Simple Summary
In a multi-species scenario, mainly in Mediterranean environments, large game species, such as wild boar and red deer, are a crucial component in the maintenance of tuberculosis, as inferred in this preliminary statistical study. From the perspective of TB mitigation in a scenario of marked closed-contact game–livestock interface, it is necessary to know the factors that allow the maintenance of the disease (it is noted that 23.3% of the analyzed areas had TB in both domestic animals and large game), including making a correct identification of the species present and the diagnosis of TB in them. This is even more important from a One Health perspective, where one of the susceptible hosts is humans, and there could be zoonotic transmission of TB at this interface. A truly inclusive strategy must be considered, encompassing all stakeholders and environmental and animal factors present that enhance the maintenance of TB in a marked game–livestock–human interface.
Abstract
Knowing the specific characteristics and animal tuberculosis risk factors present and applying good practices are crucial points in combating tuberculosis (TB) in a Mediterranean multi-species scenario. The objective of this work is to statistically analyze the association between the existence of TB in areas with a marked game–livestock interface, with various complementary factors found in 30 extensive farms in southern Portugal, such as the number of animals of each large game species present in the territory and the frequency of their sightings. Collecting this information, an inferential statistical analysis was conducted to obtain information on the association type between TB occurrence in the farms and the presence of highlighted factors. The main statistical results show an association between the presence of large game species and TB occurrence in the analyzed areas. Thus, in a multi-species scenario, large game species are a crucial component in TB maintenance, namely when stricter contact occurs. This could be one of the reasons why TB continues to circulate and why the eradication process is so difficult; the risk of zoonotic transmission is evident. It is crucial to apply biosecurity tools to improve the alignment and structure of natural resource management strategies.
1. Introduction
The management of wild animal populations has been increasingly resembling that of extensive domestic livestock management, although with limited sanitary measures, particularly in areas that include livestock development [1]. Thus, there are increased chances for interspecific interactions to occur during resource sharing, which increases the possibility of multi-host pathogenic agents being maintained and spread [2].
In a multispecies scenario, the interface between domestic and wild animals, especially large game, is defined as the continuous direct and indirect contact between them, especially at feeding and watering points marked in interface areas [3]. Human activity, environmental factors, and animal behavior patterns can all impact on this multidimensional, spatially dynamic interface [4]. According to Antolin et al. (2010) [5], the specific time and place of animal encounters are crucial elements in spreading disease [5].
Several infectious diseases impact animal health and public health. The transmission of pathogenic agents at the game–livestock interface is generally bidirectional [6]. Wild species can be infected by livestock agents while also posing a risk of persistent reinfection of cattle, constituting a barrier to eradicating diseases in domestic populations [7,8]. One of the most studied examples worldwide, and especially in the Iberian Peninsula, is tuberculosis (TB), whose epidemiological dynamics fit perfectly into a multispecies game–livestock interface scenario [9].
It is the most studied pathology at the livestock–wildlife interface, classified as requiring mandatory eradication under the Animal Health Law, with an estimate that for every 100 infected animals, 50 are domestic and the remaining are wild [10]. It is a notifiable disease with a high economic impact, demanding the culling of animals in infected livestock farms, and it can also impact the conservation of wild species. It is a zoonosis [11].
TB is a disease caused by mycobacteria from the Mycobacterium tuberculosis complex (MTC), whose main mode of transmission is direct via the oral/respiratory route; however, other routes can also be understood less frequently, such as indirect via alimentary, intrauterine, or galactophorous routes [12]. It has a wide variety of species as hosts, both domestic and wild, such as cervids (red deer, roe deer, fallow deer) and wild boar, cattle, goats, and pigs, among others, also including humans [13]. Consuming contaminated water, food, and pasture is a source of indirect transmission of great importance in game–livestock interface areas [10]. Hence, it is an important pathology in a multispecies scenario, considering that in the Iberian Peninsula, large game species such as wild boar and deer are reservoirs of mycobacteria with evidence of transmission to cattle [13].
It is difficult to strategically manage shared diseases at the livestock–wildlife interface [14]. In practice, there is a lack of integrated health surveillance in populations of wild species, such as large game [15], thus putting domestic cattle, especially those in extensive systems, at risk.
Several factors promote interactions at the game–livestock interface, making it important to understand them and strengthen their study at the in-site level [10]. The presence of diseases shared between wild and domestic species, such as TB, may indicate the persistence of inter-species transmission risk factors and their control should include all host species [16]. In general, the factors of greatest risk at the local level in the game–livestock interface depend on the availability and distribution of water; the abundance, distribution, and management of pastures; livestock handling; and the management of hunting and its by-products [10]. Any risk mitigation plan for TB at the aforementioned interface must be based on the specific risk factors found in each analyzed area, and the control measures must be based on biosecurity [14].
