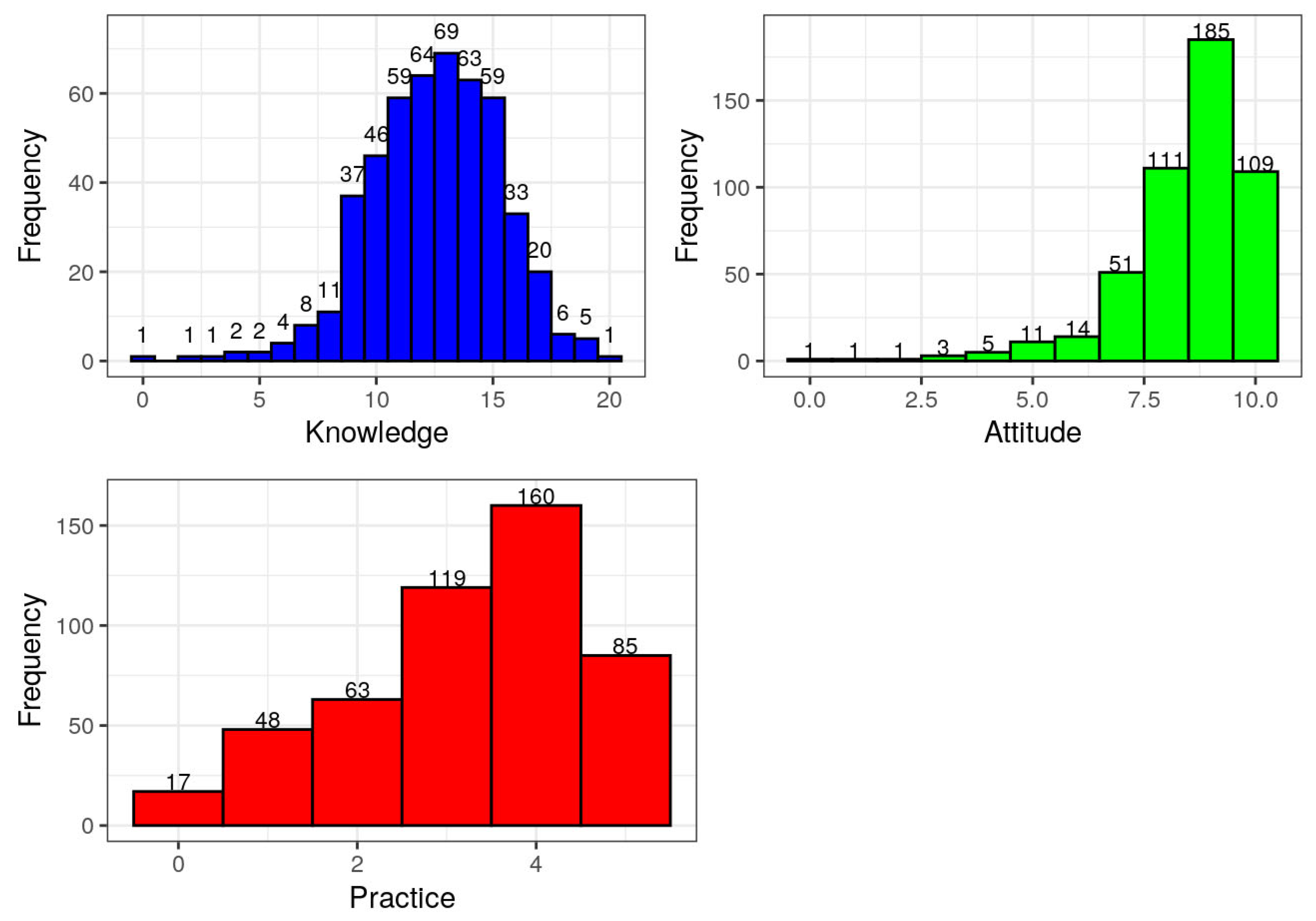
3.2. Level of Knowledge, Attitude, and Practice Among One Health Workforce During the Mpox Outbreak
The findings from the study assessing the level of knowledge, attitude, and practice (KAP) of the One Health workforce during the Mpox outbreak in Nigeria are presented in
Table 2 and
Figure 2. These results highlight distinct patterns across the three domains assessed.
In terms of knowledge, scores ranged from 0 to 20. The distribution reveals that the majority of respondents demonstrated moderate to high knowledge about Mpox. Scores of 13, 12, 14, 15, and 11 were the most frequently recorded, with proportions of 14.02%, 13.01%, 12.80%, 11.99%, and 11.99%, respectively. Conversely, only a few respondents had very low knowledge, with 0.20% each scoring 0, 2, or 3. These results suggest that the One Health workforce possessed a sound understanding of Mpox, likely reflecting previous training or experience with zoonotic disease outbreaks.
Attitude scores, which ranged from 0 to 10, revealed a generally positive outlook among respondents. The majority scored 9 (37.60%), 8 (22.56%), and 10 (22.15%), indicating a strong readiness and favorable disposition toward Mpox response activities. Only a small fraction of the workforce exhibited low attitude scores, with just 0.20% scoring 0, 1, or 2, and a cumulative 3.67% scoring between 0 and 4. This widespread positive attitude among respondents suggests a high level of commitment to public health responsibilities during the outbreak.
Practice scores, assessed on a scale of 0 to 5, displayed a slightly different pattern. The highest proportion of respondents (32.52%) scored 4; while 24.19% scored 3 and 17.28% scored 5, indicating a moderate to high level of appropriate practice. However, a notable proportion of respondents still fell into the lower practice categories, with 3.46% scoring 0 and 9.76% scoring 1. This distribution suggests that while knowledge and attitudes were generally high, actual practices lagged slightly behind. The gap between attitude and practice may reflect systemic challenges such as limited access to protective equipment, institutional constraints, or insufficient operational support in the field.
The findings depicted in
Table 2 and illustrated in
Figure 2 demonstrate a knowledgeable and motivated One Health workforce with largely positive attitudes but also underscore the need to improve practical application and support systems to enhance outbreak response effectiveness. These results reinforce the importance of continuous training, provision of adequate resources, and structural capacity-building for frontline responders during emerging infectious disease outbreaks such as Mpox.
