Computational Modelling on Gasification Processes of Municipal Solid Wastes Including Molten Slag
Abstract
1. Introduction
2. Numerical Simulation and CD-MELT Model
2.1. Fluid-Phase Equations
2.2. Solid-Phase Equations
2.3. Particle Laws and Melting Model
2.4. Solution Procedure and Inter-Phase Coupling of Mass, Momentum and Energy
3. Case Study: Prototype Gasifier
3.1. Simulation Model
3.2. Effect of Slag Recycling on Slag Flow through Packed Bed
3.3. Challenges of the Melting Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature/Abbreviations
| Nomenclature | |
| Ap | particle surface area |
| cp | specific heat capacity |
| CF | char mass fraction |
| d | diameter |
| force | |
| fraction of heat absorbed by solid particle | |
| gravitational acceleration | |
| h | enthalpy |
| convective heat transfer coefficient | |
| latent heat of melting | |
| diffusive flux | |
| interphase exchange coefficient between phase p and phase q | |
| LF | liquid mass fraction |
| m | mass |
| initial particle mass | |
| p | pressure |
| Q | heat transfer term |
| Re | Reynolds number |
| Ri | rate of production via chemical reaction or particle surface reactions |
| t | time |
| T | temperature |
| velocity | |
| VF | volatile mass fraction |
| S | source term |
| position | |
| Y | local mass fraction of species within gas mixture |
| Greek symbols | |
| α | phase volume fraction |
| β | mass loss fraction |
| ρ | density |
| stress tensor | |
| dynamic viscosity | |
| Subscripts | |
| coll | collision |
| g | gas |
| l | liquid |
| p | particle |
| ref | reference |
| Abbreviations | |
| CFD | Computational Fluid Dynamics |
| DEM | Discrete Element Method |
| DPM | Discrete Phase Model |
| FC | Fixed Carbon |
| MSW | Municipal Solid Waste |
| UDF | User-Defined Function |
| VM | Volatile Matter |
Appendix A
| Char Fraction > 0 | Char Fraction = 0 | |
|---|---|---|
| Step 1 | Obtain the new particle temperature , assuming combustion takes place. | Obtain the new particle temperature , assuming inert heating takes place. |
| Step 2 | Calculate the heat absorbed from fluid and combustion and add to particle enthalpy, . | Calculate the heat absorbed from fluid and add to particle enthalpy, . |
| Step 3 | “State 1”: Record particle mass and diameter after combustion, and particle temperature before combustion. | “State 1”: Record particle temperature and diameter before inert heating. |
| Step 4 to Step 9 are the same regardless of char fraction ** | ||
| Step 4 | Assuming that particle temperature rises to temperature , calculate theoretical particle diameter and mass based on the new temperature. is the particle diameter just before melting occurs and is saved for every particle as the diameter when the particle first reaches the solidus temperature. Particle density is assumed to be constant throughout the melting process by setting it to the particle density when the particle first reaches solidus temperature. The theoretical mass is then calculated as . | |
| Step 5 | Compare particle enthalpy with the calculated heat required for the change in mass (sum of sensible and latent heat). for char fraction > 0 is the change in mass from the particle mass after combustion. Compare with . | |
| Step 6 | If particle enthalpy is sufficient, melting occurs, and particle changes in mass, temperature, and diameter take place according to Step 4. Heat required is subtracted from particle enthalpy. | |
| Step 7 | If particle enthalpy is insufficient, no melting occurs, and particle values return to those saved in Step 3 (“State 1”). This means that for char fraction > 0, particle mass is still lost to combustion. | |
