Ruthenium Complexes, an Emerging Class of Leishmanicidal Drug Candidates
Abstract
:1. Introduction
1.1. Leishmaniasis, a Growing Concern
1.2. Clinical Forms and Treatment
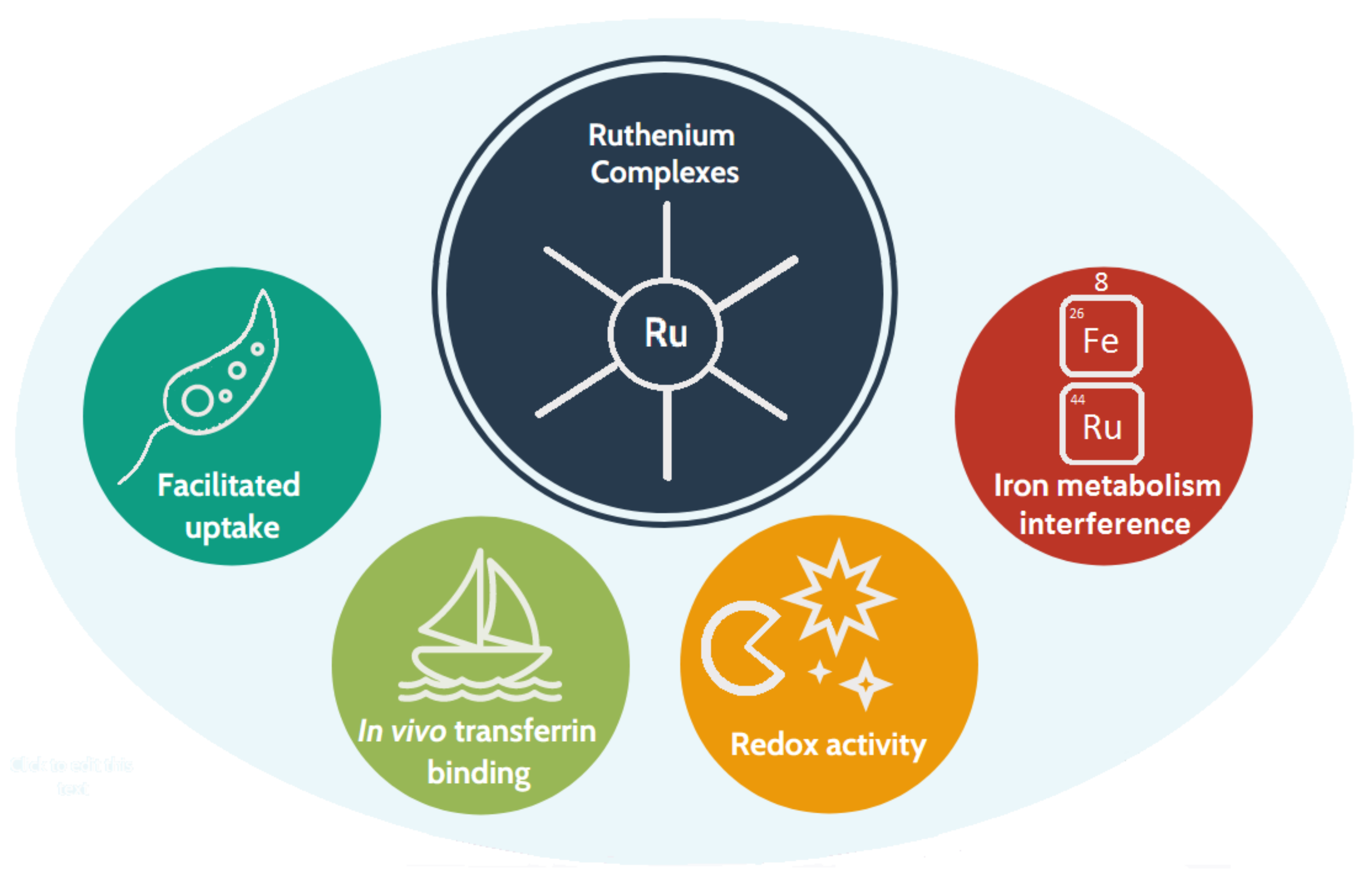
1.3. Why Ruthenium-Based Leishmanicidal Drugs?
- The redox properties of the Ru centre [32] are postulated to interfere with the parasite’s defence mechanisms against oxidation;
- Ru is less toxic than Sb(V);
- In human cells, some ruthenium complexes are taken up and metabolised through iron-related pathways [34]; thus, they are likely to enter parasitic cells by the aforementioned heme pathway.