The distribution of respondents’ levels of knowledge, attitude, and practice regarding Mpox prevention and response among the One Health workforce is presented in
Table 3. The majority of respondents (61.18%) demonstrated moderate knowledge scores (10–14), while 25.20% exhibited high knowledge (15–20), and a smaller proportion (13.62%) fell into the low knowledge category (0–9). In terms of attitude, a high proportion (59.76%) showed positive attitudes (scores 9–10), with 35.77% having moderate attitudes (6–8), and only 4.47% scoring low attitudes (0–5), reflecting a generally favorable disposition toward Mpox response. Regarding practice, over half of the participants (56.71%) had moderate levels of practice (scores 3–4), while 26.02% were classified as having low practice levels (0–2). Only 17.28% of respondents demonstrated high practice (score of 5), indicating a gap between knowledge and its translation into preventive behaviors.
3.3. Distribution of Participants’ Responses to Knowledge, Attitude, and Practice Items on Mpox
Table 4 presents the distribution of responses to twenty coded knowledge questions (k1 to k20) that assessed participants’ awareness and understanding of Mpox, using categorical variables as defined in the data dictionary (see
Appendix A). The responses reflect varying degrees of knowledge, misinformation, and uncertainty among the respondents (
n = 492).
Regarding the first time respondents heard of Mpox (k1), the majority (44.11%) indicated they were informed through school training, followed by 32.52% who learned about it during the 2022/2023 outbreak, while a smaller fraction (17.48%) had heard about it during previous outbreaks. Only 4.07% had never heard of Mpox. When asked about Mpox prevalence (k2), most participants correctly identified Central and West Africa (69.51%) as endemic regions, though some wrongly cited Europe (9.55%), North Africa (11.38%), or North America (5.28%).
On the nature of the disease (k3), a high percentage (90.24%) correctly identified Mpox as a viral disease, while small percentages mistook it for bacterial (4.47%), fungal (1.42%), or parasitic (1.63%) diseases. For modes of transmission (k4), nearly half (47.76%) correctly selected all relevant routes (direct contact, consumption of wild animals, and contact with contaminated lesions), while another 26.83% chose the more accurate but slightly restrictive option of only direct contact and lesion contact. Others selected incomplete or incorrect options.
Participants’ knowledge of human-to-human transmission (k5) was encouraging, with 78.46% answering “Yes.” Similarly, 69.31% recognized that infected monkey bites could transmit Mpox (k6), though 21.75% were unsure. Awareness of human Mpox cases in Nigeria (k7) was fairly high (68.5%), though 25% were uncertain. On the 2022/2023 outbreak’s origins (k8), 37.8% agreed and 27.44% strongly agreed that travelers from Central and West Africa were the source of imported cases, although 29.07% were not sure.
Opinions on the similarity of Mpox and smallpox symptoms (k9) were divided, with 37.4% agreeing and 13.01% strongly agreeing that they are indistinguishable, though 26.22% disagreed. Regarding early signs such as flu-like symptoms (k10), a large majority either agreed (49.39%) or strongly agreed (26.22%). High agreement was also observed for specific clinical signs: body rashes (k11) were acknowledged by 92.07% of respondents (combined agree and strongly agree), papules (k12) by 90.04%, vesicles (k13) by 79.27%, and pustules (k14) by 75.41%. However, fewer respondents recognized diarrhea (k15) as a sign, with 29.47% agreeing and 36.59% unsure.
When asked about differentiating Mpox from smallpox by lymphadenopathy (k16), 75.81% either agreed or strongly agreed. A total of 79.67% believed that antiviral drugs are required for Mpox management (k17), and 46.13% agreed or strongly agreed that a specific vaccine for Mpox is available (k18), although 42.89% were uncertain. The belief that chickenpox immunization protects against Mpox (k19) was held by 36.18%, but 35.16% were unsure and 21.14% disagreed. Finally, 57.93% either agreed or strongly agreed that men who have sex with men are at higher risk of Mpox infection (k20), while 28.25% were unsure.
In this study, the distribution of responses to Mpox knowledge items reveals that participants possess a generally good understanding of Mpox’s virology, transmission routes, clinical signs, and public health relevance. However, uncertainties remain concerning specific transmission modes, differential diagnosis, and vaccine availability, highlighting the need for targeted awareness and education campaigns.
Table 5 presents the distribution of responses to ten coded attitude questions (a1 to a10), which assess the perceptions, emotional responses, and interests of respondents regarding Mpox and related public health concerns, using five-point and three-point Likert-type scales as defined in the data dictionary (see
Appendix A).
For item a1, which asked whether Nigerian medical and animal healthcare workers play a critical role in controlling Mpox, 45.8% of respondents agreed and 33.2% strongly agreed, indicating that nearly four out of five respondents (79.0%) recognized the importance of these professionals. Only 3.4% disagreed, 1.3% strongly disagreed, and 16.3% were not sure.
Regarding item a2, which assessed beliefs in the capacity of national health institutions to control Mpox through a One Health approach, 47.6% agreed and 34.0% strongly agreed—together making up 81.6% of respondents who supported the idea. A small portion disagreed (2.6%), strongly disagreed (1.6%), or were unsure (14.2%).
On item a3, addressing whether there are currently enough Mpox prevention and control measures among Nigerian health workers, only 29.5% agreed and 11.3% strongly agreed. In contrast, 28.7% disagreed and 6.3% strongly disagreed, while 24.2% were not sure. These figures reflect a mixed perception, with a notable proportion of respondents expressing doubt or uncertainty about existing measures.
For item a4, which gauged emotional concern about Mpox becoming a worldwide pandemic, 38.7% agreed and 29.2% strongly agreed, while 20.8% were not sure. A smaller number disagreed (8.4%) or strongly disagreed (2.9%). Overall, 67.9% of respondents reported a negative emotional response to the virus’s potential global spread.
In item a5, 42.1% agreed and 40.3% strongly agreed that Mpox could add a new burden to the Nigerian medical and animal healthcare systems. Only 2.9% disagreed, 1.8% strongly disagreed, and 12.9% were not sure. These responses suggest strong awareness of the strain Mpox could place on already challenged systems, with 82.4% expressing concern.
Item a6 explored the influence of mass media on Mpox prevention. Here, 77.4% answered “Yes,” acknowledging the role of media in shaping global prevention efforts, while 16.6% were not sure and 6.0% said “No.”
