; pp. 19–27. [Google Scholar]
- Tardaguila, J.; Blanco, J.A.; Poni, S.; Diago, M.P. Mechanical Yield Regulation in Winegrapes: Comparison of Early Defoliation and Crop Thinning. Aust. J. Grape Wine Res. 2012, 18, 344–352. [Google Scholar] [CrossRef]
- Cockell, C.S.; Horneck, G. The History of the UV Radiation Climate of the Earth—Theoretical and Space-Based Observations. Photochem. Photobiol. 2001, 73, 447. [Google Scholar] [CrossRef]
- Lee, J.; Oh, M.; Son, K. Short-Term Ultraviolet (UV)—A Light-Emitting Diode (LED) Radiation Improves Biomass and Bioactive Compounds of Kale. Front. Plant Sci. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA Radiation Is Beneficial for Yield and Quality of Indoor Cultivated Lettuce. Front. Plant Sci. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Llorens, L.; Neugart, S.; Vandenbussche, F.; Castagna, A. Editorial: Ultraviolet Radiation: Friend or Foe for Plants? Front. Plant Sci. 2020, 11, 10–11. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Klinkhamer, P.G.L.; Leiss, K.A. Interactive Effects of UV-B Light with Abiotic Factors on Plant Growth and Chemistry, and Their Consequences for Defense against Arthropod Herbivores. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and Its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. Phenolic Characteristics Acquired by Berry Skins of Vitis vinifera Cv. Tempranillo in Response to Close-to-Ambient Solar Ultraviolet Radiation Are Mostly Reflected in the Resulting Wines. J. Sci. Food Agric. 2020, 100, 401–409. [Google Scholar] [CrossRef]
- Deloire, A.; Rogiers, S.; Šuklje, K.; Antalick, G.; Zeyu, X.; Pellegrino, A. Grapevine Berry Shrivelling, Water Loss and Cell Death: An Increasing Challenge for Growers in the Context of Climate Change. IVES Tech. Rev. Vine Wine 2021. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An Overview of Climate Change Impacts on European Viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under Deficit Irrigation: Hints from Physiological and Molecular Data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Changes in Grape Seed Polyphenols during Fruit Ripening. Phytochemistry 2000, 55, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zsófi, Z.; Villangó, S.; Pálfi, Z.; Tóth, E.; Bálo, B. Texture Characteristics of the Grape Berry Skin and Seed (Vitis vinifera L. Cv. Kékfrankos) under Postveraison Water Deficit. Sci. Hortic. 2014, 172, 176–182. [Google Scholar] [CrossRef]
- Garrido, I.; Uriarte, D.; Hernández, M.; Llerena, J.L.; Valdés, M.E.; Espinosa, F. The Evolution of Total Phenolic Compounds and Antioxidant Activities during Ripening of Grapes (Vitis vinifera L., Cv. Tempranillo) Grown in Semiarid Region: Effects of Cluster Thinning and Water Deficit. Int. J. Mol. Sci. 2016, 17, 1923. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Hernández-Hierro, J.M.; Rodríguez-Pulido, F.J.; Heredia, F.J. Determination of Technological Maturity of Grapes and Total Phenolic Compounds of Grape Skins in Red and White Cultivars during Ripening by near Infrared Hyperspectral Image: A Preliminary Approach. Food Chem. 2014, 152, 586–591. [Google Scholar] [CrossRef]
- Pastore, C.; Zenoni, S.; Fasoli, M.; Pezzotti, M.; Tornielli, G.B.; Filippetti, I. Selective Defoliation Affects Plant Growth, Fruit Transcriptional Ripening Program and Flavonoid Metabolism in Grapevine. BMC Plant Biol. 2013, 13, 1. [Google Scholar] [CrossRef]
- Esteban, M.A.; Villanueva, M.J.; Lissarrague, J.R. Effect of Irrigation on Changes in the Anthocyanin Composition of the Skin of Cv Tempranillo (Vitis vinifera L) Grape Berries during Ripening. J. Sci. Food Agric. 2001, 81, 409–420. [Google Scholar] [CrossRef]
- Keller, M.; Keller, M. Botany and Anatomy; Elsevier Inc.: Amsterdam, The Netherlands, 2010; ISBN 9780123748812. [Google Scholar]
- Negri, A.S.; Prinsi, B.; Rossoni, M.; Failla, O.; Scienza, A.; Cocucci, M.; Espen, L. Proteome Changes in the Skin of the Grape Cultivar Barbera among Different Stages of Ripening. BMC Genom. 2008, 9, 1–19. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The Plant Cell Wall: Biosynthesis, Construction, and Functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Cobb, F.; Tracana, S.; Costa, G.J.; Valente, I.; Goulao, L.F.; Amaîncio, S. Relating Water Deficiency to Berry Texture, Skin Cell Wall Composition, and Expression of Remodeling Genes in Two Vitis vinifera L. Varieties. J. Agric. Food Chem. 2015, 63, 3951–3961. [Google Scholar] [CrossRef] [PubMed]
