Simple Summary
American cutaneous leishmaniasis is a zoonosis transmitted by infected female sandflies that are distributed throughout all regions of Brazil. Sandflies are the vectors of the disease, carrying the protozoan Leishmania, which causes leishmaniasis, and transmitting it to humans and other animals through their bites. This study classifies Brazilian states by level of endemicity of the disease by estimating prevalence, contributing to epidemiological surveillance, planning public health actions, and directing resources.
Abstract
Brazil is the first of the 12 priority countries in Latin America with the highest number of cases of American cutaneous leishmaniasis (ACL). This study estimated the prevalence of ACL in Brazil and classified the states according to the different levels of endemicity in the period from 2014 to 2024. This is a retrospective and cross-sectional study of ACL cases registered in Brazil by the Information System for Notifiable Diseases of the Ministry of Health. The predominant cases were male (73.2%), brown (65.0%), aged between 20 and 39 (41.5%), with a low level of education (44.4%), 0.5% in pregnant women, 80% of cases were confirmed by laboratory criteria, and 90% were classified as cutaneous. A total of 182,072 autochthonous cases were recorded, with a prevalence of 89.6 cases per 100,000 population. Two states were classified as having high intense endemicity; three were classified as having medium intense endemicity; four were classified as having low intense endemicity; five states were classified as having moderate endemicity; and 13 were classified as having low endemicity. The municipality of Presidente Figueiredo, Amazonas, had the highest prevalence of ACL (5503.1%), while Belo Horizonte had the lowest prevalence (72.2%). The month with the highest average number of cases was January with 1731 (with a standard deviation of 364; upper limit of 1933; lower limit of 1572). The heterogeneity of endemicity among States suggests that social and environmental determinants influence the dynamics of ACL transmission. All sociodemographic, clinical, and epidemiological categories, when compared with the different levels of endemicity, showed significant effects (p < 0.05), except for the variable gestational status in high disease endemicity. The inclusion of these variables significantly improved the model’s ability to predict the dependent variable.
1. Introduction
In the Americas, American cutaneous leishmaniasis (ACL) is a vector-borne zoonotic disease with a complex transmission cycle involving a wide variety of parasite species, reservoirs, and vectors [1]. It is caused by several species of protozoa of the genus Leishmania and is transmitted to animals and humans through the bite of insects of the family Psychodidae [2]. Its presence is directly linked to poverty, although other social factors, in addition to environmental and climatic ones, directly influence its epidemiology [3].
Cutaneous leishmaniasis is an infectious disease that affects the skin and mucous membranes, with a worldwide distribution and endemic status in 90 countries [4]. The prevalence of ACL in Latin America is difficult to determine due to underreporting, misdiagnosis, and variability in host response, but it is an endemic disease [5]. Among the 11 countries in the world with the highest number of cases of ACL, three are in the Americas: Brazil, Colombia, and Peru [6]. Vector control actions such as chemical control [7], barrier methods [8], and ACL case management [9] in Brazil have not been fully effective due to the social and environmental heterogeneity of the regions [10]. Thus, considering the specificity and needs of each region, it is evident how important it is to study the epidemiology and transmission behavior of this disease to develop appropriate strategies [11].
The main parasite species responsible for cutaneous leishmaniasis in Brazil include Leishmania (Viannia) braziliensis, Leishmania (Leishmania) amazonensis, and Leishmania (Viannia) guyanensis [12]. The predominance of species varies considerably among different regions of Brazil, demonstrating a clear relationship between geographic location and the type of parasite present [13]. The states located in the Legal Amazon have a high rate of leishmaniasis cases, with Leishmania (Leishmania) amazonensis and Leishmania (Viannia) guyanensis being the most recurrent in these areas [14]. In contrast, Leishmania (Viannia) braziliensis is the most widely distributed species, influencing the occurrence of cases in all parts of the country, which characterizes it as the predominant species in terms of total incidence [15]. Brazil is also endemic for visceral leishmaniasis and for the species Leishmania infantum, which can also be responsible for skin lesions, as recently demonstrated in some studies [16].
The classification of areas with different endemic levels of cutaneous leishmaniasis in tropical regions such as Brazil can be considered an essential measure to facilitate the strategic planning of health services and the more efficient use of available resources, such as the distribution of medicines and supplies, sending vector control teams and the intensification of epidemiological and entomological surveillance in priority regions [17]. Areas classified as high risk can be prioritized for awareness and health education campaigns, alerting the population about forms of prevention and encouraging the early seeking of treatment [18].
Although scarce, statistical studies aimed at cutaneous leishmaniasis modeling are essential to estimate the coefficients that represent the relationship between the occurrence of the disease and the multiple groups of intervening factors such as environmental, demographic, socioeconomic, and behavioral agents of its causality [19]. These coefficients can indicate the magnitude and direction of the impact of each factor on the variable of interest, contributing to the understanding of the determinants of ACL and to the formulation of more effective control policies for the contingency of this neglected disease [20].
Thus, the present research aims to estimate the prevalence of ACL in Brazil and classify the states according to the different levels of endemicity, considering the time range from 2014 to 2024, making it possible to identify specific patterns of association between endemicity and the country’s sociodemographic characteristics.
2. Materials and Methods
2.1. Study Design
This is a retrospective and cross-sectional study with a quantitative approach. All confirmed cases of ACL that occurred in Brazil and were recorded by the Information System for Notifiable Diseases of the Ministry of Health between 2014 and 2024 were included. Imported ACL cases were excluded from all analyses performed in this study.