2. Organometallic Ruthenium Complexes
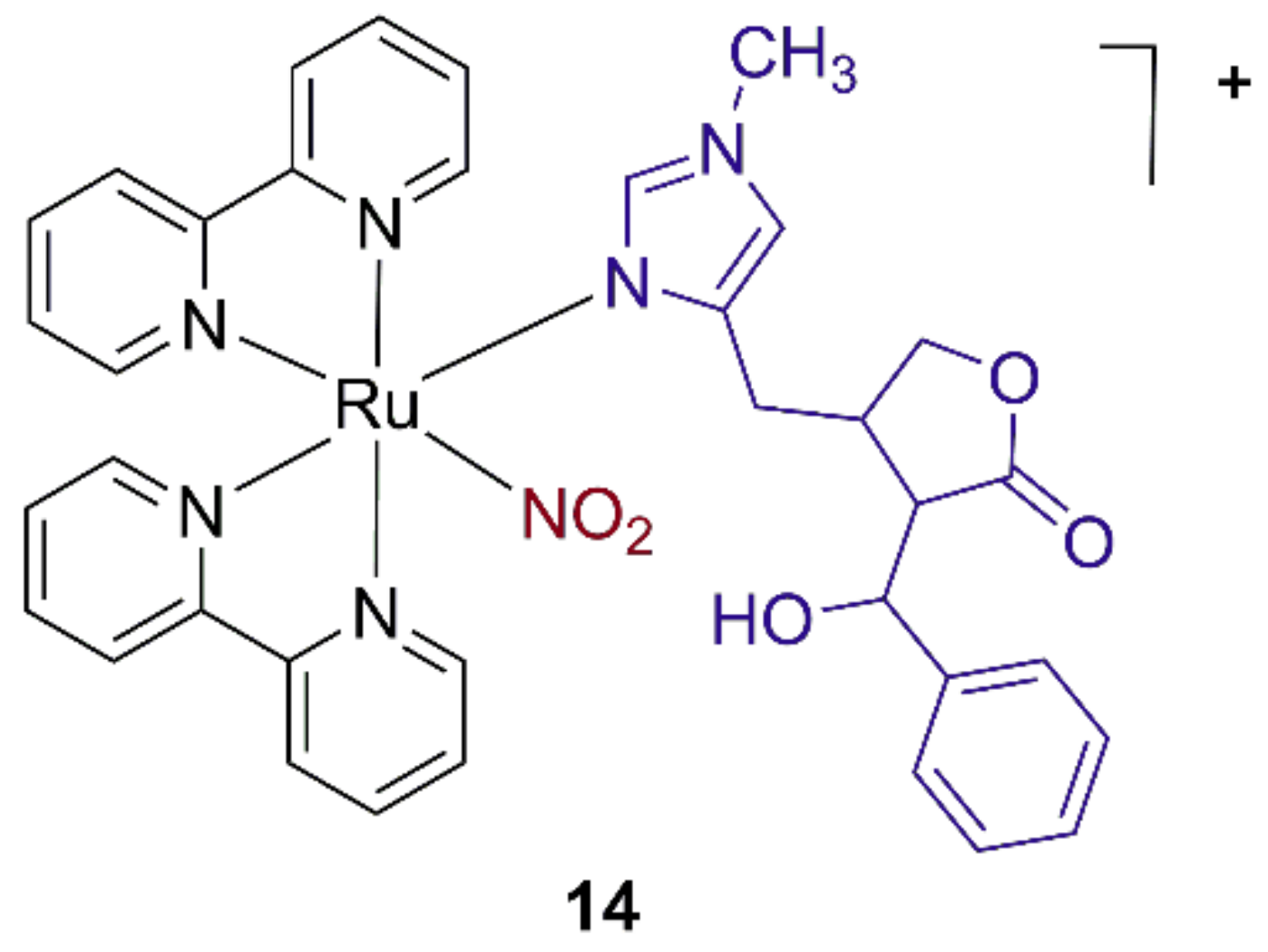
2.1. Ruthenium Azole Complexes
2.2. Ruthenium Complexes with Non-Steroidal Anti-Inflammatory Agents
3. Inorganic Ruthenium Complexes
3.1. Ruthenium Complexes with Plant Alkaloids
3.2. Ruthenium Lapachol Complexes
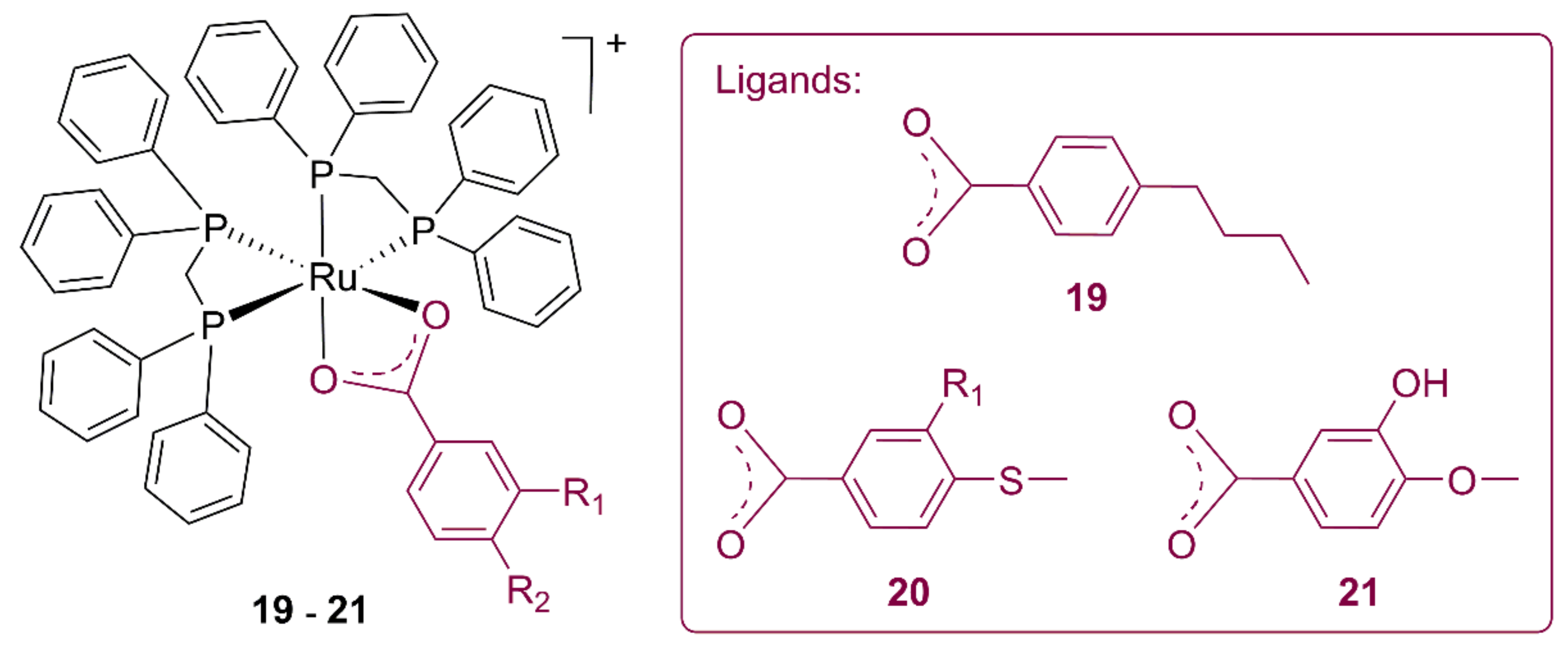