- Allegro, G.; Pastore, C.; Valentini, G.; Filippetti, I. The Evolution of Phenolic Compounds in Vitis vinifera l. Red Berries during Ripening: Analysis and Role on Wine Sensory—A Review. Agronomy 2021, 11, 999. [Google Scholar] [CrossRef]
- Kafkas, E.; Koşar, M.; Paydaş, S.; Kafkas, S.; Başer, K.H.C. Quality Characteristics of Strawberry Genotypes at Different Maturation Stages. Food Chem. 2007, 100, 1229–1236. [Google Scholar] [CrossRef]
- André, M.; Lacampagne, S.; Barsacq, A.; Gontier, E.; Petrel, M.; Mercier, L.; Courot, D.; Gény-Denis, L. Physical, Anatomical, and Biochemical Composition of Skins Cell Walls from Two Grapevine Cultivars (Vitis vinifera) of Champagne Region Related to Their Susceptibility to Botrytis Cinerea during Ripening. Horticulturae 2021, 7, 413. [Google Scholar] [CrossRef]
- Bindon, K.A.; Smith, P.A.; Holt, H.; Kennedy, J.A. Interaction between Grape-Derived Proanthocyanidins and Cell Wall Material. 2. Implications for Vinification. J. Agric. Food Chem. 2010, 58, 10736–10746. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a Versatile Polysaccharide Present in Plant Cell Walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Chang, B.-M.; Keller, M. Cuticle and Skin Cell Walls Have Common and Unique Roles in Grape Berry Splitting. Hortic. Res. 2021, 8, 168. [Google Scholar] [CrossRef]
- Nunan, K.; Sims, I.; Bacic, A.; Robinson, S.; Fincher, G. Changes in Cell Wall Composition during Ripening of Grape Berries. Plant Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef]
- Wong, D.C.J.; Lopez Gutierrez, R.; Dimopoulos, N.; Gambetta, G.A.; Castellarin, S.D. Combined Physiological, Transcriptome, and Cis-Regulatory Element Analyses Indicate That Key Aspects of Ripening, Metabolism, and Transcriptional Program in Grapes (Vitis vinifera L.) Are Differentially Modulated Accordingly to Fruit Size. BMC Genomics 2016, 17, 416. [Google Scholar] [CrossRef] [PubMed]
- Zietsman, A.J.J.; Moore, J.P.; Fangel, J.U.; Willats, W.G.T.; Trygg, J.; Vivier, M.A. Following the Compositional Changes of Fresh Grape Skin Cell Walls during the Fermentation Process in the Presence and Absence of Maceration Enzymes. J. Agric. Food Chem. 2015, 63, 2798–2810. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between Polyphenols and Polysaccharides: Mechanisms and Consequences in Food Processing and Digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between Skin Cell Wall Composition and Anthocyanin Extractability of Vitis vinifera L. Cv. Tempranillo at Different Grape Ripeness Degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Interactions between Cell Wall Polysaccharides and Polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1808–1831. [Google Scholar] [CrossRef]
- Shipp, J.; Abdel-Aal, E. Food Applications and Physiological Effects of Anthocyanins as Functional Food Ingredients. Open Food Sci. J. 2010, 4, 7–22. [Google Scholar] [CrossRef]
- Ghosh, D.; Konishi, T. Anthocyanins and Anthocyanin-Rich Extracts: Role in Diabetes and Eye Function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar]
- Andjelkovic, M.; Radovanovié, B.; Radovanović, A.; Andjelkovic, A.M. Changes in Polyphenolic Content and Antioxidant Activity of Grapes Cv Vranac during Ripening. S. Afr. J. Enol. Vitic. 2013, 34, 147–155. [Google Scholar] [CrossRef]
- García-Marino, M.; Hernandez-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Colour and Pigment Composition of Red Wines Obtained from Co-Maceration of Tempranillo and Graciano Varieties. Anal. Chim. Acta 2010, 660, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.D.; Dambergs, R.G.; Cozzolino, D.; Herderich, M.J.; Smith, P.A. Relationship between Red Wine Grades and Phenolics. 1. Tannin and Total Phenolics Concentrations. J. Agric. Food Chem. 2010, 58, 12313–12319. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.O. Antocianinas Como Fotoprotectores Naturais. Master’s Thesis, Universidade de Coimbra, Coimbra, Portugal, 2012. [Google Scholar]
- Cabrita, M.J.; Ricardo-da-Silva, J.; Laureano, O. Os Compostos Polifenólicos Das Uvas e Dos Vinhos. Available online: http://www.isa.utl.pt/riav/Pdf/Memoria%20del%20Seminario%202003.3.pdf (accessed on 28 June 2023).
