Abstract
The excessive use of agrochemicals in the field to increase production and counteract the negative effects caused by biotic and abiotic factors has led to a deterioration in soil fertility, plus an increment in negative impacts on the environment and human health. Therefore, the application of beneficial microorganisms as bioinoculants is an eco-friendly alternative to agrochemicals. Plant growth-promoting bacteria and fungi have been effective in promoting plant growth and production, as well as reducing the action of pathogens in multiple crops. However, successful application of such beneficial microorganisms in the agricultural field has faced several difficulties, such as survival, colonization efficiency and short periods of shelf storage. Therefore, it is essential to explore novel ways to encapsulate, formulate and apply bioinoculants. To obtain the expected quality in bioencapsulated products, it is essential to determine the type of polymer, capsule size, encapsulation technique and use the correct chemical and physical cofactors involved in the production process. Thus, this review highlights the various formulation types and application techniques, as well as discussing the multiple advantages of using microbial encapsulates to have better results in agricultural production.
1. Introduction
The continuous increase in the world population is accompanied by a high demand for agricultural products that must be satisfied in quantity and quality. In recent decades, and particularly since the advent of the green revolution, excessive use of chemical fertilizers has taken place to maximize production and improve the quality of crops and try to increase the productivity of nutritionally poor soils [1]. However, the benefits initially observed by the use of agrochemicals on crop productivity have been overshadowed by studies that show the adverse effects of excessive use of these products on the environment [2].
Among the damages caused to the soil are the deterioration in its structure and texture and a reduction in the populations of microflora and microfauna, which together trigger a nutritional imbalance within the soil. In addition, chemical inputs are the main source of contamination in soils used for agricultural production; they contribute significantly to the contamination of water and the atmosphere, triggering diseases in living beings [2].
In addition to the above, it has been shown that the use of chemical fertilizers by plants is inefficient, since they only take advantage of ~50% or less of the chemical doses applied, regardless of the nitrogen source with which they are formulated [3], causing an accumulation of the products used in the soil.
The damages that have been caused over time by the excessive use of agrochemicals require the development and implementation of technologies that have a minimal impact on the environment and that are designed in such a way that they can maintain and preserve the productivity of the soil. In addition, agricultural products intended for human consumption that are free of chemicals gives added value to these products [4].
One of the technological options to decrease the amount of chemicals in the soil environment is to use beneficial microorganisms that can promote plant growth and reduce synthetic fertilizers without negatively affecting crop productivity [5,6]. The use of these microorganisms, also known as biofertilizers, is one of the most important contributions of biotechnology and microbiology to modern agriculture, and it is an alternative for the reduction in production costs and the environmental impact caused by the excessive use of agrochemicals [7,8].
Therefore, the inoculation of microorganisms that promote plant growth in crops is a practical alternative to agrochemicals and can be applied directly to the soil, sprayed on plants or used as a coating for seeds [9].
2. The Role of Beneficial Microorganisms as Inoculants
The interaction of plants with microbial communities results from co-evolution over millions of years, contributing to the adaptation of plants on earth [10]. The interactions between microorganisms and plants occurs mainly in the portion of the soil that is in close contact with the plant root, known as the rhizosphere. This zone is defined as the volume of soil associated with and influenced by plant roots [11], constituting a favorable environment for the development of microorganisms in quantities that are much higher than those found in the rest of the soil. These high microorganism concentrations are a consequence of the fact that plants provide the necessary nutrients for the development of these microorganisms, which in turn provide the plants with substances that promote their growth, establishing a mutualistic relationship between both organisms [12].
The interactions between plants and beneficial microorganisms have been the subject of various scientific investigations, since this relationship provides a viable alternative for sustainable plant development and the conservation of the environment [4]. Some microorganisms that promote plant growth include mycorrhizal fungi, beneficial fungi or promoters of plant growth and certain rhizobacteria [13,14,15].
Mycorrhizal fungi are a group of root biotrophs that exchange mutual benefits with approximately 80% of plants and include arbuscular mycorrhizae and ectomycorrhizae from multiple fungal clades, such as Glomeromycota, Ascomycota and Basidiomycota [16]. Among the benefits of this mutualism is the supply of soil nutrients to plants in exchange for carbon from the host plants. This relationship results in an increase in the absorption of nutrients, the production of bioactive compounds and an increase in the production of fruits and tubers. In addition, this relationship has been highly effective as a nematicide, in addition to increasing the uptake of water to plants in certain environmental conditions [16,17].
Beneficial fungi or plant growth-promoting fungi (PGPF) have taken on great importance since it has been proven that they promote plant growth and, in turn, control numerous foliar and root pathogens by activating induced systemic resistance (ISR) in the host plant through various signaling pathways [8,18]. This group of organisms includes species of genera, such as Trichoderma, Aspergillus and Phoma [19,20].
Plant growth-promoting bacteria (PGPB) are a set of bacteria that inhabit the rhizosphere. Through different mechanisms, they promote plant growth and provide them with tolerance to both biotic and abiotic stress conditions [21]. Within this group we find species belonging to the genera Azospirillum, Azotobacter, Bacillus, Burkholderia, Enterobacter, Klebsiella and Pseudomonas, as well as some endophytic species, such as Axoarcus, Gluconacetobacter and Herbaspirillum [22,23,24].
The role of inoculants, such as PGPF and PGPB, on plants is to improve plant growth, production and resistance against several phytopathogens. These microorganisms are used as different types of formulations, prepared accordingly to the desired function or effect of the microorganism to be used. These formulations contain live or latent microorganisms (bacteria or fungi, alone or in combination) and, depending on the mechanism they use to promote plant growth (direct, indirect or both), are classified into one of three categories, i.e., biofertilizers, biostimulants or biopesticides [4,25]. The mechanisms of action of beneficial microorganisms are discussed in the next section.
Biofertilizers are formulations with one or several microorganisms that provide and improve the bioavailability of nutrients when applied to crops, biostimulants include microorganisms that promote plant growth directly through the production of hormones and biopesticides include microorganisms that are used to control phytopathogenic agents [26,27,28].
3. Mechanisms of Action of Beneficial Microorganisms
Certain microorganisms can promote plant growth through direct mechanisms or indirect mechanisms, although some mechanisms can work both directly and indirectly [29]. These mechanisms of action are briefly described below. We recommend the reader to other excellent recent reviews in this area [30,31].
3.1. Direct Mechanisms of Action
Direct mechanisms refer to the promotion of plant growth in two ways: microorganisms make it easier for plants to acquire the nutrients they need or they help to modulate the levels of plant hormones involved in the development and growth of plants [32,33,34].
With the increased use of chemicals in agriculture, much of the nutrients, such as soluble inorganic phosphorus used as a chemical fertilizer, becomes immobilized soon after its application, making it unavailable to plants [35]. Naturally, in the soil, insoluble phosphorus is found as apatite or in some organic forms, such as inositol phosphate (phytate), phosphomonoesters and phosphodiesters [36], forms which plants cannot directly assimilate. However, some microorganisms are capable of solubilizing inorganic phosphates through the production of low molecular weight organic acids that act on the inorganic phosphates making them available so that they can be used by plants [35]. Other microorganisms contain enzymes that can break down organic phosphates into a plant usable form [33].
Another important nutrient for plants is iron. The predominant form of iron in nature is Fe2+, which is not assimilable by plants. Some microorganisms can synthesize complex peptide molecules with a high affinity for Fe3+; these peptides are known as siderophores. The siderophores trap iron forming a complex; this complex may be taken up by membrane receptors of microorganisms and thus facilitates its acquisition. These iron–siderophore complexes can also be assimilated by plants and subsequently broken down inside of the plant, thus providing plants with the iron they need [37].
Nitrogen is one of the nutrients that plants require in larger concentrations, and it is found primarily in organic form in the soil. Nonetheless, plants take up inorganic nitrogen as ammonium and nitrates, rather than the organic form; thus, nitrogen mineralization from organic to inorganic form is crucial for plant growth and crop production [38,39]. Nitrogen fixing bacteria have gained attention in this regard, due to their capability to convert atmospheric nitrogen (N2) into ammonia (NH3), which plants can use, in a process called biological nitrogen fixation. Bacteria capable of such conversion encode the enzyme nitrogenase (a highly conserved enzyme complex), which catalyzes the conversion of N2 to NH3 [40,41,42].
Biological mechanisms, such as nitrogen, sulfur or phosphorous fixation [43], production of siderophores to increase iron bioavailability [44,45], phosphate and sulfates solubilization [46,47] and iron sequestration [33] help to incorporate or increase nutrients in the soil, along with their bioavailability to the plants. This increased provision of nutrients is provided by the following organisms: Rhizobium spp., Sinorhizobium spp., Mesorhizobium spp., Azotobacter spp., Azospirillum spp., Pseudomonas spp., Bacillus spp., Aureobasidium pullulans, Epicoccum nigrum, Scolecobasidium constrictum, Myrothecium cinctum and Acidianus spp., among others [43,44,46,47].
Some rhizospheric and endophytic microorganisms can produce plant hormones or induce their synthesis in plants. Many soil bacteria can produce hormones, such as cytokinins, gibberellins or auxins. In addition, some rhizosphere microorganisms produce the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase, where ACC is the immediate precursor of ethylene, a hormone related to the senescence of plants and the ripening of fruits and that very high levels of this hormone inhibit plant growth. The enzyme ACC deaminase converts ACC into α-ketobutyrate and ammonia, thus ethylene is no longer produced, and ammonia and α-ketobutyrate are compounds that can be assimilated by plants [42,48,49]. ACC deaminase production has been observed in plant beneficial organisms, such as B. subtilis, P. fluorescens, B. amyloliquefaciens, Enterobacter cloacae and Trichoderma sp., among others [50,51,52].
3.2. Indirect Mechanisms of Action
Other microorganisms can promote plant growth indirectly. A common indirect mechanism is competition for space and nutrients, where the beneficial microorganism competes with a pathogen and the one with the greatest capacity to take up nutrients and the fastest growth rate proliferating in the soil displaces the pathogen and prevents it from colonizing and infecting plants [34,53,54]. The successful competition of PGPB with pathogens provides plants with a greater opportunity to grow and develop. It is worth mentioning that the production of siderophores can also be classified as an indirect mechanism, since the microorganisms with the capacity to produce these molecules and take up siderophore–iron complexes will limit the growth of the pathogen competing for this nutrient [45,55,56].