| Step 8 | Calculate the mass loss fraction, β = 1 − , where is the particle mass recorded at the start of the melting process. | |
| Step 9 | Convert to volumetric and add the relevant source terms to the gas (energy: and molten liquid phase (mass: momentum: energy: ). | |
References
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T. Renewable energy (Gasification). Adv. Electron. Electr. Eng. 2013, 3, 1243–1250. [Google Scholar]
- Klimanek, A.; Adamczyk, W.; Katelbach-Woźniak, A.; Węcel, G.; Szlęk, A. Towards a hybrid Eulerian–Lagrangian CFD modeling of coal gasification in a circulating fluidized bed reactor. Fuel 2015, 152, 131–137. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Li, B.; Yu, X. Model development for biomass gasification in an entrained flow gasifier using intrinsic reaction rate submodel. Energy Convers. Manag. 2016, 108, 120–131. [Google Scholar] [CrossRef]
- Baruah, D.C.; Baruah, D.C.; Hazarika, M.K. Artificial neural network based modeling of biomass gasification in fixed bed downdraft gasifiers. Biomass Bioenergy 2017, 98, 264–271. [Google Scholar] [CrossRef]
- Yucel, O.; Aydin, E.S.; Sadikoglu, H. Comparison of the different artificial neural networks in prediction of biomass gasification products. Int. J. Energy Res. 2019, 43, 5992–6003. [Google Scholar] [CrossRef]
- Hu, Z.; Peng, Y.; Sun, F.; Chen, S.; Zhou, Y. Thermodynamic equilibrium simulation on the synthesis gas composition in the context of underground coal gasification. Fuel 2021, 293, 120462. [Google Scholar] [CrossRef]
- Onabanjo, T.; Patchigolla, K.; Wagland, S.T.; Fidalgo, B.; Kolios, A.; McAdam, E.; Parker, A.; Williams, L.; Tyrrel, S.; Cartmell, E. Energy recovery from human faeces via gasification: A thermodynamic equilibrium modelling approach. Energy Convers. Manag. 2016, 118, 364–376. [Google Scholar] [CrossRef]
- de Lemos, M.J.S.; Pivem, A.C. Turbulent flow with combustion in a moving bed. Int. Commun. Heat Mass Transf. 2012, 39, 1–7. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Rouboa, A. Numerical approaches and comprehensive models for gasification process: A review. Renew. Sustain. Energy Rev. 2019, 110, 188–206. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Silva, V.; Rouboa, A. Co-gasification and recent developments on waste-to-energy conversion: A review. Renew. Sustain. Energy Rev. 2018, 81, 380–398. [Google Scholar] [CrossRef]
- Xiong, Q.; Yang, Y.; Xu, F.; Pan, Y.; Zhang, J.; Hong, K.; Lorenzini, G.; Wang, S. Overview of Computational Fluid Dynamics Simulation of Reactor-Scale Biomass Pyrolysis. ACS Sustain. Chem. Eng. 2017, 5, 2783–2798. [Google Scholar] [CrossRef]
- Li, J.; Suvarna, M.; Li, L.; Pan, L.; Pérez-Ramírez, J.; Ok, Y.S.; Wang, X. A review of computational modeling techniques for wet waste valorization: Research trends and future perspectives. J. Clean. Prod. 2022, 367, 133025. [Google Scholar] [CrossRef]
- Wissing, F.; Wirtz, S.; Scherer, V. Simulating municipal solid waste incineration with a DEM/CFD method—Influences of waste properties, grate and furnace design. Fuel 2017, 206, 638–656. [Google Scholar] [CrossRef]
- Dymala, T.; Wang, S.; Jarolin, K.; Song, T.; Shen, L.; Dosta, M.; Heinrich, S. MP-PIC Simulation of Biomass Steam Gasification Using Ilmenite as an Oxygen Carrier. Atmosphere 2022, 13, 1009. [Google Scholar] [CrossRef]
- Hu, C.; Luo, K.; Wang, S.; Sun, L.; Fan, J. Influences of operating parameters on the fluidized bed coal gasification process: A coarse-grained CFD-DEM study. Chem. Eng. Sci. 2019, 195, 693–706. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, J.; Feng, D.; Zhang, X. Modeling of the molten blast furnace slag particle deposition on the wall including phase change and heat transfer. Appl. Energy 2019, 248, 288–298. [Google Scholar] [CrossRef]