Interest in further learning about Mpox (item a7) was also high, with 86.3% of respondents indicating “Yes,” while 10.0% were not sure and only 3.7% said “No.” A similar trend appeared in item a8, where 88.9% said “Yes” to wanting to learn more about emerging diseases, with 7.1% not sure and 4.0% responding “No.” In item a9, 88.4% expressed interest in learning more about reemerging diseases, 7.1% were not sure, and only 4.5% said “No.”
Lastly, item a10 evaluated perceived risk associated with international travel to Mpox-affected countries. A majority (81.8%) of respondents agreed that it is dangerous, while 13.2% were not sure and 5.0% disagreed.
In this study, the distribution of responses to Mpox attitude items reveals a broadly positive attitude among respondents toward the importance of health professionals and intersectoral collaboration in controlling Mpox, with 81.6% supporting the One Health approach. Although attitudes toward the adequacy of current control measures were mixed (only 40.8% affirming their sufficiency), there was high concern about Mpox potentially becoming a pandemic (67.9%) and overwhelming health systems (82.4%). The majority of respondents also recognized the influence of mass media (77.4%) and expressed strong interest in gaining more knowledge about Mpox (86.3%), emerging diseases (88.9%), and reemerging diseases (88.4%). These findings underscore the importance of sustained public health communication, cross-sectoral collaboration, and educational outreach in managing Mpox and similar infectious disease threats.
Table 6 presents the distribution of responses to five coded practice questions (p1 to p5) derived from the structured questionnaire. These items assess the respondents’ practical knowledge and behaviors regarding Mpox prevention, sample collection, laboratory knowledge, biosafety practices, and post-exposure response, as defined in the data dictionary for practice items in
Appendix A.
For item p1, which assessed how respondents protect themselves from contracting Mpox from suspected human or animal cases, 80.7% (n = 397) selected the comprehensive option “all of the above,” which includes creating awareness in the community, avoiding contact with suspected or confirmed cases, and practicing hand hygiene after exposure. The remaining respondents chose individual strategies, with 8.7% (n = 43) avoiding contact, 7.7% (n = 38) creating awareness, and only 2.9% (n = 14) focusing on hand hygiene alone. This overwhelming preference for a comprehensive approach indicates strong knowledge of appropriate self-protective behaviors among most respondents.
Item p2 examined respondents’ knowledge of appropriate samples for Mpox diagnosis. The majority (68.3%, n = 336) correctly selected the combination of all key diagnostic specimens except saliva, which aligns with standard laboratory protocols. Others selected only skin lesion swabs (26.0%, n = 128), while smaller percentages chose lesion roofs (2.0%, n = 10), lesion crusts (1.0%, n = 5), or saliva (2.6%, n = 13). These findings suggest that while majorities are aware of proper diagnostic sampling methods, a significant number still hold incomplete or less accurate views.
In item p3, respondents were asked to identify the location of the Mpox reference laboratory in Nigeria. Responses were nearly evenly split between Lagos (44.5%, n = 219) and Abuja (44.1%, n = 217), reflecting uncertainty or perhaps a lack of uniform national communication on laboratory network infrastructure. Fewer respondents selected Kaduna (4.9%, n = 24), Enugu (3.9%, n = 19), or Kano (2.6%, n = 13).
For item p4, which assessed proper handling of suspected Mpox cases before the confirmation of laboratory results, a large majority (76.8%, n = 378) selected “all of the above,” indicating they would observe hand hygiene, use appropriate personal protective equipment, and avoid procedures that generate infectious aerosols. Some respondents chose specific practices individually, such as PPE use (16.5%, n = 81) or hand hygiene (3.7%, n = 18), while 2.0% (n = 10) selected “none of the above.” These results indicate that the majority of respondents are familiar with and willing to adopt recommended biosafety measures in managing suspected cases.
Finally, item p5 asked respondents about their preferred action in the event of accidental exposure. Over half (54.5%, n = 268) indicated they would seek medical attention and get vaccinated, reflecting a proactive and medically sound response. A further 33.7% (n = 166) said they would seek medical attention only, while 6.1% (n = 30) opted for both medical attention and self-medication. A small number indicated vaccination alone (5.5%, n = 27), and just one respondent (0.2%) chose only self-medication. This distribution suggests that most respondents understand the importance of prompt medical intervention and the protective role of vaccination in the event of exposure.
In this study, the distribution of responses to Mpox practice items reveals that a large proportion of respondents demonstrated good practical knowledge and adherence to Mpox prevention and response protocols. Notably, 80.7% adopted all recommended self-protective measures, 68.3% correctly identified comprehensive diagnostic sample types, and 76.8% were aware of complete biosafety practices. Although responses about the location of the Mpox reference laboratory were divided almost equally between Lagos and Abuja, indicating some uncertainty, more than half of respondents (54.5%) knew the appropriate steps to take following accidental exposure, including seeking medical attention and getting vaccinated. These findings highlight the strengths and gaps in Mpox-related practical preparedness among the surveyed population.
3.5. Factors Influencing Knowledge, Attitude, and Practice Scores
3.5.1. Results from Univariable Ordinal Regression Models
Table 7 presents the results of a univariable ordinal logistic regression model examining the association between respondents’ knowledge levels on Mpox and various demographic characteristics. Gender was not significantly associated with knowledge, as females had an odds ratio (OR) of 1.02 (95% CI: 0.71–1.45;
p = 0.923), indicating no meaningful difference from males. In terms of age, individuals aged 20–29 years were significantly more likely to have higher knowledge levels compared to the 30–39 age group (OR = 1.58; 95% CI: 1.03–2.43;
p = 0.038). The 50–59 age group also showed a statistically significant association with higher knowledge (OR = 3.33; 95% CI: 1.31–8.44;
p = 0.011), suggesting that older participants in this category were substantially more knowledgeable about Mpox than those aged 30–39 years.
Marital status did not demonstrate any significant associations with knowledge, with all subcategories showing wide confidence intervals and p-values above 0.05. Regarding educational qualification, no statistically significant associations were observed. Respondents with a PhD had slightly higher odds of better knowledge compared to first-degree holders (OR = 1.06; 95% CI: 0.45–2.49; p = 0.892), but this was not statistically significant. Other qualification categories, including certificate, diploma, HND, masters, and fellowship diploma, also showed no meaningful differences.
