Simple Summary
West Nile virus (WNV) infection is a significant public health concern in Europe and Italy is one of the most affected countries since 2008. This brief report describes circulation cases of West Nile virus lineage 1 and lineage 2 strains occurring at the end of the 2022 vector season in Sicily and Tuscany, regions where no strains had ever been sequenced. The genetic characterization of the WNV strains responsible for these cases confirmed the peculiar WNV Italian scenario, marked by endemic Italian circulation spreading to new areas of the country (Tuscany case) and novel introductions from different European and Italian areas (Sicilian episode). It highlights the importance of continuous molecular surveillance for the early detection of viral circulation throughout the country and the monitoring of the arrival of new strains and the evolution of novel variants, in order to trigger adequate measures and limit its spread and human infections.
Abstract
West Nile virus (WNV) (Flaviviridae, Flavivirus) infection is a mosquito-borne zoonosis able of causing disease and death in humans and animals. Over the past decade, WNV infections have been a significant public health concern in Europe, and Italy has been among the most affected countries since 2008. The 2022 vector season has been characterized by an intense and early circulation of WNV. This report describes cases of co-circulation of WNV L1 and of WNV L2 occurring at the end of the 2022 vector season in Sicily and Tuscany, regions where no strains had ever been sequenced. The phylogenetic analysis of the detected strains confirmed the peculiar WNV scenario that has characterized the Italian West Nile disease (WND) epidemic since its appearance. The circulation observed in Tuscany was in fact a consequence of the spread of endemic strains to new areas while the Sicilian episodes were linked to new introductions of WNV L1 and L2 strains likely from other European countries.
1. Introduction
West Nile virus (WNV) (Flaviviridae, Flavivirus) infection is a mosquito-borne zoonosis capable of causing disease and death in humans and other animals. In recent decades, WNV infection has been spreading in many European countries [1], becoming a major public health concern in Europe. Italy has been severely affected since 2008 [2], and the first evidence of WNV circulation was in the Tuscany region in 1998 [3].
In 2002, the Italian Ministry of Health issued a surveillance plan aiming at monitoring the eventual introduction and spread of the virus. The plan has been updated every year according to eventual new epidemiological scenarios. In 2016, in line with a “One-Health approach”, human, veterinary and entomological surveillance have been combined in a unique plan [4]. The main goal of the program is the early detection of WNV circulation in order to trigger adequate measures and limit its spread and human infections through the substance of human origins (SoHO) [5].
In Italy, the 2022 vector season was marked by an early onset and a large number of WNV infections confirmed in mosquitoes and birds since June. The first human case was reported on 19 June [6]. At the end of 2022, the Italian surveillance system recorded 588 cases of WNV infection in humans, 82 in equids, 157 confirmed cases in residential birds (carrion crow, magpie and Eurasian jay) and 210 in wild birds. Furthermore, WNV was also detected in 246 mosquito pools [7]. Another important peculiarity of the 2022 vector season was the co-circulation of WNV lineage 1 (L1) and lineage 2 (L2) in many areas of the country [8].
This study describes cases of circulation of WNV L1 and L2 strains occurring at the end of the 2022 vector season in Sicily and Tuscany, regions where, for a long period of time, no outbreaks were reported and therefore no strains had ever been sequenced. The genetic characterization of the WNV strains responsible for these cases gave us the opportunity to update the recent phylogenetic tree of the Italian WNV strains [9] and better understand the epidemiology of WNV in these areas.