2.2. Study Location
Brazil has a population of 203,062,512 inhabitants and an area of 8,510,417.771 km2. It is divided into 5 macroregions: North, South, Central-West, Southeast, and Northeast. Its territory is divided into 26 states and the Federal District. In total, it has 5568 municipalities [21]. Its climate is predominantly hot and humid, divided into tropical and its subdivisions, Subtropical, Semiarid, and Equatorial. The vegetation is originally formed by the Amazon Rainforest, Atlantic Forest, Pine Forest, Cerrado, Caatinga, Fields, Pantanal Complex, and Coastal Vegetation [22].
The main environmental factors that influence the distribution of ACL vectors include temperature, humidity, rainfall forecast, presence of forests and dense vegetation, proximity of the urban environment to natural areas, availability of reservoirs such as dogs, foxes and marsupials, and deforestation and environmental changes caused by humans, such as the use of irrigation and inadequate waste management [23].
2.3. Data Source
The data were obtained from the Brazilian Ministry of Health database. The data source used was based on the ACL investigation forms used throughout the country. This form consists of 63 variables distributed in fields such as identification, data on the disease, test results, treatment, and case progression.
The Notifiable Diseases Information System is a fundamental tool for the surveillance of cutaneous leishmaniasis, as it allows the registration, monitoring, and analysis of all suspected and confirmed cases, providing data for the calculation of epidemiological indicators, the formulation and evaluation of health policies, and the making of timely decisions for the planning and implementation of disease prevention and control measures [24].
2.4. Variables Used
The sociodemographic variables included in this study were as follows:
- (a)
- Municipality of notification;
- (b)
- State of notification;
- (c)
- Sex (male/female);
- (d)
- Ethnicity (brown/white/black/indigenous/Asian/ignored);
- (e)
- Level of education (illiterate, incomplete 1st to 4th grade of elementary school, complete 4th grade of elementary school, incomplete 5th to 8th grade of elementary school, complete elementary school, incomplete high school, complete high school, incomplete higher education, complete higher education, not applicable, ignored);
- (f)
- Age group (under 1 year, 1 to 4 years, 5 to 9 years, 10 to 14 years, 15 to 19 years, 20 to 39 years, 40 to 59 years, 60 to 64 years, and over 65 years);
- (g)
- Gestational condition (yes, no, ignored).
The clinical-epidemiological variables included in this study were as follows:
- (a)
- Month of notification (January, February, March, April, May, June, July, August, September, October, November, December);
- (b)
- Year of notification (2014, 2015, 2016, 2017, 2018, 2019, 2020, 2021, 2022, 2023, 2024);
- (c)
- Confirmation criterion (laboratory, clinical-epidemiological);
- (d)
- Clinical form (cutaneous, mucosal).
2.5. Procedures Used
The first step consisted of building the database provided by TabNet/DATASUS, covering ACL cases from 2014 to 2024. The prevalence coefficient of confirmed cases of ACL was calculated from the number of cases multiplied by 100,000 and divided by the population of each state or municipality of interest for the study. The estimate of the resident population for each year was based on the 2022 IBGE Census (Brazilian Institute of Geography and Statistics) [25]. The Brazilian states and the Federal District were classified into different patterns of disease endemicity: low (prevalence between 1.4 and 47.2), moderate (prevalence between 47.3 and 129.2), low intensity (prevalence between 129.3 and 391.8), moderate intensity (391.9 and 672.1), and high intensity (672.2 and 1261.1), according to the natural breaks method, recommended by the Brazilian Ministry of Health [26]. The QGIS 3.40 program (Quantum Geographic Information System) was used to build the map. The results were then spatialized in a Geographic Information System environment. The first stage of mapping consisted of downloading Brazilian state boundaries provided by the IBGE, based on cartographic data from 2024. The prevalence values were entered into the attribute table for each federative unit and subsequently classified according to the methodology described above.
In addition, the 20 municipalities with the highest number of cases in the country were listed, with their respective numbers of cases, populations, territorial areas, demographic densities, and prevalence rates. We proceeded to create a control diagram by calculating the mean, upper, and lower limits, and standard deviation with a 95% confidence interval on the number of ACL cases distributed by months of the year in the period evaluated to compose the epidemiological analysis.
To classify the areas with different levels of endemicity, we followed the logic of calculating the ACL prevalence coefficient for the states and the Federal District described above, with the levels of high intense endemicity, moderate intense endemicity, and low intense endemicity being unified in the same category (high endemicity) and the others in moderate endemicity and low endemicity levels. This classification was necessary to analyze the relationship between the frequency of cases and different groups of variables (sex, ethnicity, age, level of education, gestational condition, diagnostic criteria, disease classification). The multivariate approach allowed us to identify which factors had a significant impact on the variation in endemicity, providing important insights into the patterns observed in the data.
2.6. Data Analysis
The results were generalized descriptively, using the first table as absolute values and percentages; figures representing a map showing different levels of endemicity by State; and a control diagram showing the average monthly frequency of ACL cases during the period evaluated. The second table presents the data according to the number of cases, population, territorial area, population density, and prevalence of ACL in the 20 municipalities that reported the most cases during the period. In the third table, the data are distributed according to cross-variable categories with different levels of endemicity (low, medium, and high prevalence), indicating the p-values obtained.
We collected process data, calculated the mean and standard deviation, and then determined the upper and lower control limits (UCL and LCL) that represent the expected process behavior. Confidence intervals for these limits were calculated using the sample mean, a critical value (z or t) based on the 95% confidence level, and the sample standard error (sample standard deviation divided by the square root of the sample size).
The data were exported and analyzed using statistical software R (2016), version 4.5.1. ANOVA analysis of variance was used to assess the overall significance of the variables in the model. The statistical test compared the variability explained by the independent variables with the residual variability, verifying whether the inclusion of these variables significantly improves the model’s ability to predict the dependent variable. p-value < 0.05 was considered significant for the response variable.
Linear regression was used to analyze the relationship between the frequency of cases and several explanatory variables, such as sex, gestational status, ethnicity, level of education, diagnostic criteria, disease classification, and age group. The multivariate approach allowed us to identify which factors have a significant impact on the variation in endemicity, providing important insights into the patterns observed in the data.