In terms of profession, most categories did not show statistically significant associations with knowledge levels. Although environmental scientists and medical doctors appeared to have higher odds of greater knowledge compared to registered nurses/midwives, their wide confidence intervals and high p-values suggest a lack of statistical significance. Notably, microbiologists had a reported OR close to zero (5.46 × 10−7), with no confidence interval reported beyond the lower bound, indicating a probable data sparsity issue for that category. Similarly, laboratory scientists and paravets had reduced odds of higher knowledge, though not statistically significant. Veterinary doctors had nearly equivalent odds to the reference group (OR = 1.03; 95% CI: 0.68–1.55; p = 0.902).
Overall, age was the only demographic variable with statistically significant associations with knowledge about Mpox in this univariable model, particularly among respondents aged 20–29 and 50–59 years.
Table 8 presents the univariable ordinal logistic regression results assessing the relationship between respondents’ attitudes and their demographic characteristics. Gender was marginally associated with attitude, where females had lower odds of more positive attitudes compared to males (OR = 0.70, 95% CI: 0.49–1.00,
p = 0.052), suggesting a trend toward significance. Age showed no statistically significant association, with the 20–29, 40–49, and 50–59 age groups having odds ratios of 0.89 (
p = 0.583), 1.12 (
p = 0.621), and 1.22 (
p = 0.681), respectively, compared to the 30–39 age group.
Marital status did not reveal significant associations with attitude. While single and widow respondents had odds ratios of 0.98 and 0.75, respectively, these results were not statistically significant (p = 0.908 and p = 0.835). The estimate for divorced respondents was extremely high (OR = 416,514) with a p-value of 0.981 and a confidence interval that effectively rendered the result uninterpretable due to an estimation artifact, likely from sparse data.
Regarding qualification, none of the education levels showed a statistically significant relationship with attitude. Odds ratios ranged from 1.17 for certificate holders to 1.37 for those with a master’s degree, but all p-values were above 0.05. Similarly, an implausibly high OR of 507,078 was noted for fellowship diploma holders, again likely due to sparse data or model convergence issues.
Across professional categories, there was no significant association with attitude. Compared to registered nurses/midwives, other professions such as laboratory scientists (OR = 0.76, p = 0.403), medical doctors (OR = 1.42, p = 0.491), and veterinary doctors (OR = 1.04, p = 0.852) showed no meaningful differences. As with marital status and qualification, environmental scientists showed an extreme OR (420,974) with a p-value of 0.981, indicating an unstable estimate.
In summary,
Table 8 suggests that most demographic factors did not show statistically significant associations with attitude levels in the univariable analysis, although gender approached significance, with females tending to have lower odds of more positive attitudes. Several implausibly large odds ratios suggest potential data sparsity in some categories, which may warrant caution in interpretation or the use of penalized models or data pooling for rare groups.
Table 9 presents the results of a univariable ordinal logistic regression analysis assessing the association between practice scores and various demographic characteristics. The table reports odds ratios (ORs), standard errors (SEs), z-values,
p-values, and 95% confidence intervals (CIs) for each demographic level compared to a designated reference category.
In terms of gender, females had 1.13 times the odds of a higher practice score compared to males, although this association was not statistically significant (p = 0.496, 95% CI: 0.80–1.59). For age, respondents aged 20–29 and 50–59 had higher odds of improved practice compared to those aged 30–39 (OR = 1.17 and 1.34, respectively), while those aged 40–49 had slightly lower odds (OR = 0.90). However, none of these associations reached statistical significance (p-values > 0.05), and the confidence intervals included 1.
With respect to marital status, single respondents (OR = 1.21, p = 0.315) and divorced respondents (OR = 1.37, p = 0.798) had higher odds of better practice compared to married individuals, whereas widowed respondents showed an OR of 0.00 with a corresponding confidence interval of 0, indicating sparse data and estimation issues for this group.
Educational qualification revealed a significant association for respondents with certificate-level qualifications, who had 48% lower odds of better practice compared to those with a first degree (OR = 0.52, p = 0.020, 95% CI: 0.30–0.90). Other qualification levels, including diploma, HND, master’s, and PhD, did not show statistically significant associations, although those with a PhD had lower odds (OR = 0.59, p = 0.220) and those with a master’s degree had slightly higher odds (OR = 1.11, p = 0.718) relative to first degree holders. The fellowship diploma level also showed an OR of 0.26, but with a wide confidence interval and no significance (p = 0.324).
Regarding profession, veterinary doctors had significantly higher odds of better practice compared to registered nurses or midwives (OR = 1.50, p = 0.052, 95% CI: 1.00–2.24), with the lower limit of the CI just at the threshold of 1, suggesting marginal statistical significance. Other professions such as laboratory scientists (OR = 1.26, p = 0.479), medical doctors (OR = 1.09, p = 0.850), and microbiologists (OR = 1.46, p = 0.827) did not show significant associations. Paravets had lower odds of better practice (OR = 0.19, p = 0.165), though this was not statistically significant. Notably, some professions like environmental scientists and health information managers had extremely wide confidence intervals, indicating instability in estimates likely due to small sample sizes.
In summary,
Table 9 shows that among the demographic variables assessed, only those with certificate-level qualifications were significantly less likely to report better practice scores compared to those with a first degree. Veterinary doctors also had marginally higher odds of better practice, suggesting a potential area of interest for further investigation in multivariable modeling.
3.5.2. Results from Multivariable Ordinal Regression Models
To determine the demographic variables for inclusion in the multivariable ordinal logistic regression models for each outcome—knowledge, attitude, and practice—a selection strategy based on both statistical and theoretical considerations was employed. As shown in
Table 10, the univariable ordinal logistic regression results informed the selection of variables without adjusting for confounding. Variables that were statistically significant, borderline significant, conceptually important, or likely to act as confounders were retained for further adjustment in multivariable analysis.
For the knowledge outcome, age was included due to significant associations for the 20–29 and 50–59 age groups. Although gender was not significant (p = 0.923) and had a negligible effect size, it was excluded with caution due to its theoretical relevance. Marital status was included based on its near-significant association and potential role as a confounder. Qualification and profession were both retained due to their conceptual relevance and likelihood of acting as confounders.
