Bone Lesions in a Young Dog and a NEEM (Azadirachta indica) Spray as the Only Preventive Measure against Leishmaniasis: A Case Report
Simple Summary
Abstract
1. Introduction
2. Case Report
2.1. Case History
2.2. Orthopedic Examination and Radiological Findings
2.3. Clinical Findings
2.4. Laboratory Findings
2.5. Ophthalmological Findings
2.6. Treatment
2.7. First Follow-Up Visit
2.8. Second Follow-Up Visit
2.9. Third Follow-Up Visit
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayele, A.; Seyoum, Z. Review on canine leishmaniasis; etiology, clinical sign, pathogenesis, treatment and control methods. Glob. Vet. 2016, 17, 343–352. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Michalick, M.S.M.; Da Silva, M.E.; Dos Santos, C.C.P.; Frézard, F.; Da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. BioMed. Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef] [PubMed]
- Bongiorno, G.; Habluetzel, A.; Khoury, C.; Maroli, M. Host preferences of phlebotomine sand flies at a hypoendemic focus of canine leishmaniasis in central Italy. Acta Trop. 2003, 88, 109–116. [Google Scholar] [CrossRef]
- Manzillo, V.F.; Gizzarelli, M.; Vitale, F.; Montagnaro, S.; Torina, A.; Sotera, S.; Oliva, G. Serological and entomological survey of canine leishmaniasis in Lampedusa island, Italy. BMC Vet. Res. 2018, 14, 286. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Martín-Sánchez, J.; Navarro-Mari, J.M.; Pasquau-Liaño, J.; Salomón, O.D.; Morillas-Márquez, F. Visceral leishmaniasis caused by Leishmania infantum in a Spanish patient in Argentina: What is the origin of the infection? Case report. BMC Infect. Dis. 2004, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimol, S.; Sagi, O.; Horev, A.; Avni, Y.S.; Ziv, M.; Riesenberg, K. Cutaneous leishmaniasis caused by Leishmania infantum in Southern Israel. Acta Parasitol. 2016, 61, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Salomon, O.D.; Quintana, M.G.; Mastrangelo, A.V.; Fernández, M.S. Leishmaniasis and Climate Change—Case Study: Argentina. J. Trop. Med. 2012, 2012, 601242. [Google Scholar] [CrossRef] [PubMed]
- Kholoud, K.; Denis, S.; Lahouari, B.; El Hidan, M.A.; Souad, B. Management of Leishmaniases in the Era of Climate Change in Morocco. Int. J. Environ. Res. Public Health 2018, 15, 1542. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, R.; Montoya, A.; Fontal, F.; De Murguía, L.M.; Miró, G. Controlling phlebotomine sand flies to prevent canine Leishmania infantum infection: A case of knowing your enemy. Res. Vet. Sci. 2018, 121, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Alzohairy, M.A. Therapeutics Role ofAzadirachta indica(Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid. Based Complement. Altern. Med. 2016, 2016, 7382506. [Google Scholar] [CrossRef] [PubMed]
- Islas, J.F.; Acosta, E.; G.-Buentello, Z.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Guerrini, V.; Kriticos, C. Effects of azadirachtin on Ctenocephalides felis in the dog and the cat. Vet. Parasitol. 1998, 74, 289–297. [Google Scholar] [CrossRef]
- Kilonzo, B.S.; Ngomuo, A.J.; Sabuni, C.A.; Mgode, G.F. Effects of Azadirachta indica (Neem) Extract on Livestock Fleas in Morogoro District, Tanzania. Int. J. Trop. Insect Sci. 2001, 21, 89–92. [Google Scholar] [CrossRef]
- Srivastava, R.; Ghosh, S.; Mandal, D.B.; Azhahianambi, P.; Singhal, P.S.; Pandey, N.N.; Swarup, D. Efficacy of Azadirachta indica extracts against Boophilus microplus. Parasitol. Res. 2008, 104, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.; David, M. The efficacy of neem seed extract (Azadirachta indica) to control tick infestation in Tswana, Simmentaler and Brahman cattle. South Afr. J. Anim. Sci. 2002, 32, 1–6. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Walldorf, V.; Abdel-Ghaffar, F.; Al-Quraishy, S.; Al-Rasheid, K.A.S.; Mehlhorn, J. Biting and bloodsucking lice of dogs—treatment by means of a neem seed extract (MiteStop®, Wash Away Dog). Parasitol. Res. 2011, 110, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraishy, S.; Abdel-Ghaffar, F.; Al-Rasheid, K.A.S.; Mehlhorn, J.; Mehlhorn, H. Effects of a neem seed extract (MiteStop®) on mallophages (featherlings) of chicken: In vivo and in vitro studies. Parasitol. Res. 2011, 110, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Valle Salazar, E.; Marín Rodriguez, C.; Morales, G. Control de piojos (Anoplura) con aceite de nim (Azadirachta indica a. Juss) en caprinos del estado Falcón, Venezuela. Arch. Latinoam. Prod. Anim. 2016, 24, 59–67. Available online: https://ojs.alpa.uy/index.php/ojs_files/article/view/2398/941 (accessed on 28 July 2022).