2. Materials and Methods
2.1. Sample Collection
Between late October and early November 2022, three horse samples, one serum and two tissue samples, and a pool of Culex pipiens were sent to the National Reference Center for Foreign Animal Diseases (CESME) at the Istituto Zooprofilattico Sperimentale of Abruzzo and Molise in Teramo (IZSAM) in Teramo. They were tested positive for Flavivirus by the Regional Reference Laboratories at the Istituto Zooprofilattico Sperimentale (IZS) competent for geographical area and sent to IZSAM for WNV confirmation. All horse samples were from Sicily. The serum and one of the two tissue samples were from horses from the Catania province. In particular, the tissue samples, including the spinal cord, the brain and the medulla oblongata were from a horse from the municipality of Paternò (37.451539, 14.847692) that was euthanized after showing severe neurological disorders. The serum sample was, instead, from a clinical healthy horse housed in close proximity to a WND human case in the municipality of Ramacca (37.4388, 14.8149). The second tissue sample consisted of the spinal cord of a horse in the Partinico municipality (38.01636, 13.021947) in the province of Palermo, which was euthanized due to the severity of its neurological impairment. The Cx. pipiens pool, collected during surveillance activities carried out following the occurrence of two human cases [7], originated from Chiesina Uzzanese municipality (43.83828, 10.73079), within Pistoia province in Tuscany.
2.2. Real-Time PCR Assay
Tissue samples and the mosquito pool were homogenized in sterile phosphate-buffered saline with antibiotics. Nucleic acids were then extracted from the homogenates and serum sample using the MagMAX CORE Nucleic Acid Purification Kit (Applied Biosystem, Thermo Fisher Scientific, Life Technologies Corporation, Austin, TX, USA). Amplification reactions were performed with specific one-step quantitative reverse transcription polymerase chain reactions (qRT-PCR) to detect Usutu virus (USUV) [10], WNV L1 and L2 [11], and all known lineages of WNV, as previously described [12].
2.3. Next-Generation Sequencing (NGS)
Based on qrt-PCR Ct values, WNV L1 and L2 positive samples were selected and subsequently sequenced through amplicon-based whole-genome sequencing by NGS. Specifically, cDNA was obtained through reverse transcription (RT) in a 20 μL reaction mixture, comprising 5X SSIV buffer, 50 µM Applied Biosystems™ random hexamer (Thermo Fisher Scientific, Waltham, MA, USA), 10 mM dNTPs mix, 100 mM DTT, 200 units SuperScript® IV Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA, USA), and 40 U RNAse OUT RNase inhibitor (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instructions. The reaction proceeded with incubation at 23 °C for 10 min, 50 °C for 10 min, and 80 °C for 10 min. Subsequently, 2.5 µL of the synthesized cDNA was amplified using a PCR master mix containing 5X Q5 reaction buffer, 10 mM dNTPs, 10 µM of two non-overlapping pools of WNV targeting primers as described by [13], 0.01 U/µL Q5® High Fidelity DNA polymerase (NEB, New England Biolabs, Ipswich, MA, USA), and 5X Q5 High Enhancer. The reaction was incubated at 98 °C for 30 s; 35 cycles of 95 °C for 15 s and 65 °C for 5 min; and a final cooling step at 4 °C. The resulting PCR product was purified using the Qiaquick PCR Purification kit (Qiagen, Leipzig, Germany) and quantified using the Qubit® DNA HS Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Amplified DNA was diluted to obtain a concentration of 100–500 ng. After this, library preparation ensued utilizing an Illumina DNA prep kit. Sequencing was then conducted on a NextSeq 500 (Illumina Inc., San Diego, CA, USA) using a NextSeq 500/550 Mid Output Reagent Cartridge v2 for 300 cycles with standard 150 bp paired-end reads. Quality control and trimming were performed with the FastP v0.23.1 [14] and FastQC tool v0.11.5 (Bioinformatics Group, Brabraham Institute, Cambridge, UK). WNV consensus sequences were obtained using iVar v 1.3.1 [15] after trimmed reads were mapped to specific WNV reference sequences (WNV L1 FJ483548 (Italy, 2008) and WNV L2 MN652880 (Greece, 2018)) by using Snippy (https://github.com/tseemann/snippy, accessed on 6 July 2023). A total of109 WNV L1 (including two new sequences, NRG1426_Sicily_2023 and NRG1424_Sicily_2023) and 195 WNV L2 genomes (with new L2 sequences, NRG49756_Tuscany_2022 and NRG50769_Sicily_2022) were included in this study.
