1. Introduction
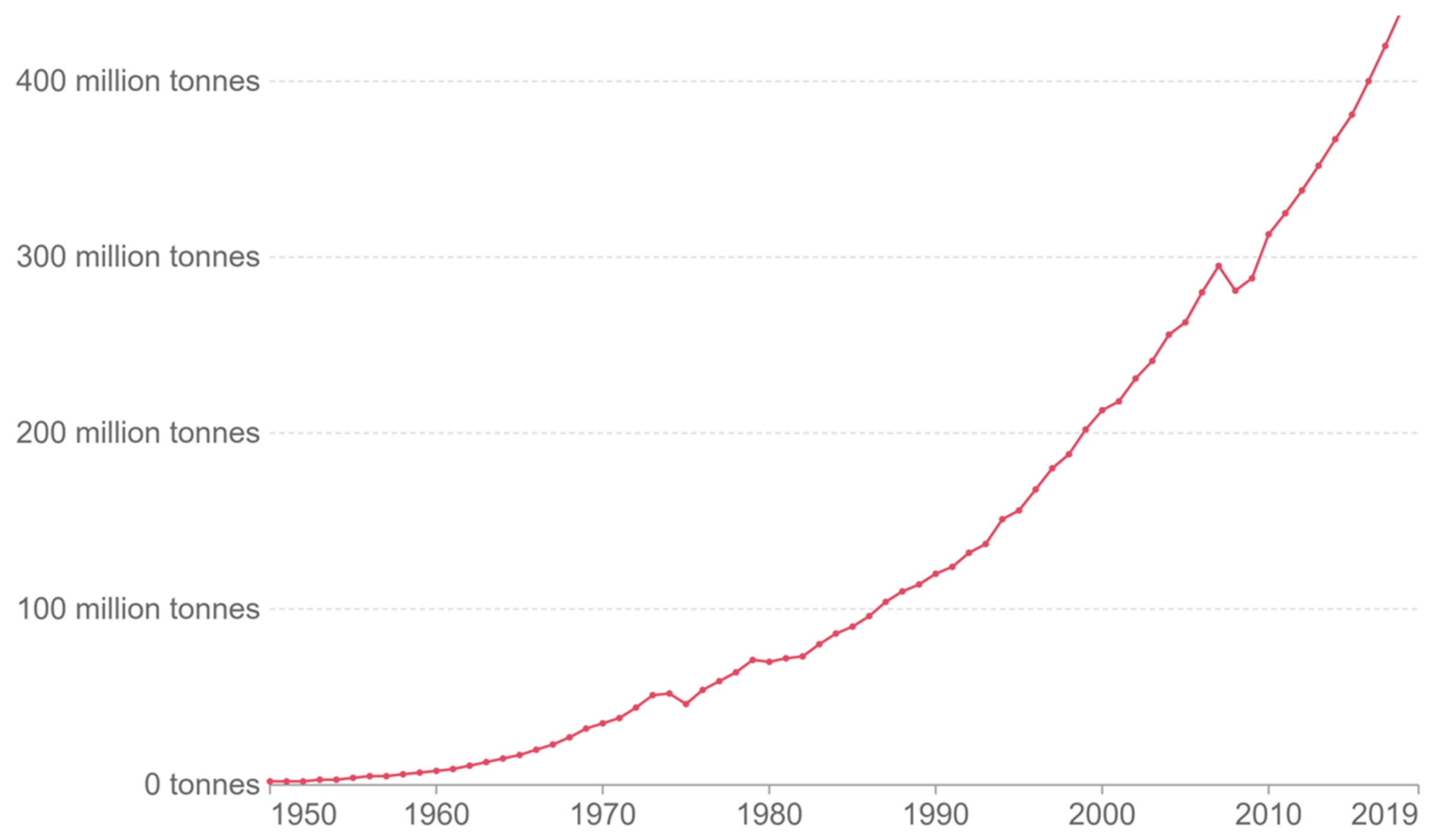
Plastic materials have gathered attention recently due to their omnipresence in the global economy. Since last century, plastics have become rapidly one of the most used materials in industry. In 2019, more than 400 million tonnes of plastics (Mt) were produced (
Figure 1) [
1].
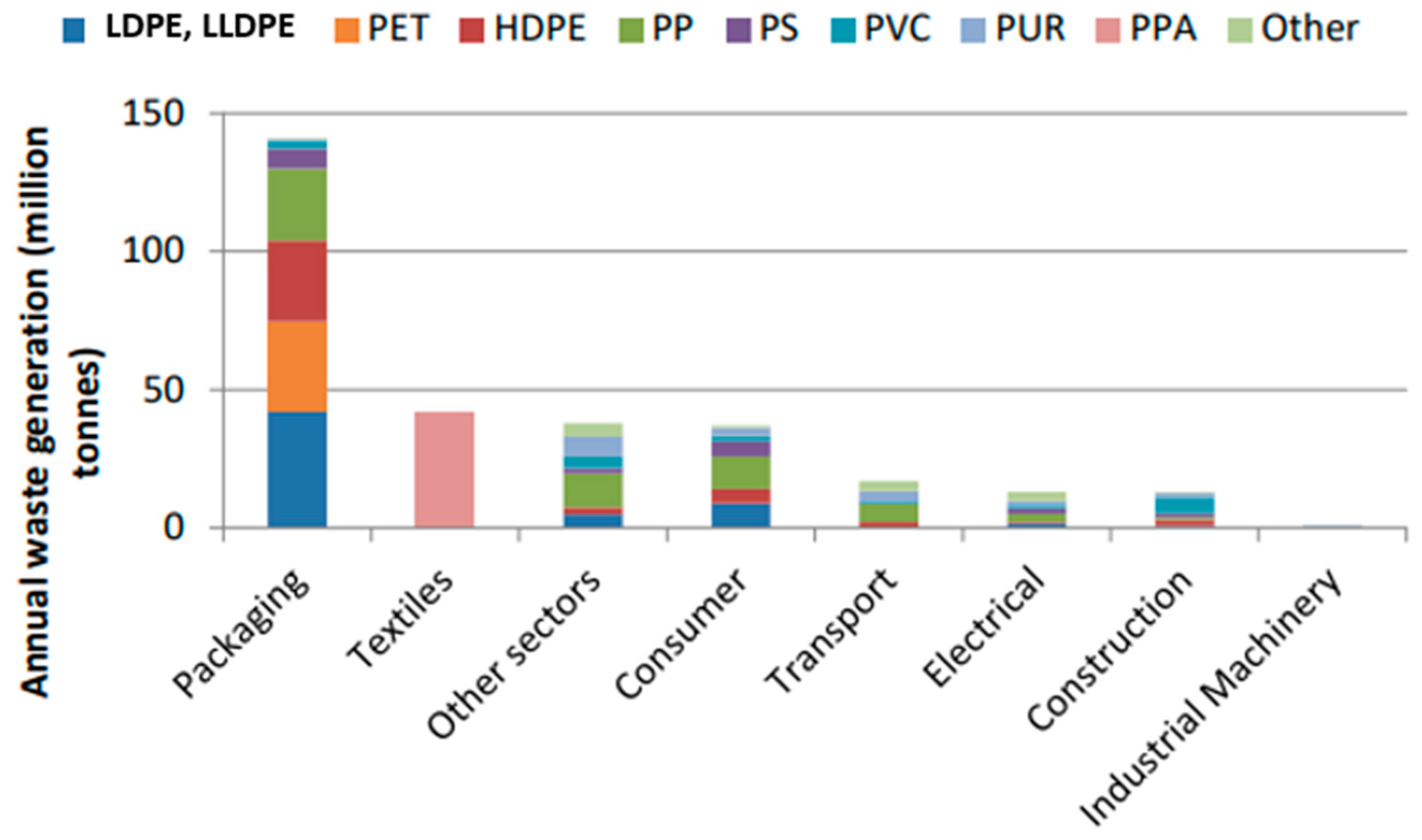
If production continues to grow at a similar rate, plastics production will reach 1600 million tons (Mt) in 2050. The rapid growth of plastics production is due to the good properties and low cost of this material. Thanks to its versatility, this material is used in several fields, such as packaging, textile, transport, and construction. Polymers are widely used, depending on the final application (
Figure 2).
The proliferation of plastic production contributes significantly to greenhouse gas emissions and generates pollution in the natural environment. Indeed, the production of virgin plastics requires the transformation of petroleum into monomers. This process is energy-intensive and generated 400 million tons (Mt) of greenhouse gas emissions in 2012 [
2].
To protect the environment, some countries adopted a new economic model that aims to revalorize post-consumed plastic and avoid landfilling. The transition toward a circular economy is unavoidable to reduce the plastic footprint and promote recycling. To manage plastic waste, there are different gates that can be classified from the most to the least preferred (
Figure 3) [
3].
Waste management places reduction as the top priority. The idea is to prevent the unnecessary consumption of resources. Direct reuse of original products is the second-best practice in waste management. The third stage is recycling products to avoid landfilling. Repurpose is about energy recovery. If the material cannot be recycled and recovered to energy, it will be landfilled, but it is the least preferred stage in the waste management hierarchy.
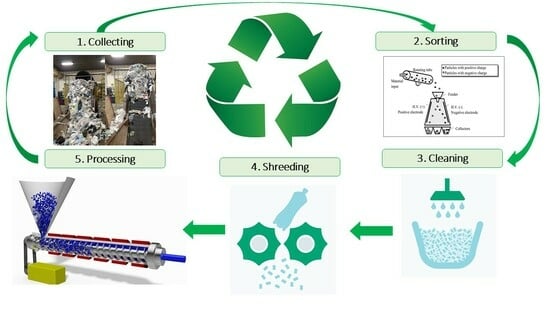
To achieve the goal of 100% recovery of plastics, the waste management system should be extended to all fields using plastics. In the industry, there are four ways to recover plastics: primary, secondary, tertiary, and quaternary recycling. This review aims to highlight the different stages of mechanical recycling: collection, sorting, cleaning, shredding, and processing (
Figure 4).
2. Sorting Technologies
Plastic separation faces a lot of challenges due to the huge quantity of plastics to collect and the complexity of identifying some types of plastics. The sorting is important to remove contaminants from plastics. This section will cover separation techniques that use density, surface charge transfer, and spectral analysis. The most used sorting methods are listed in
Table 1.
2.1. Manual Sorting
Manual sorting is a technique that allows the identification by shape and color of the plastics visually. This technique is useful if the waste plastics are large and easily identified. Otherwise, it’s very laborious and inefficient due to human errors. Moreover, it is the cheapest sorting technique [
10].
2.2. Near Infrared Radiation (NIR) and X-ray Technology
NIR (Near Infrared Radiation) involves irradiating the unsorted, unidentified plastic with near-infrared waves (600 to 2500 nm
−1 in wavelength). When exposed to light waves, different polymer reflects an identification spectrum. NIR spectroscopy allows identification thanks to the plastic signature, but it’s not adapted for dark plastics since dark pigments mask the signature of plastic material [
7].
There is another technique, like NIR, called X-ray fluorescence spectroscopy (XRF), which is used to identify flame-retardant materials (FR) and to determine the chemical composition of all kinds of materials (metal, cement, oil, and polymer).
This technology uses X-rays produced by a source to irradiate a sample. This latter produces fluorescent X-ray radiation with discrete energies that are characterized for these elements. Each element present in the sample produces a specific and unique set of characteristics of fluorescent X-rays [
11,
12].
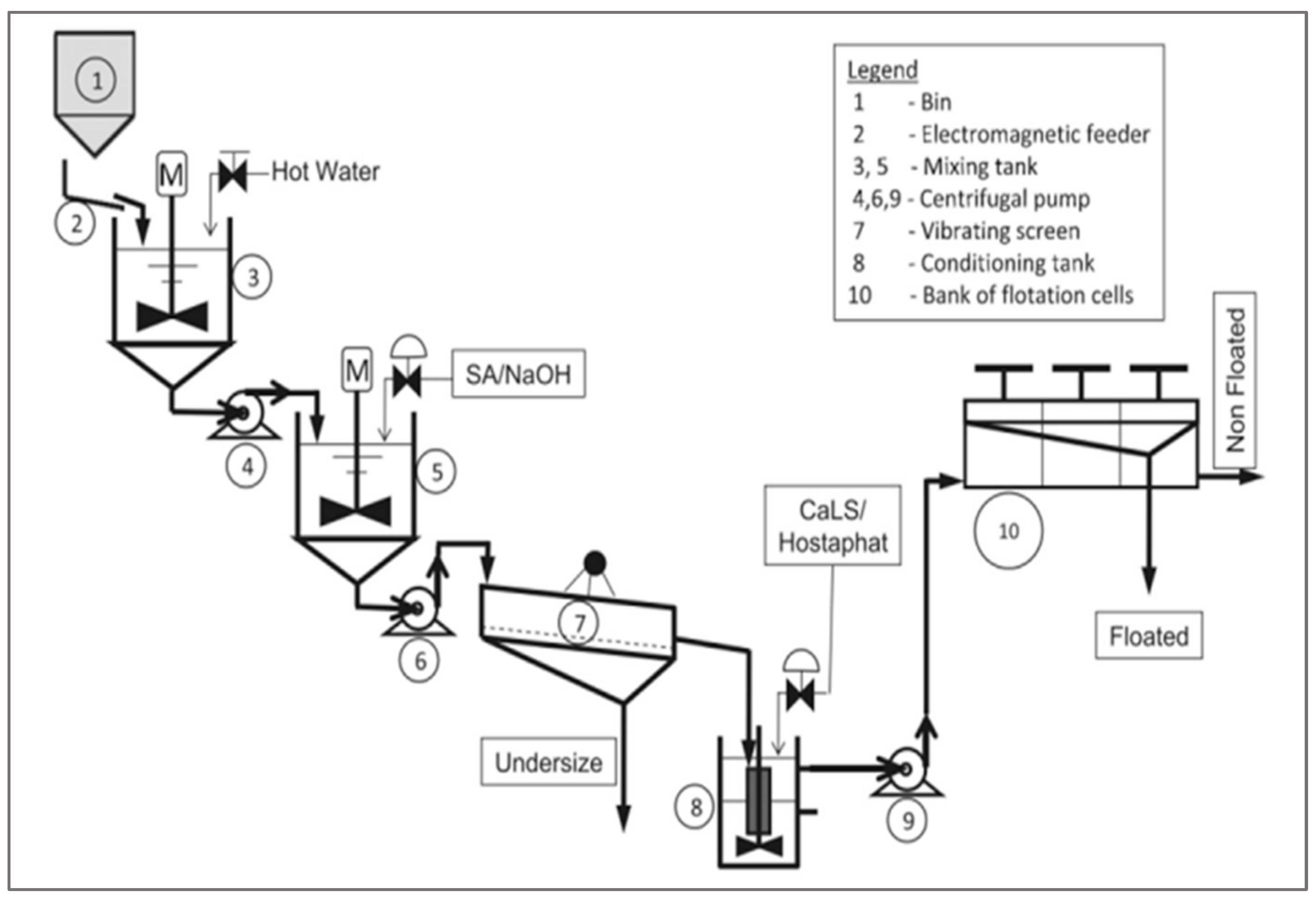
2.3. Flotation Method
The flotation method aims to separate polymers depending on specific gravity [
13]. The process is related to both the hydrophobicity and gravitational force of the material (
Figure 5). This technique can be used to separate PET (Polyethylene Terephthalate) from other plastic packaging [
14]. Furthermore, with this method, 95% of PVC (polyvinyl chloride) or PET (Polyethylene Terephthalate) can be separated [
15].
