1. Introduction
Migrant and seasonal workers in agricultural and other industries may be especially vulnerable and at high-risk for contracting tick-borne diseases (TBDs), including Tickborne Relapsing Fever, Rocky Mountain Spotted Fever, and other illnesses due to occupational environments, poverty, and inferior living conditions, along with a lack of access to preventative healthcare or infectious disease physicians. Previous scholarship suggests sleeping outdoors is particularly risky for vector-borne disease exposure in general, including malaria [
1]. For seasonal workers, whether in agriculture or other industries, occupational and immigration status often results in more time sleeping outdoors.
Among TBDs, Lyme disease (LD), Ehrlichiosis, and Rocky Mountain Spotted Fever particularly have spread south and west in the continental United States (US). TBD expansion is highly relevant in US states such as Texas, with increasing reports of LD cases, although officially perceived as non-endemic.
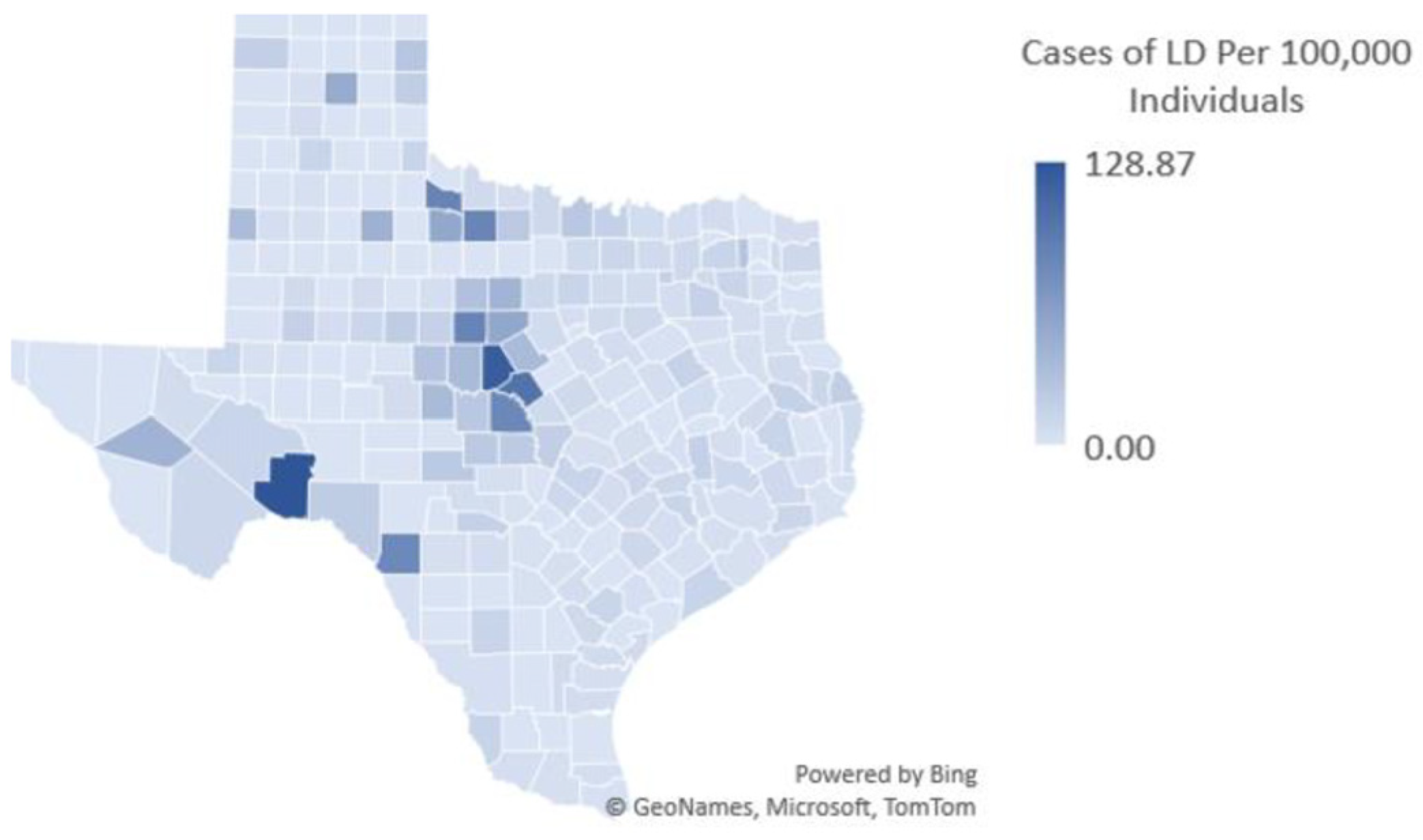
Figure 1 shows US Centers for Disease Control and Prevention (CDC) reported LD cases per 100,000 in Texas, represented by color density as indicated on the right in the figure.
