Simple Summary
Schistosomiasis, also known as bilharzia, is a major parasitic disease caused by blood flukes (trematode worms) of the genus Schistosoma that live in fresh water in tropical and subtropical zones. Over 200 million people are infected globally, 90% of whom live in sub-Saharan Africa. Nigeria has the highest burden of schistosomiasis in this region. Elimination programmes have focused on human infections, with limited attention on infections in livestock that could be transmissible to humans. Recent empirical evidence suggests that in addition to Schistosoma species for which humans are the recognized mammalian host, livestock schistosomes are infective to us. This may lead to genetic material of animal and human schistosomes becoming mixed, a phenomenon known as hybridization. In this review, we highlight current research on schistosome hybridization in Nigeria and explain its negative impact on elimination efforts. Factors promoting livestock/human schistosome interactions and strategies for addressing these are also discussed.
Abstract
Schistosomiasis is one of the leading neglected tropical diseases in sub-Saharan Africa. Recorded case numbers of this chronic and debilitating helminth disease indicate Nigeria to be the most endemic country within this region. National control efforts have focused intensively on restricting human contact with freshwater sources of intermediate host snails. However, limited attention has been paid to the role of livestock as reservoir hosts and the prevalence of transmission of schistosomes to humans via farmed animals. The West African nations of Mali, Senegal, and the neighbouring Niger, Benin, and Cameroon have all reported the hybridization of the closely related species of Schistosoma haematobium, which infects humans, and S. bovis, which infects cattle. As these countries share the Niger and Benue rivers, with their tributaries, there is a distinct possibility of aquatic snails infected with hybrid schistosomes migrating to become established in the Nigerian river system. Here, we report on the current state of research in Nigeria that aims to elucidate key aspects of zoonotic schistosomiasis epidemiology. Factors promoting the hybridization of Schistosoma species are highlighted, and how available control measures can be optimized to address the emergence of schistosome hybrids is discussed.
Keywords:
Schistosoma; schistosome; zoonosis; zoonotic; livestock; neglected tropical disease; Nigeria 1. Introduction
Schistosomiasis, also known as bilharzia, is one of the most important neglected tropical diseases (NTDs) in sub-Saharan Africa [1,2]. This parasitic disease is caused by water-borne trematode worms (blood flukes) of the genus Schistosoma, the developmental cycle of which requires infection of specific aqueous snail intermediate hosts [3]. More than 206 million people across 78 countries are currently affected, with approximately 24,000 deaths and 2.5 million disability-adjusted life years recorded annually [4]. At least six schistosome species exclusively infect humans: S. guineensis, S. haematobium, S. intercalatum, S. japonicum, S. mansoni, and S. mekongi [3,5]. Three others, namely S. bovis, S. curassoni, and S. mattheei, are known to commonly infect animals [5,6]. The geographical distribution of different species is wide, ranging across tropical and subtropical regions of Africa, the Middle East, some parts of Asia, and Latin America [3]. However, schistosomiasis remains a focal disease, with increased transmission observed among rural and marginalized urban populations that frequent surface water bodies. If these are infested with snail intermediate host species, daily water contact activities such as bathing, washing, swimming, and farming expose them to infection. Such communities have limited or no access to safe water, sanitation, and hygiene. Indiscriminate defaecation and urination in open water bodies allow schistosome eggs to encounter snail hosts. Transmission ensues when humans have sufficient contact with contaminated water bodies containing schistosome infective larvae (cercariae) [3].
Most human schistosome species locate to the inferior mesenteric veins that drain the large intestine, thereby causing intestinal schistosomiasis. In contrast, S. haematobium inhabits the vesicular and pelvic venous plexus of the bladder, manifesting clinically as urogenital schistosomiasis [7]. Urogenital schistosomiasis caused by S. haematobium is the predominant form of the disease [8]. Around 90% of individuals affected and who require treatment live in sub-Saharan Africa [3]. With over 210 million inhabitants, Nigeria is easily the most populated country in this region and harbours a major proportion of infected persons [1,2].
The World Health Organization (WHO)-led large-scale administration of praziquantel, the first-line antischistosomal drug, to vulnerable school-age children (between 5 to 14 years old) as a morbidity control strategy [9], is now into its second decade. This approach, known as preventive chemotherapy, does not require a diagnosis of individual infection before treatment. In excess of 250 million doses have been distributed since 2010 to several endemic countries including Nigeria [4]. For this prophylaxis programme to be effective at reducing morbidity due to schistosomiasis, at least 75% of a community’s school-age children must be treated consistently on an annual basis, in addition to which other health educational campaigns and snail reduction strategies should be implemented.
Over the years, little or no emphasis has been placed on the control of schistosomes that are transmissible from livestock to humans (i.e., zoonotic schistosomiasis) as a risk factor for increased transmission and recrudescence of infection. This is particularly relevant to high transmission areas where the co-existence of people and livestock overlaps with the distribution of suitable snail intermediate hosts. For many years Schistosoma species were largely considered to have limited zoonotic potential [10]. However, there are recent reports of the hybridization of two closely related species, S. haematobium, which infects humans, and S. bovis, which infects cattle [5,11,12]. This hybridization has been described in Niger [12], Senegal [13], Mali [14], Benin [15,16], and Cameroon [17,18]. The two major waterways of the Niger and Benue rivers, together with their tributaries, run through these countries as well as Nigeria. This raises the prospect of migrating snails infected with hybrid schistosomes becoming established in Nigerian waters. Besides the migration of Bulinus snails infected with Schistosoma hybrids, there is a massive influx into Nigeria of cattle, goats, and sheep from other West, Central, and Eastern African countries that are brought by nomads for trading and open grazing. Therefore, it would appear to be only a matter of time before hybrid schistosomes carried by migrating livestock become established in Nigeria.
This review discusses the progress of research in Nigeria on zoonotic schistosomiasis, its causal factors, prevalence, and the instigation of control programmes. A particular focus is given to factors that promote the hybridization of Schistosoma species and how existing control strategies can be enhanced to combat this hitherto neglected source of schistosomiasis more effectively.
2. Life Cycle of Schistosoma haematobium
The life cycle of S. haematobium passes through two phases, asexual and sexual, which involve infection of the snail intermediate host and the vertebrate definitive host, respectively. Typically, the cycle starts when eggs released with urine from an infected host come into contact with a freshwater body harbouring snails belonging to the genus Bulinus. The eggs hatch immediately to release free-swimming miracidia that penetrate the host. Upon successful establishment, these develop asexually through several stages into cercariae that leave the snail in search of the definitive host. While having a very short life span, a single miracidium established in a suitable snail host produces about 200 cercariae daily [19,20]. Cercariae penetrate the skin of suitable definitive hosts who come into contact with the contaminated water body, whereupon they transform into schistosomula. Usually, this penetration takes place in surface water, where people and livestock congregate daily for domestic and recreational purposes [21]. The schistosomula migrate through the epidermis and dermis, entering the blood to home to the lungs [20,22,23,24]. Subsequently, schistosomula exit the lungs to the left side of the heart through the pulmonary veins and enter the abdominal aorta, where they either pass through the coeliac trunk, the inferior and superior mesenteric arteries, or the iliac arteries to reach the portal veins of the liver [24]. Here, schistosomula lose their migratory ability, grow, and develop into adult male and female pairs [24]. Thereafter, a pair travels against the blood flow in the venous circulation, settles in the vesical venous plexus and produces eggs that migrate to the bladder, ureter, and other parts of the urinary tract [20,24]. These eggs permeate the walls of blood vessels, bladder, or genital organs [24]. Those that access the bladder are passed out with urine into a body of freshwater, where hatched miracidia continue the transmission cycle [20]. Other Schistosoma species follow a similar life cycle, but with different predilection sites and egg morphologies [25,26].