Multivariate linear regression was used to model the relationship between a dependent variable (level of ACL endemicity) and multiple independent variables (sex, gestational condition, ethnicity, education, diagnostic criteria, disease classification, and age group). Unlike simple regression, where there is only one explanatory variable, the multivariate approach allowed for the simultaneous consideration of multiple factors, providing a more detailed and accurate analysis. The model assumed a linear relationship between variables, meaning each independent variable influences the dependent variable proportionally. Furthermore, it estimated coefficients for each factor, indicating the magnitude and direction of each factor’s impact on the variable of interest.
2.7. Ethical Considerations
Access to the database, corrected and registered by the Ministry of Health, was made possible by the Access to Information Law (No. 12,527/2011). This is a federal law that guarantees any citizen the right to request and receive information from public agencies without needing to justify the request. This law establishes the disclosure of information of public interest, providing access to information not protected by law.
This study was exempted from review by a Research Ethics Committee, as determined by National Health Council Resolution No. 510 of 7 April 2016.
3. Results
Our results show that most confirmed cases of ACL that occurred in Brazil during the period evaluated were male (73.2%), of brown ethnicity (65.0%), aged between 20 and 39 years (41.5%), with 44.4% of the cases having not completed elementary school, and 0.5% of the women reporting being pregnant. Regarding the diagnostic confirmation criteria of the cases, 80.0% were attributed to laboratory tests, compared with 20.0% that were attributed to clinical-epidemiological diagnosis. Regarding the clinical form, most of the sample (94.0%) were classified as cutaneous, compared with 6.0% that were classified as mucosal (Table 1).

Table 1.
Sociodemographic, epidemiological, and clinical profile of cases of ACL occurring in Brazil, 2014–2024 (N = 182,072 *).
Of the total number of individuals accounted for by sex (182,667), 64 imported cases were excluded; of the total number of individuals accounted for by ethnicity (182,489), 242 imported cases were excluded; of the total number of individuals accounted for by age range, 14,837 were excluded; of the total number of individuals accounted for by education, 242 were excluded; of the total number of women accounted for who could be pregnant, 2244 were excluded; of the total number of individuals counted by confirmation criteria, 242 were excluded; and of the total number of individuals counted by clinical criteria, 320 were excluded.
Between 2014 and 2024, 182,072 confirmed cases of ACL were recorded in Brazil, corresponding to a prevalence of 89.6 cases per 100,000 inhabitants (excluding imported cases during the period). The states of Acre and Amapá had the highest prevalence of the disease, with simultaneous values of 1261.1 and 1010.5 (comprising the high intense endemicity level). Roraima (672.1), Rondônia (636.9), and Mato Grosso (567.6) were part of the medium intense endemicity level. Pará (391.8), Amazonas (390.5), Tocantins (289.0), and Maranhão (222.0) represented the low intense endemicity level. In relation to the group of states represented by the moderate level of endemicity are Bahia (129.2), Minas Gerais (79.7), Ceará (68.0), Goiás (67.5), and the Federal District (19.3). Finally, representing the states with a low level of endemicity are Mato Grosso do Sul (47.2), Espírito Santo (37.1), Pernambuco (32.9), Piauí (25.6), Paraná (25.1), Alagoas (19.7), Paraíba (17.0), São Paulo (8.0), Rio Grande do Norte (4.7), Santa Catarina (4.1), Rio de Janeiro (3.5), Sergipe (3.2), and Rio Grande do Sul (1.4) (Figure 1).
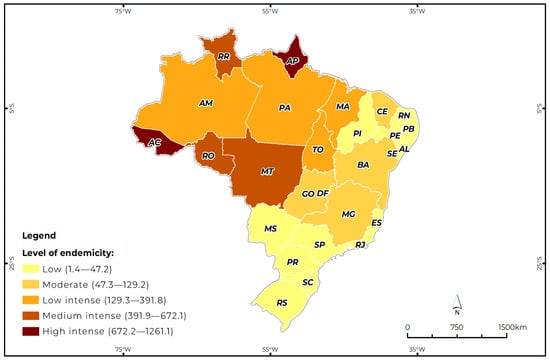
Figure 1.
Pattern of ACL endemicity in Brazil by state, according to the prevalence rate per 100,000 inhabitants, (between 2014 and 2024). AC: Acre, AL: Alagoas, AP: Amapá, AM: Amazonas, BA: Bahia, CE: Ceará, DF: Distrito Federal, ES: Espírito Santo, GO: Goiás, MA: Maranhão, MG: Minas Gerais, MS: Mato Grosso do Sul, MT: Mato Grosso, PA: Pará, PB: Paraíba, PE: Pernambuco, PI: Piauí, PR: Paraná, RJ: Rio de Janeiro, RN: Rio Grande do Norte, RS: Rio Grande do Sul, RO: Rondônia, RR: Roraima, SC: Santa Catarina, SE: Sergipe, SP: São Paulo, TO: Tocantins.
The results in Table 2 show that among the listed state capitals, Manaus had the highest number of reported cases (5619), reaching a prevalence of 272.2 cases per 100,000 inhabitants. On the other hand, the capital Belo Horizonte had 1674 reported cases, totaling a prevalence of 72.2 cases per 100,000 inhabitants. Regarding the listed municipalities, Presidente Figueiredo-AM had the highest prevalence of ACL with 5504.1 cases per 100,000 inhabitants, followed by the municipality of Rio Preto da Eva-AM, which reached a prevalence of 5197.3 cases per 100,000 inhabitants. The municipality of Belo Horizonte-MG, with 1674 notifications of the disease, had the lowest prevalence with approximately 72.2 cases per 100,000 inhabitants (Table 2).