In this method, calcium lignin sultanate and MIBC (methyl isobutyl carbinol) are used as wetting agents and frothing agents [
16]. The materials are introduced in the first bin before being mixed with hot water. After that, an alkaline treatment is applied to the material in another tank, and pulp formation will take place and fed into a vibrating screen for rinsing with cold water [
9]. Samples of different products may then be separated and extracted at predefined time intervals for analysis and approximation of the product weight. The inconvenience of this method is space; it requires a huge area, and the process is too long [
17].
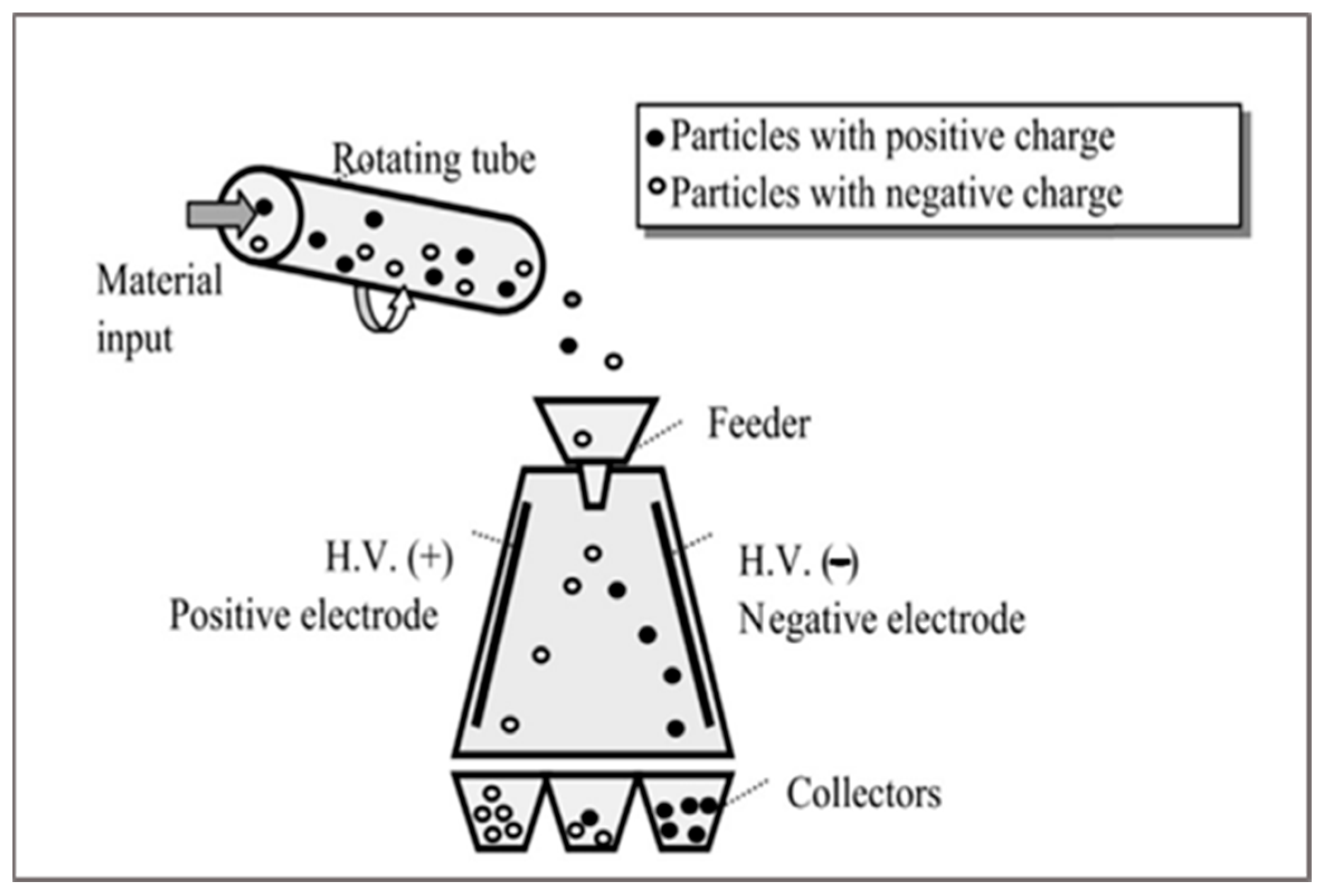
2.4. Triboelectric Sorting
This technique can be applied to complex mixtures. The separation happens due to the electrostatic charges of plastic mixture components (
Figure 6). This technique can separate metallic parts, metallic parts from plastic parts, and different plastics based on material dielectric constant [
18].
The materials are introduced on a rotating tube, and then the material presents two types of forces: the first is particle/particle forces, and the second one is particle/electrode force. When two materials stick against each other, then charge starts appearing on material particles. One gets a positive charge, and the second one gets a negative charge. Then, separation is initiated by forces acting in between them when a material particle passes through the intense electrostatic field [
9].
3. Recycling Techniques: Overview
In the industry, there are several ways to recover plastics. They are classified into four categories: primary, secondary, tertiary, and quaternary recycling. Each type has its advantages and disadvantages. It depends on the application of recycled polymer. The four ways are listed in
Table 2.
3.1. Primary Recycling
Re-extrusion [
19] is the process used for materials that are not too contaminated [
27]. Currently, it is more suitable for post-consumption than post-industrial plastics. Therefore, this technique does not require expensive equipment, and it is easy to handle in case of recovering semi-clean plastic scrap.
3.2. Secondary Recycling
Secondary or mechanical recycling is dedicated to plastic scraps that are contaminated to reuse them to produce new products [
28]. This type of recycling includes several steps, from the sorting to the pelletizing of post-consumer plastic such as Polystyrene (PS), High-Density Polyethylene (HDPE), Low-Density Polyethylene (LDPE), Poly Vinyl Chloride (PVC).
This technique faces a challenge related to the reduction of contaminants and impurities present in the resin, which affect the quality of recycled plastics [
21]. The process begins with the separation, washing, and grinding of plastics in the sorting center. After these steps, plastic materials are processed and pelletized to form pellets [
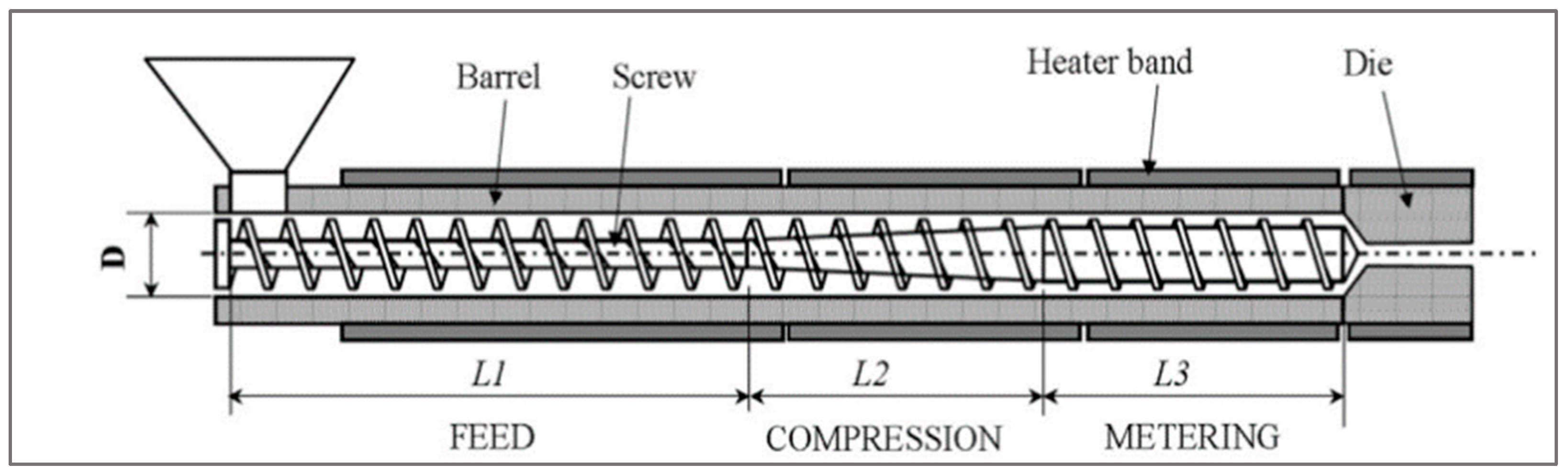
29]. Extrusion is one of the processes that is mostly used to manufacture polymers. In the industry, single or twin-screw extruders are both used to recycle used materials [
3]. An extruder uses the rotation of the screw and heating elements to soften and mix materials. The high temperature and screw apply shear force on the polymer, which induces a scission in material crosslinking [
22,
23,
24]. This chain degradation impacts the thermomechanical properties of the material. This last could be preserved by mastering the extrusion parameter and adding some additives such as carbon black and antioxidants. The machine melts the material and homogenizes it before entering the die (
Figure 7) [
9].
To reduce the contamination rate of plastics, decontamination recycling lines are often equipped with decontamination systems such as degassing system and filtration system.
3.3. Tertiary Recycling
Tertiary recycling, also known as chemical recycling, is a process that aims to convert plastics into molecules (liquids or gas) that can be used to manufacture new polymers [
26]. The products issued from chemical recycling are very profitable because they provide products with minimum waste. This type of recycling gathers: Pyrolysis, gasification, liquid-gasification, viscosity breaking, and catalytic cracking [
25].
3.4. Quaternary Recycling
The last type of recycling is energy recovery or quaternary recycling, which aims to generate energy, heat, or electricity from plastic scraps [
20]. Plastic materials have a very high calorific value after being burned compared to some oils. The burning of PSW also generates volatile organic compounds (VOCx) and smoke. Combustion is a harmful process that can be controlled by several techniques, such as acid neutralization flue gas cooling [
30]. But still, energy recovery remains the last resort to recycling plastics.
4. Sources of Contaminations
In general, ballots obtained from sorting centers are composed of three components: the desired polymer, polymeric contaminants, and some residual wastes. Contaminants can be classified into two categories volatile organic contaminants (VOCs) and solid contaminants. This last can be a polymeric contaminant or another material (metal, wood…). In the next paragraph, structural inhomogeneities and residual impurities effects will be detailed.
4.1. Structural Inhomogeneities
Compared to virgin polymers, recycled resin presents heterogeneity due to the attacking environment during its lifecycle. Irreversible structural changes can happen at both molecular and morphological levels. This modification can be induced mechano-chemically or by irradiation. The oxidation of polymeric materials creates free radicals such as carbon-centered (alkyl) and oxygen-centered (alkoxyl). This transformation is enhanced by the formation of crosslinked structures caused by the radical recombination of low molecular fragments [
31].
4.2. Impurities
Impurities are present in high concentrations in post-consumed plastics. Some of them are VOCs and depend on the polymer type. They come from additives added during the polymerization, such as phenolic antioxidants, consumed during the stabilization process. Furthermore, residues of titanium and aluminum polymerization generate colored salt. Resin absorbs contaminants, and the migration of some products to the matrix of packaging influences the quality of the material after being recycled [
5].
5. Decontamination Techniques
In general, decontamination is performed by a degassing system or/and filtration system that is linked to an extruder. The material is melted at a high temperature, which generates VOCs (Volatile Organic Compounds) [
32]. Numerous studies confirmed the effect of extruder profile heating on the extrusion performance. The ratio of VOCs was very high when heating reached 250 °C compared to 150 °C [
33].
5.1. Degassing System
5.1.1. Without Chemical Agent
To remove VOCs contained in plastics, extruders are equipped with a devolatilization system. The vacuum present inside the extruder helps to remove volatile particles. Several studies highlighted the influence of using single and multi-degassing systems in a screw extruder [
34]. The concentration of the odor was measured by dynamic olfactometry. The result shows that odor intensity decreases after one degassing step from 373 to 279 OU/m
3 and after the third degassing step to 235 OU/m
3 [
35].