2.4. Phylogenetic Analyses
Phylogenetic analyses were conducted for both WNV lineages including several genomes representative of different European and Italian geographic areas downloaded from NCBI by using a custom R script for automatic sequence retrieval. A Koutango virus (KOUTV) Senegal 2013 (EU082200) sequence was added as an outgroup for WNV L1 analysis, moreover two sequences was added as outgroups for WNV L2 analysis: WNV L1 Italy 2008 (FJ483548) and Italy 2020 (MW627239). All sequences were aligned using Unipro UGENE v.45.1 [16] with MUSCLE algorithms [17], and only coding region sequences were considered for further phylogenetic analyses. Maximum likelihood phylogenies of dataset with the 110 WNV L1 and 197 WNV L2 genomes were estimated using IQ-TREE v.1.6.12 [18] with command lines “iqtree –s “WNV_L1_CDS.fa” –st DNA –m TEST –bb 10000 –alrt 1000 –abayes” and IQ-TREE v.1.6.12 [18] with command line “iqtree –s AlignWNVL2_CDS_subalign.fa-st DNA –m TEST –bb 10000 –alrt 10000 –abayes”.
3. Results
3.1. WNV L1 Detection, Sequencing and Phylogenetic Analysis
All samples collected from the horses in Catania province were positive for WNV L1. In particular, the spinal cord from NRG1426_Sicily_2023 and the serum sample from NRG1424_Sicily_2023 showed Ct 12 and Ct 16, respectively, while brain and medulla oblongata showed a lower viral load (Ct 37). All samples were negative for WNV L2 and USUV. Based on the Ct values, the spinal cord from NRG1426_Sicily_2023 and the serum sample from NRG1424_Sicily_2023 were selected for NGS.
NGS produced a total of 489,559 and 363,558 mapped reads, respectively, for the spinal cord and serum samples, and the mapping analyses using iVar using West Nile virus strain 15217_Italy 2008 (accession no. FJ483548), recognized as the best reference by BLASTn analysis, produced a consensus sequences of 11,022 nt in length for the spinal cord sample and of 10,961 nt for the serum sample. Horizontal coverage (HCov) was 99% for both samples. The consensus sequences were published in the NCBI database under accession numbers OQ689696 (sequence name NRG1426_Sicily_2023) and OQ689697 (sequence name NRG1424_Sicily_2023).
Our maximum likelihood phylogeny identified seven distinct WNV L1 clusters, consistent with previous reports [19]. In this study, the phylogenetic analysis specifically highlighted the placement of the two newly identified Sicilian strains (accession numbers NRG1426_Sicily_2023 OQ689696 and NRG1424_Sicily_2023 OQ689697) in cluster 2 within the Western Mediterranean (W-Med) sub-cluster (Figure 1, highlighted in pale yellow). This cluster included isolates from various regions, such as Morocco (1996 and 2003), Italy (1998–2022), Israel (2000), France (2000, 2004 and 2015), Portugal (2004), Spain (2007, 2008 and 2015) and Senegal (2012–2018). Noteworthy is the close relation observed between the novel WNV strains from Sicily and those detected in the Campania region in 2020 and 2022 (Genbank MW627239 and OP850023) [20].
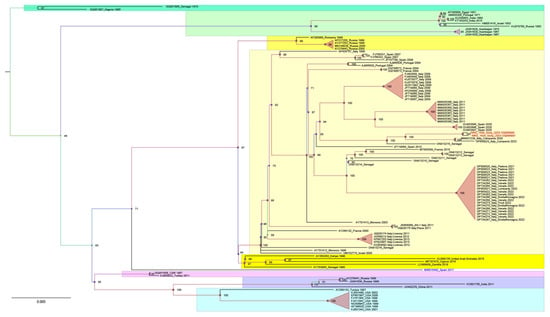
Figure 1.