Table 2.
List of Brazilian municipalities with the highest number of notifications of ACL cases, between 2014 and 2024.
Regarding the monthly average of ACL cases in Brazil, in the period evaluated, the month with the highest average of cases was January with 1731 (with a standard deviation of 364; upper limit of 1933; lower limit of 1572), while in relation to the month with the lowest number of cases, the month of June stands out with 1141 (with a standard deviation of 233; upper limit of 1256; lower limit of 1026) (Figure 2).
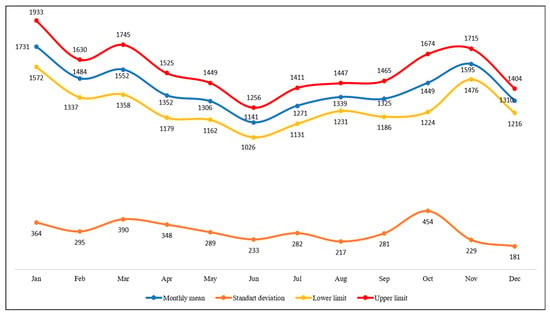
Figure 2.
Control diagram according to the average monthly frequency of ACL cases in Brazil, between 2014 and 2024.
The ANOVA results presented in Table 3 for low endemicity indicate that all variables analyzed have a significant impact on the frequency of cases. The classification of the form of the disease presents a very high F-value (90.4774) and an extremely low p-value, showing that the clinical forms strongly influence the distribution of cases. The variables age, education, and race present highly significant effects. At the level of medium endemicity, the variable clinical form, which has an F-value of 185.0971, indicates an extremely strong impact on the frequency of cases. The race variable continues to be a relevant factor, with an even greater effect than in the low endemicity group. The diagnostic criteria and age group continue to be significant factors, suggesting that age is a determining factor in the distribution of the disease. In the high endemicity group, some variables lose significance, such as pregnant women, whose p-value indicates that pregnancy has no significant impact on the frequency of cases. However, the confirmation criterion and clinical form remain highly significant, with very high F values, showing that clinical classification and diagnostic methods are essential to understanding the distribution of cases. Race remains relevant, with a significant impact on the distribution of cases, while education has a smaller effect compared with previous levels, possibly reflecting a more homogeneous pattern in regions of greater endemicity.

Table 3.
Analysis of groups of variables distributed at different levels of ACL endemicity.
4. Discussion
The highest prevalence of ACL in this sample occurred in male individuals, with the same fact being observed in other studies [27,28] except for a survey conducted in Israel, which found that among the 608 individuals screened, 51% were women from regions endemic for Leishmania major and 59% were women from regions endemic for Leishmania tropica [29]. Differences between the sexes in the incidence and severity of infection may be related to physiological or genetic constitutions [30] but may also result from differences in exposure, use of preventive strategies [31], participation in high-risk activities, attractiveness to vectors, routes of pathogen entry or pathogen processing, and cellular responses. A recent study on sex-related differences in leishmaniasis showed that environmental exposure and access to healthcare [32] alone do not explain the variance.
The fact that most ACL cases occur in brown individuals is not preferentially related to this ethnic group. This is mainly due to the predominance of this ethnicity in the Brazilian population, where 92.1 million Brazilians declared themselves brown, making up a total of 45.3% of the population [25], causing most cases to be concentrated in this ethnic group. However, the prevalence of the disease in brown ethnic groups can be explained by the greater presence of these groups in areas where the disease is more common, or by interaction with wild animals or lifestyle practices that increase the risk of contact with vectors. Our findings were also corroborated by research carried out in the northern regions [33] and northeastern Brazil [34], where cases were more prevalent in this ethnic group.
The cases of cutaneous leishmaniasis in this study were more prevalent in young adults, corroborating a study carried out in the state of Pará [11]. The age group most affected by ACL in the world, and in Brazil, has been young adults, between 20 and 39 years old. In addition, it is observed that the disease also affects, to a lesser extent, children under 10 years old and the elderly, with transmission possibly related to peri- and intra-household transmission [11].
The present sample presented a low level of education, indicating a high socioeconomic vulnerability that intensifies the risk factor for the development of this disease. Low education levels make the population unaware of the mechanisms of transmission of the disease, as well as ways to prevent it. Therefore, the theme of health education should be expanded in the basic education curriculum, especially in rural and remote areas of the country. This fact has been observed in several Brazilian municipalities, considering their socioeconomic and environmental characteristics [35].
As the highest concentration of the vector in these areas of greatest transmission depends greatly on the presence of forest formations, favorable temperatures, and humid environments typical of the states that make up the Legal Amazon and the Atlantic Forest, which favor the biological cycle of sandflies, added to the greater presence of males of working age and with low levels of education working in these regions, the probability of transmission of ACL is greatly amplified in the population group that meets these sociodemographic characteristics [36].
Exacerbation of ACL lesions during pregnancy has been reported mainly in the New World and rarely in the Old World, although maternal and child health challenges, disease severity, and manifestations of signs and symptoms have not been fully explored [37]. In the case of pregnant patients, treatment at this stage appears to be the greatest challenge, and the possibility of postponing the start of treatment until the postpartum period should be evaluated. If immediate treatment is necessary, it should be performed at a referral center, and the drug of choice is liposomal amphotericin B [26].
Most cases in Brazil are laboratory-confirmed because it is a fundamental resource for accurate diagnosis, especially in cases with atypical clinical manifestations or in immunocompromised patients [38]. Confirmation of cutaneous leishmaniasis in Brazil has been based on clinical, epidemiological, and laboratory criteria, including identification of the parasite in patient samples or positive serological tests, in addition to association with endemic areas and history of contact with the disease [26].