5.1.2. With Chemical Agent
The first type of chemical agents are adsorbent agents. They allow the control of polymer emission during extrusion. The addition of 0.30% of adsorbent based on silicate to HDPE virgin pellets reduces the amount of VOCs, and the intensities of odors also decreased [
36]. Furthermore, some studies show that the introduction of these particles with post-consumer HPDE in the extruder decreases odor by 50% [
34]. The second type of chemical agent is a stripping agent. Their addition to the melt improves the devolatilization of the VOCs contained in the polymer. Their role consists of creating a bubble inside the matrix so the free volume in the melt increases, which helps the diffusion of VOCs in the vapor so that they can easily quit the extruder through the degassing system [
36]. The most used stripping agents are water, nitrogen, and air, which enhance the devolatilization of VOCs contained in polyolefins [
37]. For example, the use of nitrogen with polyethylene during extrusion reduces VOCs by 50% [
38]. In the same context, some researchers developed a mathematical model that showed that the uses of stripping agents such as methanol toluene decrease the time required for degassing, and this number can increase by rising solvent concentration. [
39].
5.2. Filtration System
A screen changer is an important piece of equipment in a recycling line to remove solid particles from the melt. The most used screens in the extrusion process are manual and hydraulic. The common point between them is the interruption of the melt flow while changing the screen [
40]. Large-area filtration systems are available in single vessel-discontinuous or dual vessel-continuous configurations, allowing to change screens without interrupting production [
40]. The different types of screens are listed in
Table 3.
5.3. Supercritical Fluid
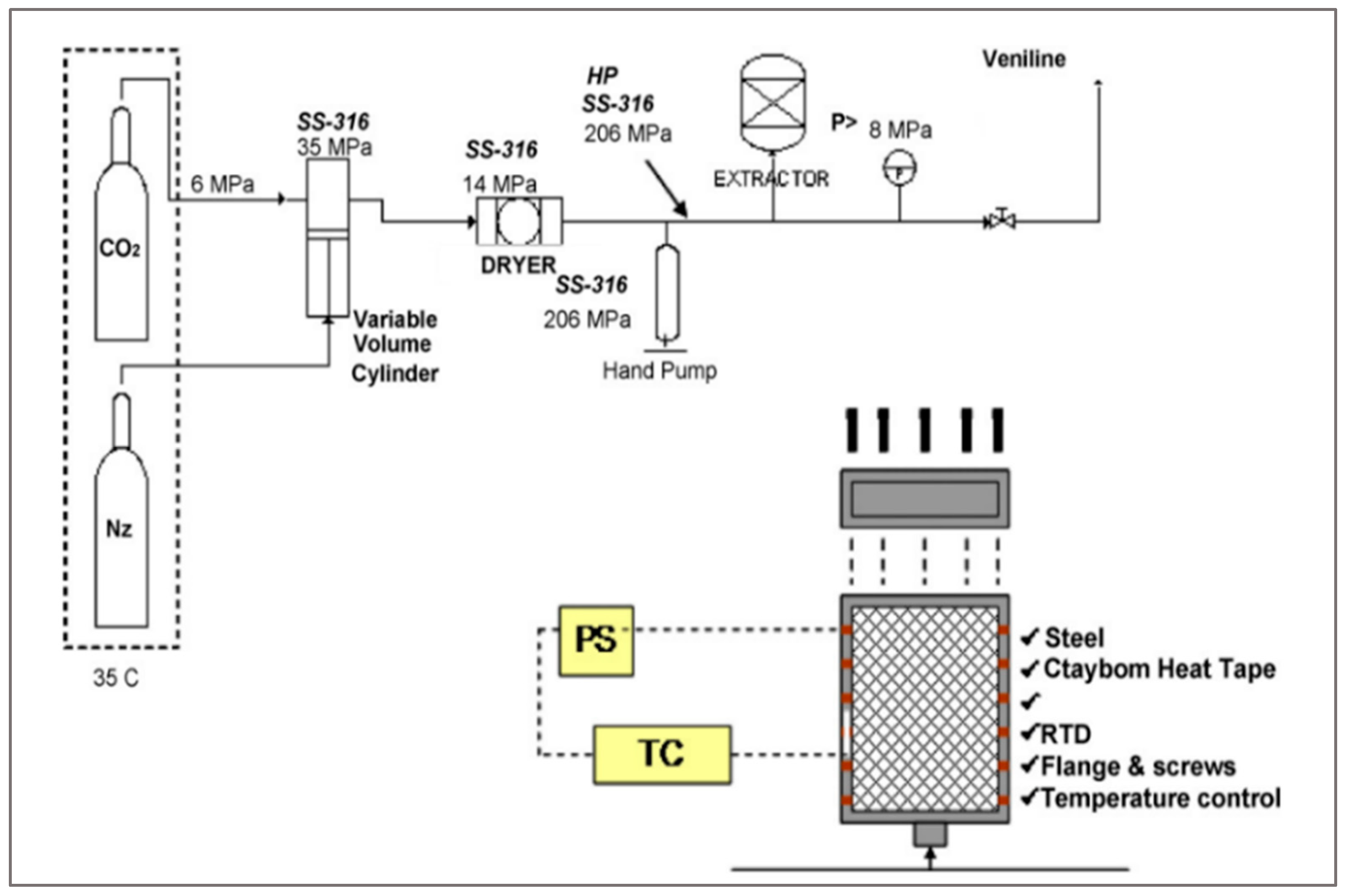
After being extruded, the polymer can be decontaminated by a purifying process such as extraction by supercritical fluid. Cristancho & Guzman [
42] studied the supercritical extraction of VOCs using CO₂ and ethane. The process was performed with pressure in the range (7.6–20.7) MPa and two temperatures, 36 and 60 °C (
Figure 8).
The result showed that using ethane was as effective as multiple extractions using CO
2. At a low pressure (7.6 Mpa) and medium temperature 60 °C, the extraction with CO
2 is effective. Higher pressure improves the extraction, but it will increase the operational cost. Both supercritical fluid help to decrease VOC concentration, but CO
2 remain safer and environment friendly than ethane. For this reason, CO
2 is the most used for the extraction of VOCs from polyethylene pellets [
42].
6. Identification and Quantification of Contamination Rate
Contaminants can be classified into two categories: polymeric contaminants and volatile organic contaminants (VOCs). To identify and quantify polymeric contaminants present in the blends, Differential scanning calorimetry (DSC) and Fourier-transform infrared spectroscopy (FTIR) can be used. Concerning volatile organic compounds, chromatographic methods are the most adapted.
6.1. Differential Scanning Calorimetry (DSC)
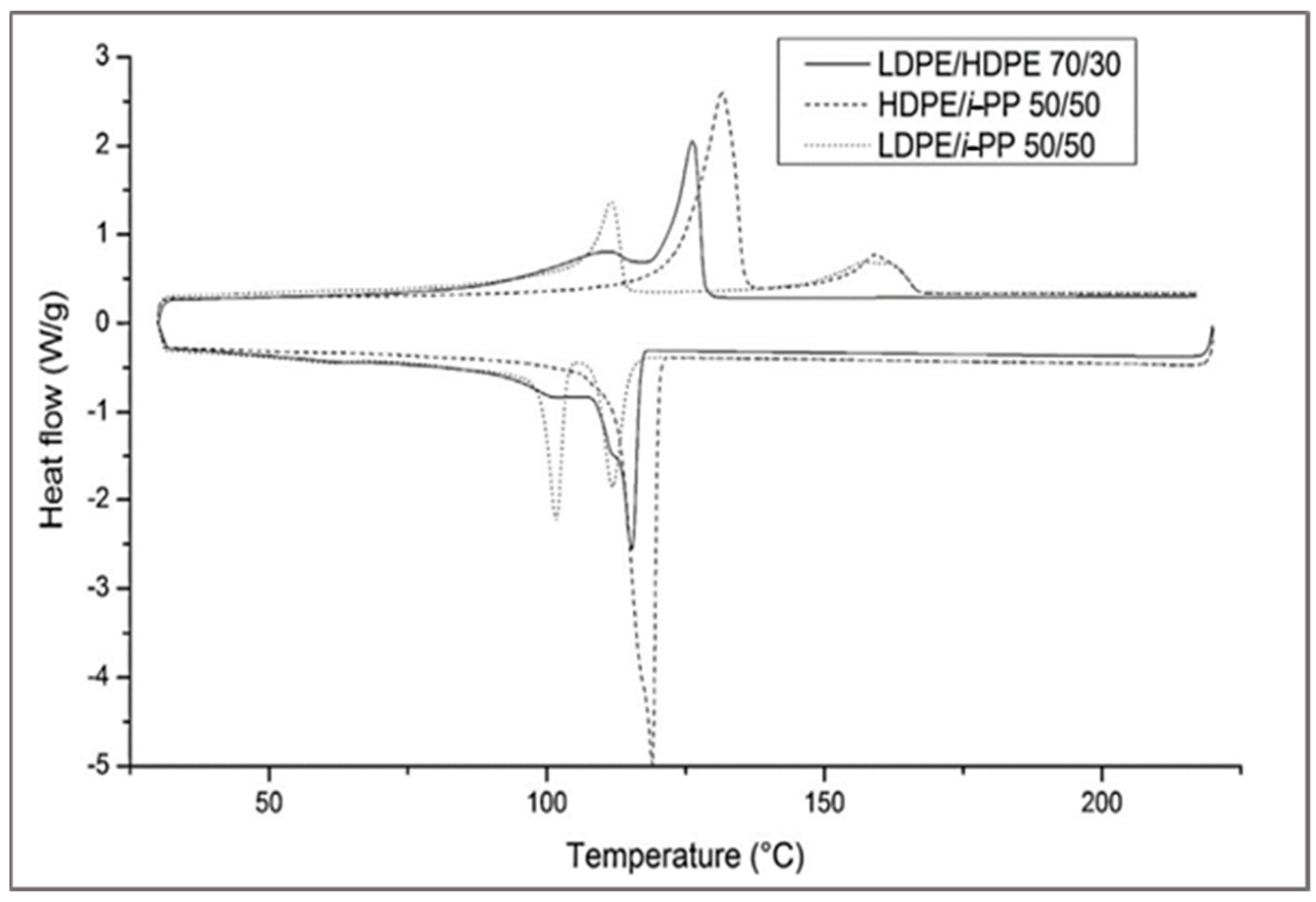
This method is adapted to determine the fraction of polymeric contaminants based on the recording of heat exchange during heating and cooling. Nevertheless, this approach is not suitable to identify LDPE/HDPE or even LLDPE/LDPE because of the similarities between their microstructures and melting temperatures. For example, to identify the fraction of HDPE in isotactic polypropylene, we can model the blend with a known ratio of the virgin polymer. These blends are extruded and analyzed by DSC (
Figure 9) [
43].
The presence of two melting peaks confirms the immiscibility of these polymers, which has been reported by many other authors [
44].
The melting enthalpies were calculated using a linear peak integration and the results for each blend (
Figure 10). This calibration curve can be used to determine the HDPE ratio in the PP/HPDE blend.
6.2. Fourier Transform Infrared Spectroscopy (FTIR)
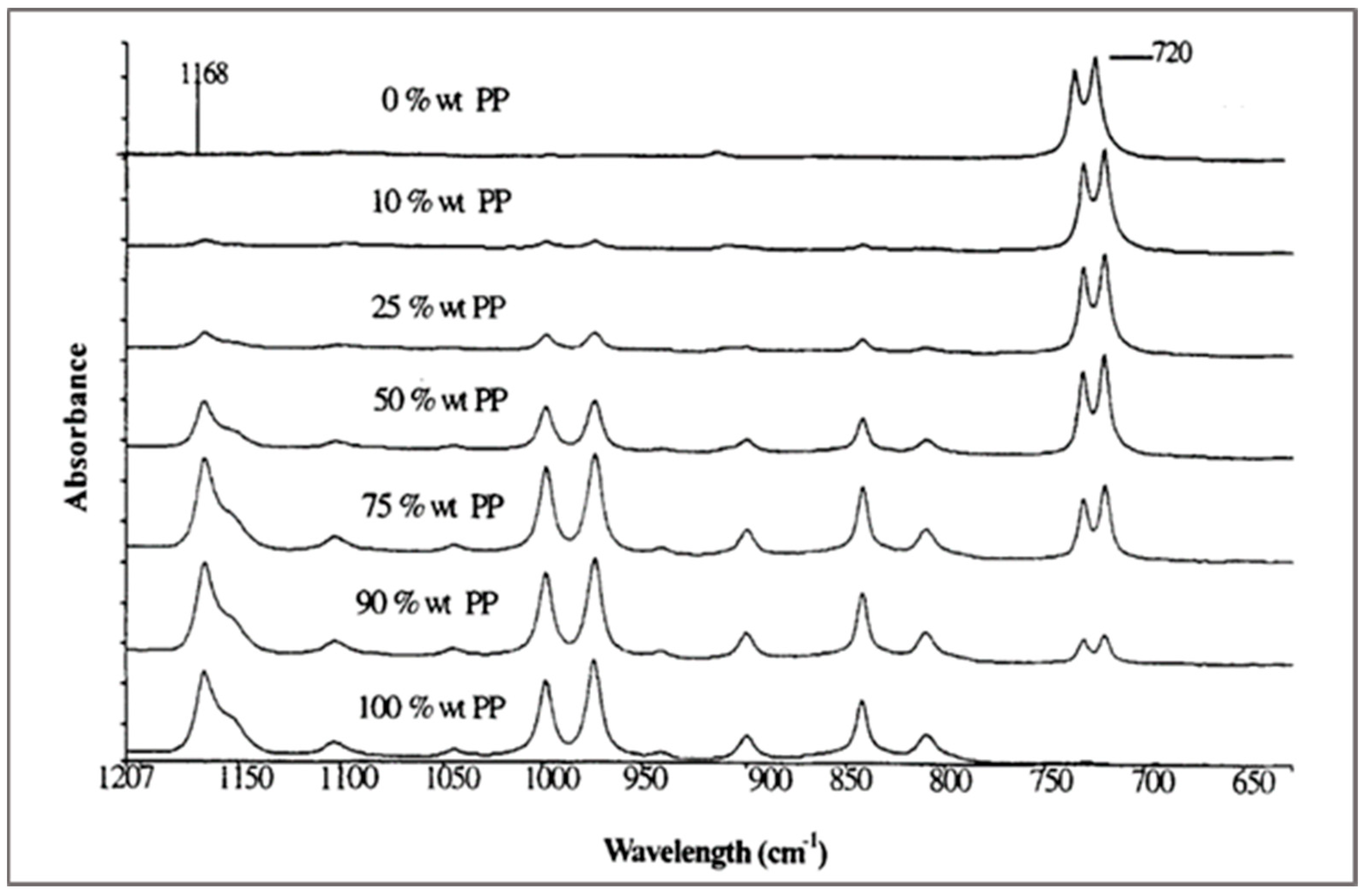
This technique is used to quantify polymeric contaminants. Light is used to track molecular translations, rotations, and vibrations. The absorbed energy is specific for each chemical bond, and a spectrum is obtained that can be used as a fingerprint to identify polymers [
43]. The spectra of the different compositions of blend based on PP and PE are shown in
Figure 11.
