Abstract
In the previous half-century, natural rock phosphates (PN) have been a valuable alternative for phosphorus (P) fertilizer for sustainable agriculture; furthermore, phosphogypsum (PG) has been widely used as a soil amendment fertilizer since it improves some soil properties, increases crop yields, and represents an environmental concern that can make a good economic profit; this research aimed to study the effects of microbial consortia of phosphate-solubilizing microorganisms (PSM) on the solubilization of PN and PG in the soil, and their effects on promoting plant growth and nutrient assimilation using ryegrass as a plant model. Local supply of PG with Pseudomonas fluorescens (MW165744) significantly increases root proliferation and plant biomass dry weight compared to other isolates, as well as improves total P uptake, with a maximum value of 62.31 mg/pot. The opposite occurred in mixing inoculation with Pseudomonas fluorescens, Pantoea agglomerans (MW165752) and Stenotrophomonas maltophilia (MW221274), with a negligible total P assimilation of 5.39 mg/pot. Whereas the addition of Pseudomonas agglomerans with PG gave outstanding total P absorption of 57.05 mg/pot when compared with PN input of 38.06 mg/pot. Finally, the results prove that the co-inoculation of Pseudomonas fluorescens with PG could be a promising and alternative option to use it as a source of P fertilizer for plants and to maintain a high level of nutrients in the soil.
1. Introduction
Soils are naturally low in phosphorus (P), and are thus readily available to support the rapid development of plants [1]. Therefore, natural rock phosphates (PN) could play a valuable role as a cheaper P source in agriculture [2]. Intensive or industrialized agriculture practices achieve high yields and allow the rapid development of cultures but require large quantities of chemical fertilizers [3]. Besides the extensive use of chemical fertilizers is a threat for human health [4], the use of phosphate-based fertilizers increased by 29% between 2004 and 2014 [5]. Nevertheless, the phosphate industry detains an essential position at the economic level in Tunisia, with four industrial units active producing phosphoric acid and phosphate fertilizers from phosphate rock exploitation; this phosphoric acid, made by the wet process, produces huge quantities of phosphogypsum (PG) [6,7,8], representing a serious concern for the fertilizer industry [9,10]. Today, the biggest problem facing the Tunisian phosphate industry is the pollution of the Gulf of Gabès by PG [11,12]. The PG contains toxic elements that could be harmful to the environment and human health as well, namely heavy metals and radionuclides [13]. The annual worldwide PG production is around 200–250 million tons [14], of which 10 million tons are produced by the Tunisian phosphoric acid companies [14].
At the industrial scale, PG can be used in some applications such as building materials, fertilizers, and soil treatment processes, but most of it is dried up in stockpiles above the ground causing serious contamination of soil and aquifers [15]. Treating and reusing PG as a fertilizer in agriculture can be considered a greener practice that is already used in many parts of the world [16,17,18]. The phosphorus and calcium present in PG make it an acceptable amendment for soils lacking organic matter [19]. Rhizoremediation has also been proposed as an innovative way of PG landfills remediation, via the establishment of a vegetative cover on PG stacks [20]; this technique can be improved by the addition of microorganisms into the soil (bioaugmentation) and may offer an attractive alternative to traditional processes towards a more economic and greener solution for PG reclamation [21]. Thus, the use of bacteria capable of stimulating plant growth could improve phytoextraction efficiency [22].
Among these microorganisms, the phosphate-solubilizing microorganisms (PSM) such as Pseudomonas, Mycobacterium, Bacillus, Pantoea, Rhizobia, and Burkholderia, are bioinoculants that have the ability to convert insoluble phosphorus into a soluble form and can be used to reduce the use of phosphate fertilizers in agriculture [23,24,25,26,27].
Tomer et al. [28] concluded that the stimulating effect of localized application of P with inoculation on the growth of ryegrass, with different amounts of PN or PG in sterile soil, improved the wheat grain yield and phosphorus uptake, while reducing the fertilizer input by 30%.
The kinetics of nutrient uptake allows comparison of uptake efficiency between species, provide insight into uptake mechanisms, and facilitate predictive modeling of nutrient uptake [29,30]. PSMs produce organic acids such as citric acid, and succinic acids; enzymes like phosphatases and phytases; and ion chelators such as siderophores that readily make P accessible for plants [28].
The purpose of this study was to explore the effects of PN, PG, and inoculation with PSM on the growth and element uptake by ryegrass grown in sterile soils. An innovative result of this work is the use of the three isolates, Pseudomonas fluorescens (P. fluorescencs), Pantoea agglomerans (P. agglomerans) and Stenotrophomonas maltophilia (S. maltophilia), which significantly enhance the biomass yield of ryegrass; this paper reviewed the impacts of waste PG addition on soil properties and plant growth to advance the utilization of PG and reduce the environmental risks, and contains hypotheses that (i) inoculum and PN inputs would enhance plant growth and nutrients uptake; (ii) by combining PSM and PG as an innovative method, and then using it as a soil amendment, could improve the quality and growth of plants, so there will be benefits for the development of agriculture practices; (iii) the use of PSM is advantageous, such as its biotechnological contributions to nutrient cycling, plant growth enhancement, and nutrients assimilation.
2. Materials and Methods
2.1. Materials
Samples of phosphate rock (PN) and phosphogypsum (PG) used in this research were provided by the Tunisian Gafsa Phosphate Company (CPG). The PG-derived waste after sodium phosphate production of the Métlaoui deposit (in southwestern Tunisia) was taken after mechanical treatment (homogenization, quartering, sieving, and grinding). In the laboratory, the two analyzed materials (PN, PG) were oven-dried for 24 h at 60 °C, subsequently homogenized, sieved, and stored for different analyses following the sampling protocol mentioned by Pérez-López et al. [31]. Thereafter, they were subjected to geochemical, mineralogical, and particle size characterization.
2.2. Characterization Methods
At the beginning of the experiment, tests were carried out to determine the main characteristics of the materials used. Different geochemical analyzes were carried out on PN and PG samples (P2O5, Na2O, CaO, MgO, SiO2, K2O, Al2O3, FeO3) [32].
The morphology of the samples was characterized with scanning electron microscopy (SEM) [32], allowing the characterization of the surface, and determination of the chemical composition of the material under study. Then, a qualitative analysis was performed by X-ray fluorescence [32], using a spectrometer of the SI-PIN Photodiode, connected to an ADMCA Multichannel spectrum analysis software. Additionally, Infra-Red spectrometry (IR) analysis was carried out using a Bruker Alpha FTIR spectrometer with Opus 7.5 software, using an ATR (attenuated total reflection) sampling device. The spectra were recorded in the spectral range of 400–4000 cm−1 at a resolution of 4 cm−1 and 24 scans [33].
2.3. Microorganisms Characterization
Soil is a hotspot of microbial diversity. Phosphate solubilizing bacteria (PSB) are known to participate in the transformation of insoluble phosphate into soluble forms, and thus improve the soil nutritional status by transforming P into a form available to plants [34]. Three bacterial species, P. fluorescens (MW165744), S. maltophilia (MW221274) and P. agglomerans (MW165752), were isolated from soil, in Tabarka, Ain Draham (Hammam Bourguiba), Tunisia, and identified using physiological, morphological, and biochemical characters, as well as 16S rRNA gene sequencing. In particular, these isolates showed high performance of phosphate solubilization in a solid or liquid medium, allowing a crucial role in the integrated management of soil nutrients.
2.4. Soil Samples
The experiment was conducted from December 2020 to 2021. The soil sample was collected from the top layer (between 0 to 15 cm deep) of the natural ecosystem in Tunisia (Tabraka) (36°58′12.8″ N; 008°52′34.7″ E). The sampling site was designated as sandy soil. The sample was homogenized, sieved (through a 2 mm mesh screen), and stored at 4 °C until use [35]. The soil sample contained 0.62% of total organic carbon, 0.28 mg/L of Kjeldahl nitrogen, 1.07% of organic matter, and 66.691 ppm of total phosphorus, and a pH of 6.6 at the beginning of the experiment.