Against this backdrop, as part of a larger ongoing effort to assess human TBD risk in different perceived endemic and non-endemic areas, we conducted an exploratory study amongst vulnerable populations living in migrant and seasonal worker communities in Texas. Survey respondents were particularly disadvantaged and vulnerable relative to occupational, immigration status, and other social factors. This brief report explores environmental, behavioral, and living conditions that may affect levels of risk among seasonal and migrant workers, among others, in agricultural communities, mostly who are Spanish-speaking and migrating from Mexico to the U.S. for employment.
2. Materials and Methods
In 2021, through a partnership with the National Center for Farmworker Health (NCFH), we conducted a survey on respondents from migrant communities, with the help of Spanish-speaking nurses and healthcare workers, across Texas. Specifically, the survey resulted in 260 validated responses. We employed the Horowitz Multisystemic Infectious Disease Syndrome Questionnaire (HMQ), recognized as a “valid, efficient, and low-cost screening tool for medical practitioners” for predicting TBDs in patients [
3]. The HMQ uses the following scoring in assessing TBD likelihood: 0–20 = Not Likely, 21–36 = Possible, 36–62 = Likely, and ≥63 = Highly Likely [
3].
Working with the NCFH, and with assistance from the Consulate General of México in Dallas in designated locations, thirteen sites were identified for survey administration (and the distribution of TBD prevention materials). The selected sites were located across nine Texas counties, covering five different ecosystems with habitats varying in suitability for relevant tick populations (
Table 1):