3. Zoonotic Schistosomiasis
The terms zoonosis or zoonotic disease are used to classify a range of infectious diseases and their causative agents that are capable of being transmitted and subsequently established between human and animal hosts [27]. Hence, zoonotic schistosomiasis refers to the disease condition in which a Schistosoma species known to affect a particular human or animal host is found in another host not specific to the schistosome group [13,28]. These species are naturally shared between humans and other animal hosts as a result of extensive mixed interactions at transmission sites [29,30] (Figure 1).
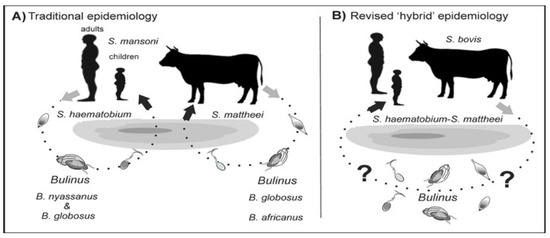
Figure 1.
Alternative models of schistosome transmission. (A) = conventional model of urogenital schistosomiasis transmission. (B) = revised model of urogenital schistosomiasis with overlapping transmission potentials between multi-specific schistosomes. ? = putative but unconfirmed transmission involving known intermediate hosts of several Schistosoma species. Modified from [5].
Hybridization could also be established in areas where schistosome species are explicitly endemic or exhibit greater geographical overlap across potential hosts [31,32]. These inter-host interactions between schistosomes can be bidirectional, whereby both the male and female schistosome pairs with the female or male of another pair to produce viable hybridized offspring [28], or unidirectional in which pairing is between either the male or female schistosomes [33,34] (Figure 2).
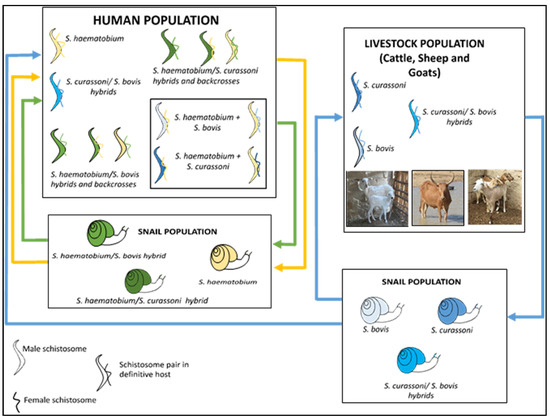
Figure 2.
Zoonotic hybrid schistosome system proposed to occur in West Africa, including Nigeria [34]. Human schistosome species are shown as yellow, animal schistosome species are shown as blue, and hybrids between animal and human schistosome species are shown as green, with infected snails also represented with corresponding colours.
4. Schistosomiasis Control Programme in Nigeria
The National Control Schistosomiasis Programme was established by the Federal Government of Nigeria in 1988, following which epidemiological surveying revealed that an estimated 20 million people were infected. Five pilot control projects were initiated in the states of Borno, Ebonyi, Katsina, Kwara, and Ondo, which have varying ecological predispositions to schistosomiasis. However, due to limited funds, the successes of these pilot projects have fallen short of initially planned targets. In 2020, the Carter Center incorporated schistosomiasis control activities into an ongoing onchocerciasis eradication programme in Plateau and Nasarawa states [35]. A decade before this, mass administration of praziquantel (MDA) commenced in other states with the support of the WHO, UNICEF, and other non-governmental development organizations. To date, planning and implementation of MDA occur within implementation units (IUs), which may be a district, province, or local government area [9]. In common with policy for combatting soil-transmitted helminth infections [36], disease prevalence and other population data generated at the community level are usually aggregated at IUs. For schistosomiasis, this information is used to determine praziquantel thresholds to be implemented [37].
MDA takes place biannually for all school-age children in districts where schistosomiasis prevalence is above 50%. MDA occurs annually when the prevalence is between 10 and 49.9%, and biennially when the prevalence is between 1 and 10% [9]. However, recent WHO guidelines on schistosomiasis risk classification have revised these recommendations as follows. In endemic communities with the prevalence of Schistosoma spp. infection ≥ 10%, in order to control schistosomiasis morbidity and advance towards eliminating the disease as a public health problem, the WHO recommends annual preventive chemotherapy with a single dose of praziquantel at ≥75% treatment coverage in all age groups from 2 years old, including adults, pregnant women after the first trimester, and lactating women [9]. In endemic communities with a prevalence of Schistosoma spp. infection ≥ 10% that demonstrate a lack of an appropriate response to annual preventive chemotherapy, despite adequate treatment coverage (≥75%), the WHO suggests consideration of preventive chemotherapy biannually (twice a year) instead of annually [38].
The review of schistosomiasis treatment data over recent years (2014–2021) shows that the number of districts treated is significantly lower than those requiring treatment. Treatment coverage was significantly lower in 2021 (during the height of the COVID-19 pandemic) than experienced in the previous 7 years. In addition, less than half of the districts met the 75% effective coverage threshold in the last 3 successive years (2019–2021). By endemicity, the proportion of districts with high prevalence (>50%) reduced from (n = 15, 3%) in 2014 to (n = 10, 2%) in 2021, and those with moderate prevalence (10–49%) reduced from (n = 305, 52%) in 2014 to (n = 294, 50%) in 2021. However, the proportion of districts with low prevalence (<10%) increased from (n = 263, 48%) in 2014 to (n = 279, 48%) in 2021 [39]. The poor coverage of praziquantel across the years is most likely connected to the availability of medicines and logistics required to drive mass treatment campaigns [40]. This is particularly worsened by the myriad of issues surrounding the availability, motivation and retention of drug distributors/health workers who are major stakeholders in the campaign [41]. These observations are in line with a 2021 global report on schistosomiasis treatment, as only 30% of people (i.e., 75.3 million of the 251.4 million Nigerians requiring praziquantel) were treated [40].
The ongoing pandemic and efforts targeted at mitigating its impacts have decreased the provision of NTD interventions, particularly praziquantel medicines for schistosomiasis. Nevertheless, there is a need for more concerted efforts towards increasing coverage of medicines in line with achieving the 2030 global targets of the WHO NTD elimination roadmap. These include: (1) reducing the proportion of moderate and heavy intensity infections to <1% in 78 countries; (2) reducing by 50% the number of tablets required during MDA; and (3) increasing domestic financial support for MDA [3,4]. Meeting these targets is largely dependent on increased community participation during MDA, with absolute geographical reach and high programme coverage [42,43,44,45].
5. Implication of Hybridization for Schistosomiasis Control Efforts
The existence of hybrid schistosomes is of great concern to the schistosomiasis control programme in Nigeria, with possible implications for widening host range, increased transmission potential, altered pathology, and drug resistance [46]. Interactions between different Schistosoma species are known to improve reproductive capacity, as manifested by an increased number of parasite offspring, a faster maturation time, and a larger intermediate host range [47,48]. A major concern is that with the emergence of hybrid schistosomes, the efficacy of praziquantel treatment might be lowered [49]. This has been reported under both field and laboratory conditions for S. mansoni [50,51,52] and was linked to the cryptic role played by hybridization. The success achieved by using this drug is therefore threatened by the outbreak of Schistosoma hybrids [5]. Furthermore, genetic diversity among the progeny of zoonotic schistosomes offers better phenotypic characteristics compared to those of either parent [53], thereby enhancing the exploitation of the host. Certain genotypic traits of the zoonotic hybrid parasite show refined adaptations to better avoid recognition and to resist the host’s adaptive immune system, potentially giving rise to greater infectivity and unusual pathologies [54]. Infection of multiple animal reservoir hosts by zoonotic hybrid schistosomes makes the elimination of the disease more challenging [15,16]. The difficulty to control and prevent Schistosoma hybrid infections in some endemic areas may be due to the ubiquitous presence of rodents, which serve as the reservoir host for many schistosome species, thereby readily enabling co-infection [55]. The magnitude of the contribution of non-human mammalian hosts to the transmission of schistosomiasis remains understudied, especially for species other than S. japonicum. This has been highlighted by the WHO, which recommends testing for Schistosoma infections in non-human mammalian hosts [38].