2.5. Experimental Design and Treatments
The experiments were composed of fifteen treatments in a randomized block split-splot design with four replications. The treatments include controls (without inoculation), bacterial isolates (P. fluorescens, S. maltophilia and P. agglomerans) with soil and bacterial isolates with soil and samples of PN and PG; these treatments were applied to commercial seed crops of allvarieties of Italian ryegrass (Lolium multiflorum). The seeds were surface disinfected and transferred to the pots [36]. The study was conducted for six months. The various tests were conducted in soil, PN, and PG sterilized by autoclaving (120 °C for 2 h), sieved (2 mm mesh), mixed, and stored at 4 °C until use [37]; furthermore, for all tests, the bacterial isolates were pre-cultivated with physiological water at 30 °C, for 24 h in an orbital shaker-incubator (180 rpm) [38].
The reference (blank) treatments with bacterial inoculation contained 8 mL of bacterial suspension (106 dilution) and 2 kg of sterile soil in individual pots. In the treatments with PN (0.5 g) and PSB inoculation was used the same relative quantities of inoculums and soil autoclaved as for the blank experiment. The treatments with PG (25 g) contained the same relative quantities of bacterial suspension and soil sterile as well. The treatments without bacterial inoculation were also carried out with the same relative quantities of autoclaved soil, PN and PG. All these treatments were repeated for different inoculums (P. fluorescens, S. maltophilia and P. agglomerans) and with inoculums containing the three isolates together. Each pot was sowed with 10 g of ryegrass seeds on 12 December 2020.
The ryegrass was maintained in parallel under natural light and temperature conditions and each pot was regularly watered with approximately 250 mL of water. On 30 May 2021, after the harvest of ryegrass plants, the soil samples were collected by hand.
2.6. Measurements
At the beginning of the experiment, ryegrass plants with similar fresh weights were taken for each treatment to obtain the initial biomass dry weight. After, the plants were divided into shoots and roots and weighted to estimate the initial biomass. At the end of the experiment, plants were harvested and rinsed in water before they were separated into roots and shoots. Then, those plant fractions were dried at 70 °C for 72 h until constant weight [39].
The measured weights were used to estimate the final dry biomass and calculate the relative growth rate. After that, the plant fractions were ground to less than 1 mm and analyzed for the mineral matter, total carbon and the total concentrations of P [40]. The plant fractions were used to determine the liberated soluble P concentration by ultraviolet spectrophotometer method. The P concentration of the shoots and roots was used to calculate the translocation factor (TF) as the shoot P concentration to the root P concentration ratio.
2.7. Statistical Analysis
The statistical analysis was performed using SAS 9.0 for Windows. Duncan’s Multiple Range test analysis method was used for separating. Significance levels were within confidence limits of 0.05 or less. Data collected were subjected to analysis of variance in triplicate and averaged. The assay was used to explore the influence of PN, PG, and bacterial inoculation concentrations, as well as their interactive effects on variation.
3. Results and Discussion
In line with the objective of this research, the results of the application of treatment PG and PN are presented and discussed in this section. The implications of the combination of different inoculums and PG, PN as a soil amendment are also examined.
3.1. Granulometric Characterization of Phosphate and Phosphogypsum
The granulometric study of the PN and PG samples allowed the determination of different granulometric classes, the results obtained are presented in Table 1.

Table 1.
Granulometry by wet sieving of PN and PG.
The mass (g) and yield weight (Rp) at a well-defined interval are shown in Table 2.

Table 2.
Granulometry of PN and PG samples.
These results allowed the determination of three granulometric classes for PN: These are fine sand [0.2–0.06], medium sand [0.2–0.6] and coarse sand [0.6–2]. The fractions [0.2–0.06], were the most important fraction of PN.
The particle size analysis revealed a marked predominance, reaching more than 63% for the amount retained in No. 140 sieve (0.106 mm) and lower than 4.5% in Sieve No. 200 (0.075 mm). The coarser PN of particle size is around 0.850 mm, it is the poorest proportion of sand about 2.6%.
The average PN with a size between 0.2 mm and 0.6 mm represents a low percentage of material retained in No. 60 and 40 sieves, reaching 5.6% for the quantity retained in No. 40 sieve (0.425 mm) and about 9.1% in the sieve No. 20 (0.850 mm). Then, for the granulometric analysis of PG, it was noted from Table 1 that the fine sands concentrate in the 0.106 mm–0.075 mm fractions, with close to 17.2% for sieve No. 140 (0.106 mm), and about 12.7% for sieve No. 200.
According to Table 2, it was found that PN has a low percentage (14.5%) of clay and silt. On the other hand, we note that most of the global mass of PG is silt and clay, constituting a very important percentage of about 70%, which causes enormous problems. Indeed, Al-Masri et al. [41] claim that it is necessary to sieve PG and remove fine particles (those smaller than 75 μm) rich in uranium, copper, cadmium, and zinc and then leach with water as copper and cadmium migrate in solution.
3.2. Composition and Properties of Phosphate Rock and Phosphogypsum
The chemical composition of Tunisian PG and PN is illustrated in Table 3. The results obtained lead to the following observations: The samples have a wide variety of chemical compositions when we move from one sample to another; Overall, the P2O5 content varies from 23% for PN to reduced levels of 0.4% for PG, with lower levels of impurities (MgO, SiO2, K2O, Al2O3, FeO3, and Na2O). In accordance with Table 3, PG was found to contain decreased concentrations of impurities (MgO, SiO2, K2O, Al2O3, P2O5, and FeO3); this difference can be explained by the variability in the PG chemical composition concerning storage time, which has been previously observed and reported to be due to element leaching because of weathering processes [42].

Table 3.
Concentrations (% weight) of major elements and impurities in PN and PG samples.
3.3. Mineralogical Analyzes
3.3.1. Microscopic Observations by SEM
Morphological observations of the PN and PG samples using light by scanning electron microscopy (SEM) were presented in Figure 1.
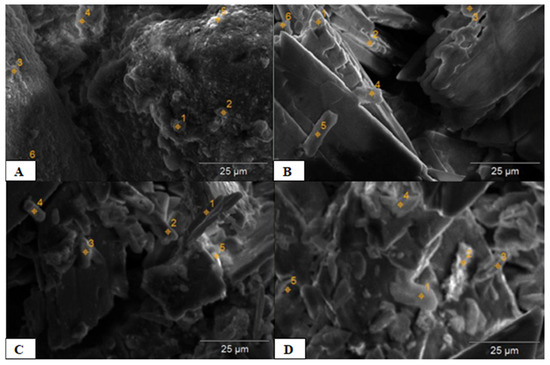
Figure 1.
SEM image of Tunisian PN (A) and PG (B–D).
The structure of natural rock phosphate is presented in Figure 1A, on which different zones are identifiable, with different magnification levels.
The images obtained by SEM with different magnifications highlighted that the analysis of each type of component appears in a particular form. Additionally, PN grains appear as fine elements.
As well, it shows the distribution of the various elements in each of the identified zones (for example point 5 showed the existence of the elements Ca, Mg, P, S, Al, C, Si, Na, and K). Subsequently, the results of the grain composition analysis of the initial PG (Figure 1B–D) demonstrate that most particles had a size range of 100–1000 μm, and presented a tabular crystalline morphology that appears as a platelet. Concerning The distribution of the elements of the example at the level of point 5 (Figure 1C) reveals the presence of Ca, S, and Si; however, the 6th point (Figure 1B) only indicates the presence of S and Ca.