Within these ecosystems, habitat suitability is affected by varying climate and related conditions. The South Texas Brushlands (Hidalgo, Starr, Bexar, and Jim Wells counties) are notable in this regard, as survey respondents report considerable disease burden in this region, also noting an ecosystem that is particularly prone to ticks. For example, white-tailed deer are known to host ticks in the Lower Rio Grande Valley and assist in the distribution of infected ticks throughout the region [
4]. Both temperature and deer density are established factors in creating suitable tick habitats [
5], and deer density has been associated with the spread of Cattle Tick Fever, or Bovine Babesiosis. As shown in
Table 2, various ticks and related causative disease agents in humans and fauna are found within Texas (note: the Cayenne is limited to only the South Texas region):
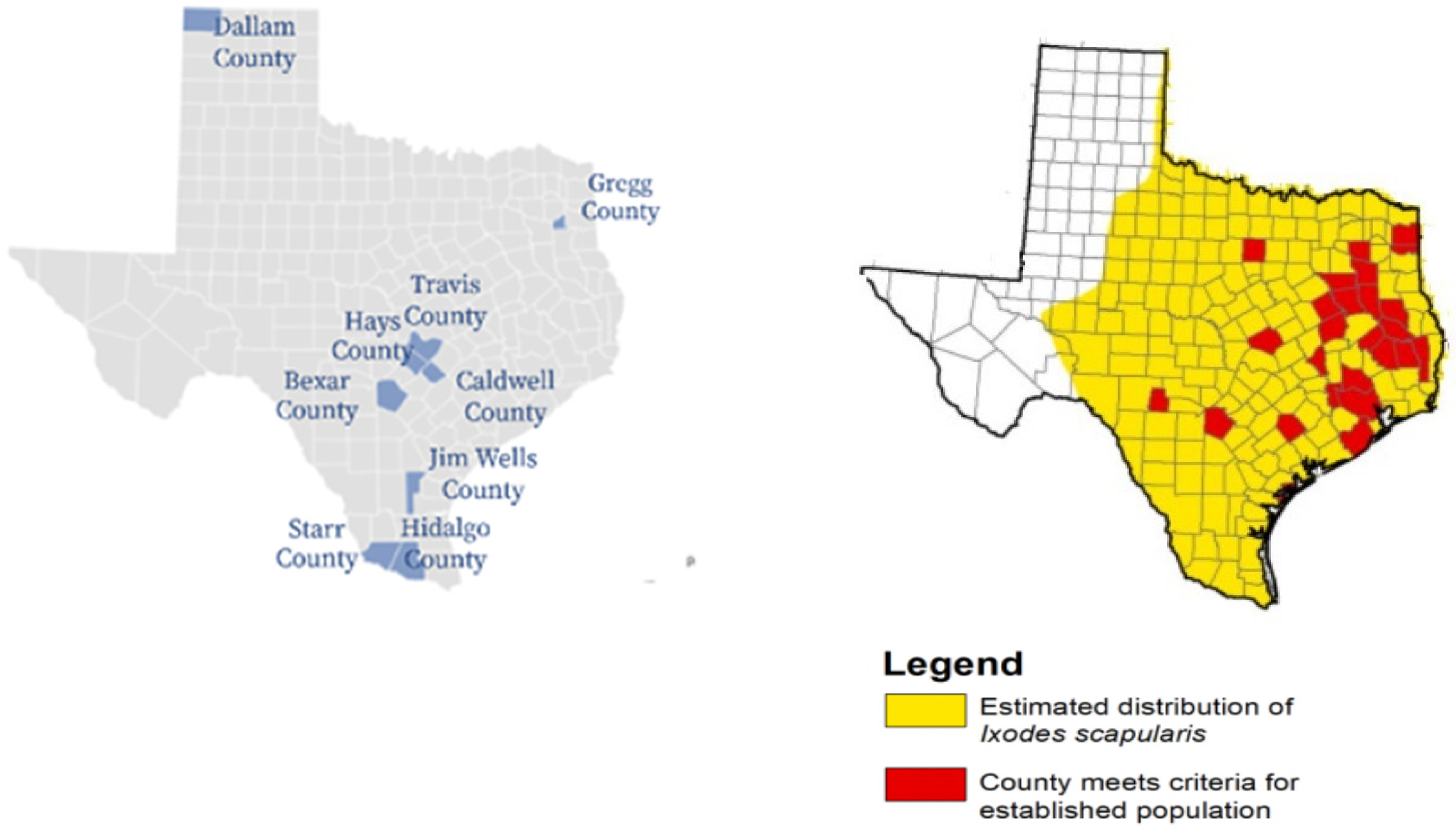
Figure 2 shows the nine survey counties compared to CDC-reported established and estimated distribution of
Ixodes scapularis tick populations. The Texas maps are shown to provide a visual representation of survey counties and known tick distribution.
3. Results
Of 260 total respondents included, 18 (7%) reported with scores ≥36 (“Likely” and “Highly Likely”); 38 (15%) scored at or between 21 and 35 (“Possibly”); and 204 (78%) scored ≤20 (“Unlikely” to have a TBD). Out of the 260, a total of 22% overall were possibly, likely, or highly likely to have a TBD. Living conditions and experiences, as reported, were compared among all scoring categories.
Of particular interest were survey respondents deemed likely-to-highly-likely to have a TBD based on the HMQ. Certain behaviors reported by those respondents, such as chances of exposure to ticks through, for example, sleeping outside, may indicate higher likelihoods of disease risk. Due to similar responses and risk categories, as well as their small sample sizes compared to lower risk categories, the highly likely and likely groups were merged for the purposes of reporting descriptive results in this report.