6. Factors Promoting Hybridization of Schistosome Species
6.1. Availability of Shared Freshwater Bodies and Natural Events
Schistosomiasis is a disease that presents as a focal transmission around localities with contaminated and slow-moving freshwater bodies that adequately support a proliferation of the snail intermediate host. This occurs especially where dams and similar water development structures are present [56,57]. However, the potential for expansion of the geographical range of the disease is attributed to changes in environmental and climatic conditions [58]. In Uganda, schistosomiasis transmission occurs at unprecedented altitudes where clinical infection would not normally be expected [59]. Rising ambient temperature was also predicted to affect schistosomiasis transmission in separate studies from eastern Africa and China [60,61]. It is believed that fluctuations in schistosomiasis transmission will more likely be seen or restricted to those areas bordering currently endemic localities [62]. However, geographically disconnected but endemic communities may share ecological links via common rivers, streams, or other freshwater bodies that pass through them. Large rivers may even transcend international boundaries and course through many communities or extend as smaller freshwater bodies. This provides an opportunity for schistosomes and their intermediate hosts to become established in new areas through weather-related events such as flooding. Research from China showed that after a major flood disaster in 1998, new populations of intermediate host snails were dispersed and established in areas where schistosomiasis was either not previously endemic or had been eliminated [61]. Similarly, in Nigeria, a schistosomiasis outbreak consequent to flooding was reported in a community of internally displaced persons [63]. The geographical range of a Schistosoma species may be extended through connected freshwater bodies. If the new area intersects with the range of other schistosomes, this increases the possibility of hybridization between species.
Schistosomiasis transmission remains unabated in some endemic foci in Nigeria despite ongoing preventive chemotherapy programmes [39,56,64]. The Niger River, which is the longest river in West Africa, extends over 4200 km and transverses nine countries (Benin, Burkina Faso, Cameroon, Chad, Côte d’Ivoire, Guinea, Mali, Niger, and Nigeria) (Figure 3). The river basin discharges into the Gulf of Guinea through a massive delta, where 80% of the country’s population lives. Hybridization of S. haematobium and S. bovis species has been reported in Niger [12], Senegal [13], Mali [14], Benin [15,16], and Cameroon [17,18]. These countries share the Niger River and/or its tributaries with Nigeria, so increasing the likelihood of snails infected with hybrid schistosomes being swept downstream during flooding to become established in the Nigerian river system. This is corroborated by a recent molecular analysis of Bulinus snails collected from Nigeria showing shared phylogeny with those from Senegal, Burkina Faso, and Niger [65]. Closer surveillance will augment monitoring for the possible emergence of schistosome hybrid species or zoonotic infections in endemic areas affected by flooding in the country.
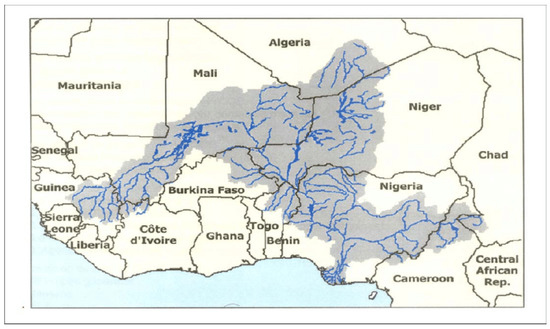
Figure 3.
Location map of the Niger River Basin, showing the associated countries [66].
6.2. Developmental Projects and Flooding
In 2017, the most recent date for which statistics are available, the Federal Ministry of Agriculture and Rural Development estimated livestock production in Nigeria to comprise 18.4 million cattle, 43.4 million sheep, 76 million goats, and 180 million poultry [67]. A significant part of this production is attributed to the husbandry activities of small-scale livestock holders and nomadic herders [68]. It was estimated that 13 million households, which make up 42% of the Nigerian population, own livestock. In common with similar regions of the developing world, in many parts of rural Nigeria, cattle and other livestock typically live close to humans and roam freely within the local community [68]. Such an intimate ecological setting is recognized to predispose to the zoonotic cycling of pathogens between animals and humans. As the transmission of schistosomiasis is focal, related to the presence of freshwater bodies to support vector aqueous snails, zoonotic transmission is associated with areas where there are close ecological interactions between humans and animals through shared resources such as water (for example, see Figure 4).

Figure 4.
Humans and livestock animals interacting at a shared freshwater source near Abeokuta, the capital city of Ogun State, southwest Nigeria (The authors, 2022).
Hybrid schistosomes have been identified in children living in Senegalese villages around the Senegal River Basin, where water development projects prompted a local increase in shared water contacts between humans and livestock [13,69]. These hybrids were due to introgressive hybridization between human and bovine schistosomes (S. haematobium and S. bovis) present at the same time in one of the two definitive hosts, thus providing evidence of zoonotic Schistosoma infection between humans and cattle. An earlier investigation of human intestinal schistosomiasis in the Senegal River Basin identified zoonotic infection of wild rodents such as the Nile rat and Hubert’s multimammate mouse by S. mansoni. This outbreak followed a major water development project, which resulted in ecological changes that led to increased interactions with humans [70,71]. A similar outbreak of schistosomiasis was reported in 1988 in Nigeria, around the Oyan River Dam [72]. However, investigations aimed at understanding the possible changes in zoonotic transmission dynamics resulting from altered human–animal interactions in the areas affected by flooding and other developmental projects are limited. Additionally, more recently, a schistosomiasis outbreak was reported during the COVID-19 pandemic in Takum, one of the northeastern districts in the country [73]. Epidemiological risk analysis highlights the predominant risky behaviour of swimming and playing among young children, and the availability of viable intermediate snail hosts as possible drivers of transmission [73]. Yet, nationwide mapping of aquatic snail populations and the parasites harboured by infective snails remains fragmented [65]. Over the years, there has been limited interest in the zoonotic transmission of schistosomes, despite the availability of studies demonstrating that locally endemic species such as S. mansoni can infect not only humans but a broad range of hosts including rodents and primates [29,74]. It is therefore important to invest in epidemiological studies targeted at mapping host range, understanding human–animal interactions, and identifying location-specific predictors of zoonotic schistosomiasis that can be modified using implementation research.
6.3. In-Country and Trans-Border Migration of Livestock
Understanding zoonotic transmission of schistosomes is particularly important in Nigeria, where nomadic pastoral practice dominates livestock production. This farming method accounts for around 82% of cattle [69] (Table 1), mainly in the ownership of the typically nomadic Fulani population [75]. They are described as the world’s largest nomadic ethnic population and the leading pastoralists in Nigeria [76,77].

Table 1.
Demographic details of Nigerian national cattle herd, 2019. Data from [68].