Regarding the attitude outcome, gender was included because of its borderline significance (p = 0.052), and age was retained due to its fundamental role as a demographic variable. Marital status, while associated with wide confidence intervals, was included cautiously for its theoretical importance, particularly with potential regrouping of sparse categories. Qualification and profession were also included, with a recommendation to merge sparse categories to improve model stability.
For the practice outcome, all five demographic variables—gender, age, marital status, qualification, and profession—were retained for multivariable modeling. Although gender was borderline non-significant, it was included for its possible explanatory value. Age was considered a possible confounder or interacting factor. Marital status was deemed relevant as a social determinant of practice behaviors. Notably, certificate-level qualification had a statistically significant negative association with practice (p = 0.020), justifying its inclusion. Profession was retained based on the borderline significance observed for veterinary doctors (p = 0.052) and the conceptual importance of professional background in shaping practice.
Table 11 presents the final multivariable ordinal logistic regression model for the association between knowledge and demographic characteristics, adjusted for potential confounders. The analysis showed that age was significantly associated with knowledge levels. Compared to respondents aged 30–39 years (reference group), those aged 20–29 years had 1.57 times higher odds of reporting better knowledge (adjusted OR = 1.57; 95% CI: 1.02–2.42;
p = 0.042), and those aged 50–59 years had significantly higher odds of higher knowledge scores (adjusted OR = 4.47; 95% CI: 1.64–12.16;
p = 0.003). However, the 40–49 age group did not differ significantly from the reference group (adjusted OR = 1.08; 95% CI: 0.69–1.69;
p = 0.745).
Regarding profession, with registered nurses/midwives serving as the reference category, laboratory scientists were significantly less likely to have higher knowledge scores (adjusted OR = 0.45; 95% CI: 0.22–0.92; p = 0.028). Other professions did not show statistically significant associations with knowledge. For example, environmental scientists had higher but non-significant odds (adjusted OR = 1.83; 95% CI: 0.10–35.02; p = 0.688), while those in health information management had lower odds (adjusted OR = 0.78; 95% CI: 0.06–10.14; p = 0.848). Similarly, medical doctors (adjusted OR = 1.47; p = 0.425), paravets (adjusted OR = 0.24; p = 0.190), and veterinary doctors (adjusted OR = 1.07; p = 0.740) were not significantly different from the reference category. Microbiologists showed an extremely low odds ratio (adjusted OR ≈ 0.00; p = 0.977), though this result was not statistically meaningful due to the absence of variation.
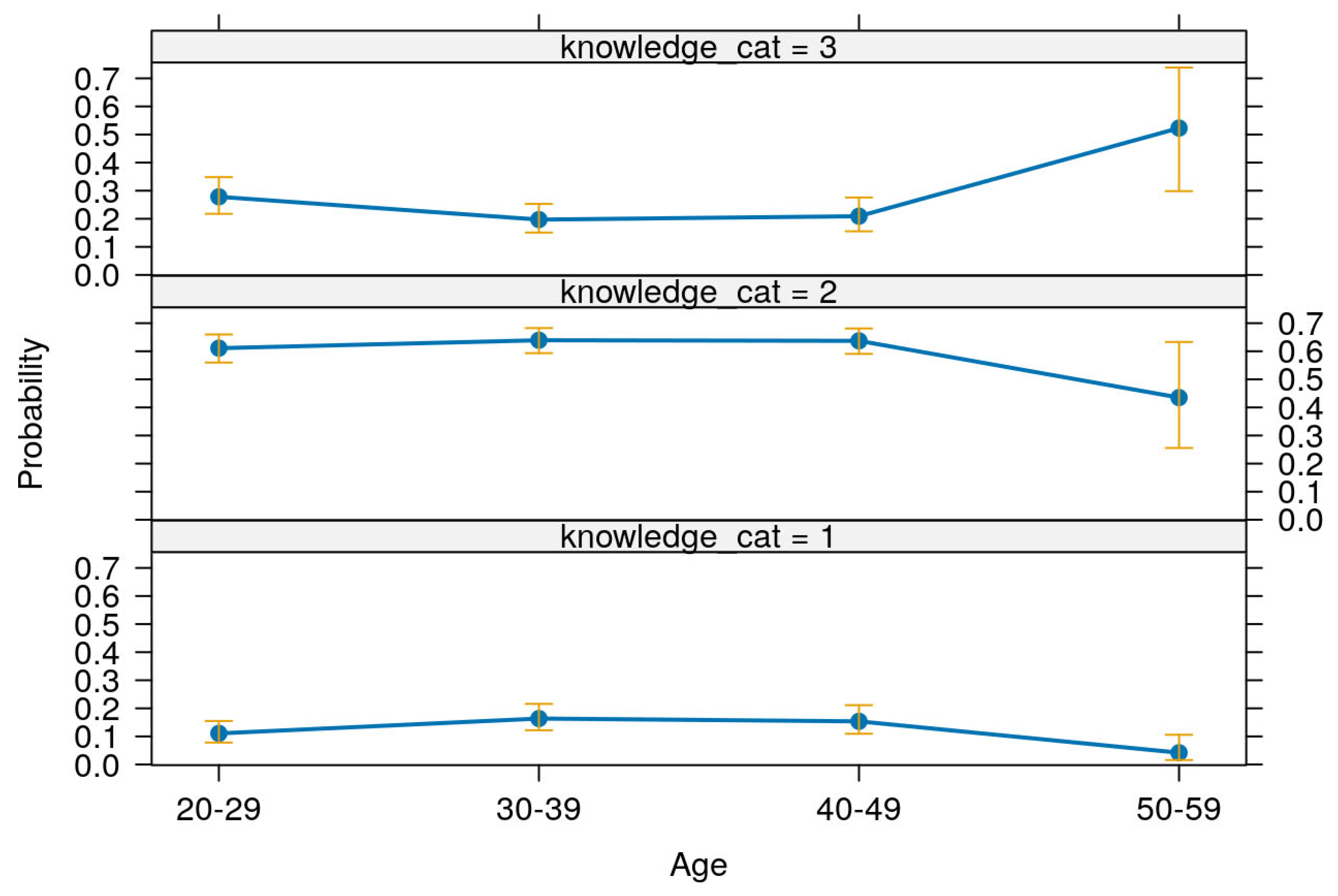
Table 12 presents the predictive margins and corresponding 95% confidence intervals for levels of knowledge across different age groups, based on the multivariable ordinal logistic regression model. These margins are further illustrated in
Figure 4.