3.3.2. Infrared Spectroscopy (IR)
The IR chromatogram shows several bands, particularly those attributable to PN (Figure 2A). Infrared spectra of PN show several main bands, in particular those attributable to the peaks at 563 cm−1 to 601 cm−1 of the phosphates, carbonates andhydroxylions OH− of apatite. The position, intensity, and identification of all bands are listed in Table 4. PG analysis was performed with the same equipment as PN (Figure 2B). PG shows different bands of vibrations (Figure 2B) which are all grouped in Table 5. The peak of the OH bond at 3399 cm−1 is caused by the symmetric and asymmetric vibration of the OH bond.
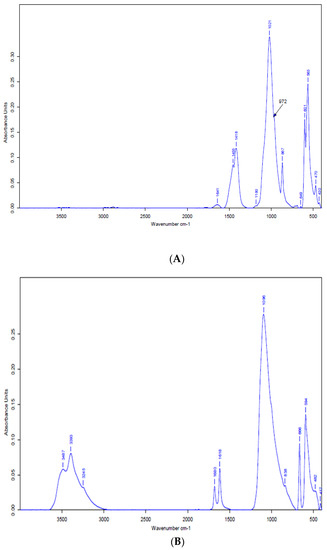
Figure 2.
Infrared spectrum of PN (A) and PG (B).

Table 4.
Position and identification of bands observed in the IR of PN.

Table 5.
Position and identification of bands observed in the IR spectrum of PG.
3.3.3. Qualitative Analysis by X-ray Fluorescence
Qualitative analysis by X-ray fluorescence has shown that the calcium employed consists mainly of Ca characterized by a peak at 23,050 Å (Kα1 Ca), (Kβ1 Ca 3300 Å), (Kα1 Fe 500 Å), (Kα1 S 400 Å), and (Kα1 P 1800 Å) (Figure 3A). PG consists of calcium (Kα1 Ca by a peak at 10,950), (Kβ1 Ca) and (Kα1 Fe) (Figure 3B).
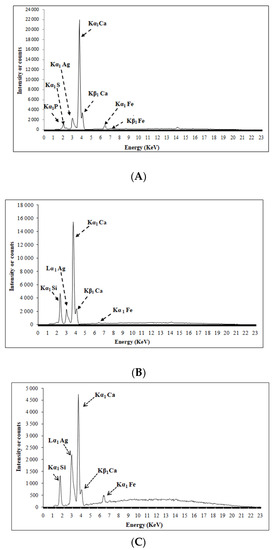
Figure 3.
Qualitative analysis by X-ray fluorescence of PN (A), PG (B) and of the lamella (C).
There are many sample preparation methods to analyze using X-ray fluorescence spectrometry, in our case we put a drop of Paraloid B-72, 50% on a coverslip then we deposited the sample which is in the form of a powder on this drop, the purpose of which is to fix, from which the result obtained is not only of the sample but also the spectra of the lamella. For this reason, a quantitative analysis of X-ray fluorescence with an empty coverslip was performed (Figure 3C). The results of X-ray fluorescence analysis of the PN, PG and coverslip are summarized in Table 6.

Table 6.
Quantitative X fluorescence analysis for all samples.
According to Table 6, it can be noticed that PN contains a very important content of calcium, whereas PG does not contain phosphorus just has a high sulfur content. Concerning the other peaks observed (Ag, Si, Fe) they are related to the lamella, neither PN nor PG includes these elements.
3.4. Plant Relative Growth
The growth of ryegrass was considerably impacted by the source and different inoculum bacteria. Regardless of isolates inoculation and PN, PG level, amendment significantly increased shoot and total biomass in comparison to treatments without additions of inoculums, PN, and PG. The three preselected bacterial isolates notice the best scores upon completion of the biochemical (solubilization of phosphates with qualitative and quantitative methods) tests used for the bioaugmentation of the mix; moreover, the comparison between P. fluorescens, S. maltophilia, and P. agglomerans in the soil showed that shoot biomass (13.23 g) and root biomass (7.17 g) increased significantly with isolate P. fluorescens, using PG as a fertilizer source. On the other hand, for the other treatments with PN, or without source of P, the increase in the total biomass was not as pronounced. Hence, this may explain that PG is mostly employed to enhance soil physicochemical properties [12,19], increase water infiltration and movement [51,52], mitigate soil acidity and toxicity [53], control soil and nutrient loss [54], and promote crop growth [55,56].
3.4.1. Effects of Isolates on Plant Growth
Studies on the use of genera Pseudomonas [57], Pantoea [58], and Stenotrophomonas [59] reported their immense potential as plant growth promoters; they perform a significant role in soil by their metabolic activities [60] and they have a remarkable role in integrated nutrient management in the soil [61]. To illustrate, with P. fluorescens the shoots biomass has avalue of 9.66 g and roots biomass of 4.28 g. With P. agglomerans the shoots present 7.58 g and the roots 3.09 g, nonetheless, the results with S. maltophilia consisted of 7.87 g for the shoots and 3.87 g for the roots, without the source of P. In fact, many possible mechanisms in which plant growth-promoting (PGPR) exert their positive effects on host plants include mitigation of nutrient deficiency via phosphorus solubilization [62], stimulation of disease-resistance mechanisms, protection against soil-borne pathogens [63,64], and production of siderophores that solubilize and sequester iron from the rhizospheric environment [65,66]. Several PGPR can provide a phytohormone involved in modulating the growth and cellular metabolism of plants [67]. Thus, PSM enhanced yield attributes like grain yield, and dry matter accumulation [61].
3.4.2. Dry Matter Yield
Various experiments have been performed to analyze the plant growth-promoting effects of inoculum and sources of P. For instance, P. fluorescens improved root and shoot biomass.
The P. fluorescens dry matter values for shoots are 0.687 g/pot with PN input and 0.782 g/pot with the source PG (see Figure 4A). The effects of P. fluorescens on ryegrass in these treatments might be due to the relatively high P level in the resource used (PN, PG) at the beginning of the experiment. For the conditions used, the P. fluorescens values for roots ranged from 0.601 g/pot to 0.93 g/pot (Figure 4B).
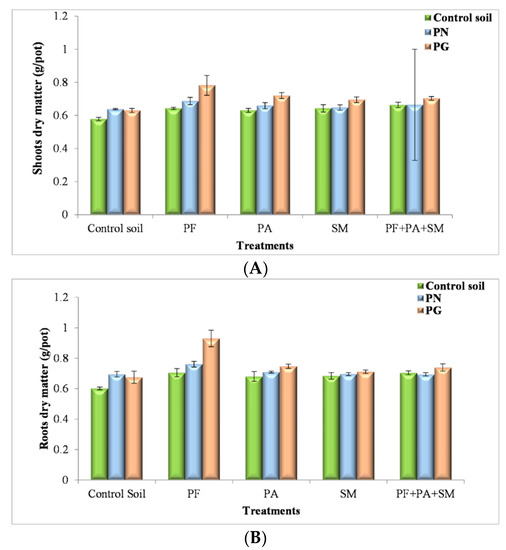
Figure 4.
Effect of PN, PG and bacterial inoculum added to the soil on shoots (A) and roots (B) dry matter (g/pot) (PF: P. fluorescens; PA: P. agglomerans; SM: S. maltophilia) (The values and the error bars represent the means ± standard deviation, n = 4).
Soil microflora can enhance crop productivity through various direct and indirect mechanisms. Even though many studies have shown these effects on plant growth resulting from inoculation, it is necessary to explain that seeds inoculated with bacterial strains generate binding to roots and endow PGPR with a beneficial effect on plant growth [68].
P. agglomerans is a bacterium that is widely distributed in natural agriculture and in environments [69]. While, in this research, the treatments with P. agglomerans showed a shoot of 0.659 g/pot and a root of 0.709 g/pot with PN input. Then, a higher value of shoot in sandy soil with only contained PG 0.72 g/pot and 0.747 g/pot in the root. A recent study [70] demonstrated that Pantoea sp. BRM17, a bacterium isolated from Tunisian PG, produces siderophores, Indole acetic acid (IAA) and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase.