Knowing the specific scenario (area, present species, facilities, daily work routine, existing diseases…) and applying good biosecurity and management practices in game and livestock are crucial points in combating TB in a multi-species scenario [14]. As a general public health problem, its implications lie specifically at the level of potential zoonotic transmission, since there are several described cases of tuberculosis in humans of animal origin, with strict epidemiological links to the management of both wild and livestock animals [17,18].
The objective of this work is to statistically analyze the association between the existence of TB in areas with a marked game–livestock interface, with various complementary factors found in extensive farms in the southern region of Portugal, such as the number of animals of each large game species present in the territory and its frequency of sightings. It is also aim of the study, clearly, to test whether higher densities of large game species are significantly associated with ongoing TB in domestic herds under extensive management.
2. Materials and Methods
This study supported an assessment of biosecurity in areas with higher densities of large game that are also dedicated to livestock farms in an extensive regime in the southern region of Portugal, known as Alentejo.
The main goals of this action are to identify risk factors that lead to the maintenance of various pathogenic agents in circulation, namely TB, among domestic and wild cohabitant species, as well as to apply biosecurity practices.
2.1. Sampling
A total of 30 farms in the southern part of Portugal that are dedicated to significant livestock farming and are located in locations with a higher density of large game species make up the sample. In 2021, the same two evaluators conducted an interview and a guided tour of each farm to gather the data used in this study.
2.2. Survey
The 195 questions in the survey are divided into several categories on both domestic and game species management, such as game, livestock, and facilities management; the history of diseases on the farm and in large game species; and the type of fencing. A relationship was also established between the survey responses and a biosecurity score (0 to 100 scale) for each evaluated area.
The data processing was divided into descriptive and quantitative statistical analyses, conducted in RStudio™ 3.6.1 and Microsoft Excel™ 2016, with some graphical representations created using Microsoft Power BI™.
2.3. Inferential Statistical Analysis
Data obtained through the responses to the survey were entered into a database created in Microsoft Excel™ 2016. The percentage of each response was then calculated for categorical variables.
The statistical analysis was performed in RStudio™ 3.6.1. The Shapiro–Wilk test was applied to verify the normality between numerical variables when the p-value was greater than 0.05. The Kruskal–Wallis test was applied to evaluate the difference in a numerical variable between categorical groups. This test compares the means of the groups with each other when at least one of the variables does not show the normality of the data. In the case of this statistical test, for p-values less than 0.05, the null hypothesis was rejected, considering that at least 2 groups present significant differences. The Dunn test was also applied when the p-value of the Kruskal–Wallis test was less than 0.05 in order to determine, by comparison between 2 groups, which groups are different.
For the analysis of the statistical association between categorical/nominal variables, the chi-square test was used. For p-values < 0.05, it means that the null hypothesis was rejected and that there are statistically significant differences between the groups.
In the results, the analyses of association between variables with a p-value < 0.05 from the applied statistical test will be highlighted, thus focusing on those considered statistically significant.
3. Results
3.1. Sample Characterization
A total of 30 randomly selected sample farms were invited and sampled in the southern region of Portugal (“Alentejo area”), of which 40% (n = 12) belong to the district of Portalegre, 36.7% (n = 11) to Beja, 20% (n = 6) to Évora, and 3.3% (n = 1) to Setúbal. About 50% of the hunting zones are located in areas considered high risk for TB in large game (Figure 1).
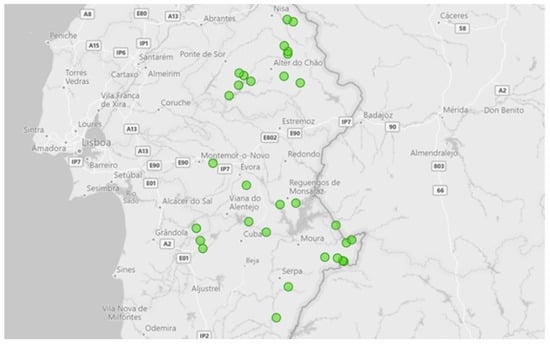
Figure 1.
Geographical distribution of the sampled farms represented by the green dots.
Regarding the dimensions of the analyzed areas, they range between 200 and 5268 hectares, with an average of 71% of the area being used for livestock activities and the entirety for hunting purposes.
On average, the number of livestock animals in the farms and analyzed area is 438 (minimum 72; maximum 1430). In turn, the density of domestic animals per area of surface used for their production varies from 0.1 to 2.7 animals/ha, with a large portion of the farms (76.7%) having a density of less than 1 animal/ha.