The production of antibiotics or antimicrobial compounds is a common and often studied indirect mechanism of plant growth promotion. For example, pyrrolnitrin produced by Pseudomanas spp. [57], iturine produced by Bacillus spp. [58] and syringomycin, produced by P. syringae [59] are some of the most common antipathogen antibiotics.
In addition to antibiotics, various microorganisms produce volatile organic compounds (VOCs) that can also be toxic to pathogens, preventing their growth or leading to their death, such as dimethyl disulfide produced by Bacillus sp. E25 [60] and B. thuringiensis CR71 [61]. Some of these VOCs, in addition to having antimicrobial action, can also promote plant growth directly, as is the case of dimethyl-hexa-decylamine [62]. Therefore, the production of VOCs can be classified as both a direct and an indirect mechanism.
Another indirect mechanism of plant growth promotion is the production of lytic enzymes related to the ability of the microorganism to parasitize and destroy the pathogen, as is the case of mycoparasitism carried out by fungi of the genus Trichoderma, adhering to the hyphae of the pathogen, where it secretes enzymes that degrade the cell wall, resulting in the death of the pathogen, or the destruction of its structure, preventing its development [50,63,64,65].
Finally, induced systemic resistance, initiated by the PGPB, is an indirect mechanism that involves activating the biochemical and molecular defense responses of the host plant, these may include the production of reactive oxygen species (ROS), phytoalexins, synthesis of proteins related to pathogenesis (PR), lignin accumulation at the site of infection, among others [66]. Some microorganisms that promote plant growth induce this defense response, preventing the colonization or the development of infection by phytopathogens [45,67,68].
4. Formulation of Microbial Inoculants
To enable plant growth-promoting microorganisms to be used and applied in agricultural practices, it is necessary to develop formulations based on these bioinoculants. Formulating a bioinoculant includes the entire series of procedures and technologies after the growth in culture of the microorganisms that promote plant growth. Bioinoculant formulation includes the mixture of a selected beneficial strain with a suitable vehicle that preserves the viability of the microorganism in either a dormant or metabolically active state during transport, storage and application [69]. To obtain a successful formulation, the microorganism must overcome the conditions of temperature, humidity, salinity, UV radiation and water stress present in the soil and during its formulation, in addition to being effective and competitive against the native microbial populations of the soil [1,70].
The compatibility of the physical form of the bioinoculant (solids in the form of powder, granules or capsules and liquids) and its incorporation through agricultural practices is a key factor determining the durability of the product and its ability to colonize plant roots [71]. According to the physical form of the inoculant, it is classified as a liquid formulation, a solid formulation or bioencapsulated [21,71].
4.1. Liquid Formulation
Liquid formulations use culture broths or formulations based mainly on water, mineral or organic oils. Seeds and seedlings can be immersed in the inoculant before sowing or transplanting [70,72], and for the biocontrol of pathogens or physiological stimulation, they can be sprinkled on the foliage of already established plants or applied directly to the soil [73]. This formulation method is the most commonly used. It is directly applied to crops without going through other processes following fermentation; most microorganisms can survive for more than one year if the containers are kept at ~4 °C, they are easy to inoculate and their application is very practical when implemented in irrigation or sprinkler systems. Liquid formulations are relatively low cost. However, even when its efficacy has been proven, its stability during storage is often limited due to its susceptibility to contamination with other microorganisms [69,74].
Liquid formulations of different microorganisms, or even in microbial consortia, and with the use of various additives, have managed to increase yields in agricultural fields. For example, when a liquid bioinoculant based on sugar and coconut water, including Pseudomonas spp., Bacillus spp., Klebsiella spp., Aspergillus spp., and Azotobacter spp., was used to inoculate soybean plants, the result was improved nutrient solubility and increased crop yield [75].
It has also been shown that the phosphate solubilization capacity and the survival rate of Pseudomonas and Pantonea strains increases and are preserved when they are used in liquid formulations up to three months after their formulation containing diluted concentrations of phosphate buffer and nutrient broth with glycerol [76]. In addition, Camelo-Rusinque et al. [77] assessed the population dynamics of the Azotobacter chrocoocum strain AC1 in MBR culture medium under bioreactor conditions after 105 days and found that both the cell viability and the biological activity of the strain was maintained, regardless of the storage temperature. This indicates that some liquid formulations can be used for a specific time, with the organisms retaining their activity and continuing to be viable for use as bioinoculants.
4.2. Solid Formulation
Solid formulations are used widely in the agricultural industry because of the advantages they offer during storage and transportation. A simple technique used for the preparation of solid formulations is adsorption, which consists of mixing the microorganisms with a solid support, such as vermiculite, perlite, sepiolite, kaolin, diatomaceous earth, natural zeolite, peat or clay, the latter being of great interest in agriculture thanks to its ability to act as a desiccant and provide excellent storage conditions for various inocula, as it has a good ability to adsorb agents dispersed or suspended in it [78,79].
Peat is one of the supports most used worldwide in commercial crops due to its low cost. However, being a complex organic matter, different batches present great chemical variability and, consequently, it is difficult to maintain the same quality in all batches. In addition, its storage is very susceptible to humidity, which decreases the inoculum cell survival [78]. According to Rose et al. [80], it is essential to be able to quantify the number of viable cells of each microorganism per unit weight of inoculant, to determine the inoculum potential at different application doses and for field results to be properly interpreted.
In a study by Quiroz Sarmiento et al. [81], the effectiveness of peat was evaluated with the following bacteria: Serratia liquefaciens CPAC53, S. plymuthica CPPC55, P. tolaasii P61 and P. yamanorum OLsSf5, in comparison with the encapsulation of the strains using alginate beads. Following a storage period of 150 days, the results showed that the encapsulated strains maintained the highest population. The effect of both types of bioinoculants on poblano chili seedlings (Capsicum annum L.) was also evaluated. In this case, the best results were observed with the encapsulated strains [81]. This suggests that the success in using peat as a support material for solid formulations depends on the conditions in which the bioinoculum will be used and the availability of other strategies.
A common technique of solid formulation is spraying or lyophilization. This technique allows for the realization of high microbial survival rates without the need to use any support, allowing for easy inoculum storage for long periods, at room temperature, without the need for refrigeration. One of the disadvantages of lyophilization is that it is necessary to protect the cell membrane and cytoplasm against dehydration during the storage period, using a cryoprotectant, such as mannitol and microcrystalline cellulose [82]. In this way, the cells remain viable and can be used long after lyophilization, for at least a year [83].
Lyophilized microorganisms may be mixed with a solid support or used directly. For example, in the laboratory, Grzegorczyk et al. [84] studied the survival and storage stability of a strain of Trichoderma hariazum, four strains of Trichoderma atroviride and two strains of Trichoderma virens, after culture lyophilization in solid wheat straw medium with and without the addition of maltodextrin. It was observed that the strains had a higher survival capacity (except for strain T. atroviride TRS40), compared to the addition of distilled water only, and in comparison with the bioformulation containing just maltodextrin. Three months after lyophilization, the strains remained stable and most still showed cellulolytic and xylanolytic activity.
Wessman et al. [85] studied the survival of the bacterial strains P. putida KT2440 and A. chlorophenolicus A6 after lyophilization in four different formulations, including (i) sucrose, (ii) Ficoll PM400 a sucrose polymer, (iii) hydroxyethylcellulose (HEC), and (iv) hydroxypropylmethylcellulose (HPMC). The polymers were chosen to obtain a monomeric structure, such as sucrose. The results of this study indicated that a key factor to help cell survival is the ability of the added ingredients to replace water during dehydration, thereby maintaining the structure of proteins and cell membranes in a dry state. Disaccharides, such as sucrose, show this property, while polymers, such as starch-based polysaccharide, do not. Thus, some polymers can facilitate cell survival to the same extent as disaccharides provided that certain physical properties of the formulation are controlled [85].
In fact, one of the techniques that have gained great importance in recent years thanks to its advantages, is the solid formulations developed based on polymers. These polymers, in the presence of ions or changes in chemical conditions, form complex matrices so that microorganisms become immobilized and encapsulated in the matrix and are gradually released as the polymer degrades. The technique of microorganism immobilization ultimately creates barriers between the microbes and the environment, improving their bioavailability and preserving their biological stability [86].
5. Bioencapsulation of Plant Growth-Promoting Microorganisms
The growing demand for the use of microorganisms as bioinoculants for use in agriculture has facilitated the use of technological tools that allow compliance with the need to develop products that can promulgate agricultural sustainability. When plants are commercially inoculated with plant growth-promoting microbes, the formulation process generates certain problems when applied in the field, given that the main physical form in which the formulation is presented as a liquid or as powders that fails to protect the survival of the microbial strains in the face of abiotic conditions (temperature, humidity, salinity, UV radiation, pH) present in the application process [87].
The use of polymers through the encapsulation technique has been proven as a highly effective alternative to increase the viability of microorganisms and, in turn, provide protection against the environmental conditions present [88,89]. Figure 1 represents the various biotic and abiotic factors that bioencapsulated microbes face when used in the field.
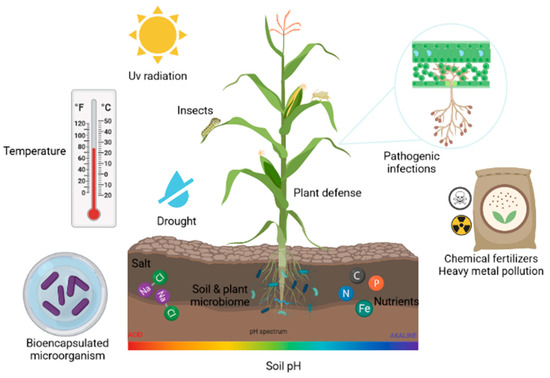
Figure 1.
Biotic and abiotic factors that cause stress in plants and affect bioencapsulated microorganisms.
6. Classification of the Well-Encapsulated
Bioencapsulated microbes can be classified according to the type of mechanism used by the microorganism to promote plant growth or according to the type of microorganisms used in their formulation, whether they are bacteria, fungi or a combination of both [69,86].
6.1. Bioencapsulated Bacteria
Bacteria, particularly PGPB, have been widely used in agricultural research and have proven to be a tool for improving plant health and growth without causing environmental pollution [89,90]. PGPB manage to mitigate abiotic stress in the soil through the production of phytohormones and associated metabolites, as well as through significant morphological changes in the roots [91,92,93]. These changes result in a better nutritional status for plants and, in turn, stimulate plant defense mechanisms to overcome unfavorable environmental conditions [10,94].