In addition, a soil amended with 2%, PG has enabled increasing the biomass of the shoots and roots of cultivated Brassica napus. A similar study [71] mentioned that inoculating Brassica napus with a bacterial consortium (Pantoea agglomerans Jp3-3) increased the dry mass as well as the chlorophyllin parts (by 24–124%) and roots (38–100%). In this context, Walterson et al. [72] reported that these species have been discovered to be associated with many different plants, frequently as epiphytic and endophytic bacteria, and have also been widely used as a potential biological control agent against fungal and bacterial plant pathogens.
Thus far, many environmental or plant-associated strains have been isolated from different sources and characterized for their plant growth-promoting traits. Although, some of these isolates are Stenotrophomonas species that have an important ecological role in the element cycle in nature [73]. Due to its potential plant growth-promoting attributes, like phosphate solubilization, S. maltophilia is considered a biotechnologically important microorganism [74].
In the present study, these species showed a shoot dry matter of 0.648 g/pot, and a root dry matter of 0.696 g/pot with a source of PN. Higher values of shoot dry matter (0.695 g/pot) and of root matter (0.711 g/pot) were also observed with PG. S. maltophilia showed the lowest value of shoot dry matter (0.643 g/pot), and a root dry matter of 0.684 g/pot (Figure 4A,B) without a source of phosphorus. The study described by Barra et al. [75], reported that Stenotrophomonas sp. RC5 with phosphorus fertilization enhanced the phosphorus content in the shoot of Lolium perenne by 29.8% compared to uninoculated control, in phosphorus-deficient soil.
Despite the fact that inoculums with three isolates were shown an important value of shoot biomass of 0.663 g/pot and a root of 0.704 g/pot (without a source of phosphorus) were conducted according to Singh and Kapoor [76] and Gull et al. [77], the significant increase in plant height and dry weight of walnut seedlings due to inoculation with PSM could be attributed to greater absorption of nutrients, exceptionally P. The above results indicate that localized fertilization with PG (as a supply of large amounts of Ca, S, P, and beneficial trace elements) enhance the biomass of roots and shoots ryegrass compared to PN input [78,79,80].
Even though, waste PG has been employed as a soil enhancer in around countries (Brazil, China, Spain, and the USA) [81,82]. Thus, the higher values of shoots and root biomass in our experience can be explained by the supplement of PG, which can increase Ca and S and diminish Mg contents in leaves [79,80,81,82,83] and shoots. Next, Nayak et al. [84] reported that an additional 10% PG had a beneficial effect on cellulose and amylase activities and microbial (fungal and bacterial) growth.
3.4.3. Shoot and Root Mineral Matter
Generally, the addition of PG or PN, the presence of bacteria and soil sterility had a significant impact on plant development (Pr > 0.0583). The mineral matter in shoots and (Pr > 0.0831) in roots after 170 days of growth, are presented in (Figure 5A,B).
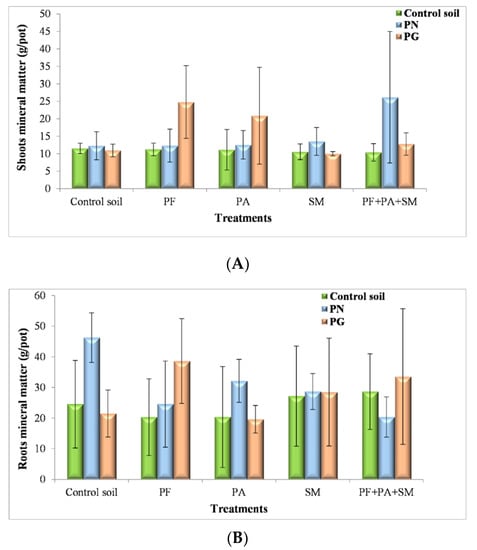
Figure 5.
Effect of PN, PG and bacterial inoculum added to the soil on shoots (A) and roots (B) mineral matter (g/pot) (PF: P. fluorescens; PA: P. agglomerans; SM: S. maltophilia) (The values and the error bars represent the means ± standard deviation, n = 4).
The presence of the mixed bacterial inoculum (P. fluorescens, S. maltophilia and P. agglomerans) and a source of PN had a significant high effect on plant shoot development (26.155 g/pot).In this context, Pratibha et al. [85] started the combined supplementation of PSMs and PN in the agricultural field as an option to improve the phosphorus use efficiency and yield of the crops. Hence, PN could play a significant role as a cheaper P source for plants.Therefore, plant-growth-promoting bacteria (PGPB) could effectively colonize plant roots and stimulate plant growth through various mechanisms that include increased mobilization of insoluble nutrients and subsequently enhanced plant uptake, as reported by Adesemoye et al. [86] and Richardson et al. [87]. Among PGPB, phosphate-solubilizing bacteria are considered prospective biofertilizers. There were more reports of growth promotion on crop plants inoculated with PSB [88,89].
On the other hand, adding PG increased the shoot and root biomass of inoculated plants by P. fluorescens (24.79 g/pot of shoot mineral matter; 38,622 g/pot of root mineral mater) (Figure 5A,B). In order to explain the role of P. fluorescens in the promotion of plant growth in the presence of PG, inoculated plants were cultivated in soil amended with 250 mg PG. Our results prove P. fluorescens to be the most efficient and had very strong growth-promoting effects on ryegrass cultivated in sterilized PG. Respectively, this effect was weaker in PG or PN with P. agglomerans and S. maltophilia shoot and root biomass. Besides, the application of PGPB is a promising method for reducing the use of chemical fertilizers and their pollution hazards and could support eco-friendly crop production as described by Requena et al. [90].
3.4.4. Nutrient Uptake
In general, according to the SAS analysis P uptake was considerably enhanced in the treatment with PG, and the bacteria present remarkably influenced plant development. In the presence of P. fluorescens and PG the P uptake had an important effect on plant root development (24.89 mg/pot) and shoot (37.42 mg/pot) (see Figure 6A,B).
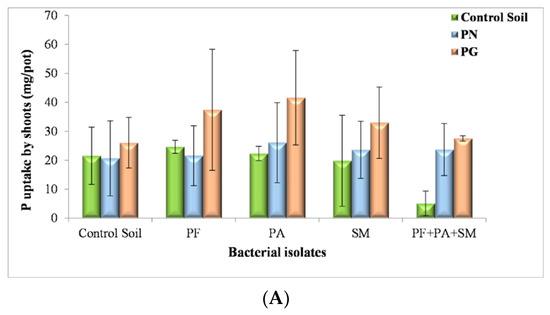
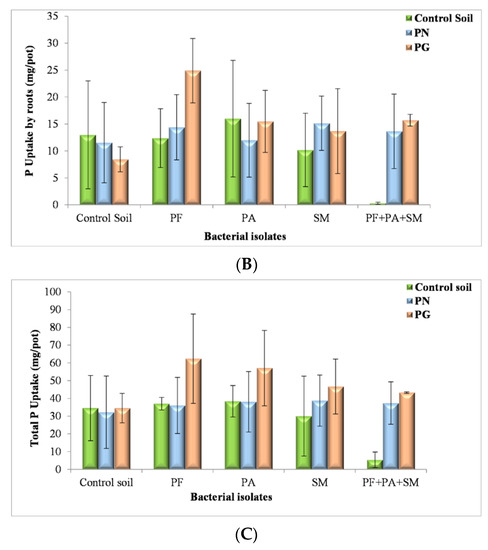
Figure 6.
Effect of PN, PG and bacterial inoculum added to the soil on P uptake by shoots (A), roots (B) and total P uptake (C) (mg/pot) of plants (shoots and roots) (PF: P. fluorescens; PA: P. agglomerans; SM: S. maltophilia) (The values and the error bars represent the means ± standard deviation, n = 4).