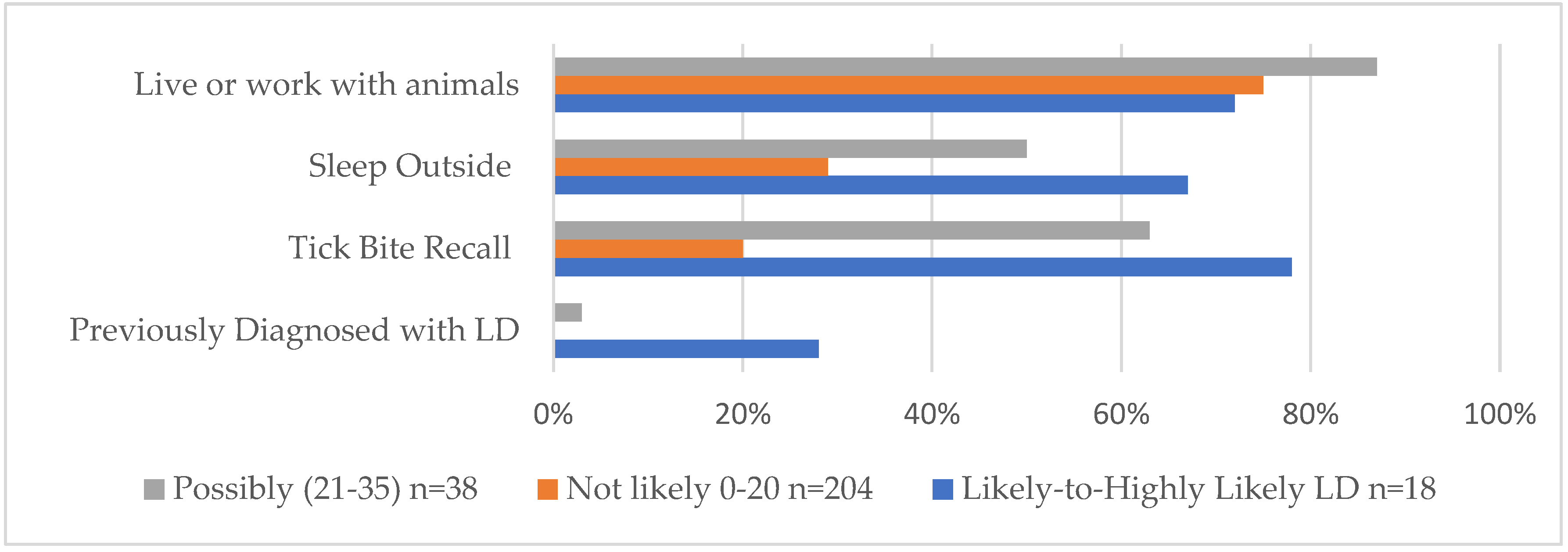
Overall, as shown in
Figure 3 and also indicated in
Table 1 to highlight key findings, the Likely-to-Highly-Likely scoring respondents—and the Possibly group, to a lesser extent—were found to have higher percentages of a “previous diagnosis of LD”, “recalled a tick bite”, and “slept outside on a regular basis” with respect to their Not-Likely counterparts. In particular, tick bite recall was considerably higher among the Likely-to-Highly-Likely and Possible groups, and a previous diagnosis of Lyme disease was reported by 28% of Likely-to-Highly-Likely scoring respondents. The Likely-to-Highly-Likely group is shown in blue in
Figure 3.
Amongst those in each risk category reporting a tick bite encounter, reactions to the tick bite were subsequently analyzed. Respondents could select if their tick bite was associated with an Erythema Migrans rash (EM), an undefined rash, and/or was followed by flu-like symptoms, amongst numerous other symptoms. Those in the Likely-to-Highly-Likely risk category reported symptomatic and clinical presentation of a TBD at considerably higher rates than those in the Possibly or Unlikely categories, e.g., 56% percent of Likely-to-Highly Likely respondents reported a tick bite with concomitant rash and flu-like symptoms (
Table 3). Almost one-third had a previous diagnosis of LD, and 67% of those most likely to have a TBD reported sleeping outdoors.