Some bacteria can migrate from the rhizosphere to the internal tissues of the plant. They do this through cracks that form in the roots as a consequence of growth, they can also enter through lenticels, due to the emergence of lateral roots or by the cells of the root hairs, among other ways. Once inside the plant, they can exert their action of promoting plant growth either by direct or indirect mechanisms and, in turn, the bacteria are protected from abiotic stress and are exempt from competing for resources and space with other soil microorganisms. The bacteria capable of colonizing the internal tissues of a plant without causing damage are known as endophytes [30,95,96].
Some bacteria can form highly stable dormant spores that can germinate, forming active bacteria when conditions are favorable [97]. Fully formed spores are recognized as one of the most resistant life forms on the planet, they protect the bacterial genome against heat, desiccation, radiation and oxidation, as well as being an efficient way to escape predation by higher organisms. Spore germination is triggered by the presence of nutrients in the environment, these are detected by membrane receptors and in a matter of minutes, the nucleus of the spores is activated, the spore rehydrates, the cortex hydrolyzes and its surface cover changes [98,99,100]. This naturally occurring process is considered to be an effective technique for the development of inoculants as it allows greater survival of the strains during the storage process, making it possible to develop encapsulation on a large scale and, in turn, is more profitable [101].
Among the bacteria that form spores are Clostridium, Sporosarcina, Thermoactinomyces and Bacillus; there are numerous reports of the beneficial effects of Bacillus strains on crops of agricultural interest. Bacillus strains promote plant growth, increase phosphorus solubilization and increase the production of growth regulators, and they are highly efficient for pest control. Within this group is the B. thuringensis species, which is widely applied worldwide as a consequence of its activity as a biological insecticide [102,103].
Many strains of bacteria have been used to make bioencapsulated bacteria based on various polymers, and their effect as biostimulants and/or biopesticides has been studied in several field or greenhouse experiments (Table 1). The bioencapsulation of spore-forming bacteria provides them with additional resistance to environmental factors; however, this type of formulation represents a greater advantage for those organisms that do not form spores, also preserving their viability, which opens the possibility of using a greater diversity of organisms that promote plant growth or biocontrol in the field.

Table 1.
Encapsulated microorganisms of agricultural importance, their beneficial effect on plant crop and application technique.
6.2. Bioencapsulated Fungi
Among the fungi that have been bioencapsulated, the entomopathogenic fungi, mycorrhizal fungi and fungi that promote plant growth and biocontrol stand out.
Entomopathogenic fungi (HEP) are part of the most important biological formulations in the microbial control of pest insects; they have also gained importance in their use as plant growth promoters and even as good plant tissue colonizers. For example, the endophyte, Metarhizum brunneum, encapsulated in alginate, was able to preserve its viability, in addition to being able to efficiently colonize tomato plants [124].
Arbuscular mycorrhizal fungi (AMF) are plant growth-promoting organisms that, following the process of absorbing mineral nutrients from the soil, transfer these nutrients to the plant. Arbuscular mycorrhizal hyphae spread widely in the soil and function as an extension of the roots, increasing the ability of plants to absorb water and nutrients from the soil [127]. Among the beneficial effects they offer are stability to the soil structure, greater host plant tolerance to water stress [128] and, as a consequence of its mycelium increasing the absorption range of the roots, it mitigates aluminum toxicity stress generated in the soil [129].
The use of encapsulation for AMF spores improves the efficiency and stability of fungal bioinocula and, therefore, it is possible to meet the optimal mechanical properties for their handling, transport and stability, following their formulation [121].
Trichoderma and Metarhizum are among the genera of fungi that promote plant growth of agronomic importance, which, through their encapsulation based on polymers, have managed to promote the plant growth of crops, such as Cajanus cajan, Lactuca sativa and Solanum tuberosum [120,123,125].
7. Bioencapsulation Process
Bioencapsulation is a cell immobilization process that consists of trapping microorganisms in a polymeric material, capable of allowing the passage of metabolites and gases to preserve cell viability and forming small capsules [86,130]. The bioencapsulation process uses different techniques, depending on the purpose and the type of microorganism being used. However, for each bioencapsulation, it is necessary to take into account: the selection of the material or polymer used, the desired size of the capsule, and the most appropriate technique to use [86]. Figure 2 illustrates the general steps of the bioencapsulation process of plant growth-promoting microorganisms (PGPM), and the most relevant points to consider for bioencapsulation are described below.
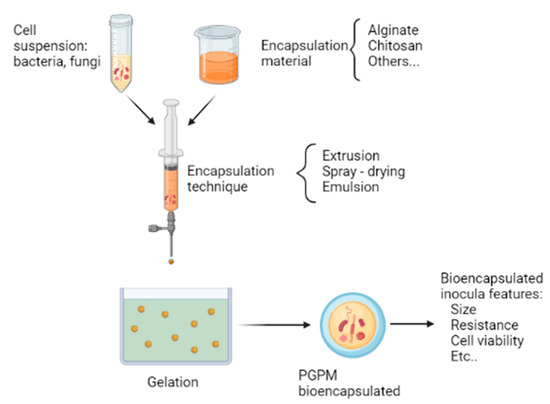
Figure 2.
General bioencapsulation process of plant growth-promoting microorganisms (PGPM).
7.1. Coating Materials in Encapsulation
The materials used for the encapsulation of the microbial cells are a key part of the formulation process. It is necessary that these materials have certain properties or characteristics, such as the ability to (i) protect cells against environmental conditions, (ii) disperse with the material to be encapsulated, (iii) release their contents under specific conditions, and (iv) cover and maintain the encapsulated organisms within its structure [78,86,131,132]. These coating materials include hydrogenated oils, waxes, maltodextrins, celluloses, starches, gums and various polymers, the latter of which play a dominant role in determining and forming capsules [69,86].
Polymers in general are chemical substances made up of many repeating units called monomers, with multiple bonds chemically linked or polarized together to build the polymer chain. Natural polymers (biopolymers) are compounds typically formed by polysaccharides, such as cellulose, chitosan or starch, and proteins, such as keratin or collagen [133]. These biopolymers have gained importance in the manufacture of bioencapsulated microorganisms because, compared to synthetic polymers, they have greater benefits for the microorganisms that are encapsulated within them. For example, greater resistance to environmental factors and increased cell viability was observed with biopolymers, compared to synthetic polymers [78,86]. Among the main polymers used in agricultural bacterial encapsulation are alginate and chitosan. In addition, other biopolymers, such as carrageenan, gelatin and laponite, have been used [106,115,118], although their use is less frequent, or they are used in combination with other polymers.
7.1.1. Alginate
Alginate is a linear polysaccharide of D-mannuronic and L-guluronic acids that is naturally present in various species of algae and some bacteria [134,135]. Alginate is the most widely used polysaccharide in the encapsulation process because it is a non-toxic, biocompatible, inexpensive and readily available material that allows the encapsulation of microorganisms in a simple way [136]. Among its main advantages is the fact that it can gel easily, has good solubility and low viscosity and can be used in mild conditions, allowing cells to be trapped with minimal losses of viability [134,137].
Calcium alginate capsules (pearls) are structured as a flexible network, and if they have been used for encapsulation, they are filled with a large amount of water (97–98% by weight). Using only alginate for cell bioencapsulation does not adequately protect the cells during the drying or solidification process and, consequently, the pearls are slightly deformed, so it is generally used in combination with other organic compounds. These compounds include starch, glycerol, chitin, skim milk or humic acids [69,131,138]. Alternatively, alginate may be mixed with clays, such as montmorillonite or halloysite, thereby increasing the mechanical resistance and improving the quality of the capsule, in addition to the survival of microorganisms [105].
Biocapsules made with alginate have many advantages for microorganisms. Glomus sp. and Acaulospora sp. alginate biocapsules increase water adsorption, thereby favoring the germination of these mycorrhizal fungi; increase colonization of plant roots; and plant resistance to water stress [121]. The concentration of nitrate and phosphorus in Eugenia stipitata plants, as well as plant biomass, increases when plants are treated with encapsulated inocula (with alginate or clay) of Azospirillum brasilense, Burkholderia cepacia, B. thuringiensis, B. megaterium, B. cereus, B. subtilis, B. subtilis strain 1411 and Trichoderma sp. [139]. Using alginate with gelatin, pectin, kaolin or bentonite to encapsulate Mesorhizobium ciceri and Bradyrhizobium japonicum increased the number of nodules formed in plants of Cicer arietinum (chickpea) and Glycine max (soybean), in comparison with non-inoculated plants [122].
The use of alginate to encapsulate microorganisms of agricultural interest improves their characteristics as biocontrol agents and as growth promoters, promoting endophytism and the content of nitrogen and phosphorus in plants [112,113,116,123,140].
7.1.2. Chitosan
Chitosan is made from glucosamine and N-acetyl-glucosamine units, is biodegradable, abundant and easy to obtain from the deacetylation of chitin, a component of the exoskeleton of crustaceans, mollusks, insects and fungi [141].
In the presence of anions and polyanions, such as alginate, chitosan can polymerize through cross-links, reducing the porosity of the alginate, and, therefore, improving its protective effect of the encapsulated microbe [141]. By combining both biopolymers to encapsulate the bacterium Bacillus licheniformis, the promotion of plant growth of Capsicum annum L. plants was improved, as well as its capacity to act as a biocontrol agent of the pathogen S. rolfsii [118]. Streptomyces fulvissimus Uts22 encapsulated in a mixture of chitosan with gellan gum promotes the development of roots and lateral shoots in wheat plants, in addition to controlling the growth of the pathogen Gaeumannomyces graminis [104] (Table 1).
In general, the use of chitosan, whether applied alone or for the introduction of other particles or microorganisms in agriculture, is highly efficient in controlling both biotic and abiotic stress, in addition to promoting growth of various plant species, thus, when used to encapsulate microbes, chitosan can have an additive effect to the benefits of the microbe [142].
7.1.3. Other Biopolymers
In addition to alginate and chitosan, there are other polymers that can be used to improve the stability and/or quality of capsules. Among these polymers is gum Arabic, which is obtained from Acacia senegal and A. seyal trees and is made up of a mixture of complex polysaccharides. In addition to oligosaccharides and glycoproteins, gum Arabic is rich in essential elements and trace elements, such as aluminum, phosphorus, magnesium, copper, zinc and iron, and acts as an emulsifier, stabilizer and protector from chemical decomposition [143,144,145].