- Lochner, E. The Evaluation of Fourier Transform Infrared Spectroscopy (FT-IR) for the Determination of Total Phenolics and Total Anthocyanins Concentrations of Grapes; Stellenbosch University: Stellenbosch, South Africa, 2006. [Google Scholar]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape Berry Flavonoids: A Review of Their Biochemical Responses to High and Extreme High Temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol Screening of Pomace from Red and White Grape Varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape Stem Extracts: Polyphenolic Content and Assessment of Their in Vitro Antioxidant Properties. LWT—Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Pereira, V.; Câmara, J.S.; Cacho, J.; Marques, J.C. HPLC-DAD Methodology for the Quantification of Organic Acids, Furans and Polyphenols by Direct Injection of Wine Samples. J. Sep. Sci. 2010, 33, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Burns, J.; Mullen, W.; Landrault, N.; Teissedre, P.L.; Lean, M.E.J.; Crozier, A. Variations in the Profile and Content of Anthocyanins in Wines Made from Cabernet Sauvignon and Hybrid Grapes. J. Agric. Food Chem. 2002, 50, 4096–4102. [Google Scholar] [CrossRef]
- Abe, L.T.; Da Mota, R.V.; Lajolo, F.M.; Genovese, M.I. Compostos Fenólicos e Capacidade Antioxidante de Cultivares de Uvas Vitis Labrusca L. e Vitis vinifera L. Ciênc. Tecnol. Aliment. 2007, 27, 394–400. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Liang, Z.; Cheng, L.; Zhong, G.Y.; Liu, R.H. Antioxidant and Antiproliferative Activities of Twenty-Four Vitis vinifera Grapes. PLoS ONE 2014, 9, e105146. [Google Scholar] [CrossRef]
- Geana, E.I.; Iordache, A.M.; Ionete, R.E. Assessing the Wine Anthocyanin Profile for Red Grape Varieties Identification. Prog. Cryog. Isot. Sep. 2011, 14, 127–133. [Google Scholar]
- Kennedy, J.A. Grape and Wine Phenolics: Observations and Recent Findings. Cienc. Investig. Agrar. 2008, 35, 77–90. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed]
- Downey, O.M.; Dokoozlian, K.N.; Krstic, P.M.; Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Vacek, J.; Ulrichová, J.; Klejdus, B.; Šimánek, V. Analytical Methods and Strategies in the Study of Plant Polyphenolics in Clinical Samples. Anal. Methods 2010, 2, 604. [Google Scholar] [CrossRef]
- Boulton, R. The Copigmentation of Anthocyanins and Its Role in the Color of Red Wine: A Critical Review. Am. J. Enol. Vitic. 2001, 21, 67–87. [Google Scholar] [CrossRef]
- McRae, J.M.; Teng, B.; Bindon, K. Factors Influencing Red Wine Color from the Grape to the Glass. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 97–106. ISBN 978-0-12-814045-1. [Google Scholar]
- Daniels, A.J.; Poblete-Echeverría, C.; Opara, U.L.; Nieuwoudt, H.H. Measuring Internal Maturity Parameters Contactless on Intact Table Grape Bunches Using NIR Spectroscopy. Front. Plant Sci. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 978-0-12-182200-2. [Google Scholar]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol Antioxidant Quantity and Quality in Foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; de Gonçalves, A.E.S.S.; Fett, R. Phenolic Compounds Content and Antioxidant Activity in Pomace from Selected Red Grapes (Vitis vinifera L. and Vitis labrusca L.) Widely Produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Gajula, D.; Verghese, M.; Boateng, J.; Walker, L.T.; Shackelford, L.; Mentreddt, S.R.; Cedric, S. Determination of Total Phenolics, Flavonoids and Antioxidant and Chemopreventive Potential of Basil (Ocimum basilicum L. and Ocimum tenuiflorum L.). Int. J. Cancer Res. 2009, 5, 130–143. [Google Scholar] [CrossRef]
- Hosu, A.; Cristea, V.-M.; Cimpoiu, C. Analysis of Total Phenolic, Flavonoids, Anthocyanins and Tannins Content in Romanian Red Wines: Prediction of Antioxidant Activities and Classification of Wines Using Artificial Neural Networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef]
- Merken, H.M.; Beecher, G.R. Measurement of Food Flavonoids by High-Performance Liquid Chromatography: A Review. J. Agric. Food Chem. 2000, 48, 577–599. [Google Scholar] [CrossRef]