3.3. Ruthenium Alkylbenzoate Complexes
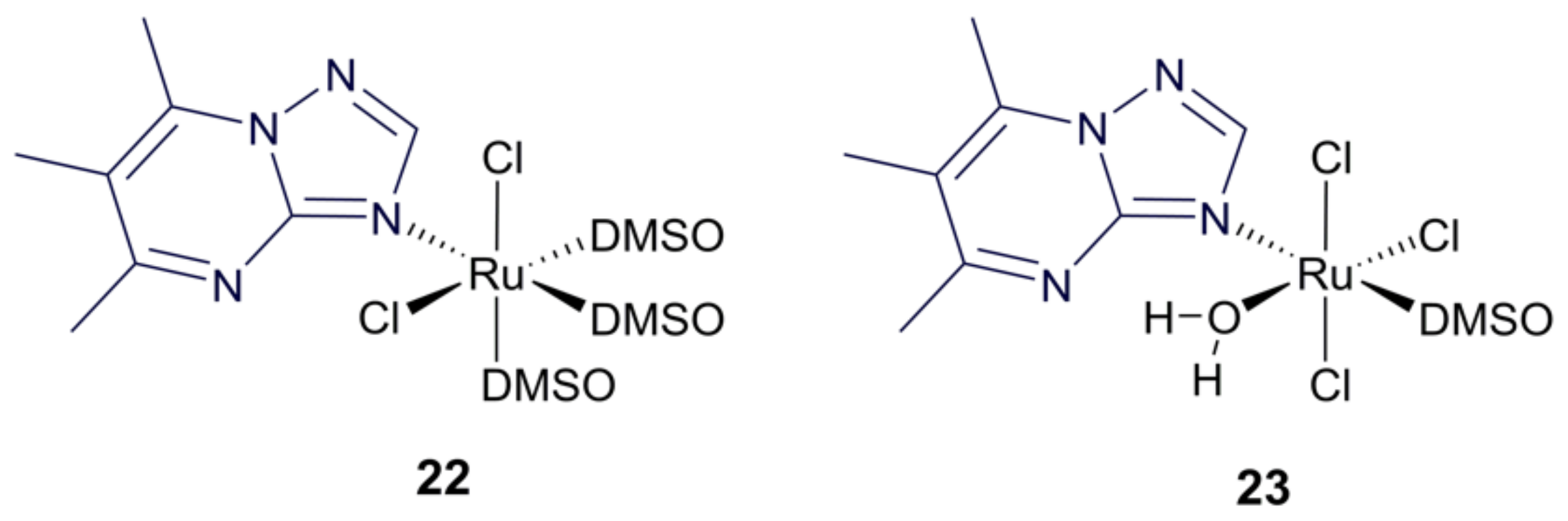
3.4. Ruthenium Purine Complexes
3.5. Ruthenium Nitrosyl Complexes
4. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Leishmaniasis: Situation and Trends. Available online: http://www.who.int/gho/neglected_diseases/leishmaniasis/en/ (accessed on 13 April 2022).