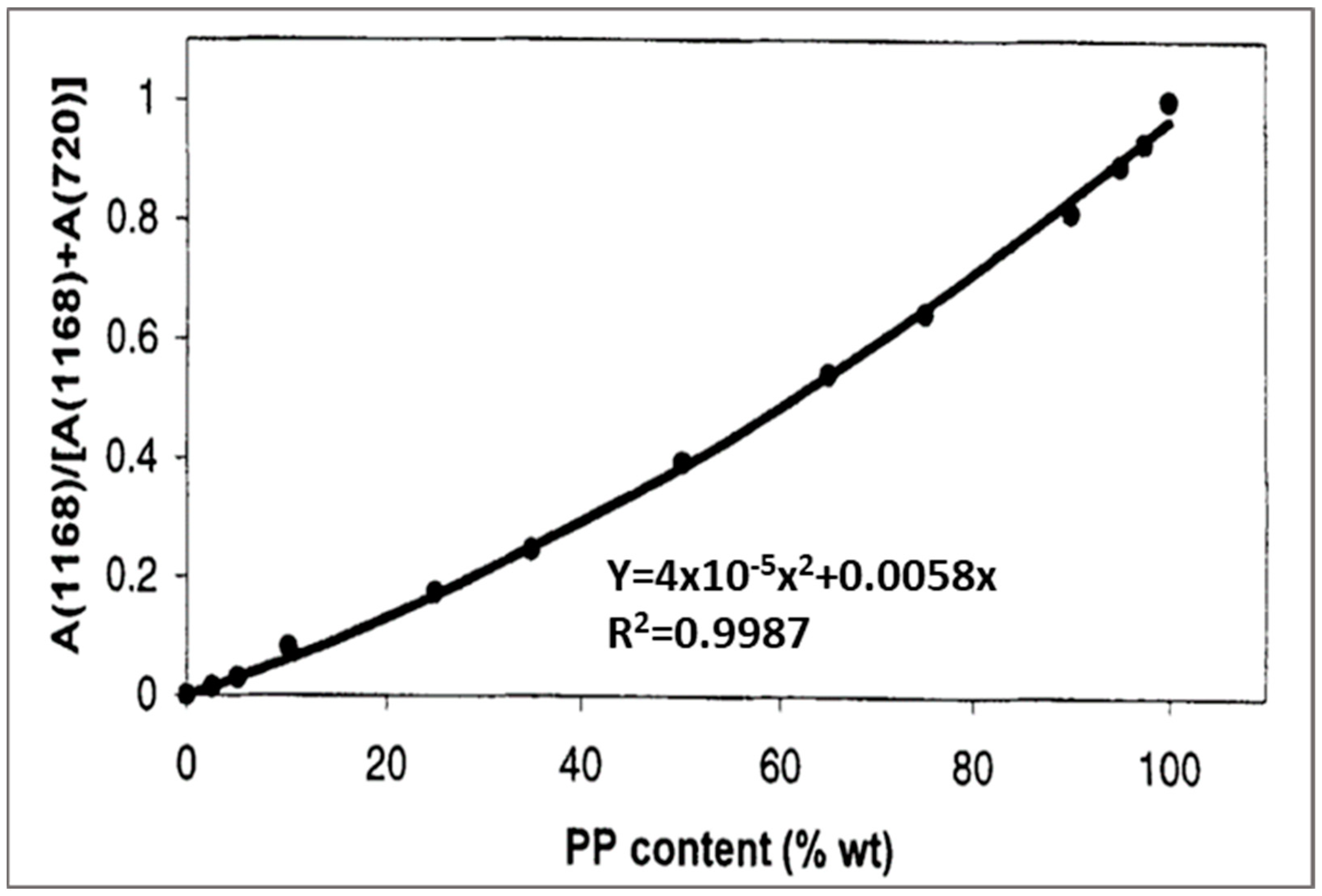
The calibration curve was plotted based on the ratio of the absorbance (integrated area) of two peaks, 1168 cm
−1 for methyl group in PP and a peak of 720 cm
−1 for methylene in HDPE (
Figure 12). A1168/(A1168 + A720) was plotted as a function of PP content. The calibration curve can be used to determine the composition of the PP/HDPE blend.
6.3. Chromatography Analysis
For the chemical analysis of recycled plastics, gas chromatography coupled with mass spectrometry (GC-MS) can be used to identify and quantify volatile organic compounds (VOCs). To detect organic contaminants, gas chromatography (GC) is equipped with a mass selective detector (MS). A capillary column with a film can be used for chromatographic separation. The GC oven can be programmed from 40 to 180 °C at 15 °C min
−1 and then to 300 °C at 5 °C min
−1, held for 12 min [
45]. Organic contaminants can be identified by consulting the mass spectra libraries. The quantification of the contaminants can be performed by using external and internal calibration curves. The external standard method creates a calibration curve for a standard sample, and unknown samples are quantified using calibration curves. The internal standard method consists of adding a fixed amount of internal standard substance to an unknown sample when creating a calibration curve using a standard sample, and a calibration curve is created with the concentration ratio vs. peak area ratio for quantification [
46,
47].
7. Mechanical Recycling: Cases Studies
Polyolefins (i.e., PE and PP), Polyethylene Terephthalate (PET), Polyamide (PA), Poly(lactic) acid (PLA), and Polyhydroxybutyrate (PHB) are widely used in industry. These materials will be developed to highlight the different degradation mechanisms and ways to improve polymer properties.
7.1. Recycling of Commodity Polymers
Commodity polymers are used when higher properties are not required. This type of material is used for packaging, food contains, and films. The most known are Polyolefins, and their recovery is complex due to their similar densities, in particular High-density polyethylene (HDPE) and low-density polyethylene (LDPE). Furthermore, the recycling process of this material decreases its thermomechanical properties. That’s why the incorporation of a stabilizer is needed to enhance the material’s properties.
7.1.1. Degradation of Polyolefins
The most used polyolefins are polyethylene (High-density polyethylene (HDPE), Low-density polyethylene (LDPE)) and polypropylene (PP) [
48].
High-Density Polyethylene: HDPE (0.952 g/cm
3) is a polymer with a high degree of crystallinity, more rigid and less elastic than LDPE. During extrusion, two mechanisms can happen: either chain scission or chain branching (crosslinking) and sometimes both. Thermo-oxidation happens, and the dissolved oxygen in the melt reacts with chains, leading to chain scission with stable carbonyl. In regions with low oxygen, the chain scission produces two reactive chain ends. These macro radicals react to produce branching with higher molecular weight [
49].
Low-density Polyethylene: LDPE is more branched than HDPE, and its structure is susceptible to crosslinking and chain branching during extrusion. After several extrusion cycles, the complex viscosity increases (
Figure 13) due to crosslinking [
50].
Figure 13.
The Effect of repeated extrusion cycle on complex viscosity [
51].
Figure 13.
The Effect of repeated extrusion cycle on complex viscosity [
51].
Polypropylene: PP has good optical and mechanical properties, which make it an essential material for packaging [
50]. Several studies investigated the degradation of Polypropylene (PP). This material was introduced in a twin screw extruder. The screw speed was fixed at 50 rpm. The extruder had five temperature control zones. After five extrusion cycles at a high temperature (Die Zone: 270 °C), the molecular weight of PP decreases due to chain scission, and consequently, the degree of crystallinity increases. At lower temperatures (Die Zone: 240 °C), PP is stable in processing even after five extrusions. Beyond five cycles and at high temperatures, chain scission happens, and PP performance decreases [
52].
7.1.2. Stabilizer Used in Polyolefins Recycling
Polyethylene: To improve the thermomechanical properties of PEs and reduce thermo-oxidation, it is necessary to introduce stabilizers during extrusion. The choice of additives depends on their solubility and dispersion of the PE matrix [
53,
54]. Phenols and phosphate-based antioxidants are both effective in stabilizing hydrogen bonding. The use of both of them at the same time showed important results [
55]. However, the combination of hindered amine light stabilizers (HALS) with phenolic antioxidants negatively influences the stabilization of nitroxyl radicals produced from photooxidation reaction with the phenolic groups. To overcome this problem, carbon black is used as an inorganic UV stabilizer [
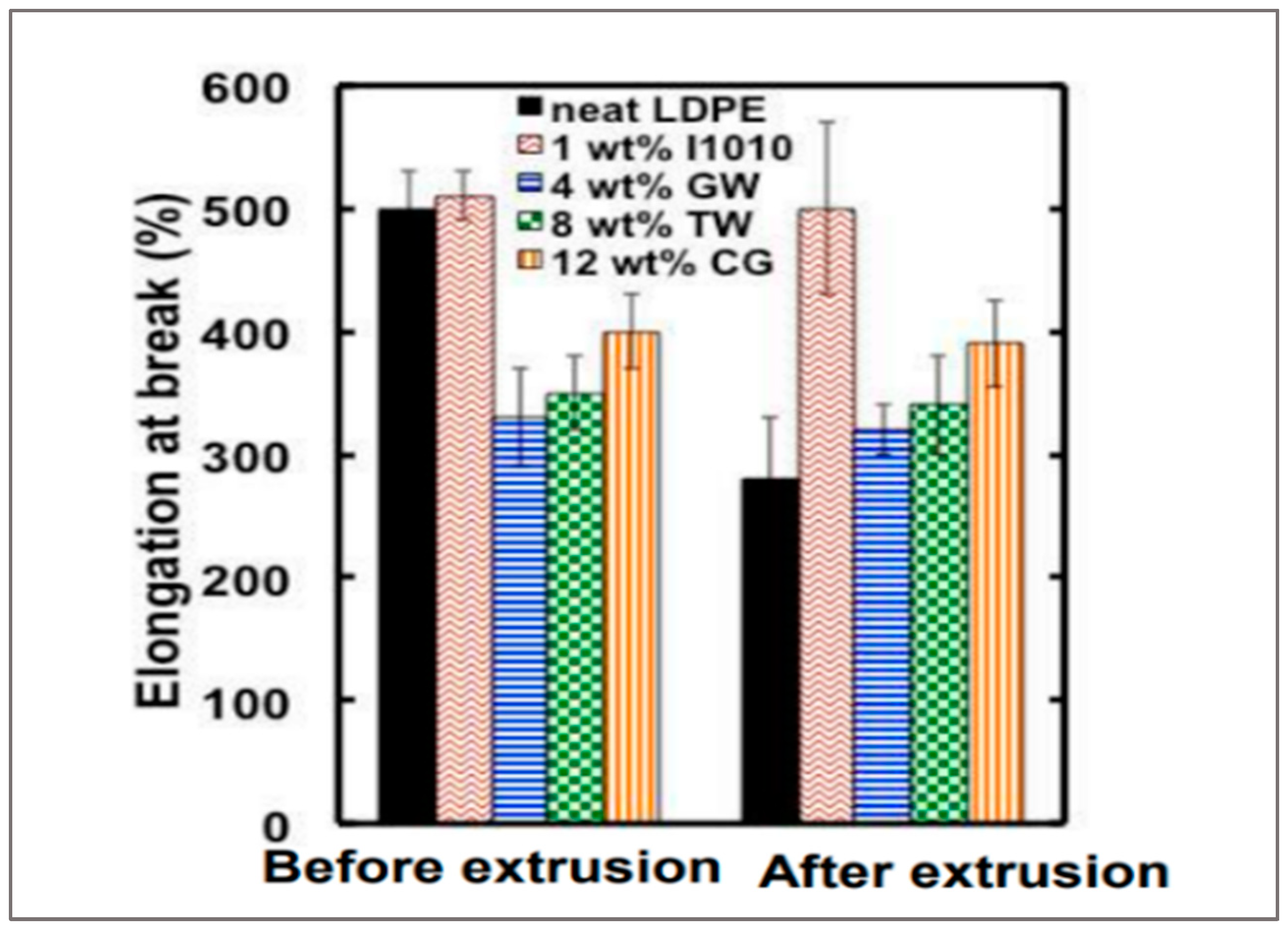
56]. Moreover, sustainable antioxidants have been used, such as caffeic acid, curcumin, and vitamin E, to prevent polymer degradation. For instance, the addition of 12 wt % of coffee ground (CG) and 8 wt % of turmeric waste (TW) increases the elongation at the break of LDPE (
Figure 14) [
55]. Moreover, the addition of antioxidants combined with carbon black improves the thermomechanical properties of PEs and protects them against thermo-oxidation during extrusion [
50].