- Owades, J.L.; Rubin, G.; Brenner, M.W. Determination of Food Tannins by Ultraviolet Spectrophotometry. Agric. Food Chem. 1958, 6, 44–46. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Comparison of Two Methods for Anthocyanin Quantification. Acta Hortic. 2009, 810, 831–834. [Google Scholar] [CrossRef]
- Kharadze, M.; Japaridze, I.; Kalandia, A.; Vanidze, M. Anthocyanins and Antioxidant Activity of Red Wines Made from Endemic Grape Varieties. Ann. Agrar. Sci. 2018, 16, 181–184. [Google Scholar] [CrossRef]
- Natividade, M.M.P.; Corrêa, L.C.; de Souza, S.V.C.; Pereira, G.E.; Lima, L.C. de O. Simultaneous Analysis of 25 Phenolic Compounds in Grape Juice for HPLC: Method Validation and Characterization of São Francisco Valley Samples. Microchem. J. 2013, 110, 665–674. [Google Scholar] [CrossRef]
- Hohnová, B.; Šťavíková, L.; Karásek, P. Determination of Anthocyanins in Red Grape Skin by Pressurised Fluid Extraction and HPLC. Czech J. Food Sci. 2009, 26, S39–S42. [Google Scholar] [CrossRef]
- Boido, E.; García-Marino, M.; Dellacassa, E.; Carrau, F.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Characterisation and Evolution of Grape Polyphenol Profiles of Vitis vinifera L. Cv. Tannat during Ripening and Vinification: Polyphenolic Profiles of Tannat. Aust. J. Grape Wine Res. 2011, 17, 383–393. [Google Scholar] [CrossRef]
- Teixeira, L.N.; Stringheta, P.C.; Oliveira, F.A. De Comparação de Métodos Para Quantificação de Antocianinas. Rev. Ceres 2008, 55, 297–304. [Google Scholar]
- De Lorenzis, G.; Rustioni, L.; Parisi, S.G.; Zoli, F.; Brancadoro, L. Anthocyanin Biosynthesis during Berry Development in Corvina Grape. Sci. Hortic. 2016, 212, 74–80. [Google Scholar] [CrossRef]
- Fernández-Novales, J.; Tardáguila, J.; Gutiérrez, S.; Diago, M.P. On-The-Go VIS + SW − NIR Spectroscopy as a Reliable Monitoring Tool for Grape Composition within the Vineyard. Molecules 2019, 24, 2795. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Methods for the Assessment of Antioxidant Activity in Foods. In Handbook of Antioxidants for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 287–333. ISBN 978-1-78242-089-7. [Google Scholar]
- Fraige, K.; Pereira-Filho, E.R.; Carrilho, E. Fingerprinting of Anthocyanins from Grapes Produced in Brazil Using HPLC-DAD-MS and Exploratory Analysis by Principal Component Analysis. Food Chem. 2014, 145, 395–403. [Google Scholar] [CrossRef]
- Stefova, M.; Stafilov, T.; Kulevanova, S. HPLC Analysis of Flavonoids. In Encyclopedia of Chromatography; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 183–195. [Google Scholar]
- Shi, P.B.; Yue, T.X.; Ai, L.L.; Cheng, Y.F.; Meng, J.F.; Li, M.H.; Zhang, Z.W. Phenolic Compound Profiles in Grape Skins of Cabernet Sauvignon, Merlot, Syrah and Marselan Cultivated in the Shacheng Area (China). S. Afr. J. Enol. Vitic. 2016, 37, 132–138. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of Polyphenols and Evaluation of Antioxidant Capacity in Grape Pomace of the Cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Mulinacci, N.; Santamaria, A.R.; Giaccherini, C.; Innocenti, M.; Valletta, A.; Ciolfi, G.; Pasqua, G. Anthocyanins and Flavan-3-Ols from Grapes and Wines of Vitis vinifera Cv. Cesanese d’Affile. Nat. Prod. Res. 2008, 22, 1033–1039. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Correlation of Two Anthocyanin Quantification Methods: HPLC and Spectrophotometric Methods. Food Chem. 2008, 110, 782–786. [Google Scholar] [CrossRef]
- Rouxinol, M.I. Determinação Das Principais Antocianinas Em Castas Tintas No Alentejo. Master’s Thesis, Universidade de Évora, Évora, Portugal, 2016. [Google Scholar]
- De Beer, P.J. Grape and Wine Phenolic Composition as a Result of Training System and Canopy Modification in Vitis vinifera L.cv Shiraz. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2015. [Google Scholar]
- Agatonovic-Kustrin, S.; Morton, D.W.; Pauzi Md Yusof, A. The Use of Fourier Transform Infrared (FTIR) Spectroscopy and Artificial Neural Networks (ANNs) to Assess Wine Quality. Mod. Chem. Appl. 2013, 1, 1–8. [Google Scholar] [CrossRef]