Regarding the number of large game animals recorded in the sampled areas, an average of 396 animals are present, with a density per area used for hunting activities of approximately 18 animals/100 ha, considered a high density as it exceeds 10 animals per 100 ha according to Rosell et al. (2001) [19]. The wild boar is the most commonly sighted species and has the highest density.
The biosecurity scores obtained from the 30 sampled areas range from 50.82 to 77.16 points out of 100.
3.2. Disease Occurrence
Regarding the disease occurrence in the 30 analyzed areas, some diseases in domestic animals have been recently diagnosed in 61.5% (n = 17) of the areas, accounting for their record since the year 2016. TB was mentioned in 36.7% (n = 11).
Regarding large game, 28.6% (n = 8) of the areas have recorded the presence of diseases in these species and another 25% (n = 7) are unaware, while the remaining 46.4% (n = 13) have not detected any diseases. The disease that was most detected in the game species was TB, in 28.6% (n = 8). In addition, 7 sampled areas (23.3%) presented TB simultaneously in cattle and in large game. However, it was assumed that the majority of respondents were not aware of the types of diseases, especially in wild animals, around the analyzed areas, due to their lack of responses.
3.3. Inferential Statistics—Complementary Factors vs. TB Occurrence
In the general statistical analysis of the survey categories, several categories have statistically significant associations (p-value < 0.05). It is worth highlighting the statistical association between the total biosecurity score and the occurrence of diseases in the animals present in the analyzed areas (Spearman rank test, p-value < 0.05). Additionally, when looking specifically for the occurrence of TB, this statistical association was also found (one-way ANOVA, p-value = 0.001).
To evaluate the association between the presence of animal TB in the biosecurity scores (BS) of the analyzed area, the T-test was used. However, it was found that there is no statistically significant difference in the average of the different scores between the group in which TB was detected and the group in which it was not detected (p > 0.05) (Figure 2).
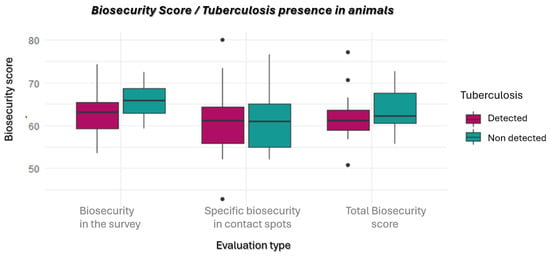
Figure 2.
Biosecurity scores according to the presence or absence of TB in domestic and/or large game in the analyzed area. It is possible to observe the distribution of the scores obtained in the survey, as well as at the observed contact spots (especially at feeding and watering points) and the total scores by the two categories: TB detected vs. not detected.
Influence of the Number of Large Game Species on the Presence of TB
The evaluation of the association between the total number of large game animals sighted in the analyzed areas, as well as the number of wild boars and the number of red deer, with the presence of animal TB, proved to be statistically significant (p < 0.05).
The categorical variable of the presence of animal TB was divided into three groups: farms with TB detected only in domestic animals; farms with TB detected in domestic and large game; and farms where TB was not detected. The associations of the total number of large game species sighted on the farm, as well as the individual numbers of wild boar and deer, with the categorical variable of the presence of animal TB were statistically significant (p < 0.05). Table 1 displays the statistical tests used and their outcomes.

Table 1.
Statistically significant differences between the means of the total number of large game animals, the number of wild boar [mean (sd): 105 (120,16)], and the number of red deer [mean (sd): 158 (387,18)] per group for the variable of animal TB presence.
The graph in Figure 3 shows the distributions of the number of large game animals, including all species (mouflon, fallow deer, red deer and wild boar), and only the average number between deer and wild boar, over the three defined groups of the animal TB presence variable.
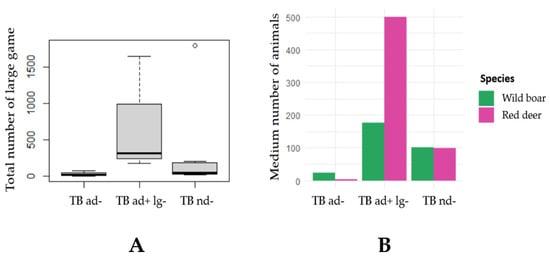
Figure 3.
Distribution of the number of large game animals according to the variable of the presence of animal TB. (A) Boxplot plot showing the distribution of the total number of large game animals on the sampled farms by the three groups of animal TB: “TB ad−”—presence of TB in domestic animals (p < 0.05); “TB ad + lg−”—presence of TB in domestic animals and large game (p < 0.05); “TB nd−”—TB not detected. (B) Bar chart showing the distribution of the average number of large game animals over the three groups of animal TB and according to species, namely wild boar and deer.