- Dhaidan, N.S.; Khalaf, A.F. Experimental evaluation of the melting behaviours of paraffin within a hemicylindrical storage cell. Int. Commun. Heat Mass Transf. 2020, 111, 104476. [Google Scholar] [CrossRef]
- Kheirabadi, A.C.; Groulx, D. The Effect of the Mushy-Zone Constant on Simulated Phase Change Heat Transfer. In Proceedings of the 6th International Symposium on Advances in Computational Heat Transfer (CHT-15), New Brunswick, NJ, USA, 25–29 May 2015. [Google Scholar]
- Mallya, N.; Haussener, S. Buoyancy-driven melting and solidification heat transfer analysis in encapsulated phase change materials. Int. J. Heat Mass Transf. 2021, 164, 120525. [Google Scholar] [CrossRef]
- Hummel, D.; Beer, S.; Hornung, A. A conjugate heat transfer model for unconstrained melting of macroencapsulated phase change materials subjected to external convection. Int. J. Heat Mass Transf. 2020, 149, 119205. [Google Scholar] [CrossRef]
- Ueda, S.; Kon, T.; Kurosawa, H.; Natsui, S.; Ariyama, T.; Nogami, H. Influence of Shape of Cohesive Zone on Gas Flow and Permeability in the Blast Furnace Analyzed by DEM-CFD Model. ISIJ Int. 2015, 55, 1232–1236. [Google Scholar] [CrossRef]
- Ueda, S.; Kon, T.; Miki, T.; Kim, S.-J.; Nogami, H. Softening, Melting, and Permeation Phenomena of CaO–FeO–SiO2 Oxide on a Coke Bed. ISIJ Int. 2015, 55, 2098–2104. [Google Scholar] [CrossRef]
- Heinze, T. A Multi-Phase Heat Transfer Model for Water Infiltration Into Frozen Soil. Water Resour. Res. 2021, 57, e2021WR030067. [Google Scholar] [CrossRef]
- Hosseini, S.; Gupta, R. Inorganic Matter Behavior during Coal Gasification: Effect of Operating Conditions and Particle Trajectory on Ash Deposition and Slag Formation. Energy Fuels 2015, 29, 1503–1519. [Google Scholar] [CrossRef]
- Kurowski, M.P.; Spliethoff, H. Deposition and slagging in an entrained-flow gasifier with focus on heat transfer, reactor design and flow dynamics with SPH. Fuel Process. Technol. 2016, 152, 147–155. [Google Scholar] [CrossRef]
- Safronov, D.; Förster, T.; Schwitalla, D.; Nikrityuk, P.; Guhl, S.; Richter, A.; Meyer, B. Numerical study on entrained-flow gasification performance using combined slag model and experimental characterization of slag properties. Fuel Process. Technol. 2017, 161, 62–75. [Google Scholar] [CrossRef]
- Montagnaro, F.; Salatino, P. Analysis of char–slag interaction and near-wall particle segregation in entrained-flow gasification of coal. Combust. Flame 2010, 157, 874–883. [Google Scholar] [CrossRef]
- Lu, X.; Wang, T. Water–gas shift modeling in coal gasification in an entrained-flow gasifier—Part 2: Gasification application. Fuel 2013, 108, 620–628. [Google Scholar] [CrossRef]
- Wang, P.; Massoudi, M. Slag Behavior in Gasifiers. Part I: Influence of Coal Properties and Gasification Conditions. Energies 2013, 6, 784–806. [Google Scholar] [CrossRef]
- Soon, G.; Zhang, H.; Yang, C.; Law, A.W.-K. Simulations of Melting in Fluid-filled Packed Media due to Forced Convection with Higher Temperature. Int. J. Heat Mass Transf. 2021, 175, 121358. [Google Scholar] [CrossRef]
- Soon, G.; Zhang, H.; Law, A.W.-K.; Yang, C. Modelling of Melting in Packed Media due to Forced Air Convection with Higher Temperature using Euler-Euler-Lagrangian approach. Int. J. Heat Mass Transf. 2022, 194, 123055. [Google Scholar] [CrossRef]
- Ansys® Academic Research Fluent, Release 20.2, Help System, ANSYS Fluent Theory Guide; ANSYS, Inc.: Canonsburg, PA, USA, 2020.