- Xiaobo, Z.; Jiewen, Z.; Povey, M.J.W.; Holmes, M.; Hanpin, M. Variables Selection Methods in Near-Infrared Spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.N.; Robertson, A.; Scotter, C.N.G. Near-Infrared Reflectance Prediction of Quality, Theaflavin Content and Moisture Content of Black Tea. Food Chem. 1988, 61–75. [Google Scholar] [CrossRef]
- Cozzolino, D.; Dambergs, R.G.; Janik, L.; Cynkar, W.U.; Gishen, M. Analysis of Grapes and Wine by near Infrared Spectroscopy. J. Infrared Spectrosc. 2006, 14, 279–289. [Google Scholar] [CrossRef]
- Skogerson, K.; Downey, M.; Mazza, M.; Boulton, R. Rapid Determination of Phenolic Components in Red Wines from UV-Visible Spectra and the Method of Partial Least Squares. Am. J. Enol. Vitic. 2007, 58, 318–325. [Google Scholar] [CrossRef]
- Teixeira Dos Santos, C.A.; Lopo, M.; Páscoa, R.N.M.J.; Lopes, J.A. A Review on the Applications of Portable Near-Infrared Spectrometers in the Agro-Food Industry. Appl. Spectrosc. 2013, 67, 1215–1233. [Google Scholar] [CrossRef]
- Cozzolino, D.; Kwiatkowski, M.J.; Parker, M.; Cynkar, W.U.; Dambergs, R.G.; Gishen, M.; Herderich, M.J. Prediction of Phenolic Compounds in Red Wine Fermentations by Visible and near Infrared Spectroscopy. Anal. Chim. Acta 2004, 513, 73–80. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Moral, F.; Carvajal-Ramirez, F.; Marques da Silva, J. Estimation of Productivity in Dryland Mediterranean Pastures: Long-Term Field Tests to Calibration and Validation of the Grassmaster II Probe. AgriEngineering 2020, 2, 15. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Antioxidant Defences in Olive Trees during Drought Stress: Changes in Activity of Some Antioxidant Enzymes. Funct. Plant Biol. 2005, 32, 45. [Google Scholar] [CrossRef]
- De Carvalho, I.S. Avaliação de Stress Oxidativo Em Bagos de Videira (Vitis vinifera L.) Da Casta “Trincadeira” Em Condições de Stress Hídrico. Master’s Thesis, Instituto Superior de Agronomia, Lisboa, Portugal, 2009. [Google Scholar]
- Alici, E.; Arabaci, G. Determination of SOD, POD, PPO and CAT Enzyme Activities in Rumex obtusifolius L. Annu. Res. Rev. Biol. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Thomas, N.O.; Shay, K.P.; Kelley, A.R.; Butler, J.A.; Hagen, T.M. Glutathione Maintenance Mitigates Age-Related Susceptibility to Redox Cycling Agents. Redox Biol. 2016, 10, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Edelmira, V.; Ferrer, S.; Carmona, G. Evolution of Grape Polyphenol Oxidase Activity and Phenolic Content During Maturation and Vinification. Vitis 1989, 28, 85–95. [Google Scholar]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Sgherri, C.; Ranieri, A.; Quartacci, M.F. Antioxidative Responses in Vitis vinifera Infected by Grapevine Fanleaf Virus. J. Plant Physiol. 2013, 170, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, O.; Denli, Y.; Aras, S.; Söylemezoğlu, G. Active Oxygen Scavenging Enzyme Activities and Glutathione, Ascorbic Acid and Lipid Peroxidation Levels in Developing Vitis vinifera l. Leaves and Berries. Biotechnol. Biotechnol. Equip. 2003, 17, 114–122. [Google Scholar] [CrossRef]
- Okuda, T.; Yokotsuka, K. Levels of Glutathione and Activities of Related Enzymes During Ripening of Koshu and Cabernet Sauvignon Grapes and During Winemaking. Am. J. Enol. Vitic. 1999, 1, 264–270. [Google Scholar] [CrossRef]
- Institut Français de la Vigne et du Vin. Enzymes in Œnology: Production, Regulation, Applications; OEnoppia: Paris, France, 2014; ISBN 2-906417-67-X. [Google Scholar]
- Troiani, E.d.P.; Tropiani, C.T.; Clemente, E. Peroxidase (POD) and Polyphenoloxidase (PPO) in Grape (Vitis vinifera L.). Ciênc. Agrotecnologia 2003, 27, 635–642. [Google Scholar] [CrossRef]
- Freitas, A.; Bernardes, J.P.; Mateus, M.P.; Braz, N. Dimensions of Mediterranean Diet—World Cultural Heritage; Universidade do Algarve: Faro, Portugal, 2015; ISBN 9789898472748. [Google Scholar]
- UNESCO—Mediterranean Diet. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 20 March 2023).