- Benelli, G.; Canale, A.; Toniolo, C.; Higuchi, A.; Murugan, K.; Pavela, R.; Nicoletti, M. Neem (Azadirachta indica): Towards the ideal insecticide? Nat. Prod. Res. 2016, 31, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Showler, A. Botanically Based Repellent and Insecticidal Effects Against Horn Flies and Stable Flies (Diptera: Muscidae). J. Integr. Pest Manag. 2017, 8, 15. [Google Scholar] [CrossRef]
- Camarda, A.; Pugliese, N.; Bevilacqua, A.; Circella, E.; Gradoni, L.; George, D.; Sparagano, O.; Giangaspero, A. Efficacy of a novel neem oil formulation (RP03™) to control the poultry red mite Dermanyssus gallinae. Med. Vet. Èntomol. 2018, 32, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Suraj, R.; Rambarran, R.; Ali, K.; Harbajan, D.; Charles, R.; Sant, C.; Georges, K.; Suepaul, S. A comparison of the efficacy of two commercial acaricides (fipronil and amitraz) with Azadirachta indica (neem) on the brown dog tick (Rhipicephalus sanguineus) from canines in Trinidad. Transbound. Emerg. Dis. 2020, 67 (Suppl. S2), 142–148. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaffar, F.; Al-Quraishy, S.; Sobhy, H.; Semmler, M. Neem seed extract shampoo, Wash Away Louse®, an effective plant agent against Sarcoptes scabiei mites infesting dogs in Egypt. Parasitol. Res. 2008, 104, 145–148. [Google Scholar] [CrossRef]
- Pasipanodya, C.N.; Tekedza, T.T.; Chatiza, F.P.; Gororo, E. Efficacy of neem (Azadirachta indica) aqueous fruit extracts against Sarcoptes scabiei var. suis in grower pigs. Trop. Anim. Health Prod. 2021, 53, 135. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.P.; Dhiman, R.C. Neem oil as a sand fly (Diptera: Psychodidae) repellent. J. Am. Mosq. Control. Assoc. 1993, 9, 364–366. Available online: https://www.biodiversitylibrary.org/content/part/JAMCA/JAMCA_V09_N3_P364-366.pdf (accessed on 28 July 2022). [PubMed]
- Dhiman, R.C.; Sharma, V.P. Evaluation of neem oil as sandfly, Phlebotomus papatasi (Scopoli) repellent in an Oriental sore endemic area in Rajasthan. Southeast Asian J. Trop. Med. Public Health 1994, 25, 608–610. Available online: https://www.tm.mahidol.ac.th/seameo/1994-25-3/1994-25-3-608.pdf (accessed on 28 July 2022).
- Srinivasan, R.; Kalyanasundaram, M. Relative efficacy of DEPA and neem oil for repellent activity against Phlebotomus papatasi, the vector of leishmaniasis. J. Commun. Dis. 2001, 33, 180–184. [Google Scholar]
- Marghinotti, M.; Bongiorno, G.; Bianchi, R.; Maroli, M. Evaluation of NeemAzal®, Neem oil and picaridin (KBR 3023) against the sandfly bites (Diptera: Psychodidae). Parassitologia 2004, 45 (Suppl. S1), 92. [Google Scholar]
- Kebede, Y.; Gebre-Michael, T.; Balkew, M. Laboratory and field evaluation of neem (Azadirachta indica A. Juss) and Chinaberry (Melia azedarach L.) oils as repellents against Phlebotomus orientalis and P. bergeroti (Diptera: Psychodidae) in Ethiopia. Acta Trop. 2010, 113, 145–150. [Google Scholar] [CrossRef]
- Vismarra, A.; Kramer, L.H.; Moschi, A.; Ciuca, L.; Genchi, M. A survey on canine leishmaniosis: Best practice and guideline awareness among Italian veterinary practitioners. Prev. Vet. Med. 2021, 195, 105450. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health. Leishmaniosis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2021; World Organization for Animal Health: Paris, France, 2021; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.11_LEISHMANIOSIS.pdf (accessed on 28 July 2022).