- Cundall, P.A.; Strack, O.D. A discrete numerical model for granular assemblies. Geotechnique 1979, 29, 47–65. [Google Scholar] [CrossRef]
- Gidaspow, D. Multiphase Flow and Fluidization: Continuum and Kinetic Theory Descriptions; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Tsuji, Y.; Kawaguchi, T.; Tanaka, T. Discrete particle simulation of two-dimensional fluidized bed. Powder Technol. 1993, 77, 79–87. [Google Scholar] [CrossRef]
- Hu, H.; Argyropoulos, S.A. Mathematical modelling of solidification and melting: A review. Model. Simul. Mater. Sci. Eng. 1996, 4, 371–396. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhou, Z.Y.; Pinson, D.; Yu, A.B. A New Approach for Studying Softening and Melting Behavior of Particles in a Blast Furnace Cohesive Zone. Metall. Mater. Trans. B 2015, 46, 977–992. [Google Scholar] [CrossRef]
- Patankar, S. Numerical Heat Transfer and Fluid Flow; Taylor & Francis: Boca Raton, FL, USA, 2018. [Google Scholar]
- Wen, C.Y. Mechanics of fluidization. Chem. Eng. Prog. Symp. Ser. 1966, 62, 100–111. [Google Scholar]
- Ergun, S. Fluid Flow through Packed Columns. J. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Ranz, W.E.; Marshall, W.R. Evaporation from drops. Chem. Eng. Prog. 1952, 48, 141–146. [Google Scholar]