Figure 14.
Elongation at break of LDPE extruded with antioxidants agro-waste [
50].
Figure 14.
Elongation at break of LDPE extruded with antioxidants agro-waste [
50].
Polypropylene: To avoid degradation of polymer chains during extrusion, PP also requires stabilization. Phenolic and hindered amine antioxidants can be used [
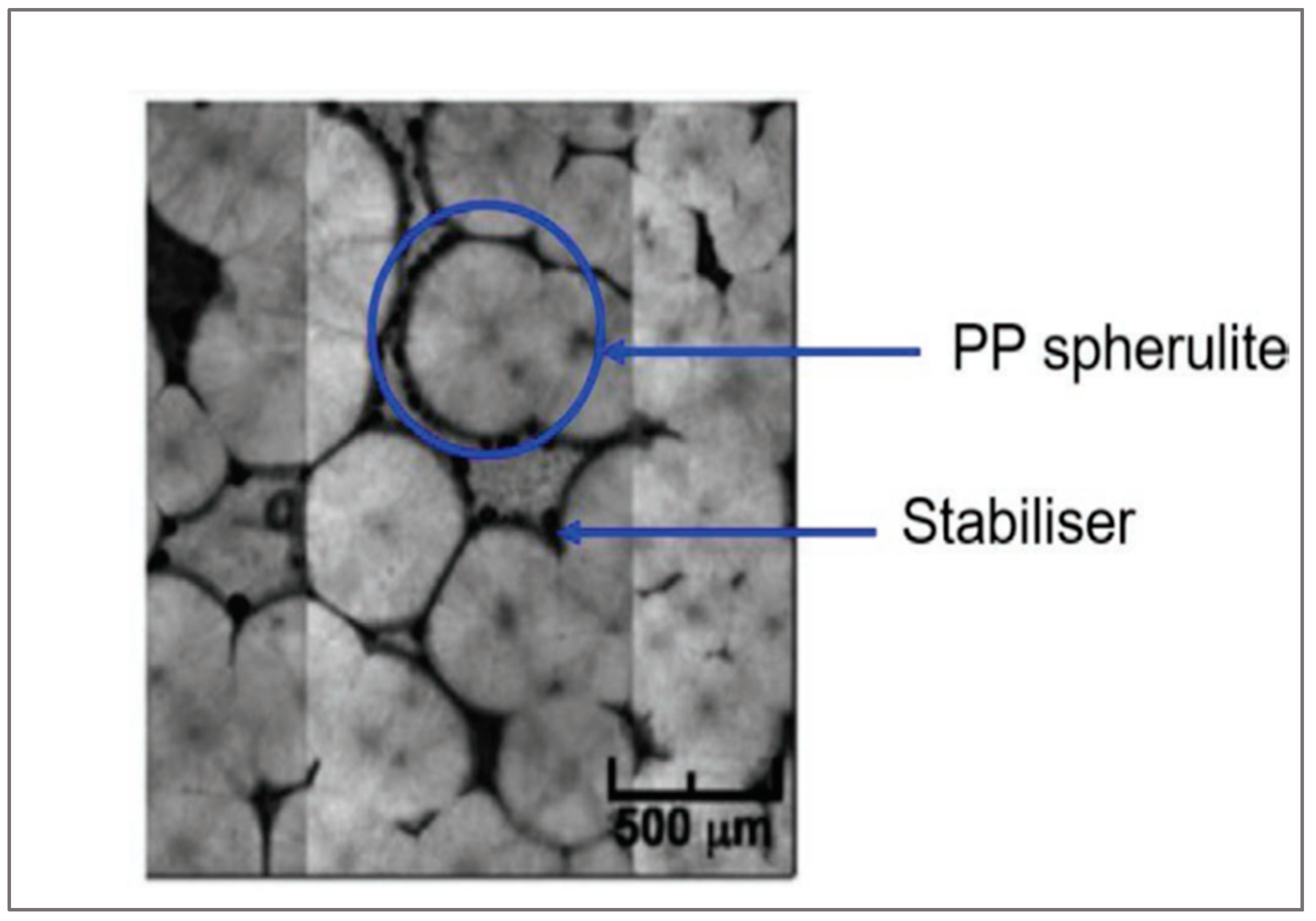
57]. The spherulitic structure of PP allows a uniform distribution of stabilizers through the polymer matrix (
Figure 15).
Figure 15.
Optical image of the spherulitic structure of PP [
50].
Figure 15.
Optical image of the spherulitic structure of PP [
50].
Lignin, which is a phenolic compound, can be used to stabilize PP at concentrations between 2–5 wt % (
Figure 16). The lignin acts as an antioxidant and filler to increase the rigidity of the polymer matrix [
58].
7.2. Recycling of Engineering Polymer
Engineering plastics are polymers that have higher mechanical and thermal properties compared to other categories of plastics, which allow them to perform under mechanical stress at high temperatures and to resist a chemical environment. Polyethylene Terephthalate (PET) and polyamides (Pa) are the most used engineering polymers [
59].
7.2.1. Polyethylene Terephthalate PET
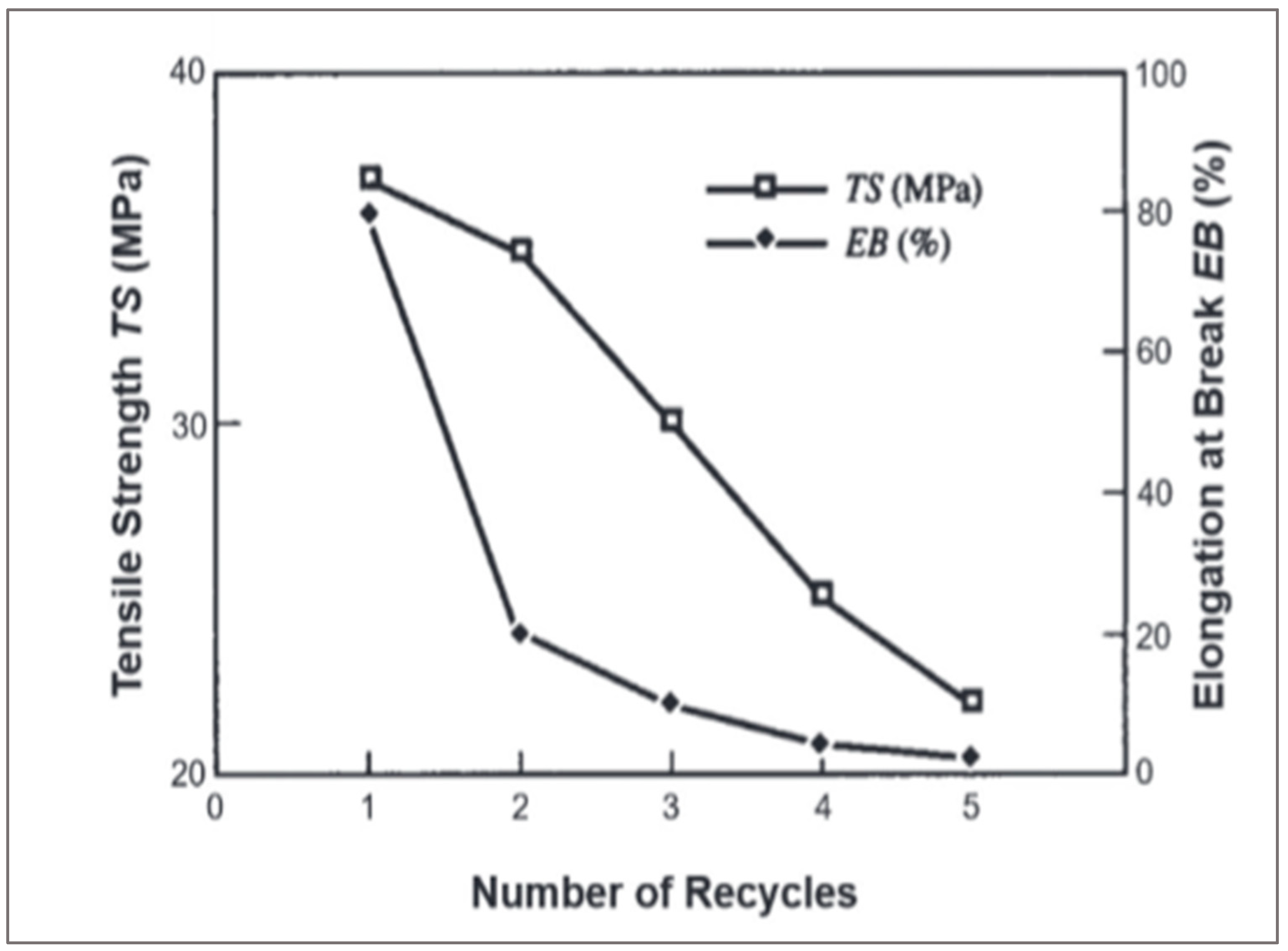
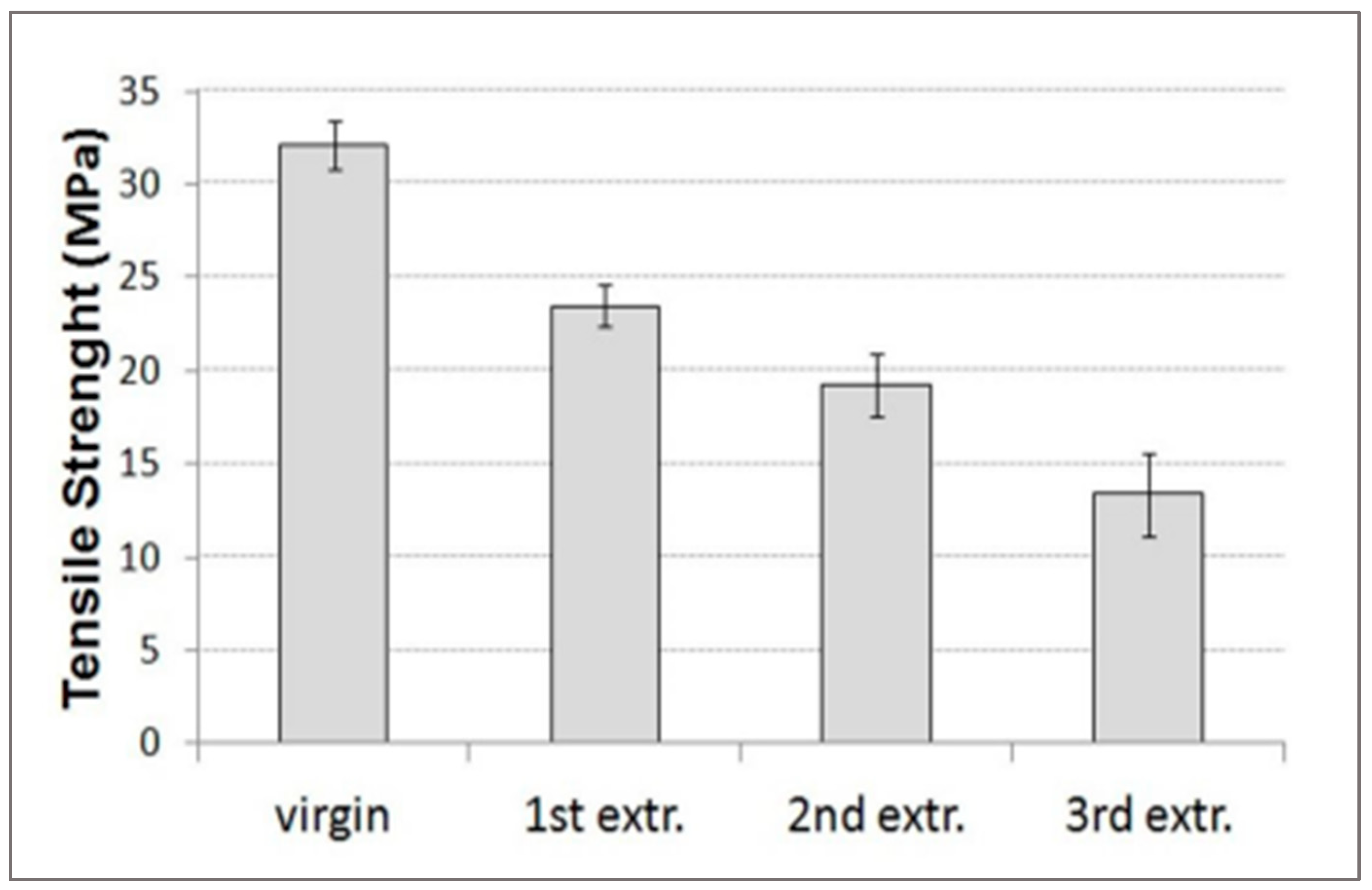
PET is a thermoplastic that has good thermomechanical properties and processability [
59]. Virgin PET has a high elongation at break values exceeding 80% and tensile strength exceeding 35 MPa. These properties decrease after several recycling cycles (
Figure 17).