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; Mazotto, A.M.; Cardoso, V.; Alves, R.L.; Amaral, A.C.F.; Silva, J.R.A.; Pinheiro, A.S.; Vermelho, A.B. Natural products: Insights into leishmaniasis inflammatory response. Mediat. Inflam. 2015, 2015, 835910. [Google Scholar] [CrossRef] [Green Version]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Available online: https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies (accessed on 13 April 2022).
- González, C.; Wang, O.; Strutz, S.E.; González-Salazar, C.; Sánchez-Cordero, V.; Sarkar, S. Climate change and risk of leishmaniasis in North America: Predictions from ecological niche models of vector and reservoir species. PLoS Negl. Trop. Dis. 2010, 4, e585. [Google Scholar] [CrossRef] [Green Version]
- Trájer, A.J.; Bede-Fazekas, Á.; Hufnagel, L.; Horváth, L.; Bobvos, J. The effect of climate change on the potential distribution of the European Phlebotomus species. Appl. Ecol. Environ. Res. 2013, 11, 189–208. [Google Scholar] [CrossRef]
- Oerther, S.; Jöst, H.; Heitmann, A.; Lühken, R.; Krüger, A.; Steinhausen, I.; Brinker, C.; Lorentz, S.; Marx, M.; Schmidt-Chanasit, J.; et al. Phlebotomine sand flies in Southwest Germany: An update with records in new locations. Parasit. Vectors 2020, 13, 173. [Google Scholar] [CrossRef] [Green Version]
- Steverding, D. The history of leishmaniasis. Parasit. Vectors 2017, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Du, R.; Hotez, P.J.; Al-Salem, W.S.; Costa-Serrano, A.A. Old world cutaneous leishmaniasis and refugee crises in the Middle East and North Africa. PLoS Negl. Trop. Dis. 2016, 10, e0004545. [Google Scholar] [CrossRef] [Green Version]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Multi-target drugs active against leishmaniasis: A paradigm of drug repurposing. Eur. J. Med. Chem. 2019, 183, 111660. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Saxena, A.; Singh, S. Chemotherapy of leishmaniasis: Past, present and future. Curr. Med. Chem. 2007, 14, 1153–1169. [Google Scholar] [CrossRef]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef] [Green Version]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Javier, F.P.; Seifert, K.; Croft, S.L.; Sundar, S.; Castanys, S.; Gamarro, F. Mechanisms of experimental resistance of Leishmania to miltefosine: Implications for clinical use. Drug Resist. Updates 2006, 9, 26–39. [Google Scholar] [CrossRef]
- Berman, J.J. Treatment of leishmaniasis with miltefosine: 2008 status. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1209–1216. [Google Scholar] [CrossRef]
- World Health Organization. Model List of Essential Medicines: 21st List 2019. Available online: https://apps.who.int/iris/handle/10665/325771 (accessed on 31 March 2020).