- Trichopoulou, A. Mediterranean Diet as Intangible Heritage of Humanity: 10 Years On. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1943–1948. [Google Scholar] [CrossRef]
- Minzer, S.; Estruch, R.; Casas, R. Wine Intake in the Framework of a Mediterranean Diet and Chronic Non-Communicable Diseases: A Short Literature Review of the Last 5 Years. Molecules 2020, 25, 5045. [Google Scholar] [CrossRef]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Faliva, M.A.; Gerbi, V.; La Vecchia, C.; Negri, E.; Opizzi, A.; Perna, S.; Pezzotti, M.; et al. Mediterranean Way of Drinking and Longevity. Crit. Rev. Food Sci. Nutr. 2016, 56, 635–640. [Google Scholar] [CrossRef]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular Aspects of Lipoic Acid in the Prevention of Diabetes Complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The Role of Glutathione Reductase and Related Enzymes on Cellular Redox Homoeostasis Network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.K.; Djanaguiraman, M.; Sudhagar, R.; Chandrashekar, C.N.; Pathmanabhan, G. Differential Antioxidative Response of Ascorbate Glutathione Pathway Enzymes and Metabolites to Chromium Speciation Stress in Green Gram (Vigna radiata (L.) R.Wilczek. Cv CO4) Roots. Plant Sci. 2004, 166, 1035–1043. [Google Scholar] [CrossRef]
- Stuart, J.A.; Robb, E.L. Bioactive Polyphenols from Wine Grapes; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9781461469681. [Google Scholar]
- Fang, J. Bioavailability of Anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Shi, J.; He, M.; Cao, J.; Wang, H.; Ding, J.; Jiao, Y.; Li, R.; He, J.; Wang, D.; Wang, Y. The Comparative Analysis of the Potential Relationship between Resveratrol and Stilbene Synthase Gene Family in the Development Stages of Grapes (Vitis quinquangularis and Vitis vinifera). Plant Physiol. Biochem. 2014, 74, 24–32. [Google Scholar] [CrossRef]
- Bouhlali, E.; Sellam, K.; Bammou, M.; Alem, C.; Filali-Zehzouti, Y. In Vitro Antioxidant and Anti-Inflammatory Properties of Selected Moroccan Medicinal Plants. J. Appl. Pharm. Sci. 2016, 6, 156–162. [Google Scholar] [CrossRef]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin Characterization, Total Phenolic Quantification and Antioxidant Features of Some Chilean Edible Berry Extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef]
- Kim, M.J.; Jun, J.G.; Park, S.Y.; Choi, M.J.; Park, E.; Kim, J.I.; Kim, M.J. Antioxidant Activities of Fresh Grape Juices Prepared Using Various Household Processing Methods. Food Sci. Biotechnol. 2017, 26, 861–869. [Google Scholar] [CrossRef]
- Mraihi, F.; Journi, M.; Chérif, J.K.; Sokmen, M.; Sokmen, A.; Trabelsi-Ayadi, M. Phenolic Contents and Antioxidant Potential of Crataegus Fruits Grown in Tunisia as Determined by DPPH, FRAP, and β-Carotene/Linoleic Acid Assay. J. Chem. 2013, 2013, 378264. [Google Scholar] [CrossRef]
- Dumitriu, D.; Peinado, R.A.; Peinado, J.; de Lerma, N.L. Grape Pomace Extract Improves the in Vitro and in Vivo Antioxidant Properties of Wines from Sun Light Dried Pedro Ximénez Grapes. J. Funct. Foods 2015, 17, 380–387. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; de Rosso, V.V. Bioavailability of Anthocyanins: Gaps in Knowledge, Challenges and Future Research. J. Food Compos. Anal. 2017, 68, 31–40. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In Vitro Antioxidant Activity of Kyoho Grape Extracts in DPPH• and ABTS• Assays: Estimation Methods for EC50 Using Advanced Statistical Programs. Food Chem. 2018, 275, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, T.K.; Wohlenberg, M.F.; Medeiros, N.; Martins, J.B.; Agostini, F.; Funchal, C.; Dani, C. Evaluation of Antioxidant Activity of Grapevine Leaves Extracts (Vitis labrusca) in Liver of Wistar Rats. An. Acad. Bras. Ciências 2016, 88, 187–196. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Blasa, M.; Gennari, L.; Angelino, D.; Ninfali, P. Fruit and Vegetable Antioxidants in Health, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; ISBN 9780123746283. [Google Scholar]