Our findings are supported by Al-Enazy et al. [91] saying that waste PG may be used to improve soil properties, promote micronutrient uptake, and increase crop yield; moreover, the decrease in micronutrients and/or their uptake by crops was concerning when PG was applied in the agricultural field.
Nevertheless, adding PN with P. fluorescens isolate reduces the shoot biomass to 21.58 mg/pot and root biomass to 14.4 mg/pot of inoculated plants; however, the shoot and root biomass of inoculated with the same isolate P. fluorescens plants decreased sharply without a source of PN or PG 37 mg/pot (Figure 6A,B). To investigate the response of ryegrass growth to localized nutrient supply, P uptake was determined in the experiment with P. agglomerans isolate. Total P uptake was significantly higher in the treatment with localized source PG and inoculum of P. agglomerans 57.05 mg/pot supply than the localized supply of inoculum P. agglomerans in the sterile soil (38.35 mg/pot) and with a source of PN (38.06 mg/pot) (Figure 6C), maybe optimization of inoculant levels should be carefully considered.
This experiment showed the role of P. agglomerans in the promotion of plant growth in the presence of PG, PN and soil indicating that P. agglomerans had really powerful growth-promoting effects on ryegrass cultivated in sterilized PG; this result was explained by Vyshpolsky et al. [92] which hypothesized that seeds germination was enhanced after PG addition.
Takasu et al. [93] reported that the addition of PG to the nursery medium resulted in rooting development and promoted plant growth in the seedling stage. One of our strains, S. maltophilia showed a high P total uptake in the treatment with a localized source of PG (46.65 mg/pot), which contributes to the solubilization of inorganic P contained in PG; this inorganic P thus becomes more available for plant nutrition, in addition to the ability of S. maltophilia to produce growth-promoting substances; this effect was weaker in soil sterilized (30 mg/pot) and with PN (38.74 mg/pot) (Figure 6C).
The results demonstrate that inoculation with S. maltophilia does have a positive effect on plant growth in soils with PG or PN amendment as compared to the control treatments. Interestingly, when ryegrass plants were grown in PG and inoculums P. fluorescens, S. maltophilia and P. agglomerans, a high P uptake was observed (43.23 mg/pot); this is a promising result for the development of bio-inoculants that were well-adapted to PG composition. In contrast, non-inoculated plants with only soil showed very poor growth and very poor P total uptake (5.39 mg/pot). Nevertheless, the P uptake had significantly increased with inoculums and a source of PN (37.33 mg/pot) (Figure 6C). Thus far, several studies have shown that PGPR growth promotion traits can be enhanced in severely polluted soils, reducing the stress in plants, as observed by Moreira et al. [94].
To sum up, these initial tests intended to choose the bacterial isolates best suited to accommodate various PG or PN concentrations under analyzing the growth of ryegrass in a condition of sterile soil. At present, the studies on mineral-microbe interactions lie at the heart of the emerging field of geomicrobiology, as minerals and rocks are the basic materials with which microbes interact at all scales [95]. Then, with the source of PG, total P uptake and three species were presented overall increasing order of isolates was: P. fluorescens > P. agglomerans > S. maltophilia > mixing inoculation with P. fluorescens, S. maltophilia and P. agglomerans. So, this important test was adapted from the one that does not employ bacterium inoculants.
As previously observed by Aransiola et al. [21] rhizoremediation can offer an attractive alternative to conventional processes to obtain an economic and ecological solution. Hussain et al. [96] also reported that PSM, (Pseudomonas, Mycobacterium, Bacillus, Pantoea Rhizobia, and Burkholderia) adopt different strategies to solubilize phosphorus, PSMs excrete organic acids such as citric acid, oxalic acid, and succinic acids; enzymes such as phosphatases and phytases; and ion chelators such as siderophores, readily make phosphorus available to plants [28]. Then, as evidenced in the findings of Rawat et al. [97] these microorganisms can contribute to ecological balance, produce safe food and meet the needs of sustainable agriculture.
Total P uptake after 170 days of growth was affected significantly (p < 0.001) by P source. Total P uptake reached a plateau in the range of 5.39 to 62.31 mg/pot (Figure 6C). The highest total P uptake was obtained with P. fluorescens (62.31 mg/pot). The difference in P uptake between treatments is due to the type of P source and isolate. The media with soil extract—PG extract in our case—serve to better approximate the composition of a complex natural medium as described by other studies [98,99].
4. Conclusions
Currently, a large amount of phosphogypsum waste is being produced due to the high demand for phosphate fertilizers; this study supports new concerns related to the application of PG as a fertilizer in agricultural soils.
In addition, microbial consortia with PSM in the soil is, therefore, a promising method without negative environmental and socio-economic impacts. PSM demonstrates an advantageous impact on the solubilization of undisclosed P present in PN and augmented plant height, dry weight, and P uptake of ryegrass. Accordingly, the application of inoculation phosphate-solubilizing with PG can be an optional alternative for introducing P nutrition to plants. An innovative result of this work is the first report of using of three isolates, P. fluorescencs, P. agglomerans and S. maltophilia that significantly enhanced the biomass yield of ryegrass; these microorganisms solubilize the unavailable phosphorus in soil and also restore the nutritional status of the soil. For this reason, this work has performed several accessible strains that can play a role in developing a host of potential benefits for field application with PG or PN as biofertilizers. So, the present search for ryegrass-associated bacteria in Tunisia regions, where this plant has agricultural importance as a range crop, discovered that several of these bacteria perform essential activities related to the promotion of plant growth and biocontrol, and some of them may act as plant growth promoters.
Author Contributions
Conceptualization, M.A., D.M., M.R.R., N.A. and C.A.; Investigation, M.A., M.G., N.A. and C.A.; Writing—original draft, M.A., D.M., N.A. and C.A.; Writing—review and editing, M.A. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zou, K.; Binkley, D.; Doxtader, K.G. New methods for estimating gross P mineralization and mobilization rates in soils. Plant Soil 1992, 147, 243–250. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Elkoca, E.; Turan, M.; Donmez, M.F. Effects of single, dual and triple inoculation with Bacillus subtilis, Bacillus megaterium and Rhizobium leguminosarumbvphase oil on nodulation, nutrient uptake, yield and yield parameters of common bean (Phaseolus vulgaris L. cv. ‘Elkoca-05′). J. Plant Nutr. 2010, 33, 2104–2119. [Google Scholar] [CrossRef]