Together with other nomadic groups, the Fulani constitute approximately 12% of the population of sub-Saharan Africa [78]. These nomad populations rely exclusively on livestock as their source of both income and food, commonly migrating in a seasonal cycle across national borders in search of pasture and water resources for their herds [79,80]. Transhumance herding, whereby livestock is moved from season to season to graze or trade, is believed to account for the expanding range of the Rhipicephalus (Boophilus) microplus tick across Africa. This explains its emergence in new areas such as Nigeria, where pasture rotation is seen as a way to manage tick populations [81]. An ectoparasite, R. microplus serves as a transmission vector for the protozoan pathogen that causes bovine babesiosis, an economically important illness of cattle. Therefore, the significance of water resources to pastoralists’ migrations in the country increases the risk of spreading both Schistosoma and Babesia to new areas of Nigeria where they were not previously a threat to public or veterinary health. Host migration also has the strong potential to disrupt the distribution of schistosome species and thereby increase the possibility of hybridization between species [48]. Besides in-country migration, the free movement of livestock into Nigeria from the other 14 countries of the Economic Community of West African States for trading and open grazing further contributes to the potential introduction and establishment of hybrid schistosomes in the Nigerian river system. This is especially relevant to those nations where hybridization has been reported. Contamination of Nigeria’s waterbodies by livestock migrating from both in-country and trans-border routes constitutes a major concern for schistosomiasis control efforts. Incorporating livestock schistosomiasis testing and subsequent care into already existing interventions at border regions and other recognized pastoralist communities, or at abattoirs, becomes very important as a first-line action towards preventing contamination of grazing land and water bodies. The integration of praziquantel treatment for bovines in human schistosomiasis elimination programmes has been implemented in China. This has resulted in achieving their elimination goal, with the majority of endemic provinces recording under 1% prevalence for infections in humans and livestock [82,83].
6.4. Livestock Schistosomiasis Diagnostic Testing Methods
In Africa, there is an ongoing discourse on the resistance of schistosomes to praziquantel, based on emerging reports on the use and misuse of praziquantel for treating uninfected livestock [84,85]. The need for a more refined control strategy for livestock schistosomiasis, therefore, becomes imperative to avert the evolution of praziquantel resistance. Consequently, discussion about which treatment strategy would be more effective in line with the elimination agenda of human schistosomiasis also becomes unavoidable. A recent study in Senegal used a transmission modelling approach to evaluate the effectiveness of a theoretical test-and-treat (TnT) strategy on bovine schistosomiasis over a nine-year period (2022–2030) [86]. The diagnostic tool, a point-of-care circulating cathodic antigen (POC-CCA), was developed to detect circulating cathodic antigens in humans [87] but has been used for detecting livestock schistosomes with moderate sensitivity [88]. Findings from the model show that the TnT strategy could be highly effective in suppressing cattle infection, with up to 85% case reduction across the years [86]. However, there are concerns that the chances of avoiding unnecessary treatments that contribute to praziquantel resistance are low since the reliability of this technique in detecting negative cases is poor, especially in low transmission areas [89]. There are also documented challenges of cross-reactivity and identification of previous infections, rather than current ones. To some extent, these issues have limited the applicability of POC-CCA in the field [90].
The identification of reliable and cost-effective diagnostic procedures is therefore important for the surveillance and eventual elimination of livestock schistosomiasis. Findings from a recent systematic review of 14 different diagnostic techniques on 9 types of non-human animals showed that formalin-ethyl acetate sedimentation-digestion (FEA-SD) and quantitative polymerase chain reaction (qPCR) are the two most reliable techniques for schistosome diagnosis in non-human animal hosts [90]. FEA-SD is a recently developed microscopic test with a high sensitivity of 0.89 (95% CI: 0.61–1.00) [90]. This is becoming a diagnostic procedure of choice owing to its similar accuracy to that of qPCR and its affordability [91]. Unlike other classical methods such as the microscopic Kato–Katz (KK) technique and the miracidium hatching test (MHT) [92,93], FEA-SD has an improved sensitivity (a shortfall of KK) [91] and is less sensitive to pH, temperature, and quality of water (a shortfall of MHT) [91]. The technique is compatible with field operations, especially when handling faecal samples of large animals, which typically contain abundant cellulose fibre and debris that normally obscure egg detection under a microscope (a common problem with KK) [91]. Implementation of the TnT strategy using FEA-SD and praziquantel in pilot schemes across fewer endemic communities could be a useful first attempt in identifying impact and inherent challenges. This could be scaled up in larger studies, with findings used to inform national policy frameworks or guidelines, especially on refined methodological details such as how a community/herd should be selected, and what treatment coverage is expected per district to meet the elimination agenda set in the country. This strategy would require investment in capacity building of technicians and community volunteers on herd selection, sample collection and processing samples using the FEA-SD technique, and administration of praziquantel to infected livestock.
6.5. Poor Access to Water, Sanitation, and Hygiene (WASH) Facilities
Schistosomiasis is classified as an NTD because it disproportionately affects the socioeconomically disadvantaged populations of the world [94]. More than 83 million Nigerians live in conditions of extreme poverty, usually characterized by lack of, or poor access to, a potable water source, sanitation, and hygiene (WASH) facilities, and other social amenities [95]. These communities depend heavily on agriculture, 70% rearing livestock to support their livelihood [96]. According to a recent analysis by the World Bank, about 39% of rural households in Nigeria lack basic access to a safe water supply, only 50% have access to improved sanitation, while one-third practice open defaecation [97]. In most settings, the principal sources of water are freshwater bodies located close to the community, which are routinely contaminated by faecal matter and other bodily waste due to poor sanitary conditions and practices [56]. The socioeconomic circumstances commonly lead to a shortfall in the capacity to develop separate sources of water to serve the needs of both humans and farmed animals. This tacitly encourages the continued co-interaction of people and livestock at available open water bodies (Figure 4), which poses a serious public health challenge. Given such a scenario, in which the combination of contamination and a common dependence on a single source of water by humans and animals is almost inevitable, the risk of zoonotic transmission of schistosomiasis between hosts is greatly elevated.
The deplorable state of WASH facilities prompted the Federal Government of Nigeria to establish water and sanitation agencies with the mandate of promoting equitable access and usage of improved WASH facilities [98,99]. One such initiative is the National Urban Water Sector Reform Program. This has constructed more than 2300 water points across the country since 2015 [97], yet urban locations have been prioritized over rural settings, where the coverage is still extremely inadequate. This compounds the reported poor progress in providing WASH facilities to rural communities through the pre-existing Rural Water Supply and Sanitation Programme [100]. In recognition of limited progress, in 2018, the Federal Government of Nigeria declared a state of emergency in the country’s drinking water, sanitation, and hygiene sector. It had been found that many water supply pumps fail shortly after installation, then remain unfixed, and so no longer serve populations in rural areas [101]. This suggests that more sustainable approaches to supplying potable water to regional communities should be considered. Studies have reported that the availability of WASH facilities is associated with a reduction in NTDs such as soil-transmitted helminth infections and schistosomiasis [102,103]. Providing WASH facilities to schistosomiasis-endemic communities not only prevents sharing of water between humans and animals, thereby reducing the risk of zoonotic infections, but also affords wider health benefits. It will be important to evaluate the impact of widening access to WASH facilities in schistosomiasis-endemic areas of Nigeria on the transmission of Schistosoma species to humans, as well as on zoonotic transmission between humans and animals.
7. Future Research Agenda
Information on the epidemiology of hybrid schistosomes in humans in Nigeria is sparse. However, there are emerging reports on morphological [104] and genetic [105] characterization of hybrid Schistosoma eggs obtained from human urine. Little is known about the spatial extent and overlapping distribution of livestock schistosomes, S. bovis and S. currasoni, in Nigeria [106]. For Nigeria to achieve the WHO NTD 2030 roadmap goal of eliminating schistosomiasis as a public health problem, national surveillance of hybrid schistosomiasis in both humans and livestock is imperative. It is also essential to stimulate further programming and policy directions, most especially on testing and treatment of non-mammalian hosts, testing of hybrid infection in snails, as well as praziquantel efficacy. These investigations would require funding, human resources, capacity development, and the networking of schistosome researchers within the One Health approach that are currently either lacking or insufficient.