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votypka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Neglected Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Alten, B.; Maia, C.; Afonso, M.O.; Campino, L.; Jiménez, M.; González, E.; Molina, R.; Bañuls, A.L.; Prudhomme, J.; Vergnes, B.; et al. Seasonal Dynamics of Phlebotomine Sand Fly Species Proven Vectors of Mediterranean Leishmaniasis Caused by Leishmania infantum. PLoS Neglected Trop. Dis. 2016, 10, e0004458. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J. Phlebotomid sandflies. Bull. World Health Organ. 1971, 44, 535–551. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2427821/pdf/bullwho00201-0064.pdf (accessed on 28 July 2022). [PubMed]
- Alexander, B. Sampling methods for phlebotomine sandflies. Med. Vet. Èntomol. 2000, 14, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, V.; Wasserberg, G.; Warburg, A. Bionomics of phlebotomine sandflies in the Galilee focus of cutaneous leishmaniasis in northern Israel. Med. Vet. Èntomol. 2004, 18, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Alten, B.; Ozbel, Y.; Ergunay, K.; Kasap, O.; Cull, B.; Antoniou, M.; Velo, E.; Prudhomme, J.; Molina, R.; Bañuls, A.-L.; et al. Sampling strategies for phlebotomine sand flies (Diptera: Psychodidae) in Europe. Bull. Èntomol. Res. 2015, 105, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Naucke, T.J.; Menn, B.; Massberg, D.; Lorentz, S. Sandflies and leishmaniasis in Germany. Parasitol. Res. 2008, 103, 65–68. [Google Scholar] [CrossRef]
- Tánczos, B.; Balogh, N.; Király, L.; Biksi, I.; Szeredi, L.; Gyurkovsky, M.; Scalone, A.; Fiorentino, E.; Gramiccia, M.; Farkas, R. First Record of Autochthonous Canine Leishmaniasis in Hungary. Vector Borne Zoonotic Dis. 2012, 12, 588–594. [Google Scholar] [CrossRef]
- Morosetti, G.; Toson, M.; Trevisiol, K.; Idrizi, I.; Natale, A.; Lucchese, L.; Michelutti, A.; Ceschi, P.; Lorenzi, G.; Piffer, C.; et al. Canine leishmaniosis in the Italian northeastern Alps: A survey to assess serological prevalence in dogs and distribution of phlebotomine sand flies in the Autonomous Province of Bolzano—South Tyrol, Italy. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100432. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: Changing distribution patterns. Parasites Vectors 2020, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Gradoni, L.; Bettini, S.; Pozio, E. Leishmaniasis in Tuscany (Italy): VI. Canine leishmaniasis in the focus of Monte Argentario (Grosseto). Acta Trop. 1981, 38, 383–393. [Google Scholar] [CrossRef]
- Orndorff, G.R.; Cooper, B.A.; Smith, W.; Ryan, J.R. Canine Visceral Leishmaniasis in Sicily. Mil. Med. 2000, 165, 29–32. [Google Scholar] [CrossRef][Green Version]
- Shang, L.-M.; Peng, W.-P.; Jin, H.-T.; Xu, D.; Zhong, N.-N.; Wang, W.-L.; Wu, Y.-X.; Liu, Q. The prevalence of canine Leishmania infantum infection in Sichuan Province, southwestern China detected by real time PCR. Parasites Vectors 2011, 4, 173. [Google Scholar] [CrossRef] [PubMed]
- Agut, A.; Corzo, N.; Murciano, J.; Laredo, F.; Soler, M. Clinical and radiographic study of bone and joint lesions in 26 dogs with leishmaniasis. Vet. Rec. 2003, 153, 648–652. [Google Scholar] [CrossRef]
- Spreng, D. Leishmanial polyarthritis in two dogs. J. Small Anim. Pract. 1993, 34, 559–563. [Google Scholar] [CrossRef]
- Wolschrijn, C.F.; Meyer, H.P.; Hazewinkel, H.A.W.; Wolvekamp, W.T.C. Destructive polyarthritis in a dog with leishmaniasis. J. Small Anim. Pract. 1996, 37, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.F.; Polizopoulou, Z.; Saridomichelakis, M.N.; Argyriadis, D.; Fytianou, A.; Plevraki, K.G. Clinical considerations on canine visceral leishmaniasis in Greece: A retrospective study of 158 cases (1989–1996). J. Am. Anim. Hosp. Assoc. 1999, 35, 376–383. [Google Scholar] [CrossRef] [PubMed]
- McConkey, S.E.; López, A.; Shaw, D.; Calder, J. Leishmanial polyarthritis in a dog. Can. Vet. J. 2002, 43, 607–609. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC339396/pdf/20020800s00025p607.pdf (accessed on 28 July 2022). [PubMed]
- Santos, M.; Marcos, R.; Assunção, M.; Matos, A.J. Polyarthritis associated with visceral leishmaniasis in a juvenile dog. Vet. Parasitol. 2006, 141, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, S.; Marchetti, V.; Mancianti, F.; Guidi, G.; Bennett, D. Retrospective study of 14 cases of canine arthritis secondary to Leishmania infection. J. Small Anim. Pract. 2014, 55, 309–313. [Google Scholar] [CrossRef]
- Yamaguchi, R.A.; French, T.W.; Simpson, C.F.; Harvey, J.W. Leishmania donovani in the synovial fluid of a dog with visceral leishmaniasis. J. Am. Anim. Hosp. Assoc. 1983, 19, 723–726. [Google Scholar]
- Söffler, C.; Winkels, P.; Hess, M.; Engelhardt, P.; Wallborn, F. Leishmania-infantum-bedingte Knochenläsionen bei einem Hund. Tierarz. Praxis Klient. 2016, 44, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Turrel, J.M.; Pool, R.R. Bone lesions in four dogs with visceral leishmaniasis. Vet. Radiol. 1982, 23, 243–249. [Google Scholar] [CrossRef]
- Buracco, P.; Abate, O.; Guglielmino, R.; Morello, E. Osteomyelitis and arthrosynovitis associated with Leishmania donovani infection in a dog. J. Small Anim. Pract. 1997, 38, 29–30. [Google Scholar] [CrossRef]
- Franch, J.; Lafuente, P.; Diaz-Bertrana, M.C.; Munilla, A.; Durall, I.; Pastor, J.; Torrent, E. Management of leishmanial osteolytic lesions in a hypothyroid dog by partial tarsal arthrodesis. Vet. Rec. 2004, 155, 559–562. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.I.; Juliano, R.S.; Gomes, T.S.; Diniz, S.D.A.; Borges, M.; Tafuri, W.L.; Santos, R.D.L. Osteolytic osteomyelitis associated with visceral leishmaniasis in a dog. Vet. Parasitol. 2005, 129, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Fondati, A.; Gradoni, L.; Lubas, G.; Paltrinieri, S.; Roura, X.; Zatelli, A.; Zini, E.; Maroli, M. Prevenzione della leishmaniosi canina: Cosa è utile sapere prima di raccomandare un prodotto. Veterinaria 2018, 32, 101–112. Available online: https://veterinaria.scivac.org/2018/year-32-n-2-april-2018/prevenzione-della-leishmaniosi-canina-cosa-e-utile-sapere-prima-di-raccomandare-un-prodotto-topico-attivo-contro-la-puntura-dei-flebotomi.html (accessed on 28 July 2022).