- USEPA. National Emission Standards for Hazardous Air Pollutants; U.S. Environmental Protection Agency, Subpart R: Washington, DC, USA, 2002. [Google Scholar]
- FAOSTAT. Food Agriculture Organization of the United Nations How Published; FAOSTAT: Roma, Italy, 2014. [Google Scholar]
- Gu, K.; Chen, B. Loess stabilization using cement, waste phosphogypsum, fly ash and quicklime for self-compacting rammed earth construction. Construct. Build. Mater. 2020, 231, 117195. [Google Scholar] [CrossRef]
- Mashifana, T.P. Chemical treatment of phosphogypsum and its potential application Chemical treatment of phosphogypsum and its potential application for building and construction. Procedia Manuf. 2019, 35, 641–648. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, H.; Zheng, L.; Wang, K.; Li, H.; Wang, Y.; Feng, H. Utilization of waste phosphogypsum to prepare hydroxyapatite nanoparticles and its application. J. Hazard. Mater. 2012, 241, 418–426. [Google Scholar] [CrossRef]
- Bisone, S.; Gautier, M.; Chatain, V.; Blanc, D. Spatial distribution and leaching behavior of pollutants from phosphogypsum stocked in a gypstack: Geochemical characterization and modelling. J. Environ. Manag. 2017, 193, 567–575. [Google Scholar] [CrossRef]
- Burnett, W.C.; Elzerman, A.W. Nuclide migration and the environmental radiochemistry of Florida phosphogypsum. J. Environ. Radioact. 2001, 54, 27–51. [Google Scholar] [CrossRef]
- Lopez, F.A.; Gazquez, M.; Alguacil, F.J.; Bolívar, J.P.; García-Díaz, I.; Lopez-Coto, I. Microencapsulation of phosphogypsum into a sulfur polymer matrix: Physicochemical and radiological characterization. J. Hazard. Mater. 2011, 192, 234–245. [Google Scholar] [CrossRef]
- Hentati, O.; Abrantes, N.; Caetano, A.L.; Bouguerra, S.; Gonçalves, F.; Rombke, J.; Pereira, R. Phosphogypsum as a soil fertilizer: Ecotoxicity of amended soil and elutriates to bacteria, invertebrates, algae and plants. J. Hazard. Mater. 2015, 294, 80–89. [Google Scholar] [CrossRef]
- Ma, B.; Lu, W.; Su, Y.; Li, Y.; Gao, C.; He, X. Synthesis of a-hemihydrate gypsum from cleaner phosphogypsum. J. Clean. Prod. 2018, 195, 396–405. [Google Scholar] [CrossRef]
- Saadaoui, E.; Ghazel, N.; Ben Romdhane, C.; Massoudi, N. Phosphogypsum: Potential uses and problems—A review. Int. J. Environ. Res. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; LÓpez, F.A.; Alguacil, F.J.; LÓpez-Delgado, A. Environmental impact and management of phosphogypsum, a review. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.; Souza, J.A.; Moreira, A.; Moraes, L.A.C. Phosphogypsum and vinasse application: Soil chemical properties and alfalfa productivity and nutritional characteristics. Rev. Caatinga 2017, 30, 213–219. [Google Scholar] [CrossRef]
- Papastefanou, C.; Stoulos, S.; Ioannidou, A.; Manolopoulou, A. The application of phosphogypsum in agriculture and the radiological impact. J. Environ. Radioact. 2006, 89, 188–198. [Google Scholar] [CrossRef]
- Prochnow, L.; Caires, E.; Rodrigues, E.C. Phosphogypsum use to improve subsoil acidity: The Brazilian experience. Better Crops Plant Food 2016, 100, 13–15. [Google Scholar]
- Elloumi, N.; Zouari, M.; Chaari, L.; Abdallah, F.B.; Woodward, S.; Kallel, M. Effect of phosphogypsum on growth, physiology, and the antioxidative defense system in sunflower seedlings. Environ. Sci. Pollut. Res. Int. 2015, 22, 14829–14840. [Google Scholar] [CrossRef]
- Komnitsas, K.; Lazar, I.; Petrisor, I.G. Application of a vegetative cover on phosphogypsum stacks. Min. Eng. 1999, 12, 175–185. [Google Scholar] [CrossRef]
- Aransiola, S.A.; Ijah, U.J.J.; Abioye, O.P.; Bala, J.D. Microbial-aided phytoremediation of heavy metals contaminated soil: A review. Eur. J. Biol. Res. 2019, 9, 104–125. [Google Scholar]
- Dorjey, S.; Dolkar, D.; Sharma, R. Plant growth promoting rhizobacteria Pseudomonas: A review. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1335–1344. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Microbial transformations in the phosphorus cycle. Adv. Microb. Ecol. 1977, 1, 95–134. [Google Scholar]
- Illmer, P.; Schinner, F. Solubilization of inorganic phosphorus by microorganisms isolated from forest soils. Soil. Biol. Biochem. 1992, 24, 389–395. [Google Scholar] [CrossRef]
- Sharma, S.N.; Ray, S.B.; Pandey, S.L.; Prasad, R. Effect of irrigation, pyrites and phospho bacteria on the efficiency of rock phosphate applied to lentil. J. Agric. Sci. 1983, 101, 467–472. [Google Scholar] [CrossRef]
- Sharma, S.N.; Prasad, R. Mussoorie rock phosphate–pyrite mixture as phosphate fertilizer. Fert. Res. 1996, 45, 187–191. [Google Scholar] [CrossRef]
- Sharma, S.N.; Prasad, R. Yield and P uptake by rice and wheat grown in a sequence as influenced by phosphate fertilization with diammonium phosphate and Mussoorie rock phosphate with or without crop residue and phosphatesolubilizing bacteria. J. Agric. Sci. 2003, 141, 359–369. [Google Scholar] [CrossRef]
- Tomer, S.; Suyal, D.C.; Goel, R. Biofertilizers: A timely approach for sustainable agriculture. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2016; pp. 375–395. [Google Scholar]
- Zhang, Z.H.; Rengel, Z.; Meney, K. Kinetics of ammonium, nitrate and phosphorus uptake by Canna indica and Schoenoplectusvalidus. Aquat. Bot. 2009, 91, 71–74. [Google Scholar] [CrossRef]
- Duan, Y.H.; Yin, X.M.; Zhang, Y.L.; Shen, Q.R. Mechanisms of enhanced rice growth and nitrogen uptake by nitrate. Pedosphere 2007, 17, 697–705. [Google Scholar] [CrossRef]
- Pérez-López, R.; Nieto, J.M.; López-Coto, I.; Aguado, J.L.; Bolivar, J.P.; Santisteban, M. Dynamics of contaminants in phosphogypsum of the fertilizer industry of Huelva (SW Spain): From phosphate rock ore to the environment. Appl Geochem. 2010, 25, 705–715. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Daghbouj, N.; Abda, H.; Castet, S.; Josse, C.; van Beek, P.; Souhaut, M.; Michel, S.; Bejaoui, N.; et al. Characterization of phosphate rock and phosphogypsum from Gabes phosphate fertilizer factories (SE Tunisia): High mining potential and implications for environmental protection. Environ. Sci. Pollut. Res. Int. 2018, 15, 14690–14702. [Google Scholar] [CrossRef]
- Zemni, S.; Hajji, M.; Triki, M.; M’nif, A.; Hamzaoui, A.H. Study of phosphogypsum transformation into calcium silicate and sodium sulfate and their physicochemical characterization. J. Clean. Prod. 2018, 198, 873–881. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indolacetic acid in development of host plant root system. Appl. Environ. Micobiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Jalali, J.; Gaudin, P.; Capiaux, H.; Ammar, E.; Lebeau, T. Fate and transport of metal trace elements from phosphogypsum piles in Tunisia and their impact on soil bacteria and wild plants. Ecotoxicol. Environ. Saf. 2019, 174, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Götz, M.; Nirenberg, H.; Krause, S.; Wolters, H.; Draeger, S.; Buchner, A.; Lottmann, J.; Berg, G.; Smalla, K. Fungal endophytes in potato roots studied by traditional isolation and cultivation-independent DNA-based methods. FEMS. Microbiol. Ecol. 2006, 58, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, T.B.; Gonçalves, M.C.; Castanheira, N.L.; Martins, J.C.; Santos, F.L.; Prazeres, A.; Fernandes, M.L. Effect of sodium and nitrogen on yield function of irrigated maize in southern Portugal. Agric.Water Manag. 2009, 96, 585–594. [Google Scholar] [CrossRef]