The reduction of the properties is related to the degradation of chains due to thermo-oxidation [
23]. The degradation of the chain induces the reduction of polymer molecular weights, and the presence of polymeric contaminants enhances this degradation. For instance, fewer traces of poly (vinyl alcohol) PVA or poly (lactic acid) lead to hydrolysis for the polymer during extrusion [
60,
61]. A macroradical is formed and reacts with oxygen due to the screw and heat of the extruder. Radical hydrogen abstraction can produce hydroperoxide that decomposes to form two new radical species that can generate another macroradical chain that enhances thermo-oxidation [
62].
Several studies show that PET can be recycled three times. Beyond this limit, the material properties decrease slowly due to the polymer size [
63]. This result is related to the relationship between molecular weight and polymer degradation. Larger polymer chains enhance degradation [
64]. The addition of additives during PET extrusion helps to improve the mechanical properties of recyclate [
65]. To reduce thermal oxidation, the use of solid-state polymerization (SSP) avoids the formation of hydroxyl and carboxyl end groups. Furthermore, chain extenders are efficient. They help repair the damage caused by chain degradation. The most common chain extenders are epoxides, carboxylic acids, and phosphates [
65,
66]. For instance, triphenyl phosphite (TPP) extends chains through its carboxyl and hydroxyl terminal groups [
65]. The addition of pyromellitic dianhydride (PMDA) branching to PET promotes increased intrinsic viscosity (ƞ*) and a decrease in melting temperature [
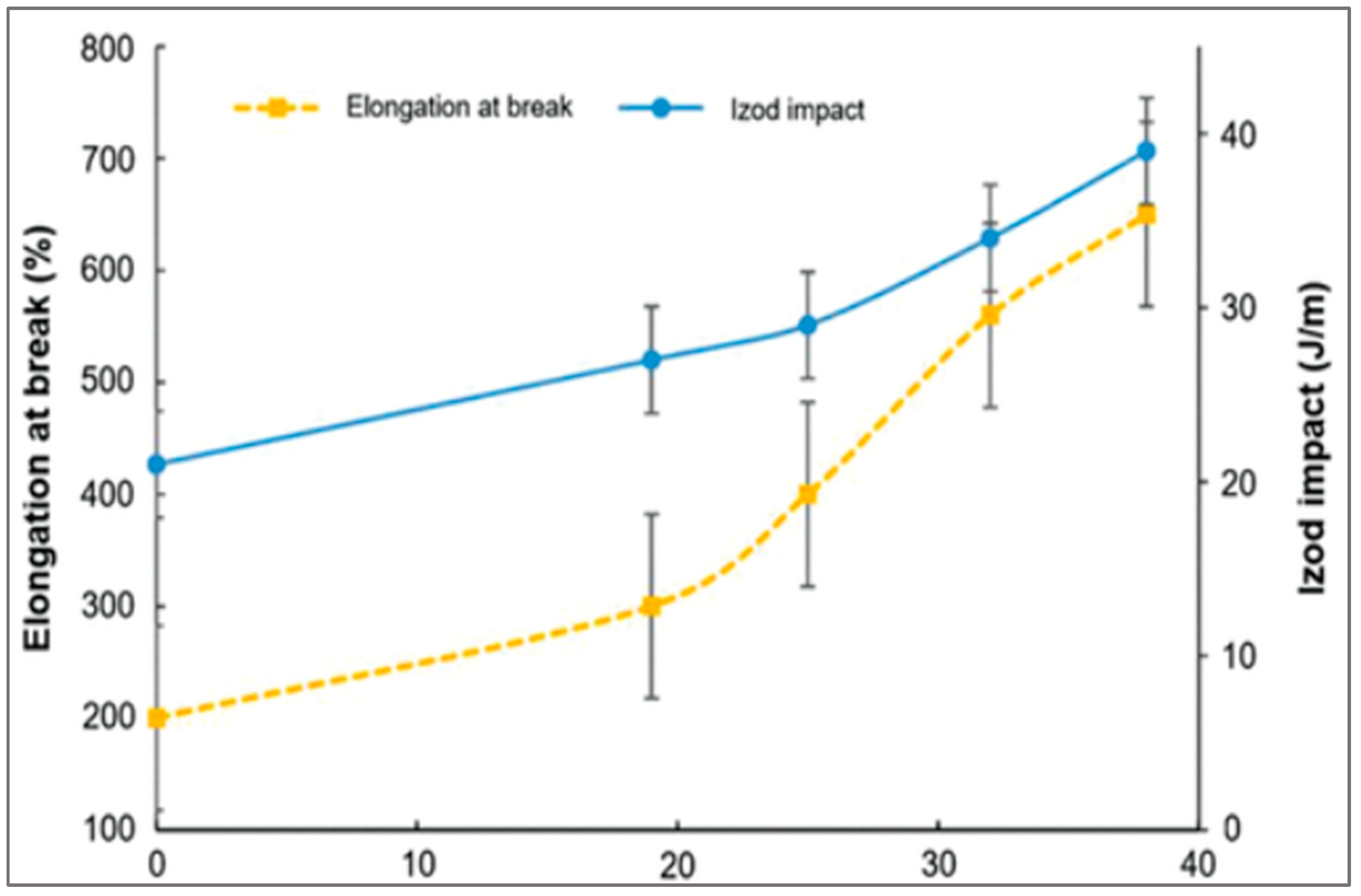
67]. The addition of an epoxide-based chain extender such as glycidyl methacrylate (GMA) increases the Elongation at break and Izod impact of recycled PET in reactive extrusion (
Figure 18).
7.2.2. Polyamides
Polyamides (PAs), known as Nylon, are one of the most important engineering polymers. PA6s are widely used thanks to their versatility, competitive price, excellent strength and stiffness, low friction coefficient, and high dimensional stability [
69].
To recycle this polymer, secondary recycling is the most widely used method, but during the extrusion process, PA6 is exposed to chemical change and degradation due to heat and shear forces. This change impacts thermomechanical properties and hence limits their use. To enhance the properties, chain extenders are used during the extrusion of recycled PA6 to increase the molecular weight of the material [
70,
71,
72]. Chain extenders react faster with either amine or carboxyl end groups of polyamides and can link polymer chains to increase the molecular weight [
70]. Selin Celebi O, Guralp O [
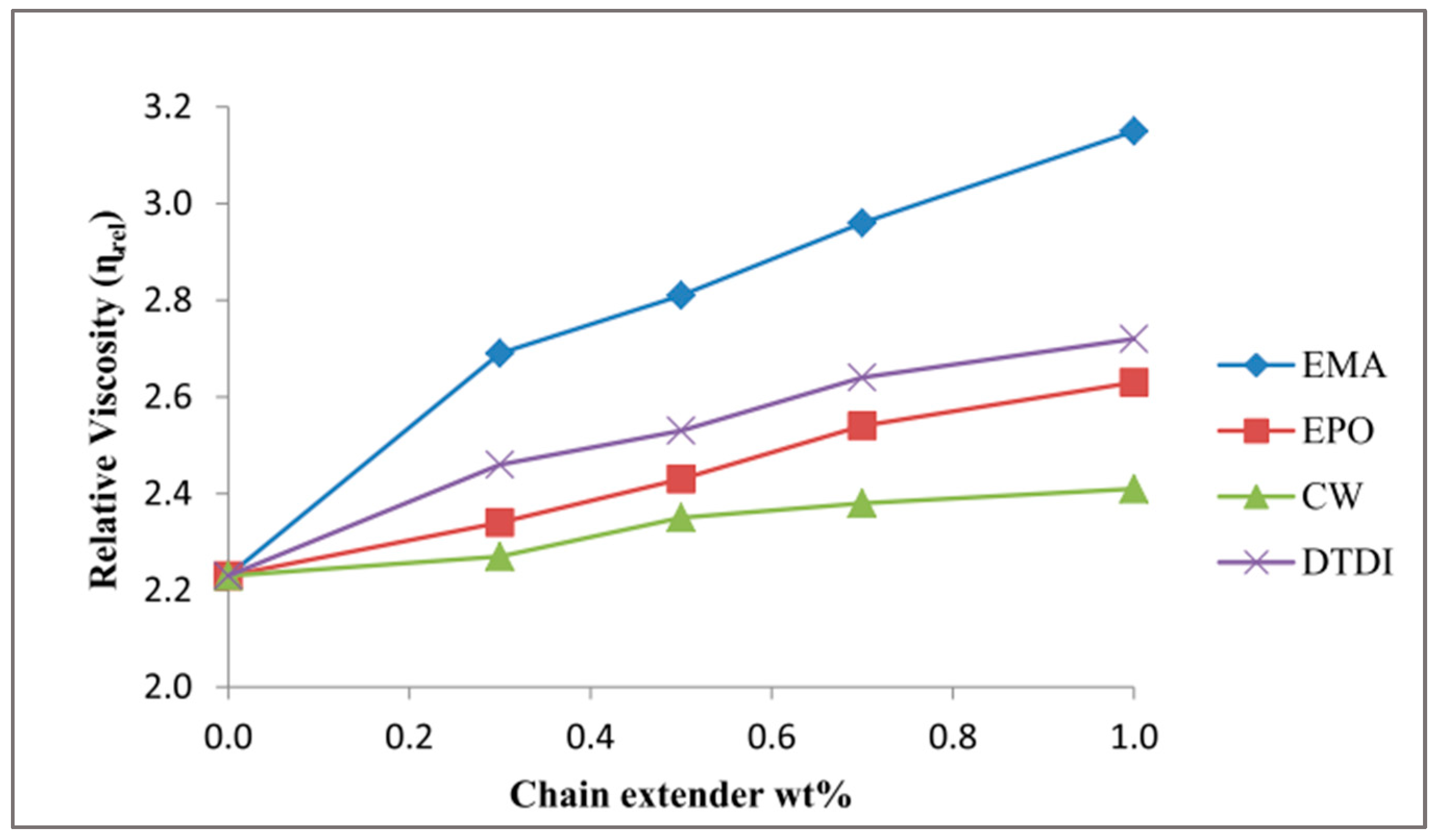
73] studied the addition of several amounts of alternating copolymer of ethylene and maleic anhydride (EMA), multi-functional epoxy-based oligomeric chain extender (EPO), polyester wax with reactive caprolactam groups (CW) and dimeric 2,4-toluene diisocyanate (DTDI) in a twin screw extruder. To evaluate the effect of the chain extender on the mechanical and physical properties of rPA6, viscosity, and tensile tests were performed. The results showed that elongation at break increased at least by 4.4 times, even at a low rate of chain extender (0.3 wt %). Ethylene and maleic anhydride (EMA) showed higher elongation at break compared to the other investigated chain extenders. The highest elongation at break was reached with 1 wt % of EMA incorporation, which increased the elongation at break by 6.3 times (
Table 4).
Elongation at break results is coherent with relative viscosity results. The addition of EMA increased relative viscosity by 41% (
Figure 19) because molecular weight is linked to elongation at break and relative viscosity. Long branches cause an increase in chain entanglement and chain straightening prior to break.
7.3. Recycling of Biodegradable Polymer
Bio-sourced plastics are manufactured from renewable resources (microorganisms, plants...). They are classed into three categories. The first type is called partially bio-based (bio-based and nonbiodegradable), such as bio-based polyolefin. The second category is bio-based biodegradable plastics such as Polylactic acids (PLA) or Polybutylene succinate (PBS). The last one is conventional plastics that are biodegradable, such as Polycaprolactone (PCL) or Polybutyrate adipate terephthalate (PBAT) [
74]. The most used bio-based and bio-degradable polymers are Poly(lactic) (PLA) and Polyhydroxybutyrate (PHB).
7.3.1. Acid Poly(lactic) (PLA)
Poly(lactic) acid is a bio-sourced material that presents an alternative to PEs. This plastic is widely used in food packaging and 3D impressions. PLA is an aliphatic polyester produced by the polymerization of lactic acid, which is obtained from the fermentation of sugar [
75]. However, the management of PLA waste has not yet been mastered. There are several valorization techniques of PLA, such as composting chemical and mechanical recycling. The first technique has some drawbacks related to the slow degradation of some PLA, which will lead to the accumulation of Plastic waste [
76]. Chemical recycling is more expensive than using virgin bio-based plastics [
77]. The mechanical is the most suitable recovery technique for PLA due to its cost and lowest environmental footprint.