- Maroli, M.; Mizzoni, V.; Siragusa, C.; D’Orazi, A.; Gradoni, L. Evidence for an impact on the incidence of canine leishmaniasis by the mass use of deltamethrin-impregnated dog collars in southern Italy. Med. Vet. Èntomol. 2001, 15, 358–363. [Google Scholar] [CrossRef]
- Brianti, E.; Gaglio, G.; Napoli, E.; Falsone, L.; Prudente, C.; Basano, F.S.; Latrofa, M.S.; Tarallo, V.D.; Dantas-Torres, F.; Capelli, G.; et al. Efficacy of a slow-release imidacloprid (10%)/flumethrin (4.5%) collar for the prevention of canine leishmaniosis. Parasites Vectors 2014, 7, 327. [Google Scholar] [CrossRef]
- Coura-Vital, W.; Leal, G.G.D.A.; Marques, L.A.; Pinheiro, A.D.C.; Carneiro, M.; Reis, A.B. Effectiveness of deltamethrin-impregnated dog collars on the incidence of canine infection by Leishmania infantum: A large scale intervention study in an endemic area in Brazil. PLoS ONE 2018, 13, e0208613. [Google Scholar] [CrossRef]
- Lopes, E.G.; Sevá, A.P.; Ferreira, F.; Nunes, C.M.; Keid, L.B.; Hiramoto, R.M.; Ferreira, H.L.; Oliveira, T.M.F.S.; Ovallos, F.G.; Galati, E.A.B.; et al. Vaccine effectiveness and use of collar impregnated with insecticide for reducing incidence of Leishmania infection in dogs in an endemic region for visceral leishmaniasis, in Brazil. Epidemiol. Infect. 2018, 146, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, O.; Dilger, E.; Calvo-Bado, L.A.; Kravar-Garde, L.; Carter, V.; Bell, M.J.; Alves, G.B.; Goncalves, R.; Makhdoomi, M.M.; González, M.A.; et al. Sand fly synthetic sex-aggregation pheromone co-located with insecticide reduces the incidence of infection in the canine reservoir of visceral leishmaniasis: A stratified cluster randomised trial. PLoS Negl. Trop. Dis. 2019, 13, e0007767. [Google Scholar] [CrossRef] [PubMed]