- Pozzan, M.S.V.; Braga, G.C.; Salibe, A.B. Teores de Antocianinas, Fenóis Totais, Taninos e Ácido Ascórbico Em Uva “bordô” Sobre Diferentes Porta-Enxertos. Rev. Ceres 2012, 59, 701–708. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.L. Evolution of Analysis of Polyhenols from Grapes, Wines, and Extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Sun, A.Y.; Simonyi, A.; Sun, G.Y. The “French Paradox” and beyond: Neuroprotective Effects of Polyphenols. Free Radic. Biol. Med. 2002, 32, 314–318. [Google Scholar] [CrossRef]
- Mossalayi, M.D.; Rambert, J.; Renouf, E.; Micouleau, M.; Mérillon, J.M. Grape Polyphenols and Propolis Mixture Inhibits Inflammatory Mediator Release from Human Leukocytes and Reduces Clinical Scores in Experimental Arthritis. Phytomedicine 2014, 21, 290–297. [Google Scholar] [CrossRef]
- Xia, E.; He, X.; Li, H.; Wu, S.; Li, S.; Deng, G. Biological Activities of Polyphenols from Grapes. Polyphen. Hum. Health Dis. 2013, 1, 47–58. [Google Scholar] [CrossRef]
- Maru, G.B.; Kumar, G.; Ghantasala, S.; Tajpara, P. Polyphenol-Mediated In Vivo Cellular Responses during Carcinogenesis; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 2, ISBN 9780123984562. [Google Scholar]
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry Anthocyanins in Health Promotion: A Metabolic Overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Bertelli, A.A.E. Wine, Alcohol and Pills: What Future for the French Paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Stanley, L.L.; Mazier, M.J.P.; Scotia, N. Potencial Explanations for the French Paradox. Science 1999, 19, 3–15. [Google Scholar]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Anthocyanins from Black Sorghum and Their Antioxidant Properties. Food Chem. 2005, 90, 293–301. [Google Scholar] [CrossRef]
- Carrieri, C.; Milella, R.A.; Incampo, F.; Crupi, P.; Antonacci, D.; Semeraro, N.; Colucci, M. Antithrombotic Activity of 12 Table Grape Varieties. Relationship with Polyphenolic Profile. Food Chem. 2013, 140, 647–653. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-weisz, U.; Carle, R. Inhibitory Effects of Polyphenols from Grape Pomace Extract on Collagenase and Elastase Activity. Fitoterapia 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Gao, Y.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A.; Moore, J.P. Dissecting the Polysaccharide-Rich Grape Cell Wall Changes during Winemaking Using Combined High-Throughput and Fractionation Methods. Carbohydr. Polym. 2015, 133, 567–577. [Google Scholar] [CrossRef]
- Kumar, V.; Nagar, S.; Tripathi, Y.C. Do Assorted Approaches Aid in Estimation of Uronic Acids? Case Studies on Tinospora Sinensis Polysaccharides. Int. J. Biol. Macromol. 2014, 70, 360–363. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Bioactive Non-Coloured Polyphenols Content of Grapes, Wines and Vinification by-products: Evaluation of the Antioxidant Activities of Their Extracts. Food Res. Int. 2010, 43, 805–813. [Google Scholar] [CrossRef]
- Estruch, R.; Sacanella, E.; Mota, F.; Chiva-Blanch, G.; Antúneza, E.; Casals, E.; Deulofeu, R.; Rotilio, D.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; et al. Moderate Consumption of Red Wine, but Not Gin, Decreases Erythrocyte Superoxide Dismutase Activity: A Randomised Cross-over Trial. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 46–53. [Google Scholar] [CrossRef]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [PubMed]