A phylogenetic analysis was conducted including 109 WNV L1 genome sequences representative of different geographic regions and Koutango virus Senegal 2013 (EU082200) was added as an outgroup. All 110 sequences were aligned using the Unipro UGENE v.45.1 and MUSCLE algorithms. For the subsequent phylogenetic analysis, only the coding region of the sequences was considered. GenBank accession numbers are indicated for each strain, with the country and year of isolation. The new strains, NRG1426_Sicily_2023 and NRG1424_Sicily_2023, are highlighted in red. Model selection was carried out on all datasets using ModelFinder implemented in IQTree v.1.6.12; the best-fit model according to the Bayesian information criterion (BIC) was GTR+F+I+G4. Maximum likelihood phylogenies were estimated using IQ-TREE v.1.6.12 with the command line: “iqtree –s “WNV_L1_CDS.fa” –st DNA –m TEST –bb 10000 –alrt 1000 –abayes”.
3.2. WNV L2 Detection, Sequencing and Phylogenetic Analysis
WNV L2 was detected in the Cx. pipiens pool (Ct 27) from Chiesina Uzzanese and in the spinal cord of the horse from Palermo (Ct 35). Both samples were negative for WNV L1 and USUV.
A total of 259,181 and 133,115 mapped reads were produced by NGS. BLASTn analysis identified West Nile virus isolate Thessaloniki_MC82m/2018 (accession no. MN652880) as the best reference (99% identity) and this sequence was used to carry out mapping using the Ivar tool. A consensus sequence of 10,905 nt was obtained from the spinal cord with an HCov of 93% and a vertical coverage (Cov) of 1821. Similarly, from the mosquito pool, a consensus sequence of 10,871 nt with an HCov of 97% and a Cov of 3237 was achieved. Both sequences were published in the NCBI database with accession no. OQ204314 (sequence name NRG49756_Tuscany_2022) and OQ204315 (sequence name NRG50769_Sicily_2022).
Phylogenetic analysis carried out with 195 sequences revealed two main clades: the Central European Clade (CEC) (Figure 1, in yellow) and the South-Eastern European Clade (SEEC) (Figure 2, green). Notably, the CEC includes Italian sequences from strains circulating in the country since 2012. Our analysis distinctly separated the two new strains from Tuscany and Sicily, assigning them to distinct clusters. Specifically, the sequence from mosquitoes (Italy_Tuscany_2022_OQ204314) clustered within the Italian clade, aligning with all WNV L2 Italian isolates used for phylogenetic analysis. In contrast, the sequence from Sicily (OQ204315_Italy_Sicily_2022) was placed within the SEEC, alongside sequences from Greece, Serbia, Hungary, Slovakia, and Bulgaria, demonstrating its distinction from the circulating WNV strains in Italy.
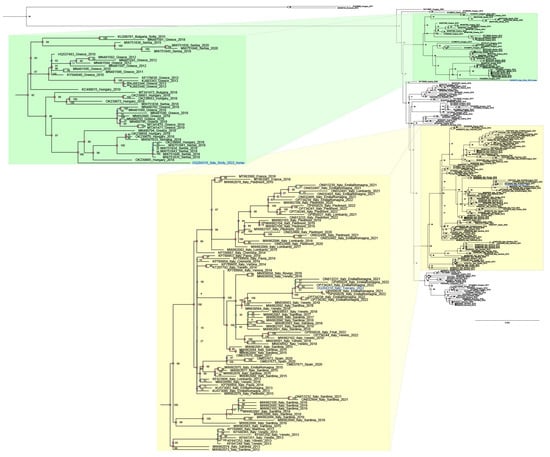
Figure 2.