- Bongiorno, G.; Meyer, L.; Evans, A.; Lekouch, N.; Doherty, P.; Chiummo, R.; Gradoni, L. Insecticidal efficacy against Phlebotomus perniciosus in dogs treated orally with fluralaner in two different parallel-group, negative-control, random and masked trials. Parasites Vectors 2022, 15, 18. [Google Scholar] [CrossRef]
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Alexander, B.; Maroli, M. Control of phlebotomine sandflies. Med. Vet. Èntomol. 2003, 17, 1–18. [Google Scholar] [CrossRef]
- Maroli, M.; Gradoni, L.; Oliva, G.; Castagnaro, M.; Crotti, A.; Lubas, G.; Paltrinieri, S.; Roura, X.; Zini, E.; Zatelli, A. Guidelines for prevention of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.D.A.; da Costa, L.M.; Pessoa, G.D.C.; Obara, M.T. Methods for detecting insecticide resistance in sand flies: A systematic review. Acta Trop. 2020, 213, 105747. [Google Scholar] [CrossRef]
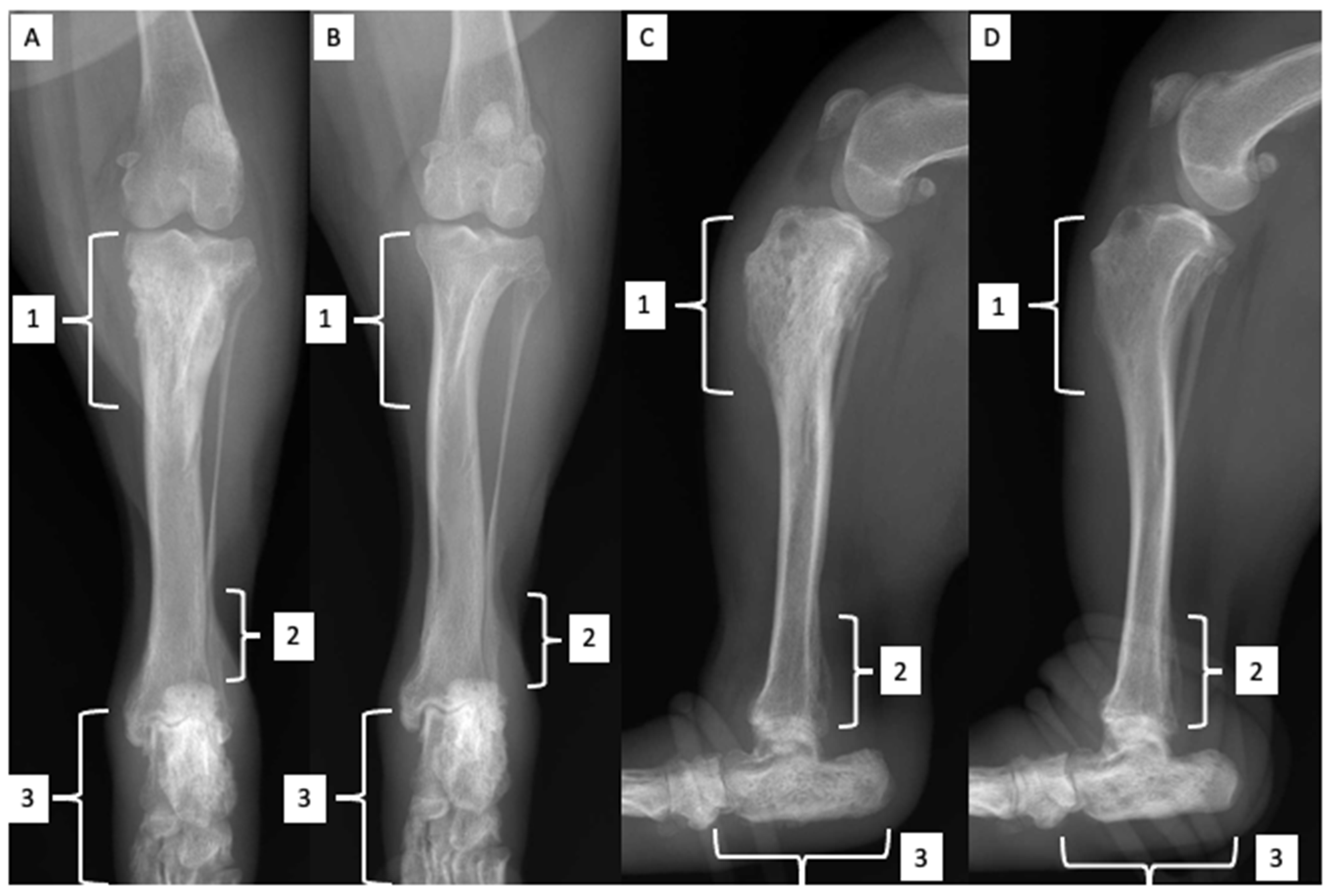


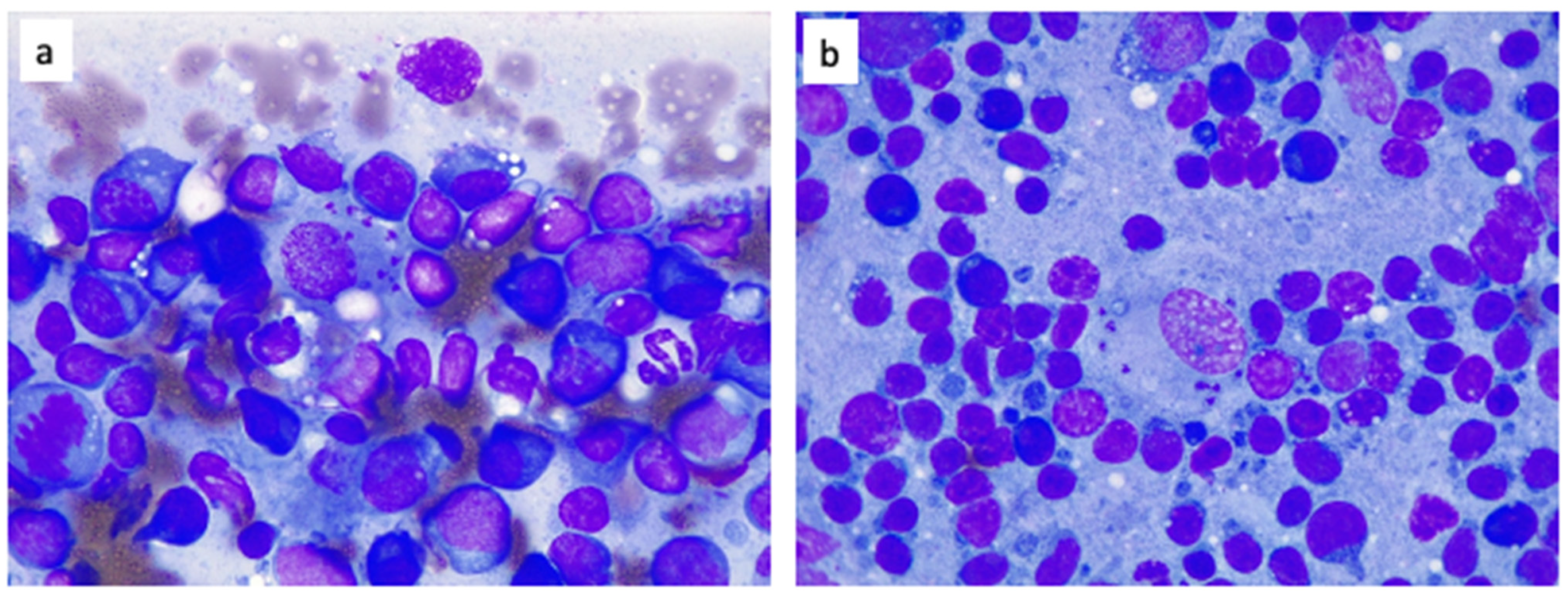
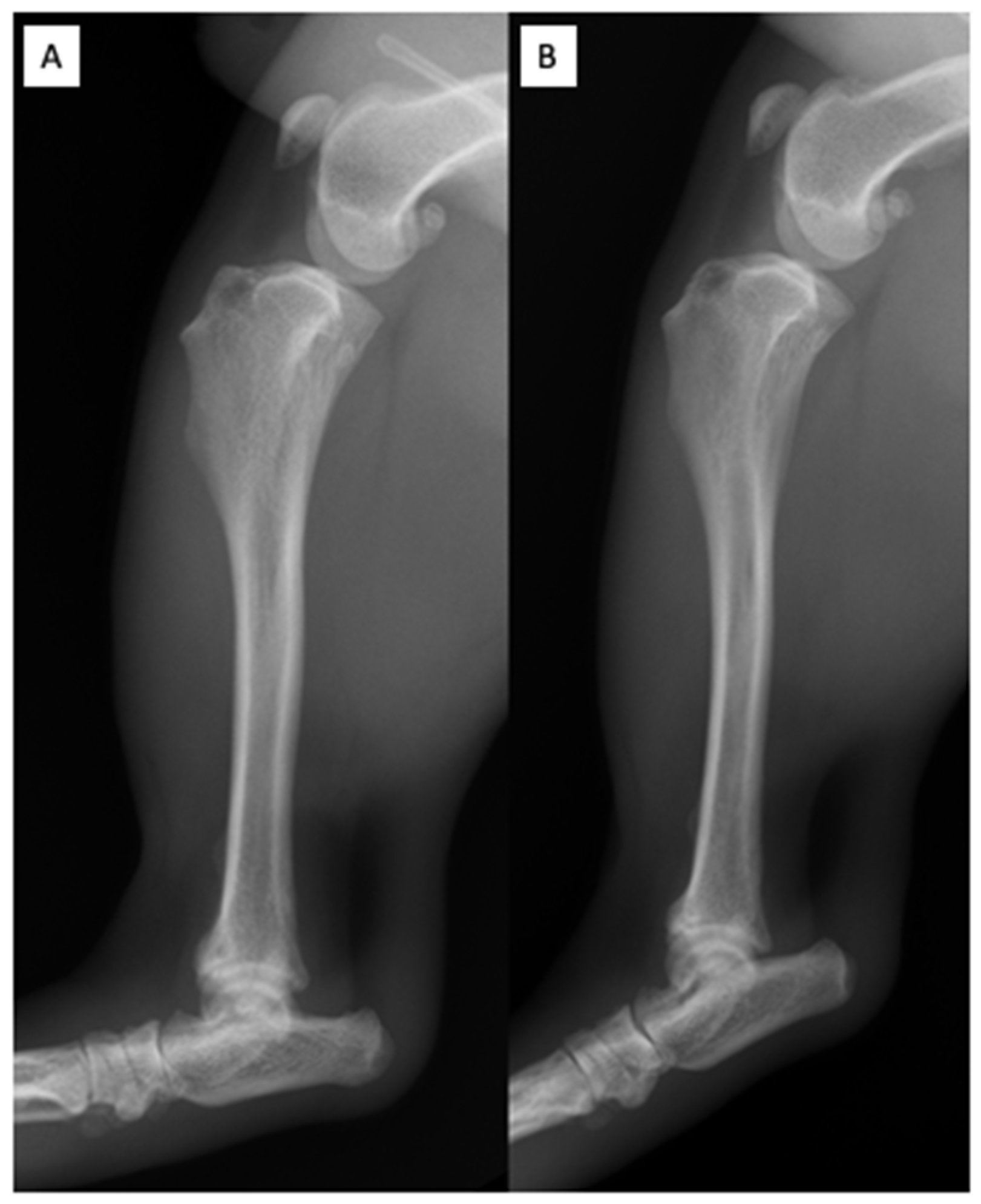
| Parameters & Units | Reference Interval | D0 * | D10 ^ | D31 | D89 |
|---|---|---|---|---|---|
| RBC M/μL | 5.65–8.87 | 4.65 | 4.31 | 4.80 | 6.05 |
| HCT % | 37.3–61.7 | 31.4 | 28.9 | 32.8 | 43.0 |
| HGB g/dL | 13.1–20.5 | 11.2 | 10.5 | 11.8 | 15.4 |
| MCV fL | 61.6–73.5 | 67.0 | 67.1 | 68.3 | 71.1 |
| MCH pg | 21.2–25.9 | 23.9 | 24.4 | 24.6 | 25.5 |
| MCHC g/dL | 32.0–37.9 | 35.7 | 36.3 | 36.6 | 35.8 |
| RDW % | 13.6–21.7 | 14.7 | 15.2 | 16.3 | 14.3 |
| Retics K/μL | 10.0–110.0 | 27.7 | 24.6 | 38.4 | 75.0 |
| Retic-HGB pg | 22.3–29.6 | 26.9 | 25.9 | 25.8 | 25.7 |
| WBC K/μL | 5.05–16.76 | 14.74 | 11.35 | 10.72 | 12.70 |
| NEU seg K/μL | 3.7–11.9 | 11.2 | 9.31 | 7.29 | 8.38 |
| NEU band K/μL | 0.0–0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| EOS K/μL | 0.1–1.35 | 1.03 | 0.23 | 0.64 | 0.76 |