- Teissedre, P.L.; Landrault, N. Wine Phenolics: Contribution to Dietary Intake and Bioavailability. Food Res. Int. 2000, 33, 461–467. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Sayago, A.; Fernández-Recamales, Á. Metabolomics: An Emerging Tool for Wine Characterization and the Investigation of Health Benefits; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128152584. [Google Scholar]
- Wang, S.-C.; Chen, Y.; Wang, Y.-C.; Wang, W.-J.; Yang, C.-S.; Tsai, C.-L.; Hou, M.-H.; Chen, H.-F.; Shen, Y.-C.; Hung, M.-C. Tannic Acid Suppresses SARS-CoV-2 as a Dual Inhibitor of the Viral Main Protease and the Cellular TMPRSS2 Protease. Am. J. Cancer Res. 2020, 10, 4538–4546. [Google Scholar] [PubMed]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and Absorption of Red Grape and Wine Anthocyanins through the Gastrointestinal Tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
| Compound Determined | Methodology Used | References |
|---|---|---|
| Total polyphenols | UV/Vis spectrophotometry: Folin Ciocalteu method | [120,122] |
| HPLC-DAD with a RP column | [125] | |
| Total flavonoids | UV/Vis spectrophotometry | [126,127] |
| HPLC | [128] | |
| Total tannins | UV/Vis spectrophotometry | [129] |
| Anthocyanins | The pH differential method | [9,130] |
| Paper chromatography, thin-layer chromatography, column chromatography, solid-phase extraction, counter-current chromatography, UV/Vis spectroscopy | [128] | |
| HPLC–DAD using a reverse phase column | [131,132] | |
| HPLC with spectrophotometer detector UV, C18 column | [133] | |
| HPLC-DAD-MS with ion trap detector, equipped with an atmospheric pressure ionization source, using an electrospray ionisation interface. | [134] | |
| High performance liquid chromatography/quadrupole time mass spctrometer with a reverse-phase C18 column | [15,16,111,135,136] | |
| FT-IR | [102] |
| Compound | Health Benefit | References |
|---|---|---|
| Phenolic compounds (Including Gallic and caffeic acids) | Can reduce coronary diseases | [206] |
| high potential to inhibit lower density lipid proteins | [124] | |
| Reduce LDL-lipoproteins and reduce ROS even in very low concentrations in vitro | [143,205] | |
| The moderate intake of wine reduced plasma SOD activity and reduced MDA levels | [205] | |
| Inhibit lower density lipid proteins | [124] | |
| Antitrombotic effect | [200] | |
| Prebiotic effects | [208] | |
| (+)-Catechin | higher plasmatic antioxidant activity | [207] |
| Grapevine leaf extracts | prevent liver disorders in Wistar rats | [186] |
| Tannic acid | anti-SARS-CoV-2 activity | [209] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouxinol, M.I.; Martins, M.R.; Barroso, J.M.; Rato, A.E. Wine Grapes Ripening: A Review on Climate Effect and Analytical Approach to Increase Wine Quality. Appl. Biosci. 2023, 2, 347-372. https://doi.org/10.3390/applbiosci2030023
Rouxinol MI, Martins MR, Barroso JM, Rato AE. Wine Grapes Ripening: A Review on Climate Effect and Analytical Approach to Increase Wine Quality. Applied Biosciences. 2023; 2(3):347-372. https://doi.org/10.3390/applbiosci2030023
Chicago/Turabian StyleRouxinol, Maria Inês, Maria Rosário Martins, João Mota Barroso, and Ana Elisa Rato. 2023. "Wine Grapes Ripening: A Review on Climate Effect and Analytical Approach to Increase Wine Quality" Applied Biosciences 2, no. 3: 347-372. https://doi.org/10.3390/applbiosci2030023
APA StyleRouxinol, M. I., Martins, M. R., Barroso, J. M., & Rato, A. E. (2023). Wine Grapes Ripening: A Review on Climate Effect and Analytical Approach to Increase Wine Quality. Applied Biosciences, 2(3), 347-372. https://doi.org/10.3390/applbiosci2030023