A phylogenetic analysis was conducted including 195 WNV_L2 genome sequences representative of different geographic regions and 2 WNV L1 sequences added as outgroups (FJ483548 Italy 2008 and MW627239 Italy 2020). The outgroups’ sequences are not included in the provided figure. All 197 sequences were aligned using the Unipro UGENE v.45.1 and MUSCLE algorithms. For the subsequent phylogenetic analysis, only the coding region of the sequences was considered. GenBank accession numbers are indicated for each strain, with the country and year of isolation. Node branches are highlighted with different colours according to their posterior probabilities value. The new strains NRG49756_Tuscany_2022 OQ204314 and NRG50769_Sicily_2022 OQ204315 are highlighted in blue Model selection was carried out on all datasets using Model Finder implemented in IQTree v.1.6.12; the best-fit model according to the Bayesian information criterion (BIC) was GTR+F+I+G4. Maximum likelihood phylogenies were estimated using IQ-TREE v.1.6.12 with the command line: iqtree -s AlignWNVL2_CDS_subalign.fa -st DNA -m TEST -bb 10000 -alrt 10000 –abayes.
4. Discussion
The 2022 Italian vector season was characterized by an earlier start of the viral circulation [7] compared to previous years [6]. This was probably due to the higher average temperatures which resulted in favorable weather and ecological conditions for WNV vectors [21]. Specifically, warm conditions during the spring (April–May) might amplify earlier WNV transmission, resulting in a higher circulation during the following months.
Unlike the previous years, when WNV lineage 1 was sporadically observed in the North-Eastern regions (2012–2014, 2017), Sardinia (2015–2016) and Campania (2020) [19], in 2022, a similar intense circulation of both WNV lineages was constantly reported in many different areas of the country [22].
The 2022 WND epidemic season also gave us the opportunity to update the recently published phylogenetic tree of the Italian WNV strains [9,23], by including strains collected from regions where no or only outdated sequences were available.
Overall, our WNV L1 and L2 phylogenetic analyses confirmed what has been previously described [20]. The sequences of the WNV L1 strains are segregated into seven well-supported clusters. Both Sicilian sequences (OQ689696 and OQ689697) are in cluster 2 (in yellow in Figure 1), along with most of the European strain sequences retrieved by Genbank for this study. They are clustered in the Western Mediterranean (W-Med) group (Figure 1, pale yellow), together with the sequences of WNV strains from Italy, Spain, France, Portugal and Morocco. It is interesting to observe how these new sequences are genetically similar to those of the 2020 (MW627239) and 2022 (OP850023) Campanian WNV strains, which in turn are closely related to some older European (Spain 2010 and France 2015) and non-European WNV sequences (Senegal 2012, 2013 and 2016). The close phylogenetic relationship might suggest a possible Campanian origin for the Sicilian strain.
Even more interesting are the results of the genome sequencing of the other Sicilian WNV strain detected in the tissue sample from the horse from Palermo province (OQ204315). The Sicilian WNV L2 strain sequence does not cluster with those of the other Italian strains but is closer to the genome sequences of the WNV L2 Balkan strains. Based on these findings, it is likely that the Sicilian WNV L2 strain originated from the Eastern Mediterranean basin. However, with regard to the WNV L2 strains, it should be said that the WNV L2 strains have played a major role in the WNV Italian epidemiological scenario. Since their first appearance in Sardinia [24] and Veneto [25] in 2011, WNV L2 strains widely spread in the country. The phylogenetic analysis performed in this study revealed the presence of two main groups. The Central European clade encloses sequences of the isolates from Austria (2008–2016), Slovakia (2013–2014), Hungary (2015–2018), Germany (2018–2020), Czech Republic (2013), Serbia (2018) and Italy. The Italian sub-clade segregates the strains circulating in the country from 2012 to 2022. The South-Eastern European clade includes all sequences of the strains originating from the first introduction in Greece (2010) and those that then spread to the Balkan region. The sequence derived from the Tuscan Cx. pipiens pool (OQ204314) is within the Italian group, closer to the 2021 and 2022 Emilia Romagna sequences, which allows us to assume a WNV spread from Emilia Romagna region.
5. Conclusions
The phylogenetic analysis of the detected strains confirmed the peculiar WNV scenario that has characterized the Italian WND epidemic since its appearance. The circulation observed in Tuscany was in fact a consequence of the spread of endemic strains to new areas while the Sicilian episodes were linked to novel introductions of WNV L1 and L2 strains, likely from Campania and the Balkans. Moreover, the detection of viral circulation up to November suggests a modification in the epidemiology of WNV characterized by longer transmission seasons, which implies an increased risk of human infection. It also highlights the importance of continuous molecular surveillance for the early detection of viral circulation throughout the country to monitor the arrival of new strains and the evolution of novel variants.