- Heberlein, S.; Chan, W.P.; Veksha, A.; Giannis, A.; Hupa, L.; Lisak, G. High temperature slagging gasification of municipal solid waste with biomass charcoal as a greener auxiliary fuel. J. Hazard. Mater. 2022, 423, 127057. [Google Scholar] [CrossRef]
- Nakai, Y.; Kikuchi, N.; Iwasa, M.; Nabeshima, S.; Kishimoto, Y. Development of Slag Recycling Process in Hot Metal Desulfurization with Mechanical Stirring. Steel Res. Int. 2009, 80, 727–732. [Google Scholar] [CrossRef]
- Diao, J.; Zhou, W.; Ke, Z.; Qiao, Y.; Zhang, T.; Liu, X.; Xie, B. System assessment of recycling of steel slag in converter steelmaking. J. Clean. Prod. 2016, 125, 159–167. [Google Scholar] [CrossRef]
- Song, W.J.; Tang, L.H.; Zhu, X.D.; Wu, Y.Q.; Zhu, Z.B.; Koyama, S. Effect of Coal Ash Composition on Ash Fusion Temperatures. Energy Fuels 2010, 24, 182–189. [Google Scholar] [CrossRef]
- Fourcault, A.; Marias, F.; Michon, U. Modelling of thermal removal of tars in a high temperature stage fed by a plasma torch. Biomass Bioenergy 2010, 34, 1363–1374. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, B.; Zhao, Z.; Zhang, L.; Chen, Y.; Luo, C.; Zheng, C. CFD modeling on char surface reaction behavior of pulverized coal MILD-oxy combustion: Effects of oxygen and steam. Fuel Process. Technol. 2020, 204, 106405. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Ouyang, J.; Cao, L.; Luo, G.; Yao, H. A kinetic study on char oxidation in mixtures of O2, CO2 and H2O. Fuel Process. Technol. 2018, 179, 250–257. [Google Scholar] [CrossRef]