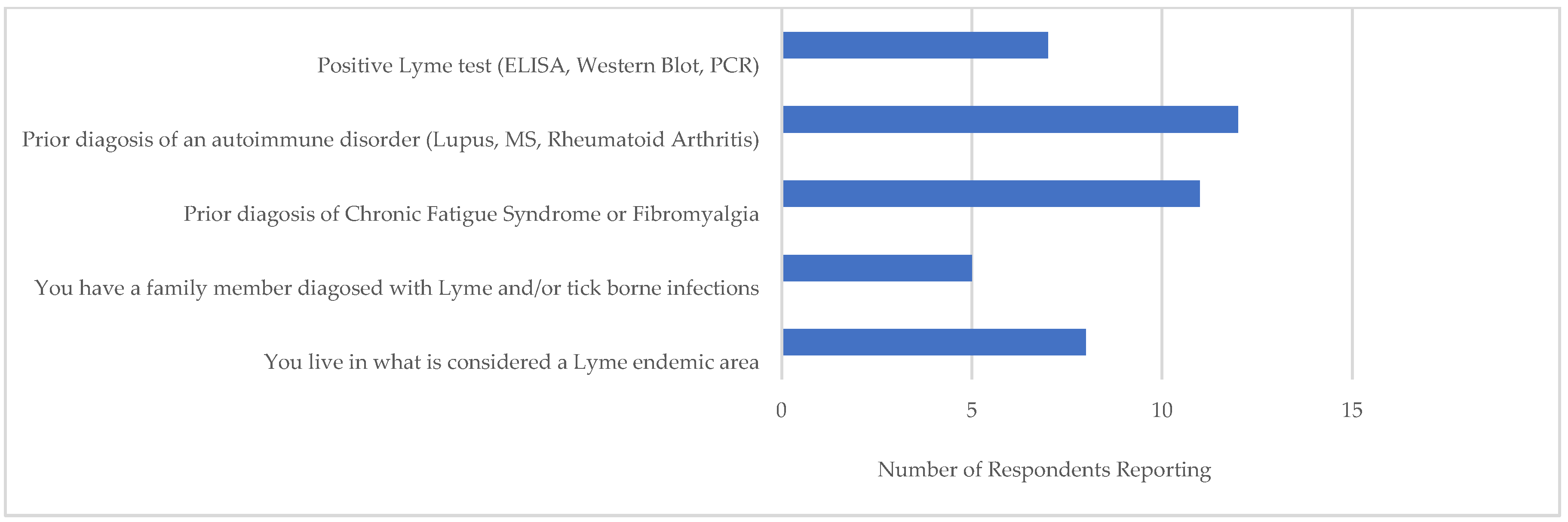
Figure 4 shows those most likely to have a TBD (i.e., a ≥ 36 score). Out of 18 Likely-to Highly-Likely respondents, more than half had previously received diagnoses for conditions associated to TBDs, i.e., an autoimmune disorder, Chronic Fatigue Syndrome, and/or fibromyalgia. A smaller number reported a positive LD serological test. Of the 8 respondents in this category who reported living in a LD endemic area, two were from Hidalgo County, two from Gregg County, and four from Hays County. According to CDC reports, these counties had prior LD cases: during 2000–2019, 34 confirmed cases were reported in Hidalgo, 24 in Hays, and 4 in Gregg [
6].
4. Discussion
This exploratory study indicates that survey respondents working in agricultural regions in Texas who scored high on the HMQ were more likely to recall a tick bite, sleep outside, and to have reported receiving a previous LD diagnosis than respondents with low scores. Living or working with animals did not appear to be associated with a higher risk of symptoms or disease in this survey. Our analysis also indicated that Likely-to-Highly-Likely respondents were found principally in the higher endemic counties amongst the survey sites. Preventative and diagnostic questions that target the main TBD symptoms, as in the HMQ survey, could assist physicians and healthcare workers in detecting and treating TBDs in these vulnerable populations, including identification of appropriate laboratories for testing. Interestingly, the highest scoring survey respondents reported previous LD diagnoses, tick bite encounters with sequala, and that they lived in TBD-endemic counties. These counties also are known for high incidences of canine TBDs such as ehrlichiosis. For example, 10.3% of all dogs tested in Hidalgo County in Texas in 2020 were positive for ehrlichiosis [
8].