ACL manifests mainly in cutaneous and mucosal forms, with cutaneous being the most frequent. In addition to these, rarer forms such as diffuse cutaneous and disseminated cutaneous may occur. The cutaneous form is the most common presentation of leishmaniasis, accounting for more than 90% of cases in Brazil. Of the reported cases, 3% to 6% present mucosal leishmaniasis; however, in some endemic municipalities, this proportion may be higher than 25%. This clinical form is mainly caused by Leishmania (Viannia) braziliensis. Diffuse cutaneous leishmaniasis is rare and its known causative agent is Leishmania (Leishmania) amazonensis [39].
Areas of Acre and Amapá considered to be highly endemic are identified mainly in regions of agricultural settlement, plant extraction, and mining. Most individuals have multiple primary lesions, suggesting multiple bites from the vector [40]. In areas described as moderately endemic, such as Roraima, Rondônia, and Mato Grosso, the greatest exposure of the population occurs in places where natural transmission of the infection occurs, where extensive forests and mild temperatures favor the reproduction of the vector, which consequently increases the number of cases [36]. In areas of low intense endemicity, such as Pará, Amazonas, Tocantins, and Maranhão, although these have the highest number of cases, proportionally, they have a lower prevalence compared with the other states due to the greater population concentration, presence of forests, and, on the other hand, due to the advance of deforestation [41].
Regarding the area represented by the region of moderate endemicity of the disease, the most prevalent states are Bahia, Ceará, and Minas Gerais, states that are strongly influenced by tropical and semiarid climates [42]. In the semiarid region, annual precipitation never exceeds 24 mm and the dry season lasts from April to October. In the semiarid region of the Northeast, where most ACL records are reported, the dry and rainy seasons are clearly defined and there is evidence that vector density is low during the dry season and increases after the end of the rainy season (December to April), reaching its highest density level around May [42]. These observations corroborate reports of peaks in leishmaniasis cases in the months of January, February, and March, when an increase in vector density favors transmission in this region [42].
Among the states that make up the region of low endemicity are states in the Central-West, Northeast, and South regions, with Mato Grosso do Sul standing out, with the highest prevalence, and Rio Grande do Sul with the lowest prevalence [43]. Despite the different epidemiological patterns, these states share a high rate of deforestation of their forest reserves and maintain a high concentration of people in rural areas with field work related to hunting, fishing, and cattle raising, causing them to have practically the same modes of transmission, since there is a closer relationship between humans when they approach the natural habitat of animals (monkeys, primates, and canines), in addition to facilitating the territorial dissemination of ACL between the vector of the pathology and humans in these regions [43].
Of the cities listed with the highest number of ACL cases, six are state capitals and another 11 are small, medium, and large inland cities located in the Legal Amazon region, considered an epicenter of ACL transmission. Due to the greater proximity of this population to the forest, transmission of the disease is generally more frequent due to the greater presence of vectors and animal reservoirs in this region [41]. The relationship between population density and disease transmission is quite complex, because even in rural regions with low population density, the prevalence of ACL can be more intense, due to the association of environmental factors such as vegetation cover, crop areas, pastures, and agricultural and pastoral activities, which certainly increase the risk of transmission of the disease to susceptible people in this region [44].
In areas where leishmaniasis is endemic, such as in practically all of Brazil, the occurrence of cases may be more constant throughout the year, with peaks in certain months, but with cases being recorded in practically every month. Cutaneous leishmaniasis does not present a fixed monthly distribution of cases but rather a seasonal pattern influenced by the activity of sand flies and climatic factors [45]. The peak of transmission usually occurs in periods of higher temperature and humidity, such as summer and early autumn. In many regions, the period of greatest transmission coincides with summer and early autumn, when climatic conditions favor the proliferation of sand flies. High temperatures and high humidity are conducive to the proliferation of the vector [46]. Heavy rainfall can also contribute to the dispersion of sand flies, increasing the risk of transmission. Phlebotomine sandflies, which transmit leishmaniasis, are more active at night and at times of lower light, which can influence the occurrence of cases in certain periods [47].
Quantitative studies with different groups of sociodemographic variables focused on the social determinants of a tropical disease such as cutaneous leishmaniasis can reveal the factors that contribute to the greater vulnerability, exposure, and susceptibility of populations. Research of this type is essential to understanding the distribution, risk, and impact of the disease, seeking to understand how these variables contribute to maintaining the endemicity of the disease [48]. In addition, ACL is a disease that requires a broad approach that goes beyond biological aspects and includes social and demographic dimensions. A study carried out among students in the Delanta district, northeastern Ethiopia, proved that sociodemographic and environmental factors are determinants of ACL [49], corroborating our thesis.
The study of models on confirmation criteria, clinical classification, and gestational condition in patients with ACL is essential to avoid misdiagnosis and unnecessary treatments. Its diagnosis usually includes clinical criteria associated with laboratory methods, such as direct parasitological examination, culture, polymerase chain reaction, and immunological tests [50]. The standardization of these criteria allows greater sensitivity and specificity, ensuring a more effective approach, especially in endemic areas where other dermatoses can mimic leishmaniasis. In endemic regions, with a high burden of the disease and limited resources, the appropriate approach to this nosology guides safe clinical care, protecting vulnerable groups such as pregnant women, strengthening leishmaniasis surveillance, and contributing greatly to equity in access to standardized and evidence-based diagnostics and treatments [51].
As a limitation of this study, we observed considerable incompleteness in the ACL notification records in the Ministry of Health’s Notifiable Diseases Information System. Although we consider complete data recording important, this lack did not compromise our analyses because it did not affect our variables of interest and did not limit our ability to answer the research questions. Therefore, the reported observation was reported to the management group, as well as the importance of implementing more comprehensive strategies to improve the quality of information collection to support more efficient surveillance and responses to ACL in this group.