Starch is one of the most widely used accompanying polymers with alginate, as it provides good protection to bacterial cells and allows optimal diffusion of micronutrients and metabolites in various formulations [146]. The starch is mainly obtained from corn, potato, barley and oats, and its amylose and amylopectin units are linked by glycosidic bonds [147]. In combination with maltodextrin and sodium alginate, the encapsulation of B. subtilis with these materials maintain cell viability and is an efficient way to control the pathogen Fusarium oxysporum f. sp. lycopersici [148].
Maltodextrin, which is obtained from starch, is a linear polysaccharide of glucosamine and N-acetyl-D-glucosamine units. It is used at low concentrations as a coating material for the production of microparticles [148].
Gelatin, which is derived from collagen and consists of glycine, proline and 4-hydroxyproline residues, is very useful as a thermoreversible gelling agent for encapsulation, either alone or in combination with other polymers. Due to its amphoteric nature, it can also form a strong interaction with anionic polymers, providing greater stability to the capsules. Gelatin, in combination with gum Arabic, has been used to encapsulate Metarhizium anisopliae, which is used to biocontrol fire ants (Solenopsis invicta) [145] or in combination with alginate to encapsulate B. subtilis SL-13, greatly increasing cell viability [74].
Carrageenan, which is extracted from red seaweed (Irish moss) and some bacteria, are polymers that have a linear structure made up of D-galactose units alternately linked by α-(1,3) and β-(1,4) bonds. These polymers can form a gel that traps microbial cells and can be used in combination with alginate. However, the gelation of carrageenan is induced by changes in temperature, which should be considered when using it to make bioencapsulated microbes, especially when it comes to organisms that may be sensitive to temperature [149,150].
Another polymer that has been used is agar/agarose. It is mainly extracted from marine red algae and is composed of alternating β-D-galactopyranosyl and 3,6-anhydro-α-L-galactopyranosyl units. One of its important characteristics is that it is resistant to degradation by most known microorganisms and is a thermosetting hydrogel, gelling in response to a reduction in temperature. However, it has the disadvantages of low mechanical strength and high cost [151,152], which should be considered when using it as a material to produce bioencapsulated microbes.
An important factor for the success of the bioencapsulated microbe formulation is the correct selection of the appropriate carrier for the microorganism of interest, providing stability and protection against environmental factors, such as UV radiation, dryness and high temperature [152]. Therefore, when choosing the appropriate polymer, factors, such as availability of the polymer, resistance to environmental factors, ability to allow cell viability, whether it will be used alone or in combination with other polymers and costs, should be considered. Table 1 shows examples of studies where various biopolymers have been used for microbial encapsulations.
7.2. Capsule Size Selection
According to the size of the capsule that is formed, capsules can be classified as macrocapsules or microcapsules. Macrocapsules range in size from millimeters to centimeters, while microcapsules range in size from 1 to 1000 µm [86]. For the preparation of bioencapsulated microorganisms of agricultural interest, the use of microcapsules is preferred, because the smaller size increases the cell concentration, is more resistant and can be better dispersed in the soil or in pots [86,131].
The size of the capsule is important at the time of application in an agricultural field; normally the most common formulation is 1–4 mm in size. However, when freely mixed with seeds and sown together, the spheres can fall far from the seed and these distances can be restrictive for many beneficial bacteria, even though their mobility in the soil has been proven. To produce smaller spheres with sizes ranging from 50 to 200 µm, it is necessary to use the appropriate technology that ensures the concentration of the biomass of the microorganisms to be encapsulated [88,131], Therefore, a small capsule size is preferred to favor its dispersion close to the plant and to ensure the interaction of beneficial microorganisms with the plants (Figure 2).
7.3. Encapsulation Techniques
There are various encapsulation techniques that allow microcapsules to be produced. However, their choice depends on various factors, such as the microorganism, to be encapsulated, the temperature, humidity, and agitation (all of which affect the microorganism’s survival), the polymer to be used and the purpose of the bioencapsulation. The most commonly used techniques for the formulation of bioencapsulated microbes for use in agriculture is extrusion, although there are other techniques that could also be used, such as the spray or emulsification technique.
7.3.1. Extrusion Encapsulation
Extrusion encapsulation is the most studied and oldest technique for producing capsules with polysaccharides, such as alginate, as it has the advantages of low cost and ease of implementation. This technique consists of mixing microbial cells in an aqueous solution of biopolymer that has gelling capabilities, and then this mixture is extruded in a gelling environment through a small nozzle or syringe to create small droplets of biopolymer containing microbial cells [86]. This technique also incorporates some methods to coat or gel the capsules, thus stabilizing the biopolymer droplets to prevent their dissociation or aggregation and provides them with better quality [133].
There are two mechanisms to carry out the gelation of capsules. The first is called external gelation and is obtained when the solution of the compound to be encapsulated is added together with the selected coating material, the mixture is forced through nozzles generating drops, and these fall into a bath of calcium ions, thereby forming a gel capsule. This mechanism is quite common and is simple to perform; however, it produces heterogeneous gels because surface gelation often occurs before core gelation, resulting in a rigid surface and a soft core [153,154]. The second mechanism, internal gelation, consists of the preparation of a solution of calcium ions and the compound of interest to be encapsulated, this mixture is forced through a nozzle and poured into a bath of sodium alginate. To carry out the release of calcium ions, the medium is acidified by adding an organic acid, such as acetic, adipic or glucono δ-lactone. In addition, a sequestering agent is added, which binds with free calcium, thus slowing down the gelling process [155,156,157].
External gelation provides larger capsule sizes (>2000 μm) and better encapsulation efficiency, therefore, it is used for the encapsulation of essential oils, bioactive compounds and plant-derived extracts [158,159]. On the other hand, internal gelation is used more frequently for the encapsulation of microorganisms due to the fact that a uniform capsule size and a smooth surface are obtained, through which agglutinations, cracks and pores are less likely to occur [154,160], resulting in better quality and more resistant capsules, without the need to use organic solvents or high temperatures to harden or coat the capsules.
The extrusion technique is gentle on microorganisms and does not require toxic solvents, and, therefore, does not cause cell damage, ensuring greater cell viability. However, this technique has the disadvantages that the production capacity is low if it is required on an industrial scale, and the size of the particles can be too large for some uses, such as agriculture [161]. However, other techniques can be adapted, along with extrusion, to obtain the desired particle size, such as the precision particle manufacturing (PPF) technique, and rotary atomization discs, among others [86,161].
Other bioencapsulation techniques have been used, such as spray-drying or emulsification, to obtain capsules of beneficial microorganisms for the food industry; however, the use and study of these techniques for the encapsulation of microorganisms of agricultural interest is not well studied.
7.3.2. Spray Drying
Spray drying is a technique that has been employed mainly in the encapsulation of microorganisms for the food industry [86,161]. It is a technique that consists of atomizing a suspension that contains the microorganism to be encapsulated and a polymeric material inside a chamber with hot gas (>100 °C up to 170 °C), generally air, which promotes the evaporation of water, causing the microorganisms to remain trapped inside the encapsulating material, giving rise to the formation of microparticles [161]. However, because this technique simultaneously dehydrates and raises the temperature of microorganisms, various microorganisms, such as non-spore-forming bacteria can suffer high mortality [69]. In addition to temperature, other variables that need to be controlled are feed flow and air flow, being key to determining the viability of microorganisms and the success of this technique for the production of bioencapsulated microbes [162,163].
7.3.3. Emulsification
Emulsification is a technique used to encapsulate different microorganisms, and it consists of mixing a disperse phase, formed by the cells and the encapsulating polymer, in a continuous phase, which is generally oil or an organic solvent. In this way, an emulsion of water in oil is obtained that is homogenized using a surfactant, such as Tween, and with constant agitation, which promotes the stability of the capsules. Alginate, carrageenan and pectin are ideal for use in this technique as encapsulating materials. Then a solidifying agent, such as calcium chloride, is added to the emulsion to form the capsules that will later be filtered. The capsules obtained can vary in size between 25 µm and 2 mm, depending on the speed of agitation, so this technique can be used to make microcapsules. However, one of the main disadvantages of this technique is the use of organic solvents that could be toxic in the subsequent use of the encapsulated microbes and removing the oil from the capsules can be difficult [86,164]. On the other hand, with this technique other types of low molecular weight materials can also be used to coat the capsules, however, the microorganisms tend to be rapidly released through the gel [133].
8. Benefits of Bioencapsulation in the Field
One of the greatest challenges in the use of plant growth promoting microorganisms in the field is their distribution in the soil and how to apply them. By directly applying microorganisms to the soil, they are exposed to environmental conditions that can be harmful to them, such as lack of water, changes in pH and temperature, in addition to the fact that their distribution area may be limited, all of which can result in the mortality of organisms and a decrease in their ability to benefit crops [69]. The use of techniques to immobilize and distribute microorganisms, such as the production of bioencapsulated microbes, has become essential to overcome the limitations encountered with the direct use of these in the field. Thus, bioencapsulation has many advantages for and the deliberate release of microorganisms to the soil. These advantages include being able to provide protection against environmental factors, increasing cell viability in the soil, favoring cell dispersion and facilitating microbial cell contact with plants, thereby increasing their effectiveness [69,86].
Among the microorganisms evaluated in the field or in greenhouse experiments, there are fungi, such as Trichoderma and Metarhizum, and a variety of bacteria, including Bacillus, Pseudomonas, Azotobacter, among others, which have been widely used as biofertilizers, biostimulators or as biopesticides and insecticides in crops of interest, such as Zea mays, Triticum sp., Solanum lycopersicum, Oryza sativa, etc., [90,105,114,121].
In a greenhouse experiment, the inoculation of wheat seeds with Streptomyces fulvissimus Uts22 microcapsules, prepared with chitosan and gellan gum, promoted the growth of plants and increased their resistance to the pathogen Gaeumannomyces graminis var. tritici, to a greater extent than inoculation with the free bacteria [104]. In a separate greenhouse experiment, with bell pepper plants, the inoculation of P. putida microcapsules significantly promoted plant growth, compared to uninoculated plants or plants inoculated with unencapsulated bacterial cultures [110]. These examples are consistent with the benefits of encapsulated microbes, compared to the application of liquid or unencapsulated cultures.
In the field, the inoculation of capsules of different bacteria of the genus Bacillus, Azospirillum and Burkholderia increased the concentration of nutrients, such as nitrogen and phosphorus, in Eugenia stipitata plants [119]. Microcapsules containing Mesorhizobium ciceri ST-282 and Bradyrhizobium japonicum M8 increased the number of nodules in roots of chickpea and soybean plants in a field experiment [122]. The encapsulation of T. viride increased the content of secondary metabolites in lettuce plants, when grown in the field and also in hydroponic culture [125], highlighting the importance of encapsulating beneficial microorganisms, including both fungi bacteria in different cultivation techniques.