8. Conclusions
Investment to provide adequate WASH facilities and effective habitat modification strategies to curtail flooding each remain essential to the control of schistosomiasis in Nigeria. However, beyond the provision of these resources, it is recommended that innovative health education programmes should be implemented towards ensuring optimal usage and sustainable maintenance by the target nomadic communities. Such outreach programmes should consult with community leaders to accommodate, where possible, local strategies for rearing livestock with a view to preventing contamination of communal water bodies with human and livestock faeces and urine. In addition, incorporating livestock schistosomiasis testing and subsequent care into already existing interventions at border regions and other recognized pastoralist communities, or at abattoirs, becomes very important as a first-line action towards preventing contamination of grazing land and water bodies. By taking these steps, the risk of hybridization of schistosome species may be significantly reduced. Finally, accurate surveillance for schistosomiasis, prompt alerts of outbreaks, identification of Schistosoma species, and regular promotion of preventive chemotherapy should be encouraged as integral activities of a nationwide Nigerian schistosomiasis control programme.
Author Contributions
Conceptualization, H.O.M., O.O.O., A.A.B., U.F.E. and A.W.T.-R.; writing—original draft preparation, H.O.M., O.O.O. and A.A.B.; writing—review and editing, O.O.O., U.F.E. and A.W.T.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hotez, P.J.; Fenwick, A. Schistosomiasis in Africa: An emerging tragedy in our new global health decade. PLoS Negl. Trop Dis. 2009, 3, e485. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Kamath, A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Schistosomiasis. 8 January 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 30 January 2023).
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Stothard, J.R.; Kayuni, S.A.; Al-Harbi, M.H.; Musaya, J.; Webster, B.L. Future schistosome hybridizations: Will all Schistosoma haematobium hybrids please stand-up! PLoS Negl. Trop. Dis. 2020, 14, e0008201. [Google Scholar] [CrossRef] [PubMed]
- Engels, D.; Chitsulo, L.; Montresor, A.; Savioli, L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002, 82, 139–146. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. DPDx—Laboratory Identification of Parasites of Public Health Concern. Schistosomiasis. Available online: https://www.cdc.gov/dpdx/schistosomiasis/index.html (accessed on 30 January 2023).
- Ekpo, U.F.; Hürlimann, E.; Schur, N.; Oluwole, A.S.; Abe, E.M.; Mafe, M.A.; Nebe, O.J.; Isiyaku, S.; Olamiju, F.; Kadiri, M.; et al. Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat. Health 2013, 7, 355–366. [Google Scholar] [CrossRef]
- World Health Organization. Helminth Control in School-Age Children: A Guide for Managers of Control Programmes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/bitstream/handle/10665/44671/9789241548267_eng.pdf (accessed on 30 January 2023).
- Rollinson, D. A wake-up call for urinary schistosomiasis: Reconciling research effort with public health importance. Parasitology 2009, 136, 1593–1610. [Google Scholar] [CrossRef]
- Webster, B.L.; Southgate, V.R. Mating interactions of Schistosoma haematobium and S. intercalatum with their hybrid offspring. Parasitology 2003, 126, 327–338. [Google Scholar] [CrossRef]
- Léger, E.; Garba, A.; Hamidou, A.A.; Webster, B.L.; Pennance, T.; Rollinson, D.; Webster, J.P. Introgressed animal schistosomes Schistosoma curassoni and S. bovis naturally infecting humans. Emerg. Infect. Dis. 2016, 22, 2212–2214. [Google Scholar] [CrossRef]
- Huyse, T.; Webster, B.L.; Geldof, S.; Stothard, J.R.; Diaw, O.T.; Polman, K.; Rollinson, D. Bidirectional introgressive hybridisation between cattle and human schistosome species. PLoS Pathog. 2009, 5, e1000571. [Google Scholar] [CrossRef]
- Soentjens, P.; Cnops, L.; Huyse, T.; Yansouni, C.; Vos, D.D.; Bottieau, E.; Clerinx, J.; Esbroeck, M.V. Diagnosis and clinical management of Schistosoma haematobium–Schistosoma bovis hybrid infection in a cluster of travelers returning from Mali. Clin. Infect. Dis. 2016, 63, 1626–1629. [Google Scholar] [CrossRef]
- Savassi, B.A.E.S.; Mouahid, G.; Lasica, C.; Mahaman, S.-D.K.; Garcia, A.; Courtin, D.; Allienne, J.-F.; Ibikounlé, M.; Moné, H. Cattle as natural host for Schistosoma haematobium (Bilharz, 1852) Weinland, 1858 x Schistosoma bovis Sonsino, 1876 interactions, with new cercarial emergence and genetic patterns. Parasitol. Res. 2020, 119, 2189–2205. [Google Scholar] [CrossRef] [PubMed]
- Savassi, B.A.E.S.; Dobigny, G.; Etougbétché, J.R.; Avocegan, T.T.; Quinsou, F.T.; Gauthier, P.; Ibikounlé, M.; Moné, H.; Mouahid, G. Mastomys natalensis (Smith, 1834) as a natural host for Schistosoma haematobium (Bilharz, 1852) Weinland, 1858 x Schistosoma bovis Sonsino, 1876 introgressive hybrids. Parasitol. Res. 2021, 120, 1755–1770. [Google Scholar] [CrossRef] [PubMed]
- Tchuenté, L.; Southgate, V.; Njiokou, F.; Njiné, T.; Kouemeni, L.; Jourdane, J. The evolution of schistosomiasis at Loum, Cameroon: Replacement of Schistosoma intercalatum by S. haematobium through introgressive hybridisation. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 664–665. [Google Scholar] [CrossRef]
- Webster, B.L.; Tchuenté, L.T.; Jourdane, J.; Southgate, V.R. The interaction of Schistosoma haematobium and S. guineensis in Cameroon. J. Helminthol. 2005, 79, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasit Vectors 2015, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Nelwan, M.L. Schistosomiasis: Life cycle, diagnosis, and control. Curr. Ther. Res. Clin. Exp. 2019, 91, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Mafiana, C.F.; Ekpo, U.F.; Ojo, D.A. Urinary schistosomiasis in preschool children in settlements around Oyan Reservoir in Ogun State, Nigeria: Implications for control. Trop. Med. Int. Health 2003, 8, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Curwen, R.S.; Wilson, R.A. Invasion of skin by schistosome cercariae: Some neglected facts. Trends Parasitol. 2003, 19, 63–66. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Salafsky, B.; Ramaswany, K. Comparison of skin invasion among three major species of Schistosoma. Trends Parasitol. 2005, 21, 201–203. [Google Scholar] [CrossRef]
- Nation, C.S.; Da’dara, A.A.; Marchant, J.K.; Skelly, P.J. Schistosome migration in the definitive host. PLoS Negl. Trop. Dis. 2020, 14, e0007951. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Parasites—Schistosomiasis. In Biology; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/parasites/schistosomiasis/biology.html (accessed on 30 January 2023).