To assess the association between the frequency of sightings of large game species in the farms with the variable of the presence of animal TB, five groups were defined and presented in Table 2.

Table 2.
Large game animal sighting frequency groups.
According to the chi-square test, there is a significant influence of the frequency of sighting of deer on the presence of TB (p = 0.005 < 0.05), with a difference detected between the very high frequency and the group in which TB is detected in domestic and large game species (p = 0.00 < 0.05) and the group in which it is not detected (p = 0.049 < 0.05), with mostly null red deer sighting frequency.
4. Discussion
The statistical results presented here provide an overview of this large game–livestock–human interface, mainly belonging to the southern region of Portugal (Alentejo), where the highest number of hunted animals of large game species in Portugal is recorded.
The present investigation is part of a broader project that describes a biosecurity scoring (BS) system based on the risk of disease transmission in this interface area between livestock and large game, integrating extensive production systems. This study indicates that the biosecurity scores among the sampled areas show some variation. The respective average scores are around 60 (out of 100), demonstrating that improvement in this area is possible.
There is a wide dispersion regarding the number of larger game species recorded on the analyzed farms, which suggests that their distribution varies greatly among them. Only 56.7% of the respondents gave an estimate of the number of wild animals that roam in their area, with some uncertainty. This indicates a gap in knowledge regarding the densities of large game species in the field. The average number of red deer recorded per area was higher than that of wild boars, despite the greater variation in their distribution, with a maximum value of 1500 recorded, unlike the wild boar whose distribution is more homogeneous among the areas.
An indirect assessment of the presence of wild species in the analyzed areas was carried out, similar to that developed by Cowie et al. (2014) [20], through their sighting frequency and activity. In most areas, it is possible to infer that there are sightings and detections of signs of wild boars and small game species, such as badgers and foxes. The wild boar was the species of large game most present in all areas, with a relatively high sighting frequency, mostly from daily to weekly.
On the other hand, the large game species less present in this sample include the fallow deer, the mouflon, and the roe deer, as observed in other Mediterranean areas such as Spain [21]. Similarly, data provided by Portuguese environmental services indicate that the major large game species with the greatest presence in Portugal is the wild boar, which is dispersed throughout the continent with an increasing density over time, followed by the red deer and the fallow deer, while hunting for mouflon and roe deer is scarce [22].
An adequate health management strategy must include correct diagnosis, isolation, and recording of diseases in all species presented in the managed area [23]; therefore, in this case, it is extremely important to know which diseases are circulating in the animals that are present. Although wild ungulates are susceptible to diseases similar to those affecting domestic animals, vaccination for these diseases in game species is still debatable, and diagnosing these species is not a common practice [24]. As previously mentioned, the most reported was TB, since this is often detected by initial examination after hunting actions.
Regarding the farms’ records concerning the detection of TB in livestock and large game, 23.3% of the analyzed areas show TB in both species. Regardless of the origin of the infection, there are considerable densities of large game species in these areas, namely wild boars and red deer, which can be a source of transmission of the mycobacteria and be associated with the detection of TB in domestic and large game animals compared to the group where TB was detected only in domestic animals (p-value = 0.03). In these areas, a higher number of red deer being associated with the presence of TB in both large game and livestock species was confirmed when compared to the group in which TB was not detected (p-value = 0.01) and in the group where it was only detected in domestics (p-value = 0.01), in addition to its high sighting frequency significantly differing between the presence of TB in both species (p-value = 0.00) and the group where it is not detected (p-value = 0.049). In this case, the transmission of TB at the domestic–wildlife interface can be explained by the possible interaction in extensive areas through the shared use of space, watering points, and feeding areas, with special emphasis on red deer, which are observed more frequently and constitute the species whose behavior more easily allows direct contact with cattle. This can be used to explain how TB transmission occurs at this large game–livestock interface. Furthermore, it has been demonstrated by Santos et al. (2015) [25] that red deer has a higher risk of being a super-shedder compared to the wild boar, meaning that it is able to excrete mycobacteria DNA in higher concentrations.
It should also be noted that the circulation of TB is not linear and depends on several factors; as previously explained in this work, there are several environmental risk/confounding factors involved in this dynamic. Human activity, environmental factors, and animal behavior patterns can all impact on this [4]. One factor is that although large game and cattle have different movement and feeding patterns (cattle more diurnal and game more nocturnal/dawn/dusk), their watering and feeding locations may be the same, leading to indirect transmission and thus influencing the dynamics of TB in this interface. Another environmental factor to consider as a confounding factor is periods of drought and rain, as it has been statistically and scientifically proven that these also influence the appearance of lesions compatible with TB in larger game and thus influence the circulation and potential transmission to cattle [26,27]. It is therefore important to always take these environmental factors into account in order to evaluate all the parameters involved in the dynamics of TB at the game–cattle–human interface.