- Mularski, J.; Pawlak-Kruczek, H.; Modlinski, N. A review of recent studies of the CFD modelling of coal gasification in entrained flow gasifiers, covering devolatilization, gas-phase reactions, surface reactions, models and kinetics. Fuel 2020, 271, 117620. [Google Scholar] [CrossRef]
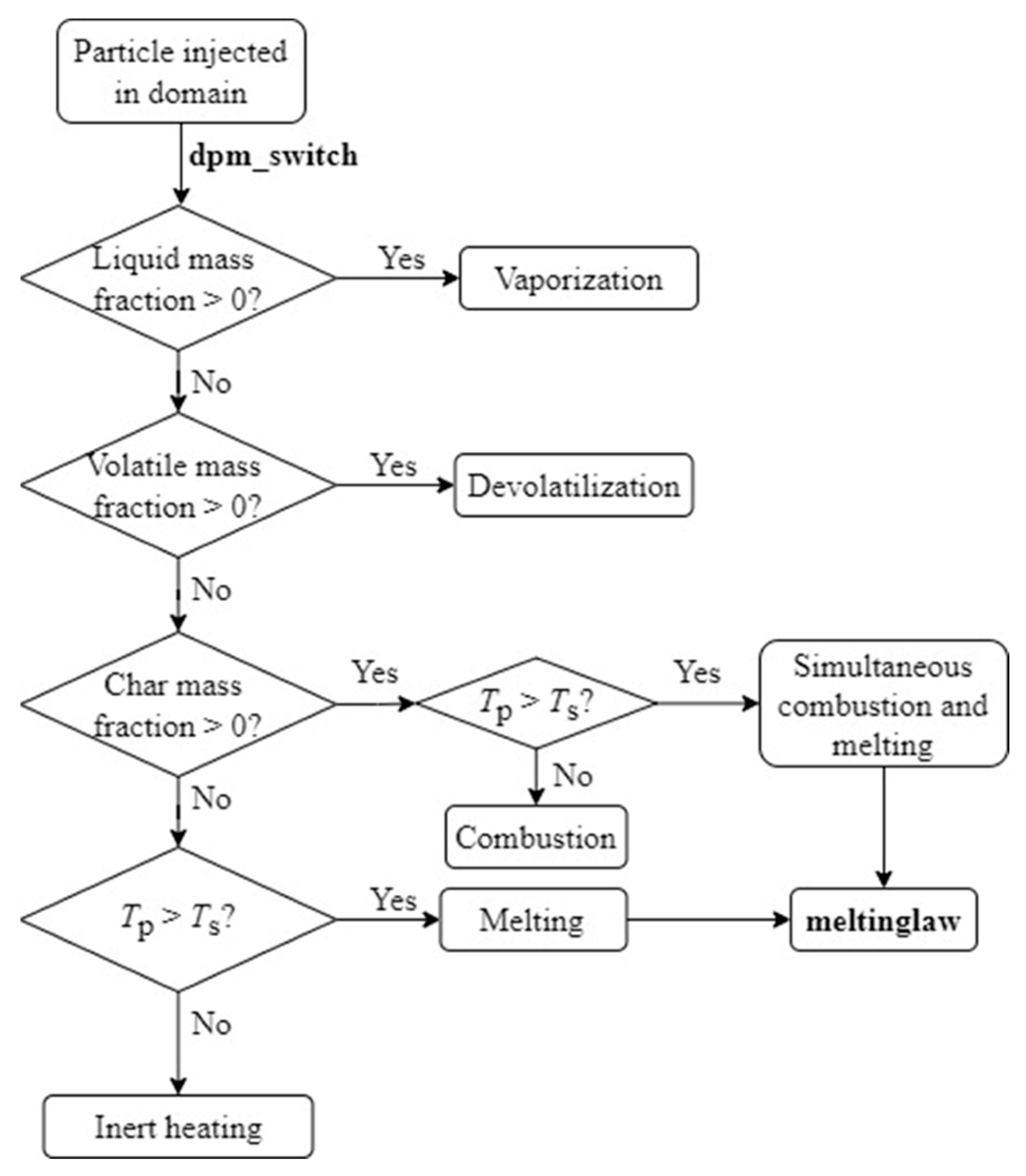

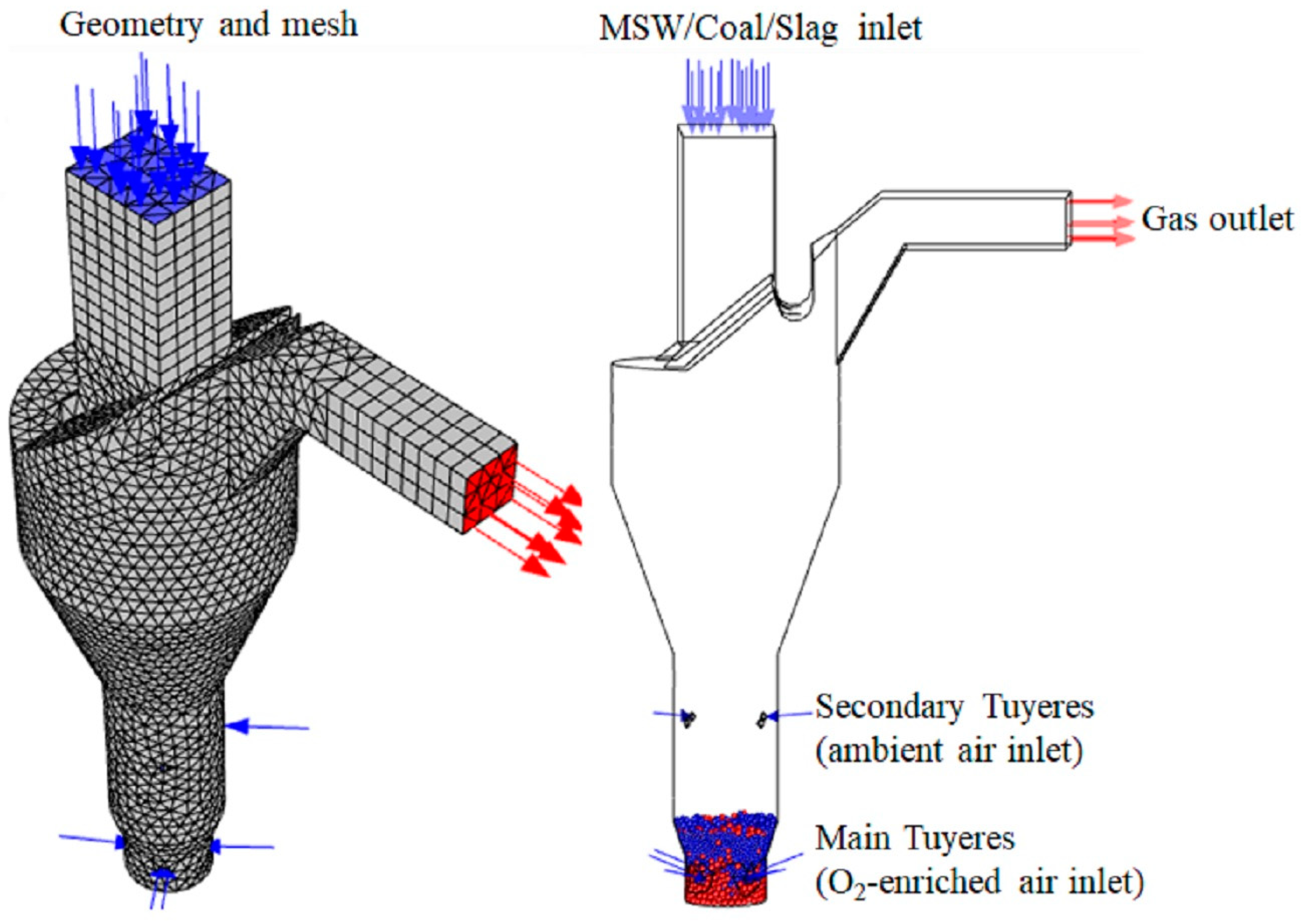



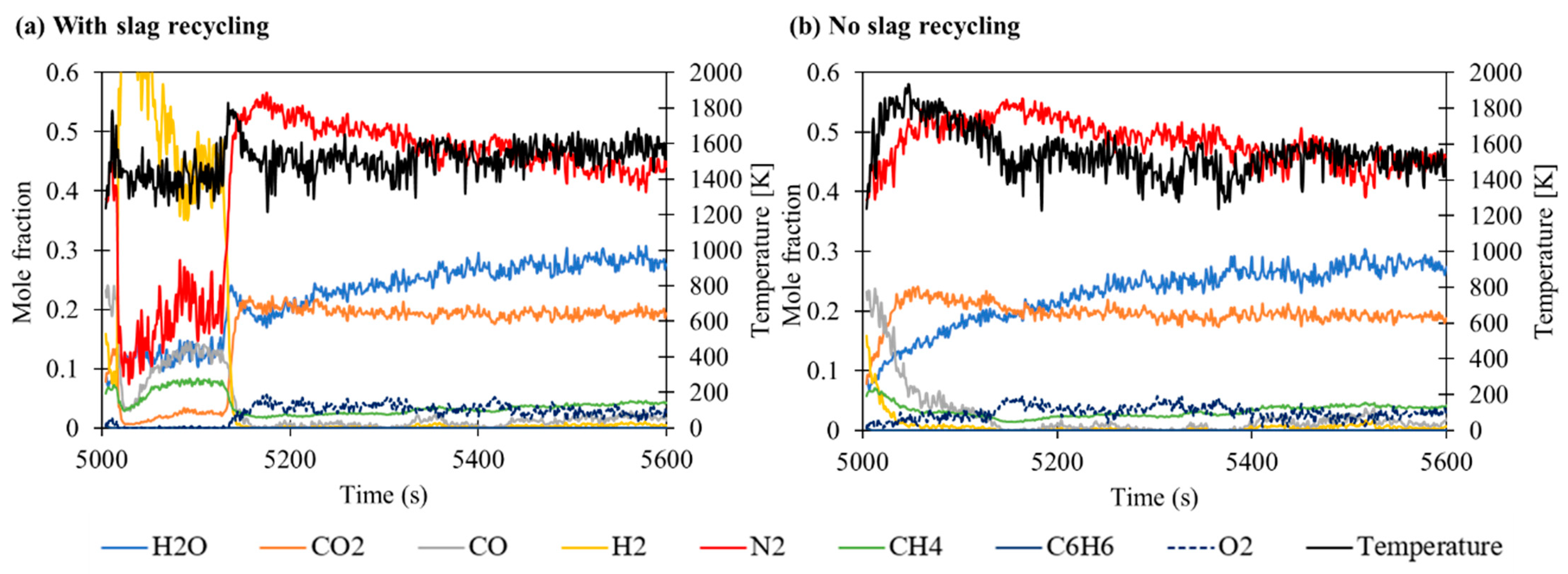

| Equation | No. |
|---|---|
| Primary Phase (Gas Mixture) | |
| Continuity: | |
| (1) | |
| Momentum: | |
| (2) | |
| Energy: | |
| (3) | |
| Species: | |
| (4) | |
| where i is used to refer to each individual species in the gas mixture. | |
| Secondary Phase (Liquid) | |
| Continuity: | |
| (5) | |
| where is the mass source added from the solid phase due to melting. | |
| Momentum: | |
| (6) | |
| Energy: | |
| (7) |
| Particle Law (s) | Particle Energy Balance Equation | No. |
|---|---|---|
| Inert Heating | (10) | |
| Vaporization | (11) | |
| Devolatilization | (12) | |
| Surface Reactions (Combustion/Pyrolysis) | (13) | |
| Simultaneous Melting and Combustion | (14) | |
| Melting | (15) |
| Properties | MSW | Coal | Slag |
|---|---|---|---|
| Chemical composition (% weight) | |||
| Moisture (W) | 43.71 | - | - |
| Volatile matter (VM) (% wt, dry basis) | 79.89 | - | - |