- Bornstein, R.S.; Yarbro, J.W. An evaluation of the mechanism of action of pentamidine isethionate. J. Surg. Oncol. 1970, 2, 393–398. [Google Scholar] [CrossRef]
- Song, J.; Baker, N.; Rothert, M.; Henke, B.; Jeacock, L.; Horn, D.; Beitz, E. Pentamidine is not a permeant but a nanomolar inhibitor of the Trypanosoma brucei aquaglyceroporin-2. PLoS Pathog. 2016, 12, e1005436. [Google Scholar] [CrossRef] [Green Version]
- Dogra, J.; Saxena, V.N. Itraconazole and leishmaniasis: A randomised double-blind trial in cutaneous disease. Int. J. Parasitol. 1996, 26, 1413–1415. [Google Scholar] [CrossRef]
- Consigli, J.; Danielo, C.; Gallerano, V.; Papa, M.; Guidi, A. Cutaneous leishmaniasis: Successful treatment with itraconazole. Int. J. Dermatol. 2006, 45, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Alrajhi, A.A.; Ibrahim, E.A.; De Vol, E.B.; Khairat, M.; Faris, R.M.; Maguire, J.H. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N. Engl. J. Med. 2002, 346, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Ghimire, R.; Lamichhane, P. Efficacy and safety of paromomycin for visceral leishmaniasis: A systematic review. J. Trop. Med. 2021, 2021, 8629039. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Fasel, N. How to master the host immune system? Leishmania parasites have the solutions! Int. Immunol. 2017, 30, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, M.; Orian, A.; Hatam, G. Immunotherapy in treatment of leishmaniasis. Immunol. Lett. 2021, 233, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.M.; Oyama, J.; Monich, M.S.T.; Brustolin, A.A.; de Souza, J.V.P.; Murase, L.S.; Lopes, L.D.G.; Santos, T.S.; Teixeira, J.J.V.; Silveira, T.G.V. Three decades of clinical trials on immunotherapy for human leishmaniases: A systematic review and meta-analysis. Immunotherapy 2021, 13, 693–721. [Google Scholar] [CrossRef]
- Goto, Y.; Cheng, J.; Omachi, S.; Morimoto, A. Prevalence, severity, and pathogeneses of anemia in visceral leishmaniasis. Parasitol. Res. 2017, 116, 457–464. [Google Scholar] [CrossRef]
- Carvalho, S.; Crus, T.; Santarém, N.; Castro, H.; Costa, V.; Tomás, A.M. Heme as a source of iron to Leishmania infantum amastigotes. Acta Trop. 2009, 109, 131. [Google Scholar] [CrossRef]
- Braga, S.S.; Lysenko, K.; Santos, N.E. Ruthenophenanthroline complexes active against tripanosomids. In A Closer Look at Phenanthroline, 1st ed.; Amies, W., Ed.; NovaScience Publishing: Hauppage, NY, USA, 2020. [Google Scholar]
- Britten, N.S.; Butler, J.A. Ruthenium metallotherapeutics: Novel approaches to combating parasitic infections. Curr. Med. Chem. 2022, 29, 1–20. [Google Scholar] [CrossRef]
- Pongratz, M.; Schluga, P.; Jakupec, M.A.; Arion, V.A.; Hartinger, C.G.; Allmaierb, G.; Keppler, B.K. Transferrin binding and transferrin-mediated cellular uptake of the ruthenium coordination compound KP1019, studied by means of AAS, ESI-MS and CD spectroscopy. J. Anal. At. Spectrom. 2004, 19, 46. [Google Scholar] [CrossRef]