- Chomel, B.B. Control and prevention of emerging parasitic zoonoses. Int. J. Parasitol. 2008, 38, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Huyse, T.; Van Den Broeck, F.; Hellemans, B.; Volckaert, F.A.M.; Polman, K. Hybridisation between the two major African schistosome species of humans. Int. J. Parasitol. 2013, 43, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Standley, C.J.; Mugisha, L.; Dobson, A.P.; Stothard, J.R. Zoonotic schistosomiasis in non-human primates: Past, present and future activities at the human–wildlife interface in Africa. J. Helminthol. 2012, 86, 131–140. [Google Scholar] [CrossRef]
- Léger, E.; Webster, J.P. Hybridizations within the Genus Schistosoma: Implications for evolution, epidemiology and control. Parasitology 2017, 144, 65–80. [Google Scholar] [CrossRef]
- Fan, P.C.; Lin, L.H. Hybridization of Schistosoma mansoni and Schistosoma japonicum in mice. Southeast Asian J. Trop. Med. Public Health 2005, 36, 89–96. [Google Scholar]
- Boissier, J.; Moné, H.; Mitta, G.; Bargues, M.D.; Molyneux, D.; Mas-Coma, S. Schistosomiasis reaches Europe. Lancet Infect. Dis. 2015, 15, 757–758. [Google Scholar] [CrossRef]
- Steinauer, M.L.; Hanelt, B.; Mwangi, I.N.; Maina, G.M.; Agola, L.E.; Kinuthia, J.M.; Mutuku, M.W.; Mungai, B.N.; Wilson, W.D.; Mkoji, G.M.; et al. Introgressive hybridization of human and rodent schistosome parasites in western Kenya. Mol. Ecol. 2008, 17, 5062–5074. [Google Scholar] [CrossRef]
- Borlase, A.; Webster, J.P.; Rudge, J.W. Opportunities and challenges for modelling epidemiological and evolutionary dynamics in a multihost, multiparasite system: Zoonotic hybrid schistosomiasis in West Africa. Evol. Appl. 2017, 11, 501–515. [Google Scholar] [CrossRef]
- The Carter Center. Schistosomiasis Control Program. 2021. Available online: https://www.cartercenter.org/resources/pdfs/factsheets/schistosomiasis-facts.pdf (accessed on 30 January 2023).
- Mogaji, H.O.; Dedeke, G.A.; Bada, B.S.; Bankole, S.; Adeniji, A.; Fagbenro, M.T.; Omitola, O.O.; Oluwole, A.S.; Odoemene, N.S.; Abe, E.M.; et al. Distribution of ascariasis, trichuriasis and hookworm infections in Ogun State, Southwestern Nigeria. PLoS ONE 2020, 15, e0233423. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for Africa. Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN). Optimizing Schistosomiasis MDA Implementation in Countries. Data Analysis Tool, June–July 2019. Available online: https://espen.afro.who.int/system/files/content/resources/Schistosomiasis%20Data%20analysis%20tool%20-%20Presentation%20%2820190724_English%29.pdf (accessed on 30 January 2023).
- WHO. Guideline on Control and Elimination of Human Schistosomiasis; World Health Organization: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/rest/bitstreams/1410449/retrieve (accessed on 30 January 2023).
- World Health Organization; Regional Office for Africa. Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN). Nigeria Overview. Available online: https://espen.afro.who.int/countries/nigeria (accessed on 30 January 2023).
- World Health Organization. Schistosomiasis and soil-transmitted helminthiases: Progress report, 2021. Wkly. Epidemiol. Rec. 2022, 97, 621–632. Available online: https://www.who.int/publications/i/item/who-wer9748-621-632 (accessed on 30 January 2023).
- Krentel, A.; Gyapong, M.; Mallya, S.; Boadu, N.Y.; Amuyunzu-Nyamongo, M.; Stephens, M.; McFarland, D.A. Review of the factors influencing the motivation of community drug distributors towards the control and elimination of neglected tropical diseases (NTDs). PLoS Negl. Trop. Dis. 2017, 11, e0006065. [Google Scholar] [CrossRef] [PubMed]
- El-Setouhy, M.; Abd Elaziz, K.M.; Helmy, H.; Farid, H.A.; Kamal, H.A.; Ramzy, R.M.R.; Shannon, W.D.; Weil, G.J. The effect of compliance on the impact of mass drug administration for elimination of lymphatic filariasis in Egypt. Am. J. Trop. Med. Hyg. 2007, 77, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Olamiju, O.J.; Olamiju, F.O.; Adeniran, A.A.; Mba, I.C.; Ukwunna, C.C.; Okoronkwo, C.; Ekpo, U.F. Public awareness and knowledge of neglected tropical diseases (NTDs) control activities in Abuja, Nigeria. PLoS Negl. Trop. Dis. 2014, 8, e3209. [Google Scholar] [CrossRef] [PubMed]
- Assaré, R.K.; N’Tamon, R.N.; Bellai, L.G.; Koffi, J.A.; Mathieu, T.-B.I.; Ouattara, M.; Hürlimann, E.; Coulibaly, J.T.; Diabaté, S.; N’Goran, E.K.; et al. Characteristics of persistent hotspots of Schistosoma mansoni in western Côte d’Ivoire. Parasit Vectors 2020, 13, 337. [Google Scholar] [CrossRef]
- Mogaji, H.O.; Odoh, I.M.; Iyeh, C.I.; Adeniran, A.A.; Oyedeji, S.I.; Okoh, H.I.; Bayegun, A.A.; Omitola, O.O.; Umunnakwe, C.U.; Olamiju, F.O.; et al. Attendee’s awareness about preventive chemotherapy neglected tropical diseases (PC-NTD) control during the first world neglected tropical diseases day in Ekiti State, Nigeria. PLoS Negl. Trop. Dis. 2021, 15, e0009315. [Google Scholar] [CrossRef]
- King, K.C.; Stelkens, R.B.; Webster, J.P.; Smith, D.F.; Brockhurst, M.A. Hybridization in parasites: Consequences for adaptive evolution, pathogenesis, and public health in a changing world. PLoS Pathog. 2015, 11, e1005098. [Google Scholar] [CrossRef]
- Moné, H.; Minguez, S.; Ibikounlé, M.; Allienne, J.-F.; Massougbodji, A.; Mouahid, G. Natural interactions between S. haematobium and S. guineensis in the Republic of Benin. Sci. World J. 2012, 2012, 793420. [Google Scholar] [CrossRef]
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Webster, J.P.; Rollinson, D. Introgressive hybridization of Schistosoma haematobium group species in Senegal: Species barrier break down between ruminant and human schistosomes. PLoS Negl. Trop. Dis. 2013, 7, e2110. [Google Scholar] [CrossRef]
- Webster, J.P.; Molyneux, D.H.; Hotez, P.J.; Fenwick, A. The contribution of mass drug administration to global health: Past, present and future. Philos. Trans./R Soc. Lond. B—Biol. Sci. 2014, 369, 20130434. [Google Scholar] [CrossRef]
- Lamberton, P.H.L.; Hogan, S.C.; Kabatereine, N.B.; Fenwick, A.; Webster, J.P. In vitro praziquantel test capable of detecting reduced in vivo efficacy in Schistosoma mansoni human infections. Am. J. Trop. Med. Hyg. 2010, 83, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Valentim, C.L.L.; Cioli, D.; Chevalier, F.D.; Cao, X.; Taylor, A.B.; Holloway, S.P.; Pica-Mattoccia, L.; Guidi, A.; Basso, A.; Tsai, I.J.; et al. Genetic and molecular basis of drug resistance and species-specific drug action in schistosome parasites. Science 2013, 342, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Faye, D.S.; Stothard, J.R.; Sousa-Figueiredo, J.C.; Rollinson, D. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: Monitoring treatment success and re-infection patterns. Acta Trop. 2013, 128, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Grigg, M.E.; Bonnefoy, S.; Hehl, A.B.; Suzuki, Y.; Boothroyd, J.C. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science 2001, 294, 161–165. [Google Scholar] [CrossRef]