The encapsulation of Ensifer fredii LP2/20 applied to soil cultivated with kale significantly modified the composition of the microbial community, also increasing the biomass of plants [109], which suggests an interaction of the encapsulated microorganisms with the soil microbiota, resulting in a positive effect for crops in general.
Beneficial microorganisms can also protect plants against different types of stress. The encapsulation of Paenibacillus polymyxa MSRH5, B. nakamurai MSRH1 and B. pacificus MSR H3 reduce the effects caused by salt stress in wheat plants, in addition to increasing plant biomass [112]. The inoculation of microcapsules of P. putida Rs-198 promotes plant growth of cotton plants when they are subjected to salt stress [108], providing resistance to this type of abiotic stress, and suggesting that encapsulated microorganisms can resist environmental factors, such as salt stress.
It is worth mentioning that most of the bioencapsulation experiments have been carried out under greenhouse conditions (Table 1) with favorable results. However, the application of bioencapsulated microbes in field experiments poses other challenges, such as greater exposure to environmental factors, that cannot be controlled. The studies mentioned above and others referring to organisms of agricultural interest that have been encapsulated and used in greenhouse or field experiments are summarized in Table 1.
9. Conclusions and Perspectives
Today the growing world population is accompanied by an increased demand for agricultural products. Thus, it is necessary to maintain food security for humans and animals without neglecting the conservation and improvement of ecosystems. The excessive use of agrochemicals to increase agrarian production has caused great damage to human health and ecosystems, deteriorating soil and water quality and in general, altering the environment. However, one of the alternatives to agrochemicals in the field is the use of beneficial microorganisms. Its use favorably contributes to increased crop yields, increased tolerance to water and saline stress conditions, and increased resistance to phytopathogens, thus potentially replacing the excessive use of chemical fertilizers and pesticides. However, the employment of microorganisms in the soil poses various challenges, such as maintaining cell viability and microbial resistance to different environmental conditions. Therefore, it is important to study tools and techniques, such as bioencapsulation, which allow these difficulties to be overcome.
Microbial encapsulation is one of the bioinoculum formulation methods that has attracted great interest in the agricultural area, thanks to the benefits it offers by safeguarding microbial cell viability during its formulation and later in its application and release in the field. To hasten the adoption of this technology, it is important to carry out in-depth studies on the appropriate bioencapsulation technique to be used, which is adjusted to the needs of the crop, the microorganism used and the specific environmental conditions. This approach should help to achieve the preservation of beneficial microorganisms and their efficient distribution in the soil, thus guaranteeing their efficacy as biostimulants, biofertilizers and biopesticides.
Author Contributions
Writing—original draft preparation, B.R.-S., L.R.M.-C. and P.G.-G.; writing—review and editing, M.d.C.O.-M., B.C.S.-M., J.M.S.-Y., A.E.F., O.O.B., B.R.G. and G.S.; Conceptualization, B.R.-S., M.d.C.O.-M. and G.S.; Supervision, Funding acquisition, M.d.C.O.-M. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
G.S. thanks CONACYT-México (Proposal: A1-S-15956) and CIC-UMSNH (2021-2022) for financial support to our research projects.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Blanca Rojas-Sánchez and Luzmaría R. Morales-Cedeño were recipients of doctoral fellowships from CONACYT, México. Paulina Guzmán-Guzmán received a postdoctoral fellowship under the research supported by CONACYT-México.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glare, T.R.; Moran-Diez, M.E. Microbial-Based Biopesticides: Methods and Protocols; Springer: Cham, Switzerland, 2016; ISBN 9781493963652. [Google Scholar]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers Function as Key Player in Sustainable Agriculture by Improving Soil Fertility, Plant Tolerance and Crop Productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef]
- Moreno Reséndez, A.; García Mendoza, V.; Reyes Carrillo, J.L.; Vásquez Arroyo, J.; Cano Ríos, P. Rizobacterias Promotoras del Crecimiento Vegetal: Una Alternativa de Biofertilización Para la Agricultura Sustentable. Rev. Colomb. Biotecnol. 2018, 20, 68–83. [Google Scholar] [CrossRef]
- Massa, F.; Defez, R.; Bianco, C. Exploitation of Plant Growth Promoting Bacteria for Sustainable Agriculture: Hierarchical Approach to Link Laboratory and Field Experiments. Microorganisms 2022, 10, 865. [Google Scholar] [CrossRef]
- Maisarah Nur Sarbani, M.; Yahaya, N. Advanced Development of Bio-Fertilizer Formulations Using Microorganisms as Inoculant for Sustainable Agriculture and Environment—A Review. Malays. J. Sci. Health Technol. 2022, 8, 92–101. [Google Scholar]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Singapore, 2017; Volume 2, pp. 135–191. ISBN 9789811065934. [Google Scholar]
- Dos Santos Lopes, M.J.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Eichmann, R.; Richards, L.; Schäfer, P. Hormones as Go-Betweens in Plant Microbiome Assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef]
- Lynch, J.M. Resilience of the Rhizosphere to Anthropogenic Disturbance. Biodegradation 2002, 13, 21–27. [Google Scholar] [CrossRef]
- Larsen, J.; Jaramillo-López, P.; Nájera-Rincon, M.; Gonzaléz-Esquivel, C.E. Biotic Interactions in the Rhizosphere in Relation to Plant and Soil Nutrient Dynamics. J. Soil Sci. Plant Nutr. 2015, 15, 449–463. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant Health: Feedback Effect of Root Exudates-Rhizobiome Interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef]
- Schirawski, J.; Perlin, M. Plant–Microbe Interaction 2017—The Good, the Bad and the Diverse. Int. J. Mol. Sci. 2018, 19, 1374. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The Role of Mycorrhizae and Plant Growth Promoting Rhizobacteria (PGPR) in Improving Crop Productivity under Stressful Environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Albuquerque da Silva Campos, M. Bioprotection by Arbuscular Mycorrhizal Fungi in Plants Infected with Meloidogyne Nematodes: A Sustainable Alternative. Crop Prot. 2020, 135, 105203. [Google Scholar] [CrossRef]
- Naziya, B.; Murali, M.; Amruthesh, K.N. Plant Growth-Promoting Fungi (Pgpf) Instigate Plant Growth and Induce Disease Resistance in Capsicum annuum L. upon Infection with Colletotrichum capsici (Syd.) Butler & Bisby. Biomolecules 2020, 10, 41. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms Underlying the Protective Effects of Beneficial Fungi against Plant Diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Rai, M.; Zimowska, B.; Shinde, S.; Tres, M.V. Bioherbicidal Potential of Different Species of Phoma: Opportunities and Challenges. Appl. Microbiol. Biotechnol. 2021, 105, 3009–3018. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Ke, X.; Feng, S.; Wang, J.; Lu, W.; Zhang, W.; Chen, M.; Lin, M. Effect of Inoculation with Nitrogen-Fixing Bacterium Pseudomonas Stutzeri A1501 on Maize Plant Growth and the Microbiome Indigenous to the Rhizosphere. Syst. Appl. Microbiol. 2019, 42, 248–260. [Google Scholar] [CrossRef]
- Pace, L.; Pellegrini, M.; Palmieri, S.; Rocchi, R.; Lippa, L.; del Gallo, M. Plant Growth-Promoting Rhizobacteria for in vitro and ex vitro Performance Enhancement of Apennines’ Genepì (Artemisia umbelliformis subsp. Eriantha), an Endangered Phytotherapeutic Plant. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 134–142. [Google Scholar] [CrossRef]
- Ali, M.; Ahmad, Z.; Ashraf, M.F.; Dong, W. Maize Endophytic Microbial-Communities Revealed by Removing PCR and 16S RRNA Sequencing and Their Synthetic Applications to Suppress Maize Banded Leaf and Sheath Blight. Microbiol. Res. 2021, 242, 126639. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of Microbial Inoculants by Encapsulation in Natural Polysaccharides: Focus on Beneficial Properties of Carrier Additives and Derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Nayana, A.R.; Joseph, B.J.; Jose, A.; Radhakrishnan, E.K. Nanotechnological Advances with PGPR Applications. In Sustainable Agriculture Review; Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 163–180. ISBN 9783030339968. [Google Scholar]
- Muñoz Rojas, J.; Molina-Romero, D.; del Rocío Bustillos, M.; Rodríguez-Andrade, O.; Morales-García, Y.E.; Santiago-Saenz, Y.; Castañeda-Lucio, M.; Muñoz-Rojas, J. Mecanismos de Fitoestimulación por Rizobacterias, Aislamientos en América y Potencial Biotecnológico. Biológicas 2015, 17, 24–34. [Google Scholar]