5. Conclusions and Recommendations
Despite the limitations inherent in the use of secondary data, our study made it possible to understand the geographic and temporal distribution of ACL cases in this representative sample associated with their sociodemographic characteristics, allowing us to contribute to the more effective targeting of public policies, epidemiological surveillance strategies, and control interventions to mitigate the spread of this zoonosis.
Future studies with spatial analyses stratified by sociodemographic profile and improvements in the quality of system data are necessary to overcome existing gaps, since regional disparities suggest that the transmission dynamics of this zoonosis are influenced by specific local contexts, which are not always captured by conventional epidemiological studies.
Author Contributions
Conceptualization, D.S.T.J., H.T.d.S., B.O.S.e.S. and A.M.R.A.; methodology, D.S.T.J. and H.K.C.T.; software, A.M.R., A.M.R.A. and B.M.G.; validation, H.T.d.S. and B.O.S.e.S.; formal analysis, E.M.d.A.; investigation, D.S.T.J.; resources, B.M.G.; data curation, H.K.C.T.; writing—original draft preparation, D.S.T.J., H.T.d.S. and B.O.S.e.S.; writing—review and editing, A.M.R.A. and M.B.L.N.; visualization, A.M.R. and A.M.R.A.; supervision, E.M.d.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the Research Ethics Committee, as determined by National Health Council Resolution No. 510 of 7 April 2016.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rangel, E.F.; Lainson, R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: Aspects of their biology and vectorial competence. Mem. Inst. Oswaldo Cruz 2009, 104, 937–954. [Google Scholar] [CrossRef]
- Anversa, L.; Tiburcio, M.G.S.; Richini-Pereira, V.B.; Ramirez, L.E. Human leishmaniasis in Brazil: A general review. Rev. Assoc. Med. Bras. 2018, 64, 281–289. [Google Scholar] [CrossRef]
- Silva, A.F.; Latorre, M.R.D.O.; Galati, E.A.B. Fatores relacionados à ocorrência de leishmaniose tegumentar no Vale do Ribeira. Rev. Soc. Bras. Med. Trop. 2010, 43, 46–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Hotez, P.J.; Woc-Colburn, L.; Bottazzi, M.E. Neglected tropical diseases in Central America and Panama: Review of their prevalence, populations at risk and impact on regional development. Int. J. Parasitol. 2014, 44, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Chan, J.V.; Valenzuela, J.; Dumonteil, E. Leishmaniasis in the Americas. In Neglected Tropical Diseases—Latin America and the Caribbean; Franco-Paredes, C., Santos-Preciado, J., Eds.; Neglected Tropical Diseases; Springer: Vienna, Austria, 2015. [Google Scholar] [CrossRef]
- Marcos-Marcos, J.; Olry de Labry-Lima, A.; Toro-Cardenas, S.; Lacasaña, M.; Degroote, S.; Ridde, V.; Bermudez-Tamayo, C. Impact, economic evaluation, and sustainability of integrated vector management in urban settings to prevent vector-borne diseases: A scoping review. Infect. Dis. Poverty 2018, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Claborn, D.M. The biology and control of leishmaniasis vectors. J. Glob. Infect. Dis. 2010, 2, 127–134. [Google Scholar] [CrossRef]
- Bamorovat, M.; Sharifi, I.; Agha Kuchak Afshari, S.; Ghasemi Nejad Almani, P. Mutual Role of Patients and the Healthcare System in the Control of Cutaneous Leishmaniasis. Transbound. Emerg. Dis. 2023, 2023, 7814940. [Google Scholar] [CrossRef]
- Wermelinger, E.D. Reflections on vector control in Brazil. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190385. [Google Scholar] [CrossRef]
- Abraão, L.S.O.; José, B.M.P.A.; Gomes, C.B.S.; Nunes, P.C.S.; dos Santos, D.R.; Varela, A.P.A.S.; Lima, C.S. Perfil epidemiológico dos casos de leishmaniose tegumentar americana no estado do Pará, Brasil, entre 2008 e 2017. Rev. Pan-Amaz. Saúde 2020, 11, e202000612. [Google Scholar] [CrossRef]
- Gonçalves, L.P.; Santos, T.V.d.; Campos, M.B.; Lima, L.V.d.R.; Ishikawa, E.A.Y.; Silveira, F.T.; Ramos, P.K.S. Further insights into the eco-epidemiology of American cutaneous leishmaniasis in the Belem metropolitan region, Pará State, Brazil. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200255. [Google Scholar] [CrossRef]
- Gontijo, B.; Carvalho, M.d.L.R.d. Leishmaniose tegumentar americana. Rev. Soc. Bras. Med. Trop. 2003, 36, 71–80. [Google Scholar] [CrossRef]
- Almeida, A.P.; Paulo, P.F.M.; Pereira Júnior, A.M.; Gujanwski, C.A.; Ferreira, V.; Costa, G.D.S.; Rodrigues, M.M.S.; Ferreira, R.G.M.; Medeiros, J.F. Occurrence of Leishmania infection in the immediate geographic region of Ji-Paraná, Rondônia State, Brazil. Rev. Soc. Bras. Med. Trop. 2021, 54, e02122021. [Google Scholar] [CrossRef]
- Santos, G.; Kückelhaus, S.; Roselino, A.; Chaer, W.; Sampaio, R. Leishmania (Viannia) braziliensis is the main species causing cutaneous leishmaniasis in the Federal District of Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 340–343. [Google Scholar] [CrossRef][Green Version]