- Schelkle, B.; Faria, P.J.; Johnson, M.B.; van Oosterhout, C.; Cable, J. Mixed infections and hybridisation in monogenean parasites. PLoS ONE 2012, 7, e39506. [Google Scholar] [CrossRef]
- Hanelt, B.; Mwangi, I.N.; Kinuthia, J.M.; Maina, G.M.; Agola, L.E.; Mutuku, M.W.; Steinauer, M.L.; Agwanda, B.R.; Kigo, L.; Mungai, B.N.; et al. Schistosomes of small mammals from the Lake Victoria Basin, Kenya: New species, familiar species, and implications for schistosomiasis control. Parasitology 2010, 137, 1109–1118. [Google Scholar] [CrossRef]
- Akinwale, O.; Ajayi, M.; Akande, D.; Gyang, P.; Adeleke, M.; Adeneye, A.; Adebayo, M.; Dike, A. Urinary schistosomiasis around Oyan Reservoir, Nigeria: Twenty years after the first outbreak. Iran J. Public Health 2010, 39, 92–95. [Google Scholar]
- Dida, G.O.; Gelder, F.B.; Anyona, D.N.; Matano, A.S.; Abuom, P.O.; Adoka, S.O.; Ouma, C.; Kanangire, C.K.; Owuor, P.O.; Ofulla, A.V. Distribution and abundance of schistosomiasis and fascioliasis host snails along the Mara River in Kenya and Tanzania. Infect. Ecol. Epidemiol. 2014, 4, 24281. [Google Scholar] [CrossRef]
- Zhou, X.N.; Yang, G.J.; Yang, K.; Wang, X.H.; Hong, Q.B.; Sun, L.P.; Malone, J.B.; Kristensen, T.K.; Bergquist, N.R.; Utzinger, J. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008, 78, 188–194. [Google Scholar] [CrossRef]
- John, R.; Ezekiel, M.; Philbert, C.; Andrew, A. Schistosomiasis transmission at high altitude crater lakes in western Uganda. BMC Infect. Dis. 2008, 8, 110. [Google Scholar] [CrossRef]
- McCreesh, N.; Nikulin, G.; Booth, M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit Vectors 2015, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Zhang, S.Q.; Xu, X.J.; Huang, Y.X.; Steinmann, P.; Utzinger, J.; Wang, T.P.; Xu, J.; Zheng, J.; Zhou, X.N. Effect of floods on the transmission of schistosomiasis in the Yangtze River valley, People’s Republic of China. Parasitol. Int. 2008, 57, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mari, L.; Ciddio, M.; Casagrandi, R.; Perez-Saez, J.; Bertuzzo, E.; Rinaldo, A.; Sokolow, S.H.; De Leo, G.A.; Gatto, M. Heterogeneity in schistosomiasis transmission dynamics. J. Theor. Biol. 2017, 432, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.E.; Egwunyenga, A.O. Schistosomiasis: The aftermath of 2012 floods in Delta State, Southern Nigeria. Int. Med. J. 2015, 22, 218–223. [Google Scholar]
- Oladejo, S.O.; Ofoezie, I.E. Unabated schistosomiasis transmission in Erinle River Dam, Osun State, Nigeria: Evidence of neglect of environmental effects of development projects. Trop. Med. Int. Health 2006, 11, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Abe, E.M.; Guo, Y.H.; Shen, H.; Mutsaka-Makuvaza, M.J.; Habib, M.R.; Xue, J.B.; Midzi, N.; Xu, J.; Li, S.Z.; Zhou, X.N. Phylogeography of Bulinus truncates (Audouin, 1827) (Gastropoda: Planorbidae) in selected African countries. Trop. Med. Infect. Dis. 2018, 3, 127. [Google Scholar] [CrossRef]
- Al-Gamal, A.S.; Sokona, Y.; Dodo, A. Climatic changes and groundwater resources in Africa. Int. J. Clim. Chang. Strat. Manag. 2009, 1, 133–145. [Google Scholar] [CrossRef]
- Federal Ministry of Agriculture and Rural Development. Agricultural Sector Food Security and Nutrition Strategy 2016–2025. Available online: https://ngfrepository.org.ng:8443/jspui/bitstream/123456789/5377/1/Agriculture-FSN-Strategy-2016-25_Printed-Version_1562696265%20%281%29.pdf (accessed on 30 January 2023).
- Food and Agriculture Organization of the United Nations. The Future of Livestock in Nigeria. Opportunities and Challenges in the Face of Uncertainty; FAO: Rome, Italy, 2019; Available online: https://www.fao.org/3/ca5464en/ca5464en.pdf (accessed on 30 January 2023).
- Léger, E.; Borlase, A.; Fall, C.B.; Diouf, N.D.; Diop, S.D.; Yasenev, L.; Catalano, S.; Thiam, C.T.; Ndiaye, A.; Emery, A.; et al. Prevalence and distribution of schistosomiasis in human, livestock, and snail populations in northern Senegal: A One Health epidemiological study of a multi-host system. Lancet Planet. Health 2020, 4, e330–e342. [Google Scholar] [CrossRef]
- Southgate, V. Schistosomiasis in the Senegal River Basin: Before and after the construction of the dams at Diama, Senegal and Manantali, Mali and future prospects. J. Helminthol. 1997, 71, 125–132. [Google Scholar] [CrossRef]
- Duplantier, J.M.; Sène, M. Rodents as reservoir hosts in the transmission of Schistosoma mansoni in Richard-Toll, Senegal, West Africa. J. Helminthol. 2000, 74, 129–135. [Google Scholar] [CrossRef]
- Ofoezie, I.E.; Imevbore, A.M.; Balogun, M.O.; Ogunkoya, O.O.; Asaolu, S.O. A study of an outbreak of schistosomiasis in two resettlement villages near Abeokuta, Ogun State, Nigeria. J. Helminthol. 1991, 65, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Olamiju, F.; Nebe, O.J.; Mogaji, H.; Marcus, A.; Amodu–Agbi, P.; Urude, R.O.; Apake, E.; Olamiju, O.; Okoronkwo, C.; Achu, I.; et al. Schistosomiasis outbreak during COVID-19 pandemic in Takum, Northeast Nigeria: Analysis of infection status and associated risk factors. PLoS ONE 2022, 17, e0262524. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P.; Gower, C.M.; Knowles, S.C.L.; Molyneux, D.H.; Fenton, A. One health–An ecological and evolutionary framework for tackling Neglected Zoonotic Diseases. Evol. Appl. 2016, 9, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, A.O. Farming households’ environment, nutrition and health interplay in southwest Nigeria. Int. J. Sci. Res. Agric. Sci. 2016, 3, 84–98. [Google Scholar] [CrossRef]
- Blench, R. Pastoral Cross-Border Movement. In Natural Resource Conflicts in North-Central Nigeria: A Handbook and Case Studies; Mandaras Publishing: London, UK, 2004; pp. 133–137. [Google Scholar]
- Umoh, N.R. Pastoralism in Nigeria’s middle-belt region: A resource or a curse? Int. J. Dev. Econ. Sustain. 2017, 5, 11–30. [Google Scholar]
- Rass, N. Policies and Strategies to Address the Vulnerability of Pastoralists in Sub-Saharan Africa. In Pro-Poor Livestock Policy Initiative; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Enwezor, F.; Umoh, J.U.; Esievo, K.; Anere, J.I. Transhumance pastoralism as risk factor in the trypanosome infections in cattle. Bull. Anim. Health Prod. Afr. 2009, 57, 44–48. [Google Scholar] [CrossRef]