- Agbodjato, N.A.; Assogba, S.A.; Babalola, O.O.; Koda, A.D.; Aguegue, R.M.; Sina, H.; Dagbenonbakin, G.D.; Adjanohoun, A.; Baba-Moussa, L. Formulation of Biostimulants Based on Arbuscular Mycorrhizal Fungi for Maize Growth and Yield. Front. Agron. 2022, 4, 894489. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant Growth-Promoting Root-Colonizing Bacterial Endophytes. Rhizosphere 2021, 20, 100433. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Defez, R.; Valenti, A.; Andreozzi, A.; Romano, S.; Ciaramella, M.; Pesaresi, P.; Forlani, S.; Bianco, C. New Insights into Structural and Functional Roles of Indole-3-Acetic Acid (IAA): Changes in DNA Topology and Gene Expression in Bacteria. Biomolecules 2019, 9, 522. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 3030443671. [Google Scholar]
- Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and Phosphate Solubilizing Bacteria: Keys for Sustainable Agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Mahdi, S.S.; Talat, M.A.; Dar, M.H.; Hamid, A.; Ahmad, L. Soil Phosphorus Fixation Chemistry and Role of Phosphate Solubilizing Bacteria in Enhancing Its Efficiency for Sustainable Cropping—A Review. J. Pure Appl. Microbiol. 2012, 66, 1905–1911. [Google Scholar]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial Siderophores in Community and Host Interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Schulten, H.R.; Schnitzer, M. The Chemistry of Soil Organic Nitrogen: A Review. Biol. Fertil. Soils 1997, 26, 1–15. [Google Scholar] [CrossRef]
- Keuper, F.; Dorrepaal, E.; van Bodegom, P.M.; van Logtestijn, R.; Venhuizen, G.; van Hal, J.; Aerts, R. Experimentally Increased Nutrient Availability at the Permafrost Thaw Front Selectively Enhances Biomass Production of Deep-Rooting Subarctic Peatland Species. Glob. Chang. Biol. 2017, 23, 4257–4266. [Google Scholar] [CrossRef] [PubMed]
- Igiehon, N.O.; Babalola, O.O. Rhizosphere Microbiome Modulators: Contributions of Nitrogen Fixing Bacteria towards Sustainable Agriculture. Int. J. Environ. Res. Public Health 2018, 15, 574. [Google Scholar] [CrossRef]
- Li, Z.; Tian, D.; Wang, B.; Wang, J.; Wang, S.; Chen, H.Y.H.; Xu, X.; Wang, C.; He, N.; Niu, S. Microbes Drive Global Soil Nitrogen Mineralization and Availability. Glob. Chang. Biol. 2019, 25, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Gupta, S. Evaluation of Pseudomonas sp. for Its Multifarious Plant Growth Promoting Potential and Its Ability to Alleviate Biotic and Abiotic Stress in Tomato (Solanum lycopersicum) Plants. Sci. Rep. 2020, 10, 20951. [Google Scholar] [CrossRef]
- Barney, B.M. Aerobic Nitrogen-Fixing Bacteria for Hydrogen and Ammonium Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 1383–1399. [Google Scholar] [CrossRef]
- Fedele, G.; Brischetto, C.; Rossi, V. Biocontrol of Botrytis cinerea on Grape Berries as Influenced by Temperature and Humidity. Front. Plant Sci. 2020, 11, 1232. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; Flores, A.; de los Santos-Villalobos, S.; Santoyo, G. Bacterias Endófitas Promotoras del Crecimiento Vegetal Como Agentes Biocontrol de Patógenos Postcosecha. In Bacterias Promotoras del Crecimiento Vegetal: Aspectos Básicos y Aplicaciones para una Agricultura Sustentable; Orzoco-Mosqueda, M.d.C., Santoyo, G., Eds.; Fontamara: Mexico City, Mexico, 2020; pp. 111–130. ISBN 9786077366591. [Google Scholar]
- Bargaz, A.; Elhaissoufi, W.; Khourchi, S.; Benmrid, B.; Borden, K.A.; Rchiad, Z. Benefits of Phosphate Solubilizing Bacteria on Belowground Crop Performance for Improved Crop Acquisition of Phosphorus. Microbiol. Res. 2021, 252, 126842. [Google Scholar] [CrossRef]
- Monachon, M.; Albelda-Berenguer, M.; Joseph, E. Biological Oxidation of Iron Sulfides. Adv. Appl. Microbiol. 2019, 107, 1–27. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Soares, C.R.F.S.; McConkey, B.J.; Glick, B.R. New Insights into 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Phylogeny, Evolution and Ecological Significance. PLoS ONE 2014, 9, e99168. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Glick, B.R.; Santoyo, G. ACC Deaminase in Plant Growth-Promoting Bacteria (PGPB): An Efficient Mechanism to Counter Salt Stress in Crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma Species: Versatile Plant Symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the Potential of Plant Growth-Promoting Rhizobacteria on Soil Health and the Sustainability of Agricultural Systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef]
- Rieusset, L.; Rey, M.; Muller, D.; Vacheron, J.; Gerin, F.; Dubost, A.; Comte, G.; Prigent-Combaret, C. Secondary Metabolites from Plant-Associated Pseudomonas Are Overproduced in Biofilm. Microb. Biotechnol. 2020, 13, 1562–1580. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Lehmann, J.; Camenzind, T.; Rauh, C. Soil Biodiversity Effects from Field to Fork. Trends Plant Sci. 2018, 23, 17–24. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Fröhlich, A.; Buddrus-Schiemann, K.; Durner, J.; Hartmann, A.; von Rad, U. Response of Barley to Root Colonization by Pseudomonas sp. DSMZ 13134 under Laboratory, Greenhouse, and Field Conditions. J. Plant Interact. 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Zhao, S.; Wei, H.; Lin, C.Y.; Zeng, Y.; Tucker, M.P.; Himmel, M.E.; Ding, S.Y. Burkholderia phytofirmans Inoculation-Induced Changes on the Shoot Cell Anatomy and Iron Accumulation Reveal Novel Components of Arabidopsis-Endophyte Interaction That Can Benefit Downstream Biomass Deconstruction. Front. Plant Sci. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Chaudhari, A.; Prabha, R.; Shukla, R.; Singh, D.P. Microbial Pyrrolnitrin: Natural Metabolite with Immense Practical Utility. Biomolecules 2019, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Fan, X.; Lou, Z.; Wang, H.; Olatunde, A.; Rengasamy, K.R.R. Iturin: Cyclic Lipopeptide with Multifunction Biological Potential. Crit. Rev. Food Sci. Nutr. 2021, 1, 13. [Google Scholar] [CrossRef]
- Bender, C.L.; Alarcón-Chaidez, F.; Gross, D.C. Pseudomonas syringae Phytotoxins: Mode of Action, Regulation, and Biosynthesis by Peptide and Polyketide Synthetases. Microbiol. Mol. Biol. Rev. 1999, 63, 266–292. [Google Scholar] [CrossRef]
- Pérez-Equihua, A.; Santoyo, G. Draft Genome Sequence of Bacillus sp. Strain E25, a Biocontrol and Plant Growth-Promoting Bacterial Endophyte Isolated from Mexican Husk Tomato Roots (Physalis ixocarpa Brot. Ex Horm.). Microbiol. Resour. Announc. 2021, 10, e01112-20. [Google Scholar] [CrossRef]
- Flores, A.; Diaz-Zamora, J.T.; Orozco-Mosqueda, M.D.C.; Chávez, A.; de los Santos-Villalobos, S.; Valencia-Cantero, E.; Santoyo, G. Bridging Genomics and Field Research: Draft Genome Sequence of Bacillus thuringiensis CR71, an Endophytic Bacterium That Promotes Plant Growth and Fruit Yield in Cucumis sativus L. 3 Biotech 2020, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Chimalhua, E.; Valencia-Cantero, E.; López-Bucio, J.; Ruiz-Herrera, L.F. N,N-Dimethyl-Hexadecylamine Modulates Arabidopsis Root Growth through Modifying the Balance between Stem Cell Niche and Jasmonic Acid-Dependent Gene Expression. Gene Expr. Patterns 2021, 41, 119201. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a Mechanism of Trichoderma-Mediated Suppression of Plant Diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayilara, S.; Akinola, S.A.; Babalola, O.O. Biocontrol Mechanisms of Endophytic Fungi. Egypt. J. Biol. Pest Control 2022, 32, 46. [Google Scholar] [CrossRef]
- Olowe, O.M.; Nicola, L.; Asemoloye, M.D.; Akanmu, A.O.; Babalola, O.O. Trichoderma: Potential Bio-Resource for the Management of Tomato Root Rot Diseases in Africa. Microbiol. Res. 2022, 257, 126978. [Google Scholar] [CrossRef]
- Stael, S.; Kmiecik, P.; Willems, P.; van der Kelen, K.; Coll, N.S.; Teige, M.; van Breusegem, F. Plant Innate Immunity–Sunny Side up? Trends Plant Sci. 2015, 20, 3–11. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities with Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Schoebitz, M.; López, M.D.; Roldán, A. Bioencapsulation of Microbial Inoculants for Better Soil-Plant Fertilization: A Review. Agron. Sustain. Dev. 2013, 33, 751–765. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Mendis, H.C.; Thomas, V.P.; Schwientek, P.; Salamzade, R.; Chien, J.T.; Waidyarathne, P.; Kloepper, J.; de La Fuente, L. Strain-Specific Quantification of Root Colonization by Plant Growth Promoting Rhizobacteria Bacillus firmus I-1582 and Bacillus amyloliquefaciens QST713 in Non-Sterile Soil and Field Conditions. PLoS ONE 2018, 13, e0193119. [Google Scholar] [CrossRef]
- Wong, C.K.F.; Saidi, N.B.; Vadamalai, G.; Teh, C.Y.; Zulperi, D. Effect of Bioformulations on the Biocontrol Efficacy, Microbial Viability and Storage Stability of a Consortium of Biocontrol Agents against Fusarium Wilt of Banana. J. Appl. Microbiol. 2019, 127, 544–555. [Google Scholar] [CrossRef]
- Tu, L.; He, Y.; Yang, H.; Wu, Z.; Yi, L. Preparation and Characterization of Alginate-Gelatin Microencapsulated Bacillus subtilis SL-13 by Emulsification/Internal Gelation. J. Biomater. Sci. Polym. Ed. 2015, 26, 735–749. [Google Scholar] [CrossRef]
- Neneng, L. Formulation of Liquid Biofertilizer for Enhance of Soil Nutrients in Peatland. Birex J. 2020, 2, 314–322. [Google Scholar]
- Goljanian-Tabrizi, S.; Amiri, S.; Nikaein, D.; Motesharrei, Z. The Comparison of Five Low Cost Liquid Formulations to Preserve Two Phosphate Solubilizing Bacteria from the Genera Pseudomonas and Pantoea. Iran. J. Microbiol. 2016, 8, 377–382. [Google Scholar] [PubMed]