Among respondents aged 20–29 years, the probability of having low knowledge was 0.11 (95% CI: 0.07–0.15), moderate knowledge was 0.60 (95% CI: 0.55–0.65), and high knowledge was 0.29 (95% CI: 0.23–0.36). For the 30–39 age group, the predicted probability of low knowledge increased slightly to 0.16 (95% CI: 0.12–0.21), moderate knowledge was 0.63 (95% CI: 0.59–0.67), and high knowledge was 0.21 (95% CI: 0.16–0.26). Respondents aged 40–49 had similar patterns, with a low knowledge margin of 0.15 (95% CI: 0.10–0.20), moderate knowledge at 0.63 (95% CI: 0.58–0.67), and high knowledge at 0.22 (95% CI: 0.16–0.28). Notably, among those aged 50–59, the predicted probability of low knowledge dropped to 0.04 (95% CI: 0.00–0.08), moderate knowledge also decreased to 0.42 (95% CI: 0.22–0.61), while the probability of high knowledge rose markedly to 0.54 (95% CI: 0.31–0.77).
These results indicate a clear age-related trend: younger respondents (20–49 years) were more likely to have moderate knowledge and less likely to have high knowledge, while older respondents (50–59 years) were significantly more likely to possess high knowledge and least likely to fall within the low knowledge category. This pattern, supported by narrow confidence intervals and statistically significant z-scores (all p < 0.001), underscores the positive association between increasing age and higher knowledge levels within the surveyed population.
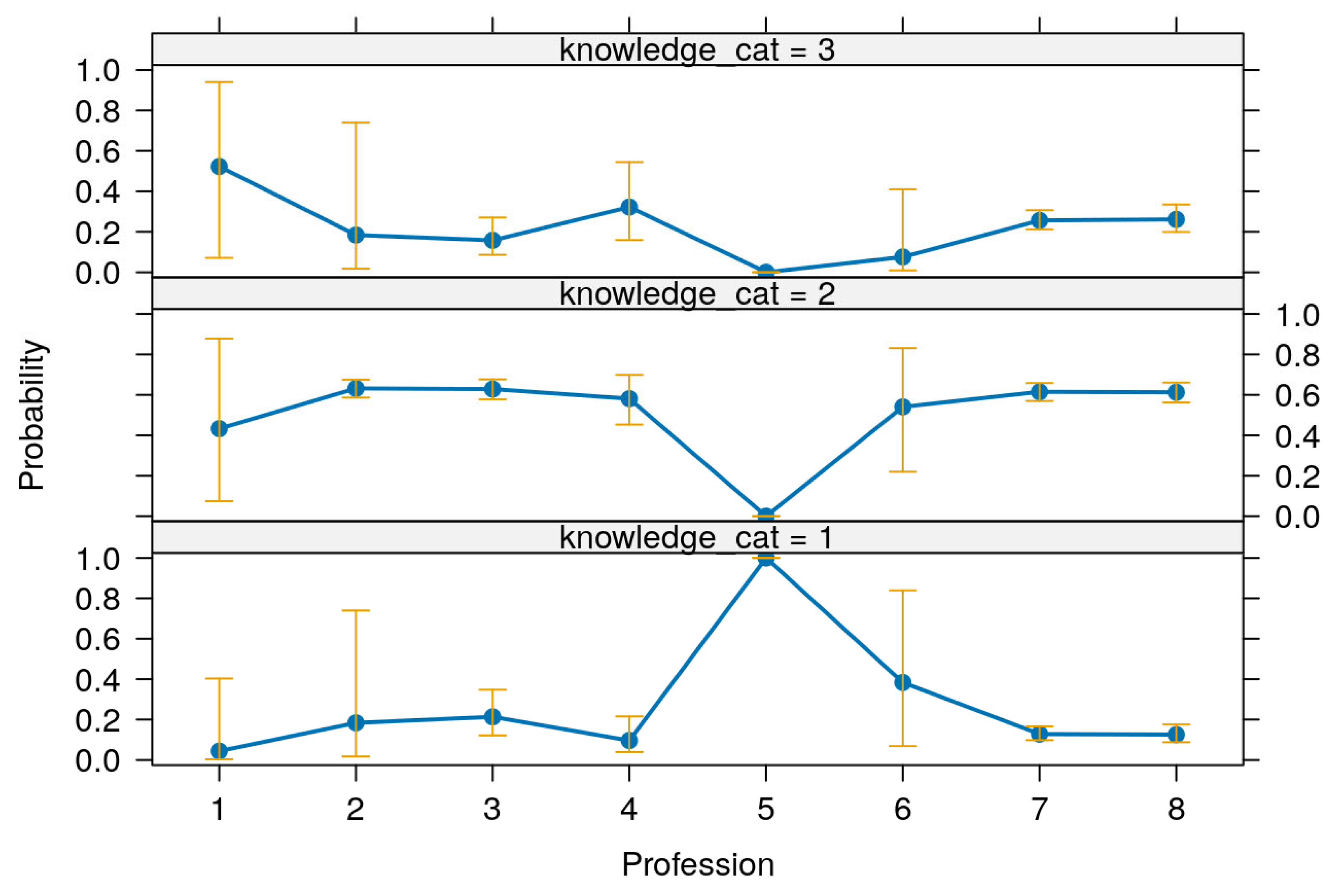
Table 13 presents the predictive margins and corresponding 95% confidence intervals for knowledge levels across various professions, offering insights into the distribution of knowledge among different professional groups. These predictive margins are visualized in
Figure 5.
The results reveal substantial variation in predicted knowledge levels among professions. For respondents identified as microbiologists, the predicted probability of having low knowledge was 1.00 (95% CI: 0.99–1.01), with corresponding margins for moderate and high knowledge being 0.00 (95% CI: −0.00–0.00), indicating a strong and exclusive prediction of low knowledge in this group. This pattern was highly statistically significant (p < 0.001). Laboratory scientists had a high probability of moderate knowledge at 0.62 (95% CI: 0.56–0.68), but also a relatively elevated margin for low knowledge at 0.24 (95% CI: 0.12–0.37), and a lower margin for high knowledge at 0.14 (95% CI: 0.06–0.22), suggesting a broad distribution across knowledge levels but skewed toward moderate knowledge.