| BAS K/μL | 0.0–0.1 | 0.0 | 0.0 | 0.11 | 0.0 |
| LYM K/μL | 0.7–5.1 | 1.18 | 0.91 | 2.04 | 3.18 |
| MON K/μL | 0.2–1.5 | 1.33 | 0.91 | 0.64 | 0.38 |
| PLT K/μL | 148–484 | 213 | 144 | 192 | 145 |
| MPV fL | 8.7–13.2 | 12.9 | 13.0 | 12.7 | 14.4 |
| PDW fL | 9.1–19.4 | 14.2 | 16.4 | 16.2 | 19.6 |
| PCT % | 0.14–0.46 | 0.27 | 0.19 | 0.24 | 0.21 |
| PLT estimate | adequate | adequate | inadequate | adequate | adequate |
| Notes on blood smear evaluation | |||||
| RBC rouleaux | Absent | +++ | +++ | Absent | Absent |
| Polychromasia | Absent/+ | Absent | Absent | Absent | + |
| Reactive LYM | Absent | +++ | +++ | ++ | Absent |
| Activated MON | Absent | + | + | ++ | Absent |
| Toxic NEU | Absent | + | Absent | Absent | Absent |
| Parameters & Units | Reference Interval | D0 * | D31 ^ | D89 |
|---|---|---|---|---|
| Creatin phosphokinase IU/L | 40–185 | 236 | ||
| Lactate dehydrogenase IU/L | 20–160 | 49 | ||
| Aspartate transaminase IU/L | 15–40 | 60 | ||
| Alanine transaminase IU/L | 20–70 | 31 | 37 | 46 |
| Alkaline phosphatase IU/L | 45–250 | 111 | 115 | 95 |
| Gamma glutamyl transferase IU/L | 2–11 | 0.8 | 1.5 | 1.6 |
| Amylase IU/L | 400–1500 | 1673 | ||
| Bilirubin Total mg/dL | 0.07–0.3 | 0.10 | ||
| Glucose mg/dL | 80–125 | 102 | 115 | 129 |
| Cholesterol mg/dL | 120–280 | 134 | 181 | 253 |
| Triglyceride mg/dL | 25–90 | 67 | ||
| Urea mg/dL | 15–55 | 40 | 37 | 57 |
| Creatinine mg/dL | 0.6–1.5 | 1.1 | 1.1 | 0.9 |
| Total Protein g/dL | 5.8–7.8 | 10.8 | 8.4 | 7.1 |
| Albumin g/dL | 2.6–4.1 | 2.1 | 2.6 | 4.0 |
| Globulin g/dL | 2.5–4.5 | 8.7 | 5.8 | 3.1 |
| A/G ratio | 0.6–1.3 | 0.24 | 0.45 | 1.3 |
| Fructosamine μmol/L | 170–430 | 255 | ||
| C-reactive protein mg/L | 0.0–0.3 | 2.0 | 0.4 | |
| Calcium mg/dL | 8.7–11.2 | 10.9 | 11.2 | 11.6 |