The recycling process of PLA has been cited in the literature in several studies [
78,
79], and all show that during the extrusion of PLA, some oligomers appear due to hydrolysis, such as hydroxyl and carboxyl [
80]. In general, during recycling, the molecular weight of PLA decreases due to chain scission [
81,
82]. Moreover, after 10 successive extrusion cycles [
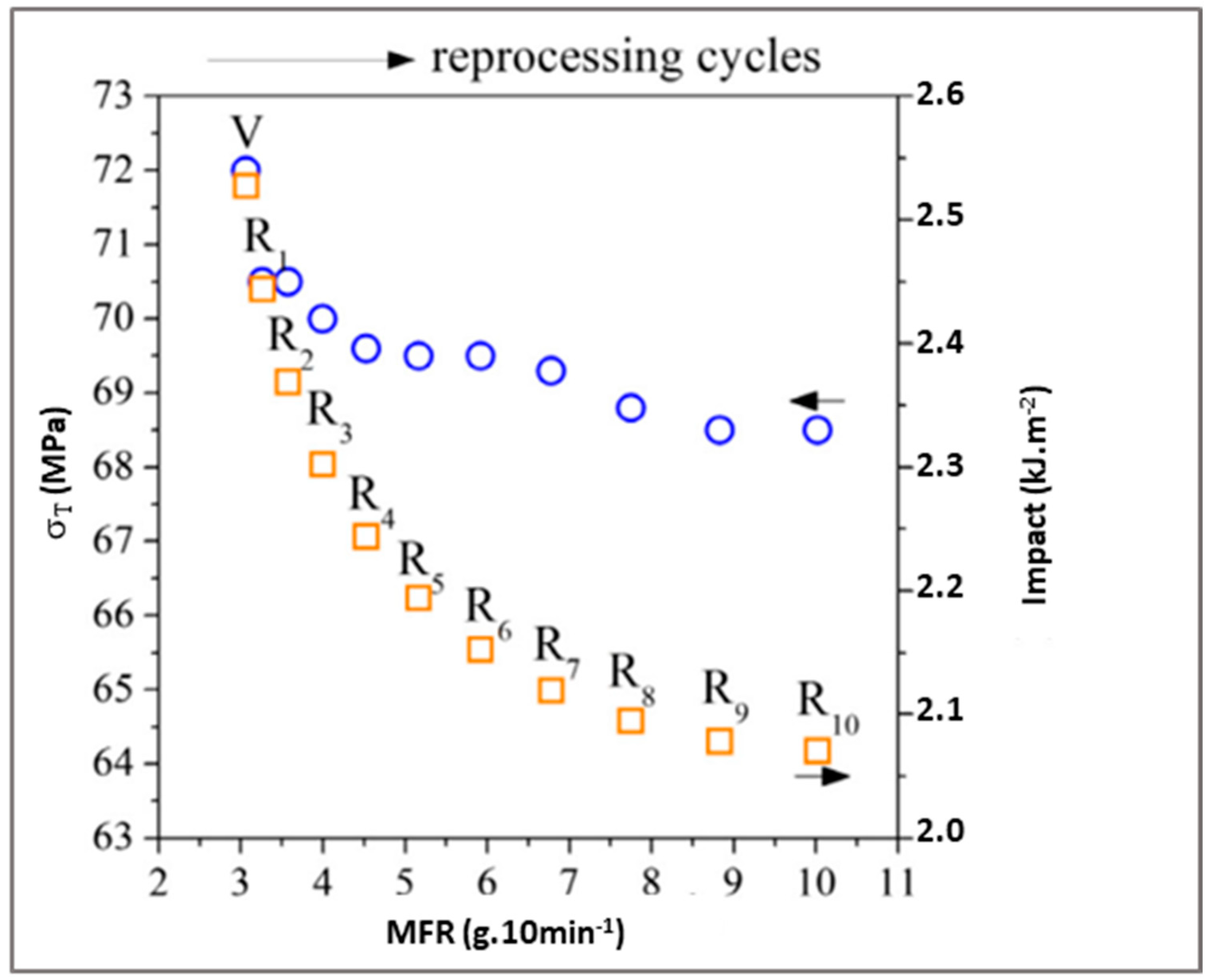
79], the results show that the mechanical and rheological properties of PLA decrease (
Figure 20). Tensile stress and impact resistance varies slowly. At the same time, the MFR (Melt flow rate) increases significantly after the third extrusion. This fact can be explained by the increase of broken chains, which raise PLA fluidity [
80].
There are several ways to improve the thermomechanical and physical properties of recycled PLA. The first technique is the annealing of recycled PLA, which is a thermal treatment that consists of exposing extruded PLA to high temperatures (120 °C) for 6 h. The goal of this treatment is to increase the material crystallinity, which has been decreasing during extrusion. This treatment improves young modulus, flexure modulus, and yield but will reduce the elongation at break [
83]. The upgrade of PLA can be reached by the addition of chemical agents. For instance, the incorporation of plasticizers helps to improve the processability [
81].
To prevent the problem of degradation during reprocessing, the incorporation of antioxidants can minimize degradation and improve properties such as impact resistance, flowability, or UV protection [
84]. Furthermore, the use of chain extenders with PLA blend improves the mechanical and rheological properties. For example, glycidyl methacrylate enhances mechanical, thermal, and rheological properties due to the stabilization of molar mass by phase dispersion [
85]. Also, the use of a chain extender based on phosphites increases the molar mass and complex viscosity of recycled PLA, Meng Xin [
86] studied the complex viscosity of raw PLA, virgin PLA, and PLA stabilized by different Phosphite chain extender PLA-TPP (Triphenylphosphite) which is PLA stabilized by TPP chain extender, PLA-168 which stabilized by Irgafos 168 chain extender and PLA-M46TBPP is PLA stabilized by M46TBPP chain extender (
Figure 21: Complex viscosity of different PLA [
86]).
The curves of complex viscosity show that all complex viscosity values of PLA stabilized by phosphites are higher than those of virgin PLA at every angular frequency. However, the complex viscosity values of PLA-TPP at every angular frequency are the best.
7.3.2. Polyhydroxybutyrate (PHB)
Polyhydroxybutyrate (PHB) is a member of the Polyhydroxyalkanoates (PHAs) family. They are bioderived aliphatic polyesters obtained by the polymerization of hydroxy alkanoic acids, which are produced from the fermentation of sugar and lipids [
87]. Although PHB is not as widely used as PLA, they are a very interesting class of biobased and biodegradable polymers, and their production capacity is expected to triple in the next five years [
88]. Several studies investigated the mechanical recycling of PHB. In one of these studies, the results show that tensile strength decreased after only two extrusions, and the degree of crystallinity increased due to chain scission (
Figure 22).
Since PHB is expensive and does not have good mechanical properties, it is generally blended with other polymers or used as an additive [
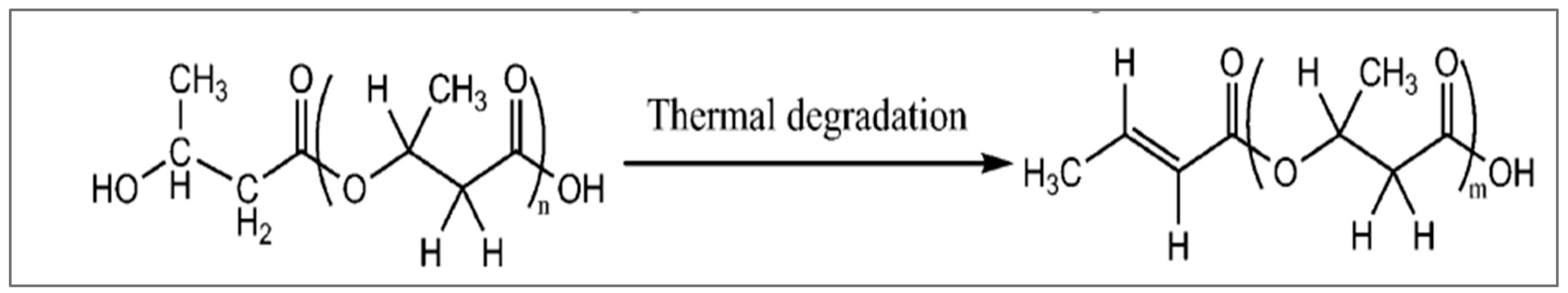
77]. X. Yang pointed out in his research [
90] that degraded PHB could be used as a plasticizer of PLA. Initially, PHB was thermally degraded in an extruder at 220 C to generate oligomers with functional groups. This last will be grafted in the main chain of PLA through a second extrusion (
Figure 23).
The results of PLA with 20% grafted PHB increased the elongation at break by 66. In parallel, WAXD measurements showed that grafting significantly increased the crystallization of PLA [
90].
7.4. Summary of Degradation Mechanism of Common Polymer
The degradation mechanism and ways to upgrade the polymer covered in the previous section are listed in
Table 5.
8. Conclusions
Polymer recycling can be performed using either mechanical or chemical processes. Mechanical recycling involves processes such as shredding, melting, and reprocessing plastics into new products. Unlike the chemical recycling process, mechanical recycling requires nearly no chemical solvents or byproducts, and it is, by far, the simplest and most affordable way to recycle plastics. It requires widespread, simple, well-established, less energy-consuming, and greenhouse gas emission equipment.
Mechanical recycling faces two main challenges: material degradation during reprocessing and the purity, regularity, and homogeneity of polymers to be recycled. The degradation phenomenon can be overcome by using suitable additives such as chain extenders and antioxidants, while the continuous improvement of sorting and decontamination technologies leads to recycled polymers with comparable properties as virgin polymers and makes them suitable for a wide range of applications.
In this paper, the mechanical recycling of commodity, engineering, and bio-based polymers is reviewed in detail to give researchers the state of the art of what has been done in the field of polymer mechanical recycling. The ultimate objective of this review paper is to convince readers that mechanical recycling is an easy and effective way to quickly reduce the amount of plastic waste going to landfills and incineration, environmental pollution, and resource depletion associated with producing new polymers.
Author Contributions
Literature revue A.L.; original draft preparation A.L.; writing—review and editing A.L. and S.E., Final reading A.L., S.E., F.M., C.D. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
Soleno Inc. and Mitacs IT23689.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the Soleno and Mitacs program for financial support.
Conflicts of Interest
The co-author Carl Diez is an employee of funding sponsor Soleno Inc. However, this sponsor has had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Geyer, R.; Jambeck, J.; Law, K. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Ellen MacArthur Foundation. The New Plastics Economy: Rethinking the Future of Plastics & Catalysing Action; Ellen MacArthur Foundation: Cowes, UK, 2017; Volume 68. [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Umeozor, E.; Vypovska, A.; Bararpour, T.; Adeyemo, T.; Zamzadeh, M. Towards a Circular Economy of Plastic Products in Canada; Canadian Energy Research Institute: Calgary, AB, Canada, 2021. [Google Scholar]
- Karlsson, S. Recycled Polyolefins. Material Properties and Means for Quality Determination. In Long Term Properties of Polyolefins; Albertsson, A.-C., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 169, pp. 201–230. [Google Scholar] [CrossRef]
- Bendimerad, S.; Tilmatine, A.; Ziane, M.; Dascalescu, L. Plastic wastes recovery using free-fall tribo electric separator. Int. J. Environ. Stud. 2009, 66, 529–538. [Google Scholar] [CrossRef]
- Masoumi, H.; Safavi, S.; Khani, Z. Identification and classification of plastics resins using Near Infrared Reflectance Spectroscopy. Int. J. Mech. Ind. Eng. 2012, 6, 213–220. [Google Scholar]
- Censori, M.; La Marca, F.; Carvalho, M.T. Separation of plastics: The importance of kinetics knowledge in the evaluation of froth flotation. Waste Manag. 2016, 54, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Wienaah, M. Sustainable Plastic Waste Management—A Case of Accra, Ghana; Kungliga Tekniska högskolan: Stockholm, Sweden, 2007. [Google Scholar]
- Ruj, B.; Pandey, V.; Jash, P.; Srivastava, V.K. Sorting of plastic waste for effective recycling. Int. J. Appl. Sci. Eng. 2015, 4, 564–571. [Google Scholar]
- Di Maio, F.; Rem, P.; Hu, B.; Serranti, S.; Bonifazi, G. The W2Plastics Project: Exploring the Limits of Polymer Separation. Open Waste Manag. J. 2010, 3, 90–98. [Google Scholar] [CrossRef]
- Alter, H. Application of the critical surface tension concept to items in our daily life. J. Adhes. 1978, 9, 135–140. [Google Scholar] [CrossRef]
- Carvalho, T.; Durão, F.; Ferreira, C. Separation of packaging plastics by froth flotation in a continuous pilot plant. Waste Manag. 2010, 30, 2209–2215. [Google Scholar] [CrossRef]
- Drelich, J.; Payne, T.; Kim, J.; Miller, J. Selective froth flotation of PVC from PVC/PET mixtures for the plastics recycling industry. Polym. Eng. Sci. 1998, 38, 1378–1386. [Google Scholar] [CrossRef]
- Barlaz, M.; Haynie, F.; Overcash, M. Framework for assesment of recycle potential applied to plastics. J. Environ. Eng. 1993, 119, 798–810. [Google Scholar] [CrossRef]
- Perrone, C. Operating experience in a washing plant for recycled LDPE film production. Resour. Conserv. Recycl. 1988, 2, 27–36. [Google Scholar] [CrossRef]
- Tilmatine, A.; Flazi, S.; Medles, K.; Ramdani, Y. Electrostatic separation of granular particles. J. Mater. Technol. 2003, 18, 207–210. [Google Scholar]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Subramanian, P. Plastics recycling and waste management in the US. Resour. Conserv. Recycl. 2000, 28, 253–263. [Google Scholar] [CrossRef]
- NAM Polymers. 2016. Products. 2016. Available online: http://nampolymers.ca/products/ (accessed on 1 January 2021).