- Ferreira Ede, C.; Cruz, I.; Cañavate, C.; de Melo, L.A.; Pereira, A.A.; Madeira, F.A.; Valério, S.A.; Cunha, H.M.; Paglia, A.P.; Gontijo, C.M. Mixed infection of Leishmania infantum and Leishmania braziliensis in rodents from endemic urban area of the New World. BMC Vet. Res. 2015, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Cosma, C.; Maia, C.; Khan, N.; Infantino, M.; Del Riccio, M. Leishmaniasis in Humans and Animals: A One Health Approach for Surveillance, Prevention and Control in a Changing World. Trop. Med. Infect. Dis. 2024, 9, 258. [Google Scholar] [CrossRef]
- Cardoso, D.T.; de Souza, D.C.; de Castro, V.N.; Geiger, S.M.; Barbosa, D.S. Identification of priority areas for surveillance of cutaneous leishmaniasis using spatial analysis approaches in Southeastern Brazil. BMC Infect. Dis. 2019, 19, 318. [Google Scholar] [CrossRef]
- Reis, E.S.D.; Paz, W.S.; Santos Ramos, R.E.; Nunes Ribeiro, C.J.; Biano, L.S.; Bezerra-Santos, M.; de Oliveira, C.I.; Lipscomb, M.W.; de Moura, T.R. Spatial and temporal modeling of the global burden of Cutaneous Leishmaniasis in Brazil: A 21-year ecological study. PLoS Negl. Trop. Dis. 2024, 18, e0012668. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.M.T.; de Araújo, V.E.M.; Barbosa, D.S.; Martins-Melo, F.R.; Werneck, G.L.; Carneiro, M. Burden of leishmaniasis in Brazil and federated units, 1990-2016: Findings from Global Burden of Disease Study 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006697. [Google Scholar] [CrossRef]
- Teresinha Schröder, N.; Fraga Da Silveira, E.; Thomasi Janhke Botton, L.; Périco, E. Neglected diseases in Brazil: Space-temporal trends and public policies. In Neglected Tropical Diseases—Unsolved Debts for the One Health Approach; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Tomasella, J.; do Amaral Cunha, A.M.; Zeri, M.; Costa, L.C.O. Changes in the aridity index across Brazilian biomes. Sci. Total Environ. 2025, 989, 179869. [Google Scholar] [CrossRef]
- Pinheiro, M.P.G.; Silva-Inacio, C.L.; Silva, M.M.M.; Araújo, P.S.F.; Ximenes, M.F.F.M. Potential vectors of Leishmania spp. in an Atlantic Forest conservation unit in northeastern Brazil under anthropic pressure. Parasites Vectors 2021, 14, 38. [Google Scholar] [CrossRef]
- Sousa Júnior, A.S.; Gonçalves, N.V.; Miranda, C.S.C.; Santos, B.O.; de Oliveira, R.A.C.; da Costa, R.J.F.; Noguchi, S.K.d.T.; Oliveira, J.S.d.S.; Matsumura, E.S.S.; Palácios, V.R.d.C.M. Cutaneous leishmaniasis spatial distribution and epidemiological and environmental risk factors in Cametá, state of Pará, Brazil. Braz. J. Infect. Dis. 2020, 24, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Cidades e Estados, Censo. 2022. Available online: https://www.ibge.gov.br/cidades-e-estados (accessed on 1 May 2025).
- Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde. Manual de Vigilância da Leishmaniose Tegumentar Americana; Ministério da Saúde: Brasília, Brazil, 2017. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar.pdf (accessed on 2 May 2025).
- Brilhante, A.F.; Melchior, L.A.K.; Nunes, V.L.B.; Cardoso, C.O.; Galati, E.A.B. Epidemiological aspects of American cutaneous leishmaniasis (ACL) in an endemic area of forest extractivist culture in western Brazilian Amazonia. Rev. Inst. Med. Trop. São Paulo 2017, 59, e12. [Google Scholar] [CrossRef]
- Medina-Morales, D.A.; Machado-Duque, M.E.; Machado-Alba, J.E. Epidemiology of Cutaneous Leishmaniasis in a Colombian Municipality. Am. J. Trop. Med. Hyg. 2017, 97, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Fuchs, I.; Glazer, Y.; Schwartz, E. Gender and Cutaneous Leishmaniasis in Israel. Trop. Med. Infect. Dis. 2022, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Stark, K.; Schneider, T.; Schoneberg, I. Diferenças sexuais na leptospirose clínica na Alemanha: 1997–2005. Clin. Infect. Dis. 2007, 44, e69–e72. [Google Scholar] [CrossRef]
- Schlagenhauf, P.; Chen, L.H.; Wilson, M.E.; Freedman, D.O.; Tcheng, D.; Schwartz, E.; Pandey, P.; Weber, R.; Nadal, D.; Berger, C.; et al. Sex and gender differences in travel-associated disease. Clin. Infect. Dis. 2010, 50, 826–832. [Google Scholar] [CrossRef]
- Lockard, R.D.; Wilson, M.E.; Rodríguez, N.E. Sex-related differences in the immune response symptomatic manifestations to infection with Leishmania species. J. Immunol. Res. 2019, 2019, 4103819. [Google Scholar] [CrossRef]
- Veiga Gonçalves, N.; Miranda, C.D.S.C.; Costa, R.J.F.D.; Guedes, J.A.; Matsumura, E.S.S.; Costa, S.B.N.D.; Noguchi, S.K.D.T.; Guimarães, L.H.R.; Coelho de Oliveira, R.A.; Simone Alves Tavares, L.; et al. Cutaneous leishmaniasis: Spatial distribution and environmental risk factors in the state of Pará, Brazilian Eastern Amazon. J. Infect. Dev. Ctries. 2019, 13, 939–944. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Pimentel, K.B.A.; Moura, M.L.; Aragão, C.F.; Guimarães-e-Silva, A.S.; Bezerra, J.M.T.; Melo, M.N.; Pinheiro, V.C.S. Clinical, epidemiological and climatic factors related to the occurrence of cutaneous leishmaniasis in an endemic area in northeastern Brazil. Braz. J. Biol. 2021, 81, 557–565. [Google Scholar] [CrossRef]