- Alessio, E. Thirty years of the drug candidate NAMI-A and the myths in the field of ruthenium anticancer compounds: A personal perspective. Eur. J. Inorg. Chem. 2017, 12, 1549–1560. [Google Scholar] [CrossRef]
- Aitken, J.B.; Antony, S.; Weekley, C.M.; Lai, B.; Spiccia, L.; Harris, H.H. Distinct cellular fates for KP1019 and NAMI-A determined by X-ray fluorescence imaging of single cells. Metallomics 2012, 4, 1051–1056. [Google Scholar] [CrossRef]
- Inigues, E.; Sánchez, A.; Vasquez, M.A.; Martínez, A.; Olivas, J.; Sattler, A.; Sánchez-Delgado, R.A.; Maldonado, R.A. Metal–drug synergy: New ruthenium(II) complexes of ketoconazole are highly active against Leishmania major and Trypanosoma cruzi and nontoxic to human or murine normal cells. J. Biol. Inorg. Chem. 2013, 18, 779–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iniguez, E.; Varela-Ramirez, A.; Martínez, A.; Torres, C.L.; Sánchez-Delgado, R.A.; Maldonado, R.A. Ruthenium-Clotrimazole complex has significant efficacy in the murine model of cutaneous leishmaniasis. Acta Trop. 2016, 164, 402–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, A.; Carreon, T.; Iniguez, E.; Anzellotti, A.; Sánchez, A.; Tyan, M.; Sattler, A.; Herrera, L.; Maldonado, R.A.; Sánchez-Delgado, R.A. Searching for new chemotherapies for tropical diseases: Ruthenium–clotrimazole complexes display high in vitro activity against Leishmania major and Trypanosoma cruzi and low toxicity toward normal mammalian cells. J. Med. Chem. 2012, 55, 3867–3877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colina-Vegas, L.; Godinho, J.L.P.; Coutinho, T.; Correa, R.S.; de Souza, W.; Rodrigues, J.C.F.; Batista, A.A.; Navarro, M. Antiparasitic activity and ultrastructural alterations provoked by organoruthenium complexes against Leishmania amazonensis. New J. Chem. 2019, 43, 1431–1439. [Google Scholar] [CrossRef]
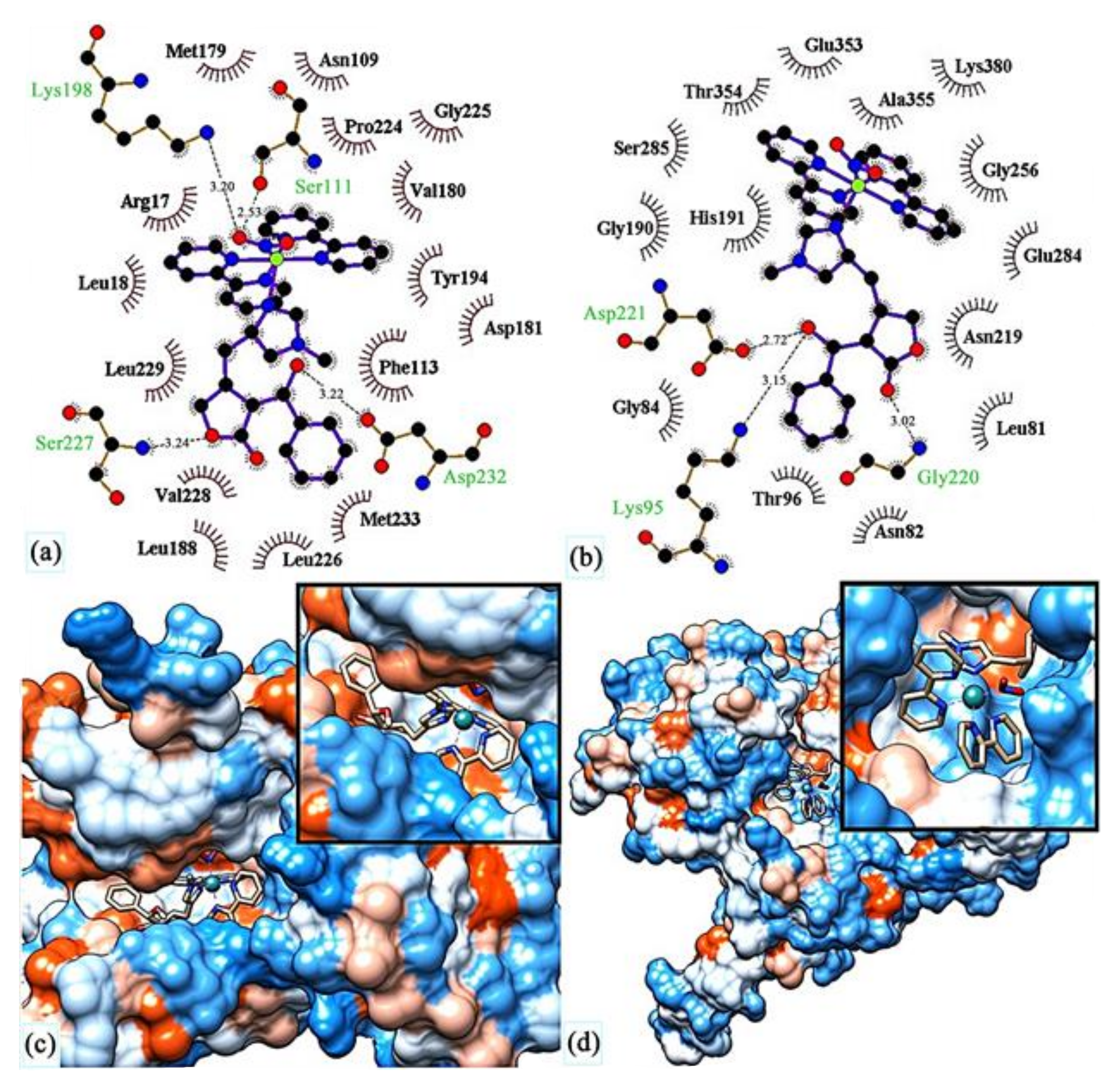
- Miranda, V.M.; Costa, M.S.; Guilardi, S.; Machado, A.E.H.; Ellena, J.A.; Tudini, K.A.G.; Von Poelhsitz, G. In vitro leishmanicidal activity and theoretical insights into biological action of ruthenium(II) organometallic complexes containing anti-inflammatories. Biometals 2018, 31, 1003–1017. [Google Scholar] [CrossRef]
- de Carvalho, L.C.; de Brito, T.V.; Júnior, J.S.D.C.; Dias Júnior, G.J.; Magalhães, D.A.; Sousa, S.G.; Silva, R.O.; da Silva, F.R.P.; Vasconcelos, D.F.P.; Véras, L.M.C.; et al. Epiisopiloturine, an imidazole alkaloid, reverses inflammation and lipid peroxidation parameters in the Crohn disease model induced by trinitrobenzenosulfonic acid in Wistar rats. Biomed. Pharmacother. 2018, 102, 278–285. [Google Scholar] [CrossRef]