Based on our preliminary statistical analysis, the presence of large game in multi-species scenarios, specifically in areas with a marked large game–livestock interface, is a key factor for the maintenance of TB in these locations. Due to Portugal’s proximity to Spain, especially in areas where there is a higher risk of TB infection, it is crucial to apply a general biosecurity tool like the one used in this project that helps to improve the alignment and structure of natural resource management strategies across borders.
4.1. Implications for the Zoonotic Potential of TB
With the results discussed in this work, it is also important to discuss the maintenance of animal tuberculosis at the game–livestock–human interface, characterizing the risk of potential zoonotic transmission of this agent [28]. There are numerous scientific publications where this problem is addressed, as it is epidemiologically known that the maintenance of TB in both wild animals and livestock is a risk for all wild animal handlers. There is a multiplicity of mycobacteria with zoonotic potential, with almost all of them having animal hosts, such as Mycobacterium bovis, M. caprae, or M. orygis [29,30]. In Portugal, the existence of various zoonotic transmission lines of TB is known, usually more linked to livestock, but the possibility of epidemiological links of human TB cases originating from wildlife is suggested [31,32]. Thus, it is of utmost importance to conduct a multi-factorial and descriptive analysis of all these multi-host communities, focusing on the marked game–livestock–human interfaces, where TB circulates and persists over several years, causing economic losses, but also where these potentially infected animals serve as a vehicle for zoonotic infection to all their handlers. These “animal/carcasses handlers” come from various backgrounds, and we can consider those who are only related to livestock, those related to hunting, and those who are exposed accidentally. In animal production, we have everything from farmers and veterinarians to visitors of farms potentially infected with TB, who can be vehicles for transporting the mycobacteria to other areas. Related to hunting, we have many more humans potentially exposed, such as hunters, game managers, veterinarians, and consumers of game meat. In this situation, consumers of meat or products derived from animals infected with TB do not play a strong role in the transmission of TB at the mentioned interface. In this case, TB is not so much a foodborne issue, but more of an occupational issue/potential risk [33,34]
However, in certain high-risk areas, particularly those with significant populations that regularly come into contact with animals, their by-products, and their carcasses, this risk cannot be understated or disregarded [35]. Various scientific investigations support the risk of occupational disease in the case of TB, in people who come into direct contact with infected animals such as farmers, veterinarians, and others, but also in game handlers, such as hunters or game managers [33,34,36]. Direct contact with live animals potentially infected with TB, such as livestock, and careless handling of carcasses of hunted species are critical points at the occupational health level. In many areas, there is a clear lack of awareness and knowledge related to these issues among these at-risk populations. The lack of application of preventive measures and the use of personal protective equipment that allows for the creation of a barrier between humans and animals/carcasses infected with TB is evident [33,37]. Focusing on these already identified flaws, more information and training actions should be promoted in order to promote more care and strategies to mitigate the potential risk of zoonotic transmission.
Potential zoonotic tuberculosis infection in these situations can be regarded as a serious public health concern. It is of great importance to alert these at-risk populations and train them to acquire protective equipment and apply biosecurity measures that ensure a true mitigation of the risk of zoonotic transmission. From a zoonotic risk mitigation perspective at the game–livestock–human interface, the cooperation of all entities, such as scientists, environmental and sanitary authorities, livestock and game associations, farmers, hunters, human health professionals, and all those who may come into direct contact with the problem daily is considered to be imperative [33].
4.2. Limitations of the Preliminary Statistical Study
The authors acknowledge some limitations in this study. In the present study, a large part of the analysis focused on descriptive aspects (observational study), and some of the results of the statistical analysis may not be conclusive due to the limited sample size and the lack of direct testing, without laboratory confirmation, as mentioned previously. We have noted several deviations in the work that may have an impact, such as underreporting of TB infection in cohabitant game species or differences in sampling effort; however, we highlight the preliminary nature of the work and the fact that this work is based on fieldwork and field/owner information.
The authors expect that in future research, the sample size will be expanded and direct observational data and farms from other regions of Portugal will be included, which would provide a more solid basis for robust statistical conclusions, thus transforming this work into a more advanced intervention study.