- Hussain, M.I.; Asghar, H.N.; Arshad, M.; Shahabaz, M. Screening of multi-traits rhizobacteria to improvemaize growth under axenic conditions. J. Anim. Plant Sci. 2013, 23, 514–520. [Google Scholar]
- Zhou, X.; Wang, G.; Yang, F. Characteristics of growth, nutrient uptake, purification effect of Ipomoea aquatica, Lolium multiflorum, and Sorghum sudanense grown under different nitrogen levels. Desalination 2011, 273, 366–374. [Google Scholar] [CrossRef]
- Shi, R. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 1986; pp. 388–389. [Google Scholar]
- Al-Masri, M.S.; Amin, Y.; Ibrahim, S.; Al-Bich, F. Distribution of some trace metals in Syrian phosphogypsum. Appl. Geochem. 2004, 19, 747–753. [Google Scholar] [CrossRef]
- Reguigui, R.; SfarFelfoul, H.; Ben Ouezdou, M.; Clastres, P. Radionuclide levels and temporal variation in phosphogypsum. J. Radioanal. Nucl. Chem. 2005, 264, 719–722. [Google Scholar] [CrossRef]
- Feki, H.E.; Savariault, J.M.; Saleh, A.B. Structure refinements by the Rietveld method of partially substituted hydroxyapatite: Ca9Na0.5(PO4)4.5(CO3)1.5(OH)2. J. Alloys Compd. 1999, 287, 114. [Google Scholar] [CrossRef]
- Gmati, N.; Boughzala, K.; Abdellaoui, M.; Bouzouita, K. Mechanochemical synthesis of strontium britholites: Reaction mechanism. C. R. Chim. 2011, 14, 896–903. [Google Scholar] [CrossRef]
- Ibrahim, N.; Mamane, A.; Zanguina, A.; Khalid, I.; Anne, B.; Michel, B. Etudes physicochimiques du phosphate Marchand de Tahoua. J. Société Ouest-Afr. Chim. 2004, 18, 137–148. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wily and Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Georgios, B.; Clément, P.; Lars, J.; Sander, B. Using FTIR-photoacoustic spectroscopy for phosphorus speciation analysis of biochars. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 5, 29–36. [Google Scholar]
- Boughzala, K.; Fattah, N.; Bouzouita, K.; Ben Hassine, H. Etude minéralogique et chimique du phosphate naturel d’Oum El Khecheb (Gafsa, Tunisie). Sci. Matériaux 2015, 6, 11–29. [Google Scholar]
- Dumitras, D.G.; Marincea, S.; Fransolet, A.M. Brushite in the bat guano deposit from the dry Cioclovina Cave (Sureanu Mountains, Romania). N. Jahrb. Miner. Abh. 2004, 180, 45–64. [Google Scholar] [CrossRef]
- El Cadi, A.; Fakih Lanjri, A.; Lalilti, A.; Chouaibi, N.; Asskali, A.; Khaddor, M. Caractérisation de la fraction lipidique du phosphogypse: Origine et évaluation du degré de transformation des polluantsorganiques (Characterization of the lipid fraction of phosphogypsum: Origin and assessment of the degree of transformation of organic pollutants). Mater. Environ. Sci. 2014, 5, 2223–2229. [Google Scholar]
- Blum, J.; Herpin, U.; Melfi, A.J.; Montes, C.R. Soil properties in a sugarcane plantation after the application of treated sewage effluent and phosphogypsum in Brazil. Agric. Water Manag. 2012, 115, 203–216. [Google Scholar] [CrossRef]
- Jackson, M.E.; Naeth, M.A.; Chanasyk, D.S.; Nichol, C.K. Phosphogypsum capping depth affects revegetation and hydrology in western Canada. J. Environ. Qual. 2011, 40, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, M.; Toma, M.; Nanzyo, M. Alleviation of subsoil acidity in nonallophanic andosols by phosphogypsum application in topsoil. Soil Sci. Plant Nutr. 1996, 42, 221–227. [Google Scholar]
- Zhang, X.C.; Miller, W.P.; Nearing, M.A.; Norton, L.D. Effects of surface treatment on surface sealing, runoff, and interrill erosion. Trans. ASAE 1998, 41, 989–994. [Google Scholar] [CrossRef]
- Bana, R.S.; Shivay, Y.S.; Sepat, S.; Rana, K.S.; Pooniya, V. Effect of summer forage crops and phosphogypsum-enriched urea on productivity of basmati rice (Oryza sativa)-wheat (Triticum aestivum) cropping system. Res. Crops 2013, 14, 649–653. [Google Scholar]
- Elloumi, N.; Belhaj, D.; Mseddi, S.; Zouari, M.; Ben Abdallah, F.; Woodward, S.; Kallel, M. Response of Nerium oleander to phosphogypsum amendment and its potential use for phytoremediation. Ecol. Eng. 2017, 99, 164–171. [Google Scholar] [CrossRef]
- Biswas, J.K.; Banerjee, A.; Rai, M.; Naidu, R.; Biswas, B.; Vithanage, M.; Dash, M.C.; Sarkar, S.K.; Meers, E. Potential application of selected metal resistant phosphate solubilizing bacteria isolated from the gut of earthworm (Metaphireposthuma) in plant growth promotion. Geoderma 2018, 330, 117–124. [Google Scholar] [CrossRef]
- Sulbaran, M.; Perez, E.; Ball, M.M.; Bahsas, A.; Yarzabal, L.A. Characterization of the mineral phosphate-solubilizing activity of Pantoeaaglomerans MMB051 isolated from an iron-rich soil in southeastern Venezuela (Bolivar state). Curr. Microbiol. 2009, 58, 378–383. [Google Scholar] [CrossRef]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; van der Lelie, D.; Dow, J.M. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 2009, 7, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Rajwar, J.; Chandra, R.; Suyal, D.C.; Tomer, S.; Kumar, S.; Goel, R. Comparative phosphate solubilizing efficiency of psychrotolerant Pseudomonas jesenii MP1 and Acinetobacter sp. ST02 against chickpea for sustainable hill agriculture. Biologia 2018, 73, 793–802. [Google Scholar] [CrossRef]
- Zaheer, A.; Malik, A.; Sher, A.; Qaisrani, M.M.; Mehmood, A.; Khan, S.U.; Ashraf, M.; Mirza, Z.; Karim, S.; Rasool, M. Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi J. Biol. Sci. 2019, 26, 1061–1067. [Google Scholar] [CrossRef]
- Kpomblekou, A.; Tabatabai, M. Effect of organic acids on release of phosphorus from phosphate rock. Soil Sci. 1994, 158, 442–453. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant. Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant growth-promoting effects of dia-zotrophs in the rhizosphere. Crit. Rev. Plant. Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Scavino, A.F.; Pedraza, R.O. The role of siderophores in plant growth-promoting bacteria. In Bacteria in Agrobiology: Crop Productivity; Maheshwari, D.K., Saraf, M., Aeron, A., Eds.; Springer-Verlag: Berlin/Heidelberg, Germay, 2013; pp. 265–285. [Google Scholar]
- Yanni, Y.G.; Rizk, R.Y.; El-Fattah, F.K.; Squartini, A.; Corich, V.; Giacomini, A.; De Bruijn, F.; Rademaker, J.; Maya-Flores, J.; Ostrom, P.; et al. The beneficial plant growth-promoting association of Rhizobium leguminosarumbv. trifolii with rice roots. Aust. J. Plant Physiol. 2001, 28, 845–870. [Google Scholar]
- Ping, L.Y.; Boland, W. Signals from the underground: Bacterial volatiles promote growth in Arabidopsis. Trends Plant Sci. 2004, 9, 263–266. [Google Scholar] [CrossRef] [PubMed]
- de-Bashan, L.E.; Hernandez, J.-P.; Bashan, Y.; Maier, R.M. Bacillus pumilus ES4: Candidate plant growth-promoting bacterium to enhance establishment of plants in mine tailings. Environ. Exp. Bot. 2010, 69, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Delétoile, A.; Decré, D.; Courant, S.; Passet, V.; Audo, J.; Grimont, P.; Arlet, G.; Brisse, S. Phylogeny and identification of Pantoea species and typing of Pantoeaagglomerans strains by multilocus gene sequencing. J. Clin. Microbiol. 2009, 47, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Trifi, H.; Ben Salem, I.; KolsiBenzina, N.; Fourati, A.; Costa, M.C.; Achouak, W.; Sghaier, H.; Saidi, M. Effectiveness of the plant growth-promoting rhizobacterium Pantoea spp. BRM17 in enhancing Brassica napus growth in phosphogypsum-amended soil. Pedosphere 2017, 17, 60454–60455. [Google Scholar]