| Fixed Carbon (FC) (% wt, dry basis) | 11.72 | 100.00 | - |
| Ash (% wt, dry basis) | 8.39 | - | 100.00 |
| Density, (kg/m3) = 1000, = 400, = 430, = 2200 | Volume-weighted average from each component | 800 | 2200 |
| Heat capacity, (J/kg K) = 4200, = 4.5099T − 101.4917, = −6.85 × 10−4T2 + 2.09T + 420, = −1.95 × 10−4T2 + 0.644T + 644 | Mass-weighted average from each component | ||
| Elemental composition (% weight) | |||
| C | 51.81 | - | - |
| H | 7.48 | - | - |
| N | 1.40 | - | - |
| O | 30.92 | - | - |
| Chemical Reactions | Reaction Rates |
|---|---|
| Homogenous reactions | |
| Combustion | |
| CO (g) + 0.5 O2 (g) → CO2 (g) | 3.165 × 1012 exp ( [CO][O2]0.25[H2O]0.5 |
| H2 (g) + 0.5 O2 (g) → H2O (g) | 1.08 × 1010exp ( [O2][H2] |
| CH4 (g) + 2O2 (g)→ 2 H2O (g) + CO2 (g) | 1.3 × 105exp ( [CH4]0.3[O2]1.3 |
| C6H6 (g) + 3 O2 (g) → 6 CO (g) + 3 H2 (g) | 1.58 × 1015exp ( [C6H6][O2] |
| Tar Cracking | |
| C6H6 (g) + 5 H2O (g) → 5 CO + 6 H2 (g) + CH4 (g) | 4.4 × 108exp ( [C6H6] |
| C6H6 (g) + 7.5 O2 (g) → 6 CO2 (g) + 3 H2O (g) | 1.783exp ( [C6H6]−0.1[O2]1.25 |
| Water-gas shift reaction | |
| CO (g) + H2O (g) → CO2 (g) + H2 (g) (forward reaction) | 2.778 × 102exp ( [CO][H2O] |
| CO2 (g) + H2 (g) → CO (g) + H2O (g) (backward reaction) | 1.263 × 104exp ( [CO2][H2] |
| Heterogenous reactions | |
| Pyrolysis | |