- Silva, V.G.; Silva, R.O.; Damasceno, S.R.B.; Carvalho, N.S.; Prudêncio, R.S.; Aragão, K.S.; Guimarães, M.A.; Campos, S.A.; Véras, L.M.C.; Godejohann, M.; et al. Anti-inflammatory and antinociceptive activity of epiisopiloturine, an imidazole alkaloid isolated from Pilocarpus microphyllus. J. Nat. Prod. 2013, 76, 1071–1077. [Google Scholar] [CrossRef]
- Véras, L.M.; Guimarães, M.A.; Campelo, Y.D.; Vieira, M.M.; Nascimento, C.; Lima, D.F.; Vasconcelos, L.; Nakano, E.; Kuckelhaus, S.S.; Batista, M.C.; et al. Activity of epiisopiloturine against Schistosoma mansoni. Curr. Med. Chem. 2012, 19, 2051–2058. [Google Scholar] [CrossRef]
- Véras, L.M.; Cunha, V.R.; Lima, F.C.; Guimarães, M.A.; Vieira, M.M.; Campelo, Y.D.; Sakai, V.Y.; Lima, D.F.; Carvalho, P.S., Jr.; Ellena, J.A.; et al. Industrial scale isolation, structural and spectroscopic characterization of epiisopiloturine from Pilocarpus microphyllus Stapf leaves: A promising alkaloid against schistosomiasis. PLoS ONE 2013, 8, e66702. [Google Scholar] [CrossRef]
- Araújo, J.; Bastos, R.; Santos, G.; de Moraes Alves, M.; Figueiredo, K.; de Sousa, L.; Passos, I.; de Amorim Carvalho, F.; das Chagas Alves Lima, F.; Rocha, J. Molecular docking and evaluation of antileishmania activity of a ruthenium complex with epiisopiloturine and nitric oxide. J. Biosci. Med. 2020, 8, 42–53. [Google Scholar] [CrossRef]
- Barbosa, M.I.F.; Corrêa, R.S.; De Oliveira, K.M.; Rodrigues, C.; Ellena, J.; Nascimento, O.R.; Rocha, V.P.C.; Nonato, F.R.; Macedo, T.S.; Barbosa-Filho, J.M.; et al. Antiparasitic activities of novel ruthenium/lapachol complexes. J. Inorg. Biochem. 2014, 136, 33–39. [Google Scholar] [CrossRef]
- Huang, X.H.; Chen, Q.X.; Wang, Q.; Song, K.K.; Wang, J.; Sha, L.; Guan, X. Inhibition of the activity of mushroom tyrosinase by alkylbenzoic acids. Food Chem. 2006, 94, 1–6. [Google Scholar] [CrossRef]
- Dogra, N.; Warburton, C.; McMaster, W.R. Leishmania major abrogates gamma interferon-induced gene expression in human macrophages from a global perspective. Infect. Immun. 2007, 75, 3506–3515. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.S.; Gonçalves, Y.G.; Nunes, D.C.O.; Napolitano, D.R.; Maia, P.I.S.; Rodrigues, R.S.; Rodrigues, V.M.; Von Poelhsitz, G.; Yoneyama, K.A.G. Anti-leishmania activity of new ruthenium(II) complexes: Effect on parasite-host interaction. J. Inorg. Biochem. 2017, 175, 225–231. [Google Scholar] [CrossRef]
- Costa, M.S.; Gonçalves, Y.G.; Teixeira, S.C.; Nunes, D.C.O.; Lopes, D.S.; da Silva, C.V.; Borges, B.C.; Silva, M.J.B.; Rodrigues, R.S.; Rodrigues, V.M.; et al. Increased ROS generation causes apoptosis-like death: Mechanistic insights into the anti-Leishmania activity of a potent ruthenium(II) complex. J. Inorg. Biochem. 2019, 195, 1–12. [Google Scholar] [CrossRef]