Veterinary doctors and registered nurses/midwives showed a similar distribution, with moderate knowledge margins of 0.61 (95% CI: 0.56–0.66) and 0.61 (95% CI: 0.57–0.66), respectively. Their high knowledge probabilities were also comparable—0.27 (95% CI: 0.20–0.34) for veterinary doctors and 0.26 (95% CI: 0.21–0.31) for nurses/midwives—indicating a balanced and strong knowledge profile across both groups. Their low knowledge probabilities were modest at 0.12 (95% CI: 0.08–0.16) for veterinary doctors and 0.13 (95% CI: 0.09–0.16) for nurses/midwives.
Medical doctors showed slightly more variation, with a low knowledge margin of 0.09 (95% CI: 0.01–0.17), a moderate knowledge margin of 0.57 (95% CI: 0.44–0.70), and a relatively high probability of possessing high knowledge at 0.34 (95% CI: 0.14–0.54), indicating better knowledge outcomes relative to several other professions. In contrast, environmental scientists and professionals in health information management exhibited broader confidence intervals, suggesting less precision. For instance, environmental scientists had a predicted moderate knowledge probability of 0.54 (95% CI: 0.06–1.02), a wide and less conclusive interval, while their high knowledge estimate of 0.39 (95% CI: −0.29–1.06) lacked statistical significance (p = 0.265), indicating uncertainty in this group’s predicted knowledge levels.
Paraveterinary professionals (paravets) had a moderate knowledge margin of 0.55 (95% CI: 0.21–0.88), and a relatively high but non-significant low knowledge margin of 0.38 (95% CI: −0.11–0.87), while their high knowledge probability remained low at 0.08 (95% CI: −0.07–0.23). This reflects a less favorable and more uncertain knowledge distribution.
Altogether,
Table 13 and
Figure 5 show that certain professional groups, particularly registered nurses, veterinary doctors, and medical doctors, tend to have higher levels of knowledge with narrower confidence intervals, whereas others like microbiologists and environmental scientists show either extreme predictions or high uncertainty, underscoring potential disparities in knowledge that could be addressed through targeted capacity building.
Table 14 presents the interaction between age groups and professional categories, showing their predictive margins (probabilities) for the knowledge score, adjusted for other variables in the model.
Figure 6 visually represents these predictive margins with corresponding 95% confidence intervals, allowing for easy comparison across groups.
The results show that the adjusted predictions (or marginal probabilities) vary significantly by both age and profession. Because effect modification by age and profession was prespecified as central to One Health interpretation, we report full interaction margins (predicted probabilities with 95% CIs) in the main text to enable transparent, decision-ready comparisons across stakeholder strata. For example, among professionals aged 20–29, Microbiologists had the highest predicted margin at nearly 1.00 (0.10; 95% CI: 0.99–1.01), indicating almost certain likelihood of the outcome occurring within this subgroup. Other professions in the same age group such as Laboratory scientists (0.201; 95% CI: 0.08–0.32) and Veterinary doctors (0.10; 95% CI: 0.05–0.14) also showed statistically significant predictions with narrow confidence intervals, suggesting more precision in these estimates. Conversely, Paravets and Health information management professionals in this age category showed wider confidence intervals (e.g., Paravets: 0.32; CI: −0.15–0.79), indicating greater uncertainty.
For the 30–39 age group, Microbiologists again had a near-certain outcome (0.10; CI: 0.10–1.00), followed by Laboratory scientists (0.28; CI: 0.14–0.43), and Veterinary doctors (0.14; CI: 0.09–0.20). These values suggest increasing margin probabilities with professional role, especially in clinical and laboratory-focused fields.
Among respondents aged 40–49, a similar trend was observed, with Microbiologists still showing extremely high predicted margins (0.10), and Laboratory scientists having a margin of 0.27 (CI: 0.12–0.41). The profession-specific differences remained significant, while professions like Environmental scientists and Paravets again showed large confidence intervals, indicating less consistent predictions in those roles.
Interestingly, the 50–59 age group revealed a general decline in predicted margins across professions. For instance, Medical doctors had a reduced margin (0.03; CI: −0.01–0.06) compared to younger counterparts, and Veterinary doctors also dropped to 0.0356 (CI: −0.00–0.07), both with wider CIs. Nevertheless, Microbiologists in this group still maintained a high margin close to 1.00 (0.10; CI: 0.98–1.02).
In sharp contrast, for outcome level 2 (presumably indicating a different or improved knowledge category), all professions saw substantial increases in predictive margins across age groups. For example, Health information management professionals aged 20–29 had a margin of 0.6244 (CI: 0.42–0.83), and Veterinary doctors in the same age group showed 0.5911 (CI: 0.52–0.66), suggesting a positive shift in outcome prediction for these professions at level 2. These differences are further visualized in
Figure 6, where the distinction in margins by age and profession is clearly delineated with vertical confidence interval bars, highlighting the significant disparities and overlaps in outcome predictions.
Overall, the interaction between age and profession plays a critical role in shaping the adjusted predictions for the knowledge outcome. Younger professionals in clinical and laboratory fields tend to show higher or more certain predicted margins at knowledge level 1, while all professions show marked improvements at knowledge level 2, emphasizing both the importance of age and professional background in influencing the knowledge outcome.
Table 15 presents the results of the final multivariable ordinal logistic regression model examining the association between gender and attitude score toward Mpox prevention and control. In the model, male respondents served as the reference group with an odds ratio (OR) of 1.00. Female respondents had lower odds of demonstrating a more positive attitude compared to males, with an adjusted OR of 0.70. The corresponding
p-value was 0.052, indicating that the association was marginally non-significant at the 5% level. The 95% confidence interval for the odds ratio ranged from 0.49 to 1.00. This suggests that, although not statistically significant, there is a trend indicating that females may be less likely than males to hold more favorable attitudes toward Mpox prevention and control after adjusting for other covariates in the model.