- Aruwayo, A.; Adeola, S.S.; Ibrahim, U. Assessment of the challenges of nomadic farming activities in Daura agricultural zone of Katsina State, Nigeria. Niger. J. Anim. Prod. 2021, 48, 200–209. [Google Scholar] [CrossRef]
- Kamani, J.; Apanaskevich, D.A.; Gutiérrez, R.; Nachum-Biala, Y.; Baneth, G.; Harrus, S. Morphological and molecular identification of Rhipicephalus (Boophilus) microplus in Nigeria, West Africa: A threat to livestock health. Exp. Appl. Acarol. 2017, 73, 283–296. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Dai, S.-M.; Xue, J.-B.; Li, Y.-L.; Lv, S.; Xu, J.; Li, S.-Z.; Guo, J.-G.; Zhou, X.-N. The epidemiological status of schistosomiasis in P. R. China after the World Bank Loan Project, 2002–2017. Acta Trop. 2019, 195, 135–141. [Google Scholar] [CrossRef]
- Wang, W.; Bergquist, R.; King, C.H.; Yang, K. Elimination of schistosomiasis in China: Current status and future prospects. PloS Negl. Trop. Dis. 2021, 15, e0009578. [Google Scholar] [CrossRef]
- Gower, C.M.; Vince, L.; Webster, J.P. Should we be treating animal schistosomiasis in Africa? The need for a One Health economic evaluation of schistosomiasis control in people and their livestock. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, P.; Léger, E.; Hollenberg, E.; Diouf, N.; Sène, M.; Webster, J.P.; Häsler, B. Estimating the financial impact of livestock schistosomiasis on traditional subsistence and transhumance farmers keeping cattle, sheep and goats in northern Senegal. Parasites Vectors 2022, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.V.; Lambert, S.; Neves, M.I.; Borlase, A.; Léger, E.; Diouf, N.D.; Sène, M.; Webster, J.P.; Walker, M. Modelling livestock test-and-treat: A novel One Health strategy to control schistosomiasis and mitigate drug resistance. Front. Trop. Dis. 2022, 3, 893066. [Google Scholar] [CrossRef]
- Colley, D.G.; Binder, S.; Campbell, C.; King, C.H.; Tchuenté, L.A.T.; N'Goran, E.K.; Erko, B.; Karanja, D.M.; Kabatereine, N.B.; van Lieshout, L.; et al. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am. J. Trop. Med. Hyg. 2013, 88, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Urbano, B.; Léger, E.; Gabain, I.; De Dood, C.J.; Diouf, N.D.; Borlase, A.; Rudge, J.W.; Corstjens, P.L.A.M.; Sène, M.; Van Dam, G.J.; et al. Sensitivity and specificity of human point-of-care circulating cathodic antigen (PO-C CCA) test in African livestock for rapid diagnosis of schistosomiasis: A Bayesian latent class analysis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhou, Y.-B.; Liang, S.; Jiang, Q.-W. Factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasit Vectors 2012, 5, 275. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Ponpetch, K.; Zhou, Y.-B.; Guo, J.; Erko, B.; Stothard, J.R.; Murad, M.H.; Zhou, X.-N.; Satrija, F.; Webster, J.P.; et al. Diagnosis of Schistosoma infection in non-human animal hosts: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010389. [Google Scholar] [CrossRef]
- Xu, B.; Gordon, C.A.; Hu, W.; McManus, D.P.; Chen, H.-G.; Gray, D.J.; Ju, C.; Zeng, X.-J.; Gobert, G.N.; Ge, J.; et al. A novel procedure for precise quantification of Schistosoma japonicum eggs in bovine feces. PLoS Negl. Trop. Dis. 2012, 6, e1885. [Google Scholar] [CrossRef]
- Yu, J.M.; de Vlas, S.J.; Jiang, Q.W.; Gryseels, B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol. Int. 2007, 56, 45–49. [Google Scholar] [CrossRef]
- Utzinger, J.; Becker, S.L.; van Lieshout, L.; van Dam, G.J.; Knopp, S. New diagnostic tools in schistosomiasis. Clin. Microbiol. Infect. 2015, 21, 529–542. [Google Scholar] [CrossRef]
- Hotez, P.J.; Asojo, O.A.; Adesina, A.M. Nigeria: “Ground zero” for the high prevalence neglected tropical diseases. PLoS Negl. Trop. Dis. 2012, 6, e1600. [Google Scholar] [CrossRef] [PubMed]
- Global Data Lab. Area Profile Report—Nigeria. Available online: https://globaldatalab.org/areadata/profiles/NGAt/ (accessed on 30 January 2023).
- Food and Agriculture Organization of the United Nations. Nigeria Agriculture at a Glance. 2022. Available online: https://www.fao.org/nigeria/fao-in-nigeria/nigeria-at-a-glance/en/ (accessed on 30 January 2023).
- The World Bank. Third National Urban Water Sector Reform Project for Nigeria. 25 November 2021. Available online: https://projects.worldbank.org/en/projects-operations/project-detail/P123513 (accessed on 30 January 2023).
- United Nations Children’s Fund. Nigeria—Water, Sanitation & Hygiene. Available online: https://www.unicef.org/nigeria/water-sanitation-and-hygiene (accessed on 30 January 2023).
- Mogaji, H.O.; Ekpo, U.F.; Yusuff, Q.A.; Yusuff, H.A.; Adeaga, D.O.; Monday, J.; Adeniran, A.A. Impacts of water, sanitation and hygiene (WASH) interventions on intestinal helminthiasis of school-aged children in Ogun State, South-Western Nigeria. Trop. Med. Int. Health 2015, 20, 233. [Google Scholar]
- Ibok, E.E.; Daniel, E.E. Rural water supply and sustainable development in Nigeria: A case analysis of Akwa Ibom State. Am. J. Rural Dev. 2014, 2, 68–73. [Google Scholar] [CrossRef]
- Ihuah, P.W.; Kakulu, I.I. Rural water supply projects and sustainable development in Nigeria. J. Sustain. Dev. Afr. 2014, 16, 56–68. [Google Scholar]
- Strunz, E.C.; Addiss, D.G.; Stocks, M.E.; Ogden, S.; Utzinger, J.; Freeman, M.C. Water, sanitation, hygiene, and soil-transmitted helminth infection: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001620. [Google Scholar] [CrossRef]
- Grimes, J.E.; Tadesse, G.; Mekete, K.; Wuletaw, Y.; Gebretsadik, A.; French, M.D.; Harrison, W.E.; Drake, L.J.; Gardiner, I.A.; Yard, E.; et al. School water, sanitation, and hygiene, soil-transmitted helminths, and schistosomes: National mapping in Ethiopia. PLoS Negl. Trop. Dis. 2016, 10, e0004515. [Google Scholar] [CrossRef]
- Bayegun, A.A.; Omitola, O.O.; Umunnakwe, C.U.; Akande, F.A.; Akinwale, O.P.; Mogaji, H.O.; Ademolu, K.O.; Gyang, V.P.; Odoemene, S.N.; Stothard, J.R.; et al. Morphometric analysis of schistosome eggs recovered from human urines in communities along the shoreline of Oyan River Dam in Ogun State, Nigeria. J. Helminthol. 2023, 96, e89. [Google Scholar] [CrossRef]
- Onyekwere, A.M.; Rey, O.; Allienne, J.F.; Nwanchor, M.C.; Alo, M.; Uwa, C.; Boissier, J. Population genetic structure and hybridization of Schistosoma haematobium in Nigeria. Pathogens 2022, 11, 425. [Google Scholar] [CrossRef]
- Ndifon, G.T.; Betterton, C.; Rollinson, D. Schistosoma curassoni Brumpt, 1931 and S. bovis (Sonsino, 1876) in cattle in northern Nigeria. J. Helminthol. 1988, 62, 33–34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).