- Oblak, P.; Gonzalez-Gutierrez, J.; Zupančič, B.; Aulova, A.; Emri, I. Processability and mechanical properties of extensively recycled high density polyethylene. Polym. Degrad. Stab. 2015, 114, 133–145. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Vinci, M. Recycling of heterogeneous plastics wastes. II—The role of modifier agents. Polym. Degrad. Stab. 1994, 42, 213. [Google Scholar] [CrossRef]
- Vlachopoulos, J.; Polychronopoulos, N.D. Polychonopoulos, Understanding Rheology and Technology of Polymer Extrusion, 1st ed.; Polydynamics Inc.: Dundas, On, Canada, 2019. [Google Scholar]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Mastellone, M.L. Thermal Treatments of Plastic Wastes by Means of Fluidized Bed Reactors; Department of chemical Engineering University of Naples: Naples, Italy, 1999. [Google Scholar]
- Al-Salem, S. Establishing an integrated databank for plastic manufacturers and converters in Kuwait. Waste Manag. 2009, 29, 479–484. [Google Scholar] [CrossRef]
- Tilmatine, A.; Bendimerad, S.; Younes, M.; Dascalescu, L. Dascalescub, Experimental analysis and optimisation of a free-fall triboelectric separator of granular plastic particles. Int. J. Sustain. Eng. 2009, 2, 184–191. [Google Scholar] [CrossRef]
- Aznar, M.P.; Caballero, M.A.; Sancho, J.A.; Francés, E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process. Technol. 2006, 87, 409–420. [Google Scholar] [CrossRef]
- Yassin, L.; Lettierim, P.; Simons, S.; Germana, A. Energy recovery from thermal processing of waste: A review. Eng. Sustain. Process 2005, 158, 97–103. [Google Scholar] [CrossRef]
- Denisov, E.T. Mechanism of regeneration of hindered nitroxyl and aromatic amines. Polym. Degrad. Stab. 1989, 25, 209–215. [Google Scholar] [CrossRef]
- Liu, S.S.; Hu, J.Y.; Qin, D.; Gao, L.L.; Chen, Y.; Ke, F.Y.; Wang, C.S.; Wang, H.P. Analysis of the Volatile Organic Compounds (VOCs) during the Regeneration of Post-Consumed Poly(Ethylene Terephthalate) Using HS-GC-MS Method. Mater. Sci. Forum 2019, 944, 1208–1214. [Google Scholar] [CrossRef]
- Yamashita, K.; Kumaga, K.; Noguchi, M.; Yamamoto, N.; Mizukoshi, A.; Yanagisawa, Y.; Ni, Y. Voc Emssion from waste plastics during melting process, présenté à International conference indoor air quality. In Proceedings of the 6th International Conference on Indoor Air Quality, Ventilation and Energy Conservation in Buildings: Sustainable Built Environment, Sendai, Japan, 28–31 October 2007; pp. 407–412. [Google Scholar]
- Bledzki, A.K.; Kessler, A.; Lieser, J. Odour reduction on plastics and its measurement. Polym. Test. 1999, 18, 63–71. [Google Scholar] [CrossRef]
- Cabanes, A.; Valdes, F.J.; Fullana, A. A review on VOCs from recycled plastics. Sustain. Mater. Technol. 2020, 25, e00179. [Google Scholar]
- Villberg, K.; Veijanen, A.; Gustafsson, I. Identification of off-flavor compounds in high-density polyethylene (HDPE) with different amounts of abscents. Polym. Eng. Sci. 1998, 38, 922–925. [Google Scholar] [CrossRef]
- Ravindranath, K.; Mashelkar, R.A. Analysis of the role of stripping agents in polymer devolatilization. Chem. Eng. Sci. 1988, 43, 429–442. [Google Scholar] [CrossRef]
- Yang, C.T.; Smith, T.G.; Bigio, D.I.; Anolick, C. Polymer trace Devolatilization: I. foaming experiments and model development. Aiche J. 1997, 43, 1861–1872. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L.; Ling, H.-C. Enhancement of impurity removal from polymer films. J. Appl. Polym. Sci. 1985, 30, 4499–4516. [Google Scholar] [CrossRef]
- Darley, D. Polymer filtration options: Screen Changers or large area. In Proceedings of the Third International Polymer Filtration Conference, Stuttgart, Germany. 1997. Available online: http://www.extrusionauxiliary.com/sitebuildercontent/sitebuilderfiles/screenchangers.pdf (accessed on 18 July 2023).
- Available online: https://psi-polymersystems.com/products/screen-changer-overview/manual-screen-changer/ (accessed on 1 January 2021).
- Cristancho, D.E.; Guzman, J.D.; Taylor, C.; Ortiz-Vega, D.; Acosta, H.; Hall, K.R. Supercritical extraction of volatile organic components from polyethylene pellets. J. Supercrit. Fluids 2012, 69, 124–130. [Google Scholar] [CrossRef]
- Luijsterburg, B.; Goossens, H. Assessment of plastic packaging waste: Material origin, methods, properties. Ressour. Conserv. Recycl. 2013, 85, 88–97. [Google Scholar] [CrossRef]
- Camacho, W.; Karlsson, S. NIR, DSC, and FTlR as Quantitative Methods for Compositional Analysis of Blends of Polymers Obtained From Recycled Mixed Plastic Waste. Polym. Eng. Sci. 2001, 41, 1626–1635. [Google Scholar] [CrossRef]
- Kleine-Benne, E.; Rose, B. Versatile Automated Pyrolysis GC Combining a Filament Type Pyrolyzer with a Thermal Desorption Unit. Gerstel Appl.note 11. 2011. Available online: www.gerstel.de/pdf/p-gc-an-2011-04.pdf (accessed on 18 July 2023).
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Rood, D. Gas chromatography problem solving and troubleshooting. J. Chromatogr. A 1995, 33, 347. [Google Scholar]
- Applied Plastics Engineering Handbook: Processing, Materials, and Applications; Plastics Design Library: Chadds Ford, PA, USA, 2017.
- Pinheiro, L.A.; Chinekatto, M.A.; Canevarolo, S.V. The role of chain scission and chain branching in high density polyethylene during thermo-mechanical degradation. Polym. Degrad. Stab. 2004, 86, 445–453. [Google Scholar] [CrossRef]
- Schyns, Z.O.; Shaver, M.P. Mechanical recycling of packaging plastics. Macromol. Rapid Commun. 2020, 42, 2000415. [Google Scholar] [CrossRef]
- Jin, H.; Gonzalez-Gutierrez, J.; Oblak, P.; Zupančič, B.; Emri, I. The effect of extensive mechanical recycling on the properties of low density polyethylene. Polym. Degrad. Stab. 2012, 97, 2262–2272. [Google Scholar] [CrossRef]
- Borysiak, S. The thermo-oxidative stability and flammability of wood/polypropylene composites. J. Therm. Anal. Calorim. 2014, 119, 1955–1962. [Google Scholar] [CrossRef]
- Rosales-Jasso, A.; Allen, N.S.; Sasaki, M. Evaluation of novel 4,4-dimethyloxazolidine derivatives as thermal and UV stabilisers in linear low density polyethylene (LLDPE) film. Polym. Degrad. Stab. 1999, 64, 277. [Google Scholar] [CrossRef]
- Craig, I.H.; White, J.R. Mechanical properties of photo-degraded recycled photo-degraded polyolefins. J. Mater. Sci. 2006, 41, 993–1006. [Google Scholar] [CrossRef]
- Iyer, K.A.; Zhang, L.; Torkelson, J.M. Direct Use of Natural Antioxidant-rich Agro-wastes as Thermal Stabilizer for Polymer: Processing and Recycling. ACS Sustain. Chem. Eng. 2015, 4, 881–889. [Google Scholar] [CrossRef]
- Peña, J.; Allen, N.; Edge, M.; Liauw, C.; Valange, B. Studies of synergism between carbon black and stabilisers in LDPE photodegradation. Polym. Degrad. Stab. 2001, 72, 259–270. [Google Scholar] [CrossRef]
- Grabmann, M.K.; Gernot, W.; Maringer, L. Hot air aging behavior of polypropylene random copolymers. J. Appl. Polym. Sci. 2018, 136, 47350. [Google Scholar] [CrossRef]
- Liao, J.; Brosse, N.; Pizzi, A.; Hoppe, S.; Xi, X.; Zhou, X. Polypropylene Blend with Polyphenols through Dynamic Vulcanization: Mechanical, Rheological, Crystalline, Thermal, and UV Protective Property. Polymers 2019, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.F. Extrusion of Rubber and Plastics. Compr. Polym. Sci. Suppl. 1989, 7, 303–354. [Google Scholar]
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recyling of PET. Eur. Polym. J. 2005, 41, 1453. [Google Scholar] [CrossRef]
- Ragaert, K.; Huysveld, S.; Vyncke, G.; Hubo, S.; Veelaert, L.; Dewulf, J. Design from recycling: A complex mixed plastic waste case study. Resour. Conserv. Recycl. 2020, 155, 104646. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Vinci, M. Recycling poly(ethyleneterephthalate). Polym. Degrad. Stab. 1994, 45, 121–125. [Google Scholar] [CrossRef]
- Liu, W.-C.; Halley, P.J.; Gilbert, R.G. Mechanism of Degradation of Starch, a Highly Branched Polymer, during Extrusion. Macromolecules 2010, 43, 2855–2864. [Google Scholar] [CrossRef]
- Cavalcanti, F.N.; Teofilo, E.T.; Rabello, M.S.; Silva, S.M.L. Chain extension and degradation during reactive processing of PET in the presence of triphenyl phosphite. Polym. Eng. Sci. 2007, 47, 2155. [Google Scholar] [CrossRef]
- Raffa, P.; Coltelli, M.B.; Savi, S.; Bianchi, S.; Castelvetro, V. Chain extension and branching of poly(ethylene terephthalate) (PET) with di- and multifunctional epoxy or isocyanate additives: An experimental and modelling study. React. Funct. Polym. 2012, 72, 50–60. [Google Scholar] [CrossRef]
- Incarnato, L.; Scarfato, P.; Di Maio, L.; Acierno, D. Structure and rheology of recycled PET modified by reactive extrusion. Polymer 2000, 41, 6825–6831. [Google Scholar] [CrossRef]
- Tapia, J.J.B.; Valdez, M.H.; Cortez, J.C.; García, V.M.D.; Barrios, H.L. Improving the Rheological and Mechanical Properties of Recycled PET Modified by Macromolecular Chain Extenders Synthesized by Controlled Radical Polymerization. J. Polym. Environ. 2018, 26, 4221–4232. [Google Scholar] [CrossRef]
- Hsiao, S.; Wang, H.; Chou, J.; Guo, W.; Tsai, T. Synthesis and characterization of novel organosoluble and thermally stable polyamides bearing triptycene in their backbones. J. Polym. Res 2012, 19, 9902–9911. [Google Scholar] [CrossRef]
- Murphy, J. Additives for Plastics Handbook; Elsevier Advanced Technology; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Tuna, B.; Benkreira, H. Chain extension of recycled PA6. Polym. Eng. Sci 2017, 58, 1037–1042. [Google Scholar] [CrossRef]
- Lu, C.; Chen, L.; Ye, R.; Cai, X. Chain Extension of Polyamide 6 Using Bisoxazoline Coupling Agents. J. Macromol. Sci. Part B 2008, 47, 986–999. [Google Scholar] [CrossRef]
- Selin Celebi, O.; Ozkoc, G.; Serhatli, E. Thermal, mechanical and physical properties of chain extended recycled polyamide 6 via reactive extrusion: Effect of chain extender types. Polym. Degrad. Stab. 2019, 162, 76–84. [Google Scholar]
- Saurabh, S.; Rahul, S.M.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar]
- Auras, R.A.; Loong-Tak, T.; Selke, S.E.M.; Tsuji, H. Poly(lactic acid): Synthesis, Structures, Properties, Processing, Applications, and End of Life, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Niaounakis, M. Biopolymers Reuse, Recycling, and Disposal, 1st ed.; William Andrew: Cary, NC, USA, 2013. [Google Scholar]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- Lesprit, U. Étude Expérimentale du Chargement Triboélectrique d’une Bille de Verre en Interaction avec des Matériaux Polymères. Doctoral Dissertation, Université de Poitiersp, Poitiers, France, 2020; p. 161. [Google Scholar]
- Zenkiewicz, M.; Richert, J.; Rytlewski, P.; Moraczewski, K.; Stepczynska, M.; Karasiewicz, T. Characterisation of multi-extruded poly(lactic acid). Polym. Test. 2009, 28, 412–418. [Google Scholar] [CrossRef]
- Badiaa, J.D.; Ribes-Greusa, A. Mechanical recycling of polylactide, upgrading trends and combination of valorization techniques. Eur. Polym. J. 2016, 84, 22–39. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gamez-Perez, J.; Santana, O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. Material valorisation of amorphous polylactide. Influence of thermo-mechanical degradation on the morphology, segmental dynamics, thermal and mechanical performance. Polym. Degrad. Stab. 2012, 97, 670–678. [Google Scholar] [CrossRef]
- Pantani, R.; De santis, F.; Sorrentino, A.; De Maio, F.; Titomanlio, G. Crystallization kinetics of virgin and processed poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 1148–1159. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Tsubakihara, S.; Sugimoto, Y.; Nakafuku, C. Thermal analysis of the double-melting behavior of poly(L-lactic acid). J. Polym. Sci. Part B Polym. Phys. 2003, 42, 25–32. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Iura, K.; Dan, Y. Melting behavior of poly(l-lactic acid): Effects of crystallization temperature and time. Polymer 2007, 48, 5398–5407. [Google Scholar] [CrossRef]
- Meng, X.; Shi, G.; Chen, W.; Wu, C.; Xin, Z.; Han, T.; Shi, Y. Structure effect of phosphite on the chain extension in PLA. Polym. Degrad. Stab. 2015, 120, 283–289. [Google Scholar] [CrossRef]
- Giulia, F.; Dorigato, A. Recycling of bioplastic waste: A review. Adv. Ind. Eng. Polym. Res. 2021, 4, 159–177. [Google Scholar]
- European Bioplastics. Bioplastics Facts and Figures; European Bioplastics Association: Berlin, Germany, 2019. [Google Scholar]
- Rivas, L.F.; Casarin, S.A.; Nepomuceno, N.C.; Alencar, M.I.; Agnelli, J.A.M.; Medeiros, E.S.D.; Wanderley, A.D.O.; Oliveira, M.P.D.; Medeiros, A.M.D. Reprocessability of PHB in extrusion: ATR-FTIR, tensile tests and thermal studies. Polimeros 2017, 27, 122–128. [Google Scholar] [CrossRef]
- Yang, X.; Clénet, J.; Xu, H.; Odelius, K.; Hakkarainen, M. Two Step Extrusion Process: From Thermal Recycling of PHB to Plasticized PLA by Reactive Extrusion Grafting of PHB Degradation Products onto PLA Chains. Macromolecules 2015, 48, 2509–2518. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).