- Zhang, Y.F.; He, L.Y.; Chen, Z.J.; Zhang, W.H.; Wang, Q.Y.; Qian, M.; Sheng, X.F. Characterization of lead-resistant and ACC deaminase-producing endophytic bacteria and their potential in promoting lead accumulation of rape. J. Hazard. Mater. 2011, 186, 1720–1725. [Google Scholar] [CrossRef]
- Walterson, A.M.; Starinides, J. Pantoea: Insights into a highly versatile and diverse genus within the Enterobacteriaceae. FEMSMicrobiol. Rev. 2015, 39, 968–984. [Google Scholar]
- Ikemoto, S.; Suzuki, K.; Kaneko, T.; Komagata, K. Characterization of strains of Pseudomonas maltophilia which do notrequire methionine. Int. J. Syst. Bacteriol. 1980, 30, 437–447. [Google Scholar] [CrossRef]
- Berg, G.; Knaape, C.; Ballin, G.; Seidel, D. Biological control of Verticillium dahliae KLEB by naturally occurring rhizosphere bacteria. Arch. Phytopathol. Dis. Prot. 1994, 29, 249–262. [Google Scholar] [CrossRef]
- Barra, P.J.; Pontigo, S.; Delgado, M.; Parra-Almuna, L.; Duran, P.; Valentine, A.J.; Jorquera, M.A.; de la Luz Mora, M. Phosphobacteria inoculation enhances the benefit of P–fertilization on Lolium perenne in soils contrasting in P–availability. Soil Biol. Biochem. 2019, 136, 107516. [Google Scholar] [CrossRef]
- Singh, S.; Kapoor, K.K. Inoculation with phosphate-solubilizing microorganisms and a vesicular-arbuscular mycorrhizal fungus improves dry matter yield and nutrient uptake by wheat grown in a sandy soil. Biol. Fertil. Soils 1999, 28, 139–144. [Google Scholar] [CrossRef]
- Gull, M.; Hafeez, F.Y.; Saleem, M.; Malik, K.A. Phosphorus uptake and growth promotion of chickpea by co-inoculation of mineral phosphate solubilising bacteria and a mixed rhizobial culture. Aust. J. Exp. Agric. 2004, 44, 623–628. [Google Scholar] [CrossRef]
- Lloyd, G.M. Phosphogypsum—A sulfur resource. Abstr. Pap. Am. Chem. Soc. 1989, 198, 31-FERT. [Google Scholar]
- Soratto, R.P.; Costa Crusciol, C.A. Nutrition and grain yield of black oats affected by surface application of lime and phosphogypsum at the establishment of no-tillage system. Rev. Bras. Ciencia Solo 2008, 32, 715–725. [Google Scholar] [CrossRef]
- Rechcigl, J.E.; Mislevy, P. Stargrass response to lime and phosphogypsum. J. Prod. Agric. 1997, 10, 101–105. [Google Scholar] [CrossRef]
- Enamorado, S.; Abril, J.M.; Delgado, A.; Más, J.L.; Polvillo, O.; Quintera, J.M. Implications for food safety of the uptake by tomato of 25 trace-elements from a phosphogypsum amended soil from SW Spain. J. Hazard. Mater. 2014, 226, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Mishra, V.K.; Sharma, D.K.; Jha, S.K.; Singh, C.S.; Shahabuddin, M.; Shahid, M. Efficiency of phosphogypsum and mined gypsum in reclamation and productivity of rice-wheat cropping system in sodic soil. Commun. Soil Sci. Plant Anal. 2013, 44, 909–921. [Google Scholar] [CrossRef]
- Vicensi, M.; Muller, M.M.L.; Kawakami, J.; do Nascimento, R.; Michalovicz, L.; Lopes, C. Do rates and splitting of phosphogypsum applications influence the soil and annual crops in a No-tillage system? Rev. Bras. Ciência Solo 2016, 40. [Google Scholar] [CrossRef]
- Nayak, S.; Mishra, C.S.; Guru, B.C.; Rath, M. Effects of phosphogypsum amendment on soil physico-chemical properties, microbial and soil enzyme activities. J. Environ. Biol. 2011, 32, 613–617. [Google Scholar]
- Pratibha, R.; Sudeshna, D.; Deepti, S.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Increased plant uptake of nitrogen from 15N-depleted fertilizer using plant growth-promoting rhizobacteria. Appl. Soil Ecol. 2010, 46, 54–58. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquistion of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Chabot, R.; Antoun, H.; Cescas, M.P. Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar. phaseoli. Plant Soil 1996, 184, 311–321. [Google Scholar] [CrossRef]
- Morales Dr, A.; Alvear, M.; Valenzuela, E.; Rubio, R.; Borie, F. Effect of inoculation with Penicillium albidum, a phosphate-solubilizing fungus, on the growth of Trifolium pratense cropped in a volcanic soil. J. Basic Microb. 2007, 47, 275–280. [Google Scholar] [CrossRef]
- Requena, B.N.; Jimenez, I.; Toro, M.; Barea, J.M. Interactions between plant growth promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytiisoides, a model legume for revegetation in Mediterranean semi-arid ecosystem. New Phytol. 1997, 136, 667–677. [Google Scholar] [CrossRef]
- Al-Enazy, A.A.R.; Al-Oud, S.S.; Al-Barakah, F.N.; Usman, A.R.A. Role of microbial inoculation and industrial by-product phosphogypsum in growth and nutrient uptake of maize (Zea mays L.) grown in calcareous soil. J. Sci. Food Agric. 2017, 97, 3665–3674. [Google Scholar] [CrossRef]
- Vyshpolsky, F.; Mukhamedjanov, K.; Bekbaev, U.; Ibatullin, S.; Yuldashev, T.; Noble, A.D.; Mirzabaev, A.; Aw-Hassan, A.; Qadir, M. Optimizing the rate and timing of phosphogypsum application to magnesium-affected soils for crop yield and water productivity enhancement. Agric. Water Manag. 2010, 97, 1277–1286. [Google Scholar] [CrossRef]
- Takasu, E.; Yamada, F.; Shimada, N.; Kumagai, N.; Hirabayashi, T.; Saigusa, M. Effect of phosphogypsum application on the chemical properties of Andosols, and the growth and Ca uptake of melon seedlings. Soil Sci. Plant Nutr. 2006, 52, 760–768. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.A.; Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Mine land valorization through energy maize production enhanced by the application of plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi. Environ. Sci Pollut. Res. 2015, 23, 6940–6950. [Google Scholar] [CrossRef]
- Dong, H. Mineral-microbe interactions: A review. Front. Earth Sci. 2010, 4, 127–147. [Google Scholar] [CrossRef]
- Hussain, A.; Adnan, M.; Iqbal, S.; Fahad, S.; Saeed, M.; Mian, I.A.; Muhammad, M.W.; Romman, M.; Perveez, R.; Wahid, F.; et al. Combining phosphorus (P) with phosphate solubilizing bacteria (PSB) improved wheat yield and P uptake in alkaline soil. Pure Appl. Biol. 2019, 8, 1809–1817. [Google Scholar] [CrossRef]
- Rawat, P.; Shankhdhar, D.; Shankhdhar, S.C. Plant growth-promoting rhizobacteria: A booster for ameliorating soil health and agriculture production. In Soil Health; Giri, B., Verma, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 59, pp. 47–68. [Google Scholar]
- Braud, A.; Hubert, M.; Gaudin, P.; Lebeau, T. A quick rhizobacterial selection tool to be used in phytoextraction-assisted bioaugmentation of metal contaminated soils. J. Appl. Microbiol. 2015, 119, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Liebeke, M.; Brozel, V.S.; Hecker, M.; Lalk, M. Chemical characterization of soil extract as growth media for the ecophysiological study of bacteria. Appl. Microbiol. Biotechnol. 2009, 83, 161–173. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).