- Gonçalves, N.V.; Alcântara, R.C.C.; Sousa, A.S.; Pereira, A.L.R.R.; Miranda, C.S.C.; Oliveira, J.S.S.; Melo, A.C.B.V.; Guedes, J.A.; Costa, R.J.F.; Costa, S.B.N.; et al. Leprosy in an administrative district of Belém, Pará State, Brazil: Relations between territory, socioeconomics, and public health policy, 2007–2013. Rev. Pan-Amaz. Saúde 2018, 9, 2176–6223. [Google Scholar] [CrossRef]
- Lima, C.C.M.; Grisotti, M.; Santos, F.S. Os desafios no controle das leishmanioses no contexto da cidade de Montes Claros (MG). Rev. Unimontes Científica 2017, 18, 131–147. [Google Scholar]
- Morgan, D.J.; Guimaraes, L.H.; Machado, P.R.; D’OLiveira, A., Jr.; Almeida, R.P.; Lago, E.L.; Faria, D.R.; Tafuri, W.L.; Dutra, W.O.; Carvalho, E.M. Cutaneous leishmaniasis during pregnancy: Exuberant lesions and potential fetal complications. Clin. Infect. Dis. 2007, 45, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Santa Catarina, Secretaria Estadual de Saúde, Superintendência de Vigilância em Saúde. Guia de Orientação da Vigilância da Leishmaniose Tegumentar; Superintendência de Vigilância em Saúde: Florianópolis, Brazil, 2021. [Google Scholar]
- Vasconcelos, J.M.; Gomes, C.G.; Sousa, A.; Teixeira, A.B.; Lima, J.M. Leishmaniose tegumentar americana: Perfil epidemiológico, diagnóstico e tratamento. Rev. Bras. Análises Clínicas 2018, 50, 221–227. [Google Scholar]
- Almeida ANFde Nascimento Lde CSdo Sousa ESMde, M.; de Oliveira, A.J.D.; de Sena, M.G.; de Resende, B.M.; Chaves, R.C.G.; Garcez, L.M. Vigilância da leishmaniose cutânea em amostras clínicas: Distribuição da Leishmania guyanensis no estado do Amapá, 2018. Epidemiol. Serv. Saúde 2020, 29, e2018504. [Google Scholar] [CrossRef]
- Santos, M.F.D.; Lorenz, C.; Chiaravalotti-Neto, F.; Lima-Camara, T.N. Spatial analysis of American cutaneous leishmaniasis in the state of Amazonas. Rev. Saude Publica 2024, 58, 11. [Google Scholar] [CrossRef]
- Rodgers, M.S.M.; Bavia, M.E.; Eichold, B.; Shipman, C.; Owen, N.; Winstanley, H.; Gordon, M.; Karapetyan, M.; Silva, M.M.N.; Carneiro, D.D.M.T.; et al. Environmental risk factors of leishmaniasis in Bahia State, Brazil using NASA Earth observation satellites. Rev. Inst. Adolfo Lutz 2018, 77, e1775. [Google Scholar] [CrossRef]
- Yarzon RMde, G.B.; Dorval, M.E.C.; Freitas HGde Oshiro, E.T. Leishmaniose Tegumentar Americana (LTA) em Mato Grosso do Sul. Rev. Soc. Bras. Med. Trop. 2003, 36, 41–42. [Google Scholar] [CrossRef]
- Temponi, A.O.D.; de Brito, M.G.; Ferraz, M.L.; Diniz, S.d.A.; Silva, M.X.; da Cunha, T.N. Ocorrência de casos de leishmaniose tegumentar americana: Uma análise multivariada dos circuitos espaciais de produção, Minas Gerais, Brasil, 2007 a 2011. Cad Saúde Pública 2018, 34, e00165716. [Google Scholar] [CrossRef]
- Lana, R.S.; Michalsky, E.M.; Fortes-Dias, C.L.; França-Silva, J.C.; Lara-Silva, F.O.; Lima, A.C.V.M.R.; de Avelar, D.M.; Martins, J.C.D.; Dias, E.S. Phlebotomine sand fly fauna and Leishmania infection in the vicinity of the Serra do Cipó National Park, a natural Brazilian heritage site. BioMed Res. Int. 2015, 2015, 385493. [Google Scholar] [CrossRef]
- Osmari, V.; Fernandes, F.D.; Tatto, M.; Souza, G.D.; Ratzlaff, F.R.; Vasconcellos, J.S.d.P.; Botton, S.d.A.; Machado, D.W.N.; Vogel, F.S.F.; Sangioni, L.A. Fauna and seasonality of sand flies (Diptera: Psychodidae: Phlebotominae) from a leishmaniasis transmission area in the central region of Rio Grande do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2024, 33, e000824. [Google Scholar] [CrossRef]
- Pereira, N.C.L.; Michalsky, E.M.; Lara-Silva, F.O.; Lana, R.S.; Paula, A.J.V.; Pereira, D.M.; Lopes, J.V.; Fortes-Dias, C.L.; Dias, E.S. Ecology of phlebotomine sand flies in a Brazilian area with recent leishmaniasis transmission (Itaúna, in Minas Gerais state). Rev. Soc. Bras. Med. Trop. 2020, 53, e20190538. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa Fde, A.; Ximenes, R.A. Sociodemographic and environmental risk factors for American cutaneous leishmaniasis (ACL) in the State of Alagoas, Brazil. Am. J. Trop. Med. Hyg. 2009, 81, 195–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dires, A.; Gedamu, S.; Kumar, P.; Yimam, W.; Ademe, S.; Dires, T. Determinants of cutaneous leishmaniasis among students in Delanta district, Northeast Ethiopia: A case-control study. Health Sci. Rep. 2022, 5, e917. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef]
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.M.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin. Infect. Dis. 2016, 63, e202–e264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).