Author Contributions
Conceptualization, F.V., F.M. and G.S.; methodology, F.V, O.P., M.P., V.C., L.F.M., G.A., I.D.L., A.C., A.G. and F.G.; software, A.P.; validation, F.M., G.S. and D.M.; formal analysis, F.V. and F.I.; investigation, F.V., F.M., A.P. and F.I.; resources, G.P., F.M. and D.M.; data curation, A.P.; writing—original draft preparation, F.V. and F.I.; writing—review and editing, F.M., G.S., C.D.L. and G.P.; visualization, F.M.; supervision, F.M. and G.S.; project administration, F.M., G.P. and C.D.L.; funding acquisition, F.M. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
The Italian Ministry of Health, the law on 19 January 2001, has funded this research. This research has been partially supported by the Italian Ministry of Health research grant no. IZS AM 02/22 RC, and by the research grant no IZS SI 06/21 RC.
Institutional Review Board Statement
The collection of data used for this manuscript is compulsory in Italy in accordance with the Italian West Nile Virus PNA guidelines (https://westnile.izs.it/j6_wnd/ministeriale, accessed on 6 July 2023) as part of routine clinical surveillance activities. This system is designed to document and register the occurrence and progression of significant infectious animal diseases in accordance with Council Directive 82/894/EC. The consensus for the collection of this data is not mandatory, based on Guideline 12 of the WHO on ethical issues in public health surveillance.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated for this study are presented within the manuscript and are available on request from the corresponding author (F.I., f.iapaolo@izs.it).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rizzoli, A.; Jiménez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Eurosurveillance 2015, 20, 21135. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Monaco, F.; Calistri, P.; Lelli, R. Phylogenetic analysis of West Nile virus isolated in Italy in 2008. Eurosurveillance 2008, 13, 19048. [Google Scholar] [CrossRef] [PubMed]
- Autorino, G.L.; Battisti, A.; Deubel, V.; Ferrari, G.; Forletta, R.; Giovannini, A.; Lelli, R.; Murri, S.; Scicluna, M.T. West Nile virus Epidemic in Horses, Tuscany Region, Italy. Emerg. Infect. Dis. 2002, 8, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- National Integrated Surveillance and Response Plan for West Nile Virus. 2016. Available online: https://westnile.izs.it/j6_wnd/docMinisteriale?annoDocumento=2016 (accessed on 6 July 2023).
- Velati, C.; Angelini, P.; Pupella, S. State of the art: Vest Nile Virus circulation surveillance in Italy and transfusion risk early prevention methods. Transfus. Clin. Biol. 2017, 24, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Bella, A.; Monaco, F.; Ferraro, F.; Petrone, D.; Mateo-Urdiales, A.; Andrianou, X.D.; Del Manso, M.; Venturi, G.; Fortuna, C.; et al. Rapid increase in neuroinvasive West Nile virus infections in humans, Italy, July 2022. Eurosurveillance 2022, 27, 2200653. [Google Scholar] [CrossRef] [PubMed]
- West Nile Disease. Available online: https://westnile.izs.it/j6_wnd/home (accessed on 6 July 2023).