On the other hand, the weights assigned to the biosecurity parameters evaluated in the questionnaire consist of a subjective estimate of their importance, which may be debatable. Also, the data provided by the interviewees in the analyzed areas may be biased, as they may not have provided all the answers according to reality due to the pressure of being evaluated or due to lack of knowledge. Future work will consider the biosecurity questionnaire, its validation, and the evaluation of the results.
However, it is a relevant piece of work, even as a preliminary statistical study, to bring the discussion of maintenance hosts of tuberculosis in a multi-host community and the potential risk of inter-species and zoonotic transmission to the attention of the scientific community.
5. Conclusions
A higher number of large game species, including red deer and wild boar, was confirmed on the farms with TB detected in both domestic and large game species. This group also showed a higher frequency of red deer sightings which highlights the role of red deer in spreading TB to livestock. We believe that in a multi-species scenario, large game species are a crucial component in the maintenance of TB.
It is also important to note that the risk of disease transmission may vary depending on the region and the specific wildlife and livestock populations involved. Therefore, targeted surveillance and management strategies should be developed based on local conditions and the specific risks involved. The existence of various management strategies based on in vivo tests is known. These should play a crucial role in the management and fight against TB at the interface studied in this work. There are several recently published works that address the interferon gamma assay as being quite a reliable test, with a lower impact of human error than the intradermal tuberculin test. It is necessary to evaluate these pre-slaughter surveillance tests as points of interest in the mitigation of TB at the interface.
Various measures to mitigate the risk of multi-species TB transmission at the aforementioned interface should be based on wild species. Several perspectives must be taken into account, such as a robust sampling of wildlife or better movement controls for red deer or wild boar.
Apart from everything discussed, this issue should also be addressed from the perspective of TB mitigation in a multi-host animal community, human intervention, and the potential zoonotic transmission between animals and handlers. The strategies to be considered in this highly marked interface are more than just measures of animal health, they are also ones of community/human health. Thus, a truly inclusive strategy must be considered, encompassing all stakeholders and factors present that enhance the maintenance of TB in a marked game–livestock–human interface.
Author Contributions
Conceptualization, M.P.F. and A.C.A.; methodology, M.P.F., Y.V., M.V.-P. and A.C.A.; software, M.P.F.; validation, Y.V. and A.C.A.; formal analysis, A.C.A.; investigation, M.P.F.; data curation, M.P.F.; writing: M.P.F. and A.C.A.; visualization, M.P.F.; supervision, M.V.-P., Y.V. and A.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Technical project C3C project (number 2020403001002) by Fundo Florestal Permanente (FFP) from the Portuguese Institute of Nature Conservation and Forests (ICNF). And scientific support by UIDP/00772/2020 and LA/P/0059/2020 of Fundação da Ciência e Tecnologia de Portugal.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data from this study can be obtained by requesting it from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gortázar, C.; Ferroglio, E.; Höfle, U.; Frölich, K.; Vicente, J. Diseases shared between wildlife and livestock: A European perspective. Eur. J. Wildl. Res. 2007, 53, 241–256. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, S.; Laguna, E.; Vicente, J.; García-Bocanegra, I.; Martínez-Guijosa, J.; Cano-Terriza, D.; Risalde, M.A.; Acevedo, P. Characterization and management of interaction risks between livestock and wild ungulates on outdoor pig farms in Spain. Porc. Health Manag. 2022, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Huyvaert, K.P.; Russell, R.E.; Patyk, K.A.; Craft, M.E.; Cross, P.C.; Garner, M.G.; Martin, K.M.; Nol, P.; Walsh, D.P. Challenges and opportunities developing mathematical models of shared pathogens of domestic and wild animals. Vet. Sci. 2018, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- VanderWaal, K.; Enns, E.A.; Picasso, C.; Alvarez, J.; Perez, A.; Fernandez, F.; Gil, A.; Craft, M.; Wells, S. Optimal surveillance strategies for bovine tuberculosis in a low-prevalence country. Sci. Rep. 2017, 7, 4140. [Google Scholar] [CrossRef] [PubMed]
- Antolin, M.F.; Biggins, D.E.; Gober, P. Symposium on the ecology of plague and its effects on wildlife: A model for translational research. Vector-Borne Zoonotic Dis. 2010, 10, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Bengis, R.G.; Kock, R.A.; Fischer, J. Infectious animal diseases: The wildlife/livestock interface. Rev. Sci. Tech. (Int. Off. Epizoot.) 2002, 21, 53–65. [Google Scholar]
- Conner, M.M.; Ebinger, M.R.; Blanchong, J.A.; Cross, P.C. Infectious disease in cervids of North America: Data, models, and management challenges. Ann. N. Y. Acad. Sci. 2008, 1134, 146–172. [Google Scholar] [CrossRef] [PubMed]
- OIE. Guidelines for Wildlife Disease Surveillance: An Overview; World Organization for Animal Health: Paris, France, 2015; Available online: https://www.woah.org/app/uploads/2021/03/oie-guidance-wildlife-surveillance-feb2015.pdf (accessed on 8 December 2021).