| Phosphate mg/dL | 2.5–5.0 | 5.7 | 4.8 | |
| Na mEq/L | 146–156 | 142 | 146 | |
| K mEq/L | 3.9–5.5 | 4.3 | 4.0 | |
| Na/K ratio | 26.5–40 | 33 | 37 | |
| Cl mEq/L | 10–122 | 117 | 108 | |
| HCO3 mEq/L | 21–31 | 25 | 25 | |
| Anion Gap mEq/L | 12–24 | 4.3 | 17 | |
| Mg mg/dL | 1.6–2.7 | 2.0 | 1.7 | |
| Iron mcg/dL | 80–190 | 132 | ||
| Serum aspect | Clear | Clear | Clear | Mild lipemia & hemolysis |
| Parameters & Units | Reference Interval | D0 * | D31 ^ | D89 |
|---|---|---|---|---|
| Sampling | Free catch | Free catch | Free catch | |
| Color | Yellow | Dark yellow | Yellow | Light yellow |
| Aspect | Clear | Clear | Cloudy | Clear |
| Specific gravity | 1015–1045 | 1049 | 1038 | 1058 |
| pH | 5.0–7.5 | 7.0 | 6.0 | 5.0 |
| Glucose | Neg. | Neg. | Neg. | Neg. |
| Ketones | Neg. | Neg. | Neg. | Neg. |
| HGB-RBC/mcL | Neg. | 250 | Neg. | Neg. |
| Bilirubin mg/dL | Neg. | 1 | Neg. | Neg. |
| Urobilinogen mg/dL | Neg. | 1 | Neg. | Neg. |
| Protein mg/dL | 50 | 100 | Neg. | Neg. |
| UPC ratio | < 0.5 | 0.62 | 0.06 | 0.23 |
| Sediment | ||||
| RBC/HPF | 0–5 | 10 | 0 | 0 |
| WBC/HPF | 0–5 | 0 | 0 | 0 |
| Epithelial cells/HPF | 0-rare | 0 | 1 | 1 |
| Casts/LPF | 0 | 1 hyaline | 0 | 0 |
| Crystals | 0-rare | 0 | 0 | 0 |
| Bacteria | Absent | Absent | Absent | Absent |
| Parameters & Units | Reference Interval | D0 * | D31 ^ |
|---|---|---|---|
| Prothrombin time s | 5.5–11.4 | 8.2 | 7.9 |
| Activated partial thromboplastin time s | 10.6–19.9 | 16.2 | 14.8 |
| Fibrinogen mg/dL | 125–335 | 527 | 263 |
| D-dimer mcg/mL | 0.1–0.35 | 0.11 |
| Parameters & Units | Reference Interval | D0 * | D31 ^ | D89 |
|---|---|---|---|---|
| Albumin % | 51–65 | 20.1 | 31.8 | 50.3 |
| α1 globulin % | 2–6 | 4.7 | 4.0 | 4.8 |
| α2 globulin % | 7–15 | 11.7 | 6.7 | 10.4 |
| β1 globulin % | 2–5 | 5.1 | 3.4 | 3.3 |