- Barzon, L.; Montarsi, F.; Quaranta, E.; Monne, I.; Pacenti, M.; Michelutti, A.; Toniolo, F.; Danesi, P.; Marchetti, G.; Gobbo, F.; et al. Early start of seasonal transmission and co-circulation of West Nile virus lineage 2 and a newly introduced lineage 1 strain, northern Italy, June 2022. Eurosurveillance 2022, 24, 2200548. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Iapaolo, F.; Polci, A.; Marcacci, M.; Di Gennaro, A.; Teodori, L.; Curini, V.; Di Lollo, V.; Secondini, B.; Scialabba, S.; et al. West Nile Virus Lineage 2 Overwintering in Italy. Trop. Med. Infect. Dis. 2022, 7, 160. [Google Scholar] [CrossRef]
- Cavrini, F.; Della Pepa, M.E.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. A rapid and specific real-time RT-PCR assay to identify Usutu virus in human plasma, serum, and cerebrospinal fluid. J. Clin. Virol. 2011, 50, 221–223. [Google Scholar] [CrossRef]
- Del Amo, J.; Sotelo, E.; Fernández-Pinero, J.; Gallardo, C.; Llorente, F.; Agüero, M.; Jiménez-Clavero, M.A. A novel quantitative multiplex real-time RT-PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. J. Virol. Methods 2013, 189, 321–327. [Google Scholar] [CrossRef]
- Vázquez, A.; Herrero, L.; Negredo, A.; Hernández, L.; Sánchez-Seco, M.P.; Tenorio, A. Real time PCR assay for detection of all known lineages of West Nile virus. J. Virol. Methods 2016, 236, 266–270. [Google Scholar] [CrossRef]
- Diagne, M.M.; Ndione, M.H.D.; Mencattelli, G.; Diallo, A.; Ndiaye, E.H.; Di Domenico, M.; Diallo, D.; Kane, M.; Curini, V.; Top, N.M.; et al. Novel Amplicon-Based Sequencing Approach to West Nile Virus. Viruses 2023, 15, 1261. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Gangavarapu, K.; Quick, J.; Matteson, N.L.; De Jesus, J.G.; Main, B.J.; Tan, A.L.; Paul, L.M.; Brackney, D.E.; Grewal, S.; et al. An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol. 2019, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; The UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Ndione, M.H.D.; Rosà, R.; Marini, G.; Diagne, C.T.; Diagne, M.M.; Fall, G.; Faye, O.; Diallo, M.; Faye, O.; et al. Epidemiology of West Nile virus in Africa: An underestimated threat. PLoS Negl. Trop. Dis. 2022, 16, e0010075. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Iapaolo, F.; Monaco, F.; Fusco, G.; de Martinis, C.; Portanti, O.; Di Gennaro, A.; Curini, V.; Polci, A.; Berjaoui, S.; et al. West Nile Virus Lineage 1 in Italy: Newly Introduced or a Re-Occurrence of a Previously Circulating Strain? Viruses 2021, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Farooq, Z.; Rocklöv, J.; Wallin, J.; Abiri, N.; Sewe, M.O.; Sjödin, H.; Semenza, J.C. Artificial intelligence to predict West Nile virus outbreaks with eco-climatic drivers. Lancet Reg. Health Eur. 2022, 17, 100370. [Google Scholar] [CrossRef]
- Story Map Integrated Surveillance of West Nile and Usutu Virus. Available online: https://storymaps.arcgis.com/collections/5f04c28b7a264d31b53d9cc676b8a12b (accessed on 6 July 2023).
- Veo, C.; della Ventura, C.; Moreno, A.; Rovida, F.; Percivalle, E.; Canziani, S.; Torri, D.; Calzolari, M.; Baldanti, F.; Galli, M.; et al. Evolutionary Dynamics of the Lineage 2 West Nile Virus That Caused the Largest European Epidemic: Italy 2011–2018. Viruses 2019, 11, 814. [Google Scholar] [CrossRef]
- Bagnarelli, P.; Marinelli, K.; Trotta, D.; Monachetti, A.; Tavio, M.; Del Gobbo, R.; Capobianchi, M.R.; Menzo, S.; Nicoletti, L.; Magurano, F.; et al. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Eurosurveillance 2011, 16, 20002. [Google Scholar] [CrossRef]
- Savini, G.; Capelli, G.; Monaco, F.; Polci, A.; Russo, F.; Di Gennaro, A.; Marini, V.; Teodori, L.; Montarsi, F.; Pinoni, C.; et al. Evidence of West Nile virus lineage 2 circulation in Northern Italy. Vet. Microbiol. 2012, 158, 267–273. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).