- Camelo-Rusinque, M.; Moreno-Galván, A.; Romero-Perdomo, F.; Bonilla-Buitrago, R. Development of a Liquid Fermentation System and Encystment for a Nitrogen-Fixing Bacterium Strain Having Biofertilizer Potential. Rev. Argent. Microbiol. 2017, 49, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, T.; Dixit, M.; Gera, R.; Shukla, A.K.; Prakash, A.; Gupta, G.; Shukla, P. Techniques for Improving Formulations of Bioinoculants. 3 Biotech 2020, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Surampalli, R.Y.; Prévost, D. Bio-Encapsulation of Microbial Cells for Targeted Agricultural Delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef]
- Rose, M.T.; Deaker, R.; Potard, S.; Tran, C.K.T.; Vu, N.T.; Kennedy, I.R. The Survival of Plant Growth Promoting Microorganisms in Peat Inoculant as Measured by Selective Plate Counting and Enzyme-Linked Immunoassay. World J. Microbiol. Biotechnol. 2011, 27, 1649–1659. [Google Scholar] [CrossRef]
- Quiroz Sarmiento, V.F.; Almaraz Suarez, J.J.; Sánchez Viveros, G.; Argumedo Delira, R.; González Mancilla, A. Biofertilizantes de Rizobacterias en el Crecimiento de Plántulas de Chile Poblano. Rev. Mex. Cienc. Agríc. 2019, 10, 1733–1745. [Google Scholar] [CrossRef]
- Berger, B.; Patz, S.; Ruppel, S.; Dietel, K.; Faetke, S.; Junge, H.; Becker, M. Successful formulation and application of plant growth-promoting Kosakonia radicincitans in maize cultivation. BioMed Res. Int. 2018, 2018, 6439481. [Google Scholar] [CrossRef]
- King, V.A.E.; Lin, H.J.; Liu, C.F. Accelerated Storage Testing of Freeze-Dried and Controlled Low-Temperature Vacuum Dehydrated Lactobacillu acidophilus. J. Gen. Appl. Microbiol. 1998, 44, 161–165. [Google Scholar] [CrossRef][Green Version]
- Grzegorczyk, M.; Kancelista, A.; Łaba, W.; Piegza, M.; Witkowska, D. The Effect of Lyophilization and Storage Time on the Survival Rate and Hydrolytic Activity of Trichoderma Strains. Folia Microbiol. 2018, 63, 433–441. [Google Scholar] [CrossRef]
- Wessman, P.; Håkansson, S.; Leifer, K.; Rubino, S. Formulations for Freeze-Drying of Bacteria and Their Influence on Cell Survival. J. Vis. Exp. 2013, 78, 4058. [Google Scholar] [CrossRef]
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of Microbial Cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Bashan, Y. Alginate Beads as Synthetic Inoculant Carriers for Slow Release of Bacteria That Affect Plant Growth. Appl. Environ. Microbiol. 1986, 51, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ismail, S.; Dadrasnia, A. Encapsulation of Plant Growth Promoting Rhizobacteria—Prospects and Potential in Agricultural Sector: A Review. J. Plant Nutr. 2019, 42, 2600–2623. [Google Scholar] [CrossRef]
- Reed, M.L.E.; Glick, B.R. Applications of Plant Growth-Promoting Bacteria for Plant and Soil Systems. In Applications of Microbial Engineering; Gupta, V.K., Schmoll, M., Maki, M., Tuohy, M., Mazutti, M.A., Eds.; Taylor & Francis: Enfield, CT, USA, 2013; pp. 181–229. ISBN 9781466585782. [Google Scholar]
- Gamalero, E.; Glick, B.R. Ethylene and Abiotic Stress Tolerance in Plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 395–412. ISBN 9781461408154. [Google Scholar]
- Gepstein, S.; Glick, B.R. Strategies to Ameliorate Abiotic Stress-Induced Plant Senescence. Plant Mol. Biol. 2013, 82, 623–633. [Google Scholar] [CrossRef]
- Ali, S.; Glick, B.R. Plant-Bacterial Interactions in Management of Plant Growth under Abiotic Stresses. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbes in Soil, Crop and Environmental Sustainability; Singh, J.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–45. ISBN 9780128182581. [Google Scholar]
- Goswami, M.; Deka, S. Plant Growth-Promoting Rhizobacteria—Alleviators of Abiotic Stresses in Soil: A Review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; Orozco-Mosqueda, M.d.C.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de los Santos-Villalobos, S.; Santoyo, G. Plant Growth-Promoting Bacterial Endophytes as Biocontrol Agents of Pre- and Post-Harvest Diseases: Fundamentals, Methods of Application and Future Perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef]
- Errington, J. Regulation of Endospore Formation in Bacillus Subtilis. Nat. Rev. Microbiol. 2003, 1, 117–126. [Google Scholar] [CrossRef]
- Klobutcher, L.A.; Ragkousi, K.; Setlow, P. The Bacillus subtilis Spore Coat Provides “Eat Resistance” during Phagocytic Predation by the Protozoan Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 2006, 103, 165–170. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus Endospores to Extreme Terrestrial and Extraterrestrial Environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spore Germination. Curr. Opin. Microbiol. 2003, 6, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Menaker Raskin, M. Polymeric Micelles in Mucosal Drug Delivery: Challenges towards Clinical Translation. Biotechnol. Adv. 2015, 33, 1380–1392. [Google Scholar] [CrossRef]
- Sauka, D.H.; Benintende, G.B. Bacillus thuringiensis: Generalidades. Un Acercamiento a Su Empleo En El Biocontrol de Insectos Lepidópteros Que Son Plagas Agrícolas. Rev. Argent. Microbiol. 2008, 40, 124–140. [Google Scholar] [PubMed]
- Corrales Ramírez, L.C.; Caycedo Lozano, L.; Gómez Méndez, M.A.; Ramos Rojas, S.J.; Rodríguez Torres, J.N. Bacillus spp.: Una Alternativa para la Promoción Vegetal por dos Caminos Enzimáticos. Nova 2017, 15, 45. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M. A Novel Encapsulation of Streptomyces fulvissimus Uts22 by Spray Drying and Its Biocontrol Efficiency against Gaeumannomyces graminis, the Causal Agent of Take-All Disease in Wheat. Pest Manag. Sci. 2021, 77, 4357–4364. [Google Scholar] [CrossRef]
- Meftah Kadmiri, I.; El Mernissi, N.; Azaroual, S.E.; Mekhzoum, M.E.M.; Qaiss, A.E.K.; Bouhfid, R. Bioformulation of Microbial Fertilizer Based on Clay and Alginate Encapsulation. Curr. Microbiol. 2021, 78, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Tapias, D.; Sierra, O.O.; Botía, D.R.; Bonilla, R. Preservation of Azotobacter chroococcum Vegetative Cells in Dry Polymers. Univ. Sci. 2015, 20, 201–207. [Google Scholar] [CrossRef]
- Tu, L.; He, Y.; Shan, C.; Wu, Z. Preparation of Microencapsulated Bacillus subtilis SL-13 Seed Coating Agents and Their Effects on the Growth of Cotton Seedlings. BioMed Res. Int. 2016, 2016, 3251357. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Ye, B.C.; Wang, J.; Guan, X.; Zhang, J. Viability Evaluation of Alginate-Encapsulated Pseudomonas putida Rs-198 under Simulated Salt-Stress Conditions and Its Effect on Cotton Growth. Eur. J. Soil Biol. 2016, 75, 135–141. [Google Scholar] [CrossRef]
- Pongsilp, N.; Nimnoi, P. Inoculation of Ensifer fredii Strain LP2/20 Immobilized in Agar Results in Growth Promotion and Alteration of Bacterial Community Structure of Chinese Kale Planted Soil. Sci. Rep. 2020, 10, 15857. [Google Scholar] [CrossRef] [PubMed]
- Hernández Montiel, L.G.; Chiquito Contreras, R.G.; Castillo Rocha, D.G.; Chiquito Contreras, C.J.; Vidal Hernández, L.; Beltrán Morales, F.A. Efecto de Microcápsulas de Pseudomonas putida Sobre Crecimiento y Rendimiento de Pimiento Morrón. Rev. Mex. Cienc. Agríc. 2018, 20, 4223–4233. [Google Scholar] [CrossRef]
- Bhise, K.K.; Dandge, P.B. Alleviation of Salinity Stress in Rice Plant by Encapsulated Salt Tolerant Plant Growth Promoting Bacteria Pantoea agglomerans Strain KL and Its Root Colonization Ability. Arch. Agron. Soil Sci. 2019, 65, 1955–1968. [Google Scholar] [CrossRef]
- Saad, M.M.; Abo-Koura, H.A.; Bishara, M.M.; Gomaa, I.M. Microencapsulation: Toward the Reduction of the Salinity Stress Effect on Wheat Plants Using NPK Rhizobacteria. Biotechnol. J. Int. 2020, 23, 1–18. [Google Scholar] [CrossRef]
- Pour, M.M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Investigating the Formulation of Alginate-Gelatin Encapsulated Pseudomonas fluorescens (VUPF5 and T17-4 Strains) for Controlling Fusarium solani on Potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Pacheco-Aguirre, J.; Ruiz-Sánchez, E.; Reyes-Ramírez, A.; Cristóbal-Alejo, J.; Tun-Suárez, J.; Borges-Gómez, L. Polymer-Based Encapsulation of Bacillus subtilis and Its Effect on Meloidogyne incognita in Tomato. Phyton 2016, 85, 1–6. [Google Scholar]
- Snigdha, S.; Kalarikkal, N.; Thomas, S.; Radhakrishnan, E.K. Laponite® Clay/Poly(Ethylene Oxide) Gel Beads for Delivery of Plant Growth-Promoting Rhizobacteria. Bull. Mater. Sci. 2021, 44, 107. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Rocha, M.; Rocha, I.; Ma, Y.; Freitas, H.; Oliveira, R.S. Encapsulation of Pseudomonas libanensis in Alginate Beads to Sustain Bacterial Viability and Inoculation of Vigna unguiculata under Drought Stress. 3 Biotech 2021, 11, 293. [Google Scholar] [CrossRef]
- Panichikkal, J.; Prathap, G.; Nair, R.A.; Krishnankutty, R.E. Evaluation of Plant Probiotic Performance of Pseudomonas sp. Encapsulated in Alginate Supplemented with Salicylic Acid and Zinc Oxide Nanoparticles. Int. J. Biol. Macromol. 2021, 166, 138–143. [Google Scholar] [CrossRef]
- Panichikkal, J.; Puthiyattil, N.; Raveendran, A.; Nair, R.A.; Krishnankutty, R.E. Application of Encapsulated Bacillus licheniformis Supplemented with Chitosan Nanoparticles and Rice Starch for the Control of Sclerotium rolfsii in Capsicum annuum (L.) Seedlings. Curr. Microbiol. 2021, 78, 911–919. [Google Scholar] [CrossRef]
- Nascimento, F.C.; Santos, C.H.B.; Kandasamy, S.; Rigobelo, E.C. Efficacy of Alginate- and Clay-Encapsulated Microorganisms on the Growth of Araçá-Boi Seedlings (Eugenia stipitata). Acta Sci.-Biol. Sci. 2019, 41, e43936. [Google Scholar] [CrossRef]