| β2 globulin % | 2–9 | 2.6 | 7.0 | 8.8 |
| β3 globulin % | 6–12 | 4.1 | 4.7 | 8.3 |
| γ globulin % | 5–15 | 51.7 | 42.4 | 14.1 |
| Albumin g/dL | 3.0–4.2 | 2.0 | 2.7 | 3.6 |
| α1 globulin g/dL | 0.2–0.3 | 0.5 | 0.3 | 0.3 |
| α2 globulin g/dL | 0.6–0.8 | 1.2 | 0.6 | 0.7 |
| β1 globulin g/dL | 0.2–0.3 | 0.5 | 0.3 | 0.2 |
| β2 globulin g/dL | 0.3–0.4 | 0.3 | 0.6 | 0.6 |
| β3 globulin g/dL | 0.5–0.7 | 4.1 | 0.4 | 0.6 |
| γ globulin g/dL | 0.6–0.8 | 5.2 | 3.6 | 1.0 |
| A/G ratio | 0.6–1.3 | 0.25 | 0.47 | 1.0 |
| Total Protein g/dL | 5.5–7.6 | 10.1 | 8.4 | 7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Feo, G.; Lubas, G.; Citi, S.; Puccinelli, C.; Papini, R.A. Bone Lesions in a Young Dog and a NEEM (Azadirachta indica) Spray as the Only Preventive Measure against Leishmaniasis: A Case Report. Zoonotic Dis. 2022, 2, 95-110. https://doi.org/10.3390/zoonoticdis2030010
De Feo G, Lubas G, Citi S, Puccinelli C, Papini RA. Bone Lesions in a Young Dog and a NEEM (Azadirachta indica) Spray as the Only Preventive Measure against Leishmaniasis: A Case Report. Zoonotic Diseases. 2022; 2(3):95-110. https://doi.org/10.3390/zoonoticdis2030010
Chicago/Turabian StyleDe Feo, Giulia, George Lubas, Simonetta Citi, Caterina Puccinelli, and Roberto Amerigo Papini. 2022. "Bone Lesions in a Young Dog and a NEEM (Azadirachta indica) Spray as the Only Preventive Measure against Leishmaniasis: A Case Report" Zoonotic Diseases 2, no. 3: 95-110. https://doi.org/10.3390/zoonoticdis2030010
APA StyleDe Feo, G., Lubas, G., Citi, S., Puccinelli, C., & Papini, R. A. (2022). Bone Lesions in a Young Dog and a NEEM (Azadirachta indica) Spray as the Only Preventive Measure against Leishmaniasis: A Case Report. Zoonotic Diseases, 2(3), 95-110. https://doi.org/10.3390/zoonoticdis2030010