- Santos, N.; Richomme, C.; Nunes, T.; Vicente, J.; Alves, P.C.; de La Fuente, J.; Gortázar, C. Quantification of the animal tuberculosis multi-host community offers insights for control. Pathogens 2020, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Guijosa, J.; Acevedo, P.; Balseiro, A.; García-Bocanegra, I.; Sáez-Llorente, J.L.; Vicente, J.; Gortázar, C. Medidas de Bioseguridad en Explotaciones Extensivas de Ganado Bovino. In Manual para la Actuación Frente a la Tuberculosis en Fauna Silvestre; GOSTU: Gotsu, Japan, 2021; ISBN 978-84-09-31694-6. Available online: https://www.irec.es/wp-content/uploads/2021/07/Manual-Tuberculosis-explotaciones-ganaderas-ganado_compressed.pdf (accessed on 16 July 2025).
- Azami, H.Y.; Zinsstag, J. Economics of bovine tuberculosis: A one health issue. In Bovine Tuberculosis; CAB International: Wallingford, UK, 2018; pp. 31–42. [Google Scholar]
- Ferreira, E.M.; Duarte, E.L.; Cunha, M.V.; Mira, A.; Santos, S.M. Disentangling wildlife–cattle interactions in multi-host tuberculosis scenarios: Systematic review and meta-analysis. Mammal Rev. 2023, 53, 287–302. [Google Scholar] [CrossRef]
- DGAV. Estratégia Sanitária para as Espécies Cinegéticas; Proposta: Portugal; Direção Geral de Alimentação e Veterinária: Lisbon, Portugal, 2015. [Google Scholar]
- Abrantes, C.; Vieira-Pinto, M. Interfaz Ganado-Fauna: Enfermidades Compartidas, Factores de Riesgo y Control. Ruminews Junio. 2021. Available online: https://rumiantes.com/interfaz-ganado-fauna-enfermedades-compartidas-factores-de-riesgo-y-control/ (accessed on 18 September 2021).
- Martinez-Guijosa, J.; Acevedo, P.; Balseiro, A.; García-Bocanegra, I.; Sáez-Llorente, J.L.; Vicente, J.; Gortázar, C. Programas de Mejora Sanitaria en Terrenos Cinegéticos para el Control de la Tuberculosis en Fauna Silvestre. In Manual para la Actuación Frente a la Tuberculosis en Fauna Silvestre; GOSTU: Gotsu, Japan, 2021; ISBN 978-84-09-31694-6. Available online: https://www.irec.es/wp-content/uploads/2021/07/Manual-Tuberculosis-explotaciones-ganaderas-ganado_compressed.pdf (accessed on 16 July 2025).
- Barroso, P.; Barasona, J.A.; Acevedo, P.; Palencia, P.; Carro, F.; Negro, J.J.; Torres, M.J.; Gortázar, C.; Soriguer, R.C.; Vicente, J. Long-term determinants of tuberculosis in the ungulate host community of doñana national park. Pathogens 2020, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Sánchez, L.P.; Pérez, S.; Herrera, L.; Jiménez, M.S.; Samper, S.; Iglesias, M. Human tuberculosis due to Mycobacterium bovis and M. caprae in Spain, 2004–2007. Int. J. Tuberc. Lung Dis. 2009, 13, 1536–1541. [Google Scholar] [PubMed]
- De la Rua-Domenech, R. Human Mycobacterium bovis infection in the United Kingdom: Incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis 2006, 86, 77–109. [Google Scholar] [CrossRef] [PubMed]
- Rosell, C.; Fernández-Llario, P.; Herrero, J. El Jabalí (Sus scrofa Linnaeus, 1758). Galemys. 2001. Available online: https://www.researchgate.net/publication/228522052_El_Jabali_Sus_scrofa_Linnaeus_1758 (accessed on 10 May 2022).