- Venkata Raju, N.; Sukumar, K.; Reddy, G.B.; Busetty, M.; Paritala, V.; Praveena, K. Usage of Encapsulated Plant Growth Promoting- Microbial Consortia and Testing Its Efficacy on Cajanus cajan. J. Microb. Biochem. Technol. 2021, 12, 452. [Google Scholar] [CrossRef]
- Pitaktamrong, P.; Kingkaew, J.; Yooyongwech, S.; Cha-Um, S.; Phisalaphong, M. Development of Arbuscular Mycorrhizal Fungi-Organic Fertilizer Pellets Encapsulated with Alginate Film. Eng. J. 2018, 22, 65–79. [Google Scholar] [CrossRef]
- Shcherbakova, E.N.; Shcherbakov, A.V.; Rots, P.Y.; Gonchar, L.N.; Mulina, S.A.; Yahina, L.M.; Lactionov, Y.V.; Chebotar, V.K. Inoculation Technology for Legumes Based on Alginate Encapsulation. Agron. Res. 2018, 16, 2156–2168. [Google Scholar] [CrossRef]
- Krell, V.; Unger, S.; Jakobs-Schoenwandt, D.; Patel, A.V. Endophytic Metarhizium brunneum Mitigates Nutrient Deficits in Potato and Improves Plant Productivity and Vitality. Fungal Ecol. 2018, 34, 43–49. [Google Scholar] [CrossRef]
- Krell, V.; Jakobs-Schoenwandt, D.; Vidal, S.; Patel, A.V. Encapsulation of Metarhizium brunneum Enhances Endophytism in Tomato Plants. Biol. Control 2018, 116, 62–73. [Google Scholar] [CrossRef]
- Jurić, S.; Sopko Stracenski, K.; Król-Kilińska, Ż.; Žutić, I.; Uher, S.F.; Đermić, E.; Topolovec-Pintarić, S.; Vinceković, M. The Enhancement of Plant Secondary Metabolites Content in Lactuca sativa L. by Encapsulated Bioactive Agents. Sci. Rep. 2020, 10, 3737. [Google Scholar] [CrossRef]
- Kamel, S.M.; Ebtsam, M.M.; Massoud, O.N. Potentiality of Some Yeast Species as Biocontrol Agents against Fusarium oxysporum f. sp. Cucumerinum the Causal Agent of Cucumber Wilt. Egypt. J. Biol. Pest Control 2016, 26, 299–307. [Google Scholar]
- Barrer, S.E. El Uso de Hongos Micorrizicos arbusculares como una Alternativa para la Agricultura. Fac. Cienc. Agropecu. 2009, 7, 124–132. [Google Scholar]
- Brundrett, M. Diversity and Classification of Mycorrhizal Associations. Biol. Rev. Camb. Philos. Soc. 2004, 79, 473–495. [Google Scholar] [CrossRef]
- Gomes, E.A.; Oliveira, C.A.; Lana, U.G.P.; Noda, R.W.; Marriel, I.E.; de Souza, F.A. Arbuscular Mycorrhizal Fungal Communities in the Roots of Maize Lines Contrasting for Al Tolerance Grown in Limed and Non-Limed Brazilian Oxisoil. J. Microbiol. Biotechnol. 2015, 25, 978–987. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; Bučko, M.; Gemeiner, P.; Navrátil, M.; Švitel, J.; Faas, M.; Strand, B.L.; Skjak-Braek, G.; Morch, Y.A.; Vikartovská, A.; et al. Multiscale Requirements for Bioencapsulation in Medicine and Biotechnology. Biomaterials 2009, 30, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; Hernandez, J.P.; Leyva, L.A.; Bacilio, M. Alginate Microbeads as Inoculant Carriers for Plant Growth-Promoting Bacteria. Biol. Fertil. Soils 2002, 35, 359–368. [Google Scholar] [CrossRef]
- Nava Saucedo, J.E.; Barbotin, J.N. Bioencapsulation Revisited. Biomater. Artif. Cells Immobil. Biotechnol. 1993, 21, 383–389. [Google Scholar] [CrossRef]
- Park, S.; Oh, K.K.; Lee, S.H. Biopolymer-Based Composite Materials Prepared Using Ionic Liquids. Adv. Biochem. Eng. Biotechnol. 2019, 168, 133–176. [Google Scholar] [CrossRef] [PubMed]
- Szczech, M.; Maciorowski, R. Microencapsulation Technique with Organic Additives for Biocontrol Agents. J. Hortic. Res. 2016, 24, 111–122. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of Encapsulation Techniques of Probiotics for Yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of Alginate Microspheres for Drug Delivery: A Review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef]
- Dos Santos, G.F.; Locatelli, G.O.; Coêlho, D.A.; Botelho, P.S.; de Amorim, M.S.; de Vasconcelos, T.C.L.; Bueno, L.A. Factorial Design, Preparation and Characterization of New Beads Formed from Alginate, Polyphosphate and Glycerol Gelling Solution for Microorganism Microencapsulation. J. Sol-Gel Sci. Technol. 2015, 75, 345–352. [Google Scholar] [CrossRef]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.; Sagis, L.M. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Tavares, M.J.; Franck, J.; Ali, S.; Glick, B.R.; Rossi, M.J. ACC Deaminase Plays a Major Role in Pseudomonas fluorescens YsS6 Ability to Promote the Nodulation of Alpha- and Betaproteobacteria Rhizobial Strains. Arch. Microbiol. 2019, 201, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montiel, L.G.; Chiquito-Contreras, C.J.; Murillo-Amador, B.; Vidal-Hernández, L.; Quiñones-Aguilar, E.E.; Chiquito-Contreras, R.G. Efficiency of Two Inoculation Methods of Pseudomonas putida on Growth and Yield of Tomato Plants. J. Soil Sci. Plant Nutr. 2017, 17, 1003–1012. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological Effects of Gum Arabic: A Review of Some Recent Research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Cheng, J.; Nie, X.; Yu, X.; Zhao, Y.; Wang, W. Microencapsulation of Bacillus subtilis B99-2 and Its Biocontrol Efficiency against Rhizoctonia solani in Tomato. Biol. Control 2015, 90, 34–41. [Google Scholar] [CrossRef]
- Qiu, H.L.; Fox, E.G.P.; Qin, C.S.; Zhao, D.Y.; Yang, H.; Xu, J.Z. Microcapsuled Entomopathogenic Fungus against Fire Ants, Solenopsis invicta. Biol. Control 2019, 134, 141–149. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Ebrahimi-Zarandi, M.; Gholizadeh Vazvani, M.; Skorik, Y.A. Reducing Drought Stress in Plants by Encapsulating Plant Growth-Promoting Bacteria with Polysaccharides. Int. J. Mol. Sci. 2021, 22, 12979. [Google Scholar] [CrossRef]
- Cummings, J.H.; Stephen, A.M. Carbohydrate Terminology and Classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef]
- Estefania, C.A.; Ligia, S.L. Immobilized Bacillus Subtilis by Ionic Gelation as Biocontrol Alternative of Fusarium oxysporum f. sp. Lycopersici. Indian J. Anim. Res. 2018, 52, 655–660. [Google Scholar] [CrossRef]
- Gaaloul, S.; Turgeon, S.L.; Corredig, M. Influence of Shearing on the Physical Characteristics and Rheological Behaviour of an Aqueous Whey Protein Isolate–Κappa-Carrageenan Mixture. Food Hydrocoll. 2009, 23, 1243–1252. [Google Scholar] [CrossRef]
- Choińska-Pulit, A.; Mituła, P.; Śliwka, P.; Łaba, W.; Skaradzińska, A. Bacteriophage Encapsulation: Trends and Potential Applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in Agriculture: An Overview on Concepts, Strategies and Effects on Soil Microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, A.V. Review of Encapsulation Methods Suitable for Microbial Biological Control Agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Poncelet, D.; Lencki, R.; Beaulieu, C.; Halle, J.P.; Neufeld, R.J.; Fournier, A. Production of Alginate Beads by Emulsification/Internal Gelation. I. Methodology. Appl. Microbiol. Biotechnol. 1992, 38, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Rauth, A.M.; Wu, X.Y. Immobilization and Bioactivity of Glucose Oxidase in Hydrogel Microspheres Formulated by an Emulsification-Internal Gelation-Adsorption-Polyelectrolyte Coating Method. Int. J. Pharm. 2007, 339, 148–156. [Google Scholar] [CrossRef]
- Secchi, E.; Munarin, F.; Alaimo, M.D.; Bosisio, S.; Buzzaccaro, S.; Ciccarella, G.; Vergaro, V.; Petrini, P.; Piazza, R. External and Internal Gelation of Pectin Solutions: Microscopic Dynamics versus Macroscopic Rheology. J. Phys. Condens. Matter 2014, 26, 464106. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of Alginate Microspheres in Therapeutics Delivery and Cell Culture: Past, Present and Future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liang, H.F.; Chung, C.K.; Chen, M.C.; Sung, H.W. Physically Crosslinked Alginate/N,O-Carboxymethyl Chitosan Hydrogels with Calcium for Oral Delivery of Protein Drugs. Biomaterials 2005, 26, 2105–2113. [Google Scholar] [CrossRef]
- De Moura, S.C.S.R.; Berling, C.L.; Germer, S.P.M.; Alvim, I.D.; Hubinger, M.D. Encapsulating Anthocyanins from Hibiscus sabdariffa L. Calyces by Ionic Gelation: Pigment Stability during Storage of Microparticles. Food Chem. 2018, 241, 317–327. [Google Scholar] [CrossRef]
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Hermosín-Gutiérrez, I.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Bog Bilberry Phenolics, Antioxidant Capacity and Nutrient Profile; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 201, ISBN 9046237737. [Google Scholar]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending Viability of Bifidobacterium longumin Chitosan-Coated Alginate Microcapsules Using Emulsification and Internal Gelation Encapsulation Technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Chavarri, M.; Maranon, I.; Carmen, M. Encapsulation Technology to Protect Probiotic Bacteria. In Probiotics; IntechOpen: London, UK, 2012; pp. 501–540. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.; Serpa, V.G.; Felipe dos Santos, A.C.; Marinês da Costa, S.; Dalpubel, V.; Lehn, D.N.; Volken de Souza, C.F. Microencapsulation of Lactobacillus plantarum ATCC 8014 through Spray Drying and Using Dairy Whey as Wall Materials. LWT-Food Sci. Technol. 2017, 82, 176–183. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Prévost, D.; Surampalli, R.Y. Effect of emulsion formulation of Sinorhizobium meliloti and pre-inoculated seeds on alfalfa nodulation and growth: A pouch study. J. Plant Nutr. 2012, 36, 231–242. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).