- Fandzloch, M.; Arriaga, J.M.M.; Sánchez-Moreno, M.; Wojtczak, A.; Jezierska, J.; Sitkowski, J.; Wiśniewska, J.; Salas, J.M.; Łakomska, I. Strategies for overcoming tropical disease by ruthenium complexes with purine analog: Application against Leishmania spp. and Trypanosoma cruzi. J. Inorg. Biochem. 2017, 176, 144–155. [Google Scholar] [CrossRef]
- Negri, L.B.; Martins, T.J.; Ramos, L.C.B.; da Silva, R.S. Nitric oxide derivative ruthenium compounds as NO-based chemotherapeutic and phototherapeutic agents Nitric Oxide Donors. In Novel Biomedical Applications and Perspectives, 1st ed.; Seabra, A.B., Ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Da Silva, F.O.N.; Araújo, S.X.B.; Holanda, A.K.M.; Meyer, E.; Sales, F.A.M.; Diógenes, I.C.N.; Carvalho, I.M.M.; Moreira, I.S.; Lopes, L.G.F. Synthesis, characterization, and NO release study of the cis- and trans-[Ru(Bpy)2(SO3)(NO)]+ complexes. Eur. J. Inorg. Chem. 2006, 2006, 2020–2026. [Google Scholar] [CrossRef]
- Do Nascimento, N.R.F.; Aguiar, F.L.N.; Santos, C.F.; Costa, A.M.L.; Hardoim, D.J.; Calabrese, K.S.; Almeida-Souza, F.; de Sousa, E.H.S.; Lopes, L.G.F.; Teixeira, M.J.; et al. In vitro and in vivo leishmanicidal activity of a ruthenium nitrosyl complex against Leishmania (Viannia) braziliensis. Acta Trop. 2019, 192, 61–65. [Google Scholar] [CrossRef]


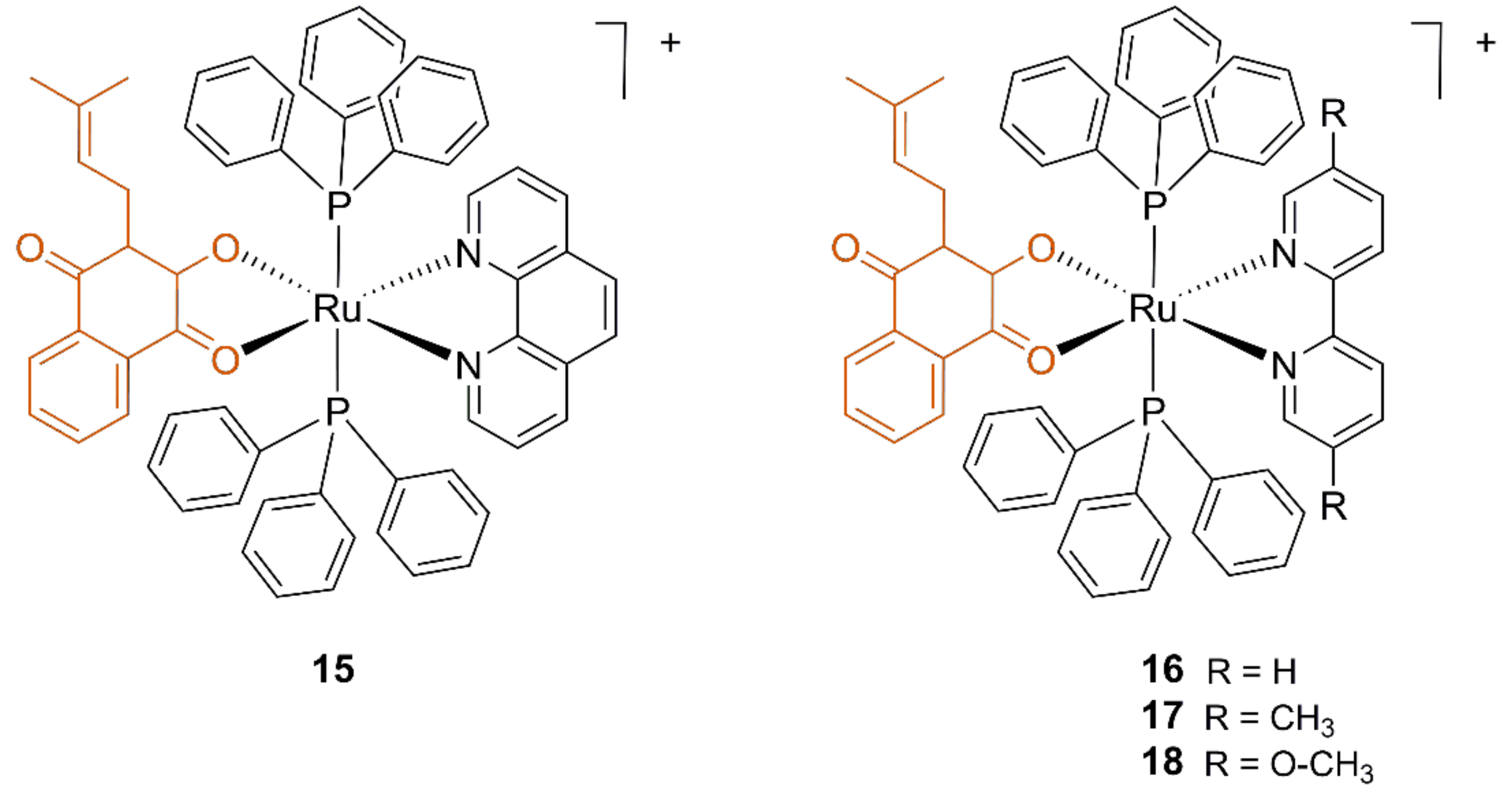

| Compound | L. amazonensis IC50 (μM) | Healthy Mouse J774 Macrophagues IC50 (μM) | |
|---|---|---|---|
| Promastigotes | Amastigotes | ||
| 15 | >10 | 0.07 ± 0.002 | 0.33 ± 0.08 |
| 16 | 0.18 ± 0.04 | 0.17 ± 0.01 | 1.0 ± 0.46 |
| 17 | 0.42 ± 0.03 | >10 | 6.7 ± 1.3 |
| 18 | 1.60 ± 0.44 | n.d. | 1.9 ± 1.3 |
| Lapachol | 12.4 ± 0.69 | >10 | >10 |
| Compound | L. Amazonensis IC50 (μM) | L. Braziliensis IC50 (μM) | L. Infantum IC50 (μM) | Healthy Mouse RAW 264 Macrophagues IC50 (μM) |
|---|---|---|---|---|
| 19 | 7.52 | 9.09 | 12.59 | 8.73 |
| 20 | 0.70 | 3.28 | 3.17 | 1.85 |
| 21 | 0.52 | 0.86 | 1.75 | 2.14 |
| Ru precursor | 15.48 | 3.93 | 19.46 | 62.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, S.S. Ruthenium Complexes, an Emerging Class of Leishmanicidal Drug Candidates. Appl. Biosci. 2022, 1, 129-142. https://doi.org/10.3390/applbiosci1020009
Braga SS. Ruthenium Complexes, an Emerging Class of Leishmanicidal Drug Candidates. Applied Biosciences. 2022; 1(2):129-142. https://doi.org/10.3390/applbiosci1020009
Chicago/Turabian StyleBraga, Susana Santos. 2022. "Ruthenium Complexes, an Emerging Class of Leishmanicidal Drug Candidates" Applied Biosciences 1, no. 2: 129-142. https://doi.org/10.3390/applbiosci1020009
APA StyleBraga, S. S. (2022). Ruthenium Complexes, an Emerging Class of Leishmanicidal Drug Candidates. Applied Biosciences, 1(2), 129-142. https://doi.org/10.3390/applbiosci1020009