- Cowie, C.E.; Beck, B.B.; Gortazar, C.; Vicente, J.; Hutchings, M.R.; Moran, D.; White, P.C.L. Risk factors for the detected presence of Mycobacterium bovis in cattle in south central Spain. Eur. J. Wildl. Res. 2014, 60, 113–123. [Google Scholar] [CrossRef]
- Bencatel, J.; Sabino-Marques, H.; Álvares, F.; Moura, A.E.; Barbosa, A.M. (Eds.) Atlas de Mamíferos de Portugal, 2ª Edição; Universidade de Évora: Évora, Portugal, 2019. [Google Scholar]
- Carvalho, J.; Hipólito, D.; Teixeira, D.; Fonseca, C.; Torres, R.T. Hunting bag statistics of wild mammals in Portugal (1989–2022): On the need to improve data report and compilation. Eur. J. Wildl. Res. 2024, 70, 96. [Google Scholar] [CrossRef]
- Dewulf, J.; Immerseel, F.V. (Eds.) General principles of biosecurity in animal production and veterinary medicine. Biosecurity in animal production and veterinary medicine: From principles to practice. In Biosecurity in Animal Production and Veterinary Medicine: From Principles to Practice; ACCO: Hague, The Netherlands, 2018; pp. 63–76. [Google Scholar]
- Balseiro, A.; Thomas, J.; Gortázar, C.; Risalde, M.A. Development and Challenges in Animal Tuberculosis Vaccination. Pathogens 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Almeida, V.; Gortázar, C.; Correia-Neves, M. Patterns of Mycobacterium tuberculosis-complex excretion and characterization of super-shedders in naturally infected wild boar and red deer. Vet. Res. 2015, 46, 129. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, A.C.; Serejo, J.; Vieira-Pinto, M. Drought as a Risk Factor to the Occurrence of Tuberculosis-like Lesions Detected during Red Deer Post-Mortem Inspection: A Pilot Study. REDVET-Rev. Electrónica Vet. 2022, 23. Available online: https://www.veterinaria.org/index.php/REDVET/article/view/155 (accessed on 16 July 2025).
- Abrantes, A.C.; Serejo, J.; Vieira-Pinto, M. The association between Palmer Drought Severity index data and tuberculosis-like lesions occurrence in Mediterranean hunted wild boars. Animals 2021, 11, 2060. [Google Scholar] [CrossRef] [PubMed]
- Kock, R.; Michel, A.L.; Yeboah-Manu, D.; Azhar, E.I.; Torrelles, J.B.; Cadmus, S.I.; Zumla, A. Zoonotic tuberculosis–the changing landscape. Int. J. Infect. Dis. 2021, 113, S68–S72. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Saleem, M.H.; Yasmeen, R.; Safdar, A.; Ahmad, W.; Asif, M.; Bokhari, A.; Aslam, I. Tuberculosis in Wild Animals a Great Threat for Wildlife Conservation and Public Health—A Review. J. Wildl. Biodivers. 2023, 7, 129. [Google Scholar]
- Thoen, C.O.; Steele, J.H.; Kaneene, J.B. (Eds.) Zoonotic Tuberculosis: Mycobacterium Bovis and Other Pathogenic Mycobacteria; Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Canhão-Dias, M.; Pires, T.M.; Henriques, R.; Lopes, D.G.; Madeira de Carvalho, L.M. Zoonoses as important causes of hospital admissions: A 15-year study in Portugal. Port. J. Public Health 2022, 40, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Quelhas, C.; Mendes, M.; Rosa, P.; Cavaco, S. Mycobacterium caprae: Um agente zoonótico ignorado da tuberculose humana. Bol. Epidemiológico Obs. 2023, 12, 34–37. [Google Scholar]
- Abrantes, A.C.; Serejo, J.; Vieira-Pinto, M. Risk Practices for Occupational Zoonotic Exposure to Tuberculosis in a High-Risk Population in Portugal. Trop. Med. Infect. Dis. 2023, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Khattak, I.; Mushtaq, M.H.; Ahmad, M.U.D.; Khan, M.S.; Haider, J. Zoonotic tuberculosis in occupationally exposed groups in Pakistan. Occup. Med. 2016, 66, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Marcotty, T.; Matthys, F.; Godfroid, J.; Rigouts, L.; Ameni, G.; Gey van Pittius, N.; Kazwala, R.; Muma, J.; van Helden, J.; Walravens, K.; et al. Zoonotic tuberculosis and brucellosis in Africa: Neglected zoonoses or minor public-health issues? The outcomes of a multi-disciplinary workshop. Ann. Trop. Med. Parasitol. 2009, 103, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Adesokan, H.K.; Akinseye, V.O.; Sulaimon, M.A. Knowledge and practices about zoonotic tuberculosis prevention and associated determinants amongst livestock workers in Nigeria; 2015. PLoS ONE 2018, 13, e0198810. [Google Scholar] [CrossRef] [PubMed]
- Sharan, M.; Vijay, D.; Yadav, J.P.; Bedi, J.S.; Dhaka, P. Surveillance and response strategies for zoonotic diseases: A comprehensive review. Sci. One Health 2023, 2, 100050. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).