| C1.088H1.732N0.023O0.361 (s) → 0.088 C (char, s) + 0.03 CO2 (g) + 0.065 H2 (g) + 0.061 H2O (g) + 0.08 C6H6 (g) + 0.24 CO (g) + 0.25 CH4 (g) + 0.0115 N2 (g) | 2.0 × 1011exp ( |
| C (s) + O2 (g) → CO2 (g) | MSW: 5.88 × 10−6exp ( [O2] Coal: 4.53 × 10−6exp ( [O2] |
| C (s) + CO2 (g) → 2 CO (g) | MSW: 5.88 × 10−6exp ( [CO2] Coal: 4.13 × 10−6exp ( [CO2] |
| C (s) + H2O (g) → CO (g) + H2 (g) | MSW: 5.0 × 10−6exp ( [H2O] Coal: 4.13 × 10−6exp ( [H2O] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soon, G.; Zhang, H.; Law, A.W.-K.; Yang, C. Computational Modelling on Gasification Processes of Municipal Solid Wastes Including Molten Slag. Waste 2023, 1, 370-388. https://doi.org/10.3390/waste1020023
Soon G, Zhang H, Law AW-K, Yang C. Computational Modelling on Gasification Processes of Municipal Solid Wastes Including Molten Slag. Waste. 2023; 1(2):370-388. https://doi.org/10.3390/waste1020023
Chicago/Turabian StyleSoon, Genevieve, Hui Zhang, Adrian Wing-Keung Law, and Chun Yang. 2023. "Computational Modelling on Gasification Processes of Municipal Solid Wastes Including Molten Slag" Waste 1, no. 2: 370-388. https://doi.org/10.3390/waste1020023
APA StyleSoon, G., Zhang, H., Law, A. W.-K., & Yang, C. (2023). Computational Modelling on Gasification Processes of Municipal Solid Wastes Including Molten Slag. Waste, 1(2), 370-388. https://doi.org/10.3390/waste1020023