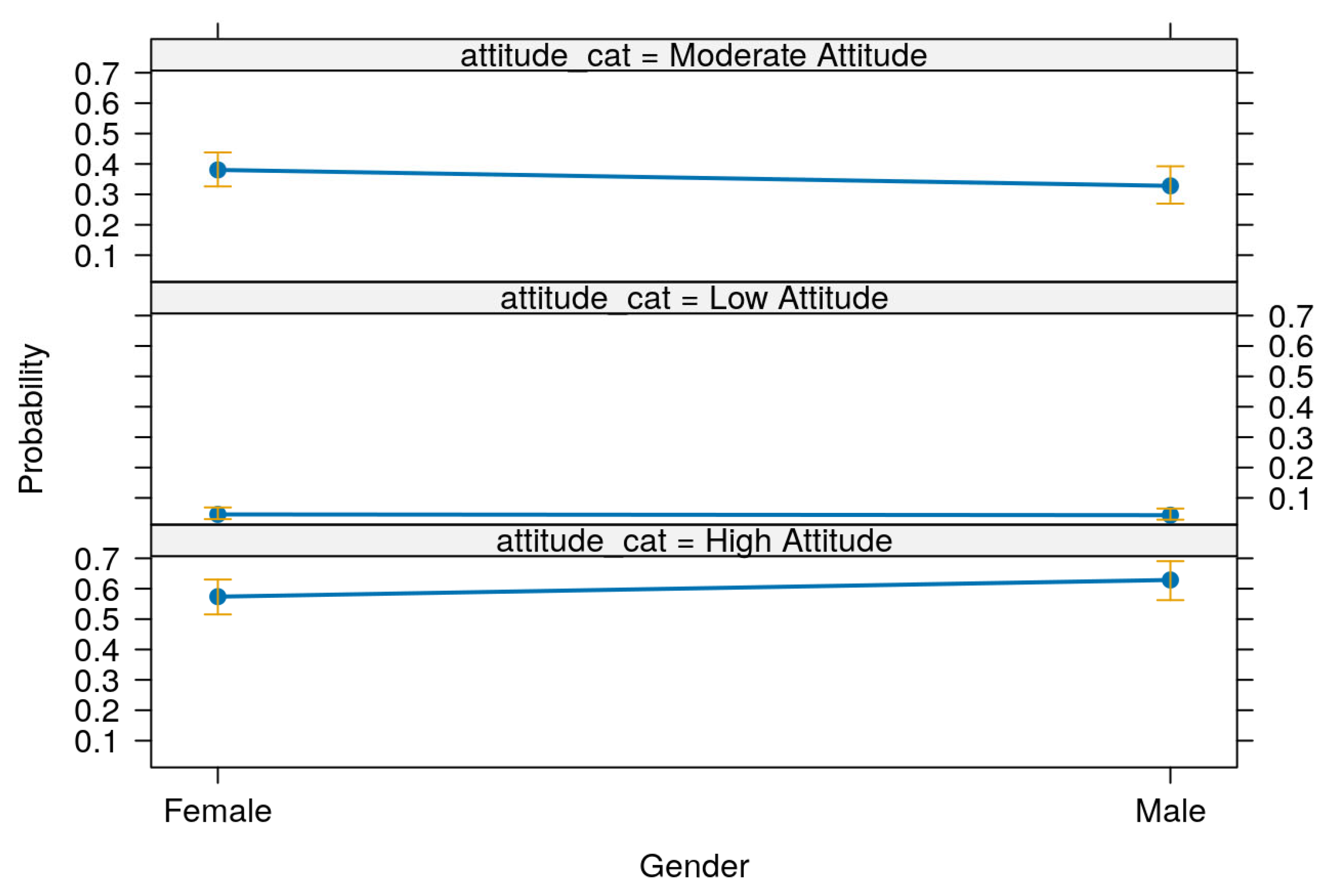
Table 16 and
Figure 7 present the predictive margins of attitude toward Mpox prevention and control across gender groups. The margins represent the predicted probabilities of respondents falling into each attitude category—low, moderate, or high—based on their gender, with 95% confidence intervals.
In
Table 16, the probability of having a low attitude score was slightly higher among females (margin = 0.05, 95% CI: 0.03–0.07) than males (margin = 0.04, 95% CI: 0.02–0.05). For the moderate attitude category, females also showed a higher predicted probability (0.39, 95% CI: 0.34–0.44) compared to males (0.32, 95% CI: 0.26–0.38). However, the trend reversed for the high attitude category, where males had a higher predicted probability (0.64, 95% CI: 0.58–0.71) than females (0.56, 95% CI: 0.50–0.62). All differences across gender and attitude levels were statistically significant (
p < 0.001).
Figure 7 visually illustrates these predictive margins along with their 95% confidence intervals, reinforcing the statistical findings in
Table 16. The figure clearly shows that males are more likely to have a high attitude score, while females are more likely to be in the low or moderate categories, further supporting the marginally non-significant association found in the regression model (
Table 15).
Given the observed results, we decided not to present the multivariable ordinal logistic regression model for the association of practice with demographics as the final model. The added complexity of the multivariable approach did not yield meaningful results, with no significant predictors retained in the model. Instead, we focused on the univariable analyses, where significant associations were observed and could be more reliably interpreted. The lack of significance in the multivariable model may be attributed to potential collinearity between predictors, limitations in sample size, or the absence of a true association between the explanatory variables and the outcome. Since the multivariable model did not improve upon the univariable model in terms of explanatory power or model fit, we chose to present and interpret only the univariable results. Although the multivariable model was explored, it did not offer additional insights and thus was not retained as the final model.
Table 17 presents the model fit statistics for the final ordinal logistic regression models assessing the association of demographic variables with knowledge and attitude regarding Mpox among respondents. The model for knowledge included 492 observations, with a log-likelihood value of −452.382 for the null model and −441.435 for the fitted model, yielding an Akaike Information Criterion (AIC) of 906.869 and a Bayesian Information Criterion (BIC) of 957.2508 based on 12 degrees of freedom. For the final model on attitude, also based on 492 observations, the null and fitted model log-likelihoods were −400.671 and −398.776, respectively, with an AIC of 803.5509 and a BIC of 816.1463 based on 3 degrees of freedom. Lower AIC and BIC values in the attitude model compared to the knowledge model suggest that the attitude model has a comparatively better fit to the data, especially given its more parsimonious structure with fewer parameters. These results support the suitability of both models for ordinal logistic regression analysis, with better model fit indicated by improvements in log-likelihood and lower AIC/BIC values compared to the respective null models.