Vulnerable populations, especially those who may not seek treatment due to poverty, low literacy, immigration status, etc., may be at a heightened risk of TBDs in areas with reported cases. Interestingly, however, some respondents with more severe symptoms reported a prior diagnosis of LD, indicating rural and migrant clinicians may have knowledge of TBD risk in these geographic areas. Numerous TBDs with similar or overlapping symptoms are present in Texas, including Southern Tick-Associated Rash Illness (STARI), caused by the bite of an infected lone star tick, Amblyomma Americanum, found throughout the south-central states with distribution extending into central Texas. Along with occupational exposure, living conditions and exposure factors as reported here—such as sleeping outside or experiencing a tick bite encounter with subsequent clinical manifestations of TBDs—should be noted by migrant and rural healthcare providers and integrated into the broader epidemiological surveying infrastructure.
5. Conclusions
This research, while preliminary, suggests that vulnerable populations, in particular those whose living conditions result in increased exposure to ticks (and other disease-carrying vectors), should be considered as high risk for related diseases. Also, Lyme disease is often used as a catch-all term for TBDs and findings here suggest that the highest scoring respondents could have other tick-borne diseases that cause nonspecific and multi-system symptoms that could be mistaken for Lyme disease. The safety and health of vulnerable populations such as migrant and seasonal workers in cases of TBDs is not recognized in proportion to its scope and magnitude of risk and requires more in-depth detection and analysis at both local and broader system levels.
Author Contributions
Conceptualization, S.P.M.; methodology, S.P.M., C.B., C.L.M. and K.T.; formal analysis, S.P.M. and C.B.; investigation, S.P.M. and C.L.M.; resources, S.P.M.; data curation, S.P.M., C.B., C.L.M. and K.T.; writing—original draft preparation, S.P.M. and C.L.M.; writing—review and editing, S.P.M., C.L.M. and K.T.; visualization, S.P.M. and C.B.; project administration, S.P.M., C.L.M. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. However, opportunities to collect data for this publication were available while working on a grant titled, “Nursing in the Fields: Vector-borne Illness Prevention and Detection among Migrant and Seasonal Farmworkers” funded by the Rita & Alex Hillman Foundation.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of University of Texas at Dallas (protocol code IRB-21-149 and 8 December 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Monroe, A.; Asamoah, O.; Lam, Y.; Koenker, H.; Psychas, P.; Lynch, M.; Ricotta, E.; Hornston, S.; Berman, A.; Harvey, S.A. Outdoor-Sleeping and Other Night-Time Activities in Northern Ghana: Implications for Residual Transmission and Malaria Prevention. Malar. J. 2015, 14, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, S.P.; McNeely, C.L.; Thomas, K.; Brooks, C. Tick-Borne Surveillance Patterns in Perceived Non-Endemic Geographic Areas: Human Tick Encounters and Disease Outcomes. Healthcare 2021, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Citera, M.; Freeman, P.R.; Horowitz, R.I. Empirical Validation of the Horowitz Multiple Systemic Infectious Disease Syndrome Questionnaire for Suspected Lyme Disease. Int. J. Gen. Med. 2017, 10, 249–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pound, J.M.; George, J.E.; Kammlah, D.M.; Lohmeyer, K.H.; Davey, R.B. Evidence for Role of White-Tailed Deer (Artiodactyla: Cervidae) in Epizootiology of Cattle Ticks and Southern Cattle Ticks (Acari: Ixodidae) in Reinfestations Along the Texas/Mexico Border in South Texas: A Review and Update. J. Econ. Entomol. 2010, 103, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Gabriele-Rivet, V.; Arsenault, J.; Badcock, J.; Cheng, A.; Edsall, J.; Goltz, J.; Kennedy, J.; Lindsay, L.R.; Pelcat, Y.; Ogden, N.H. Different Ecological Niches for Ticks of Public Health Significance in Canada. PLoS ONE 2015, 10, e0131282. [Google Scholar] [CrossRef]
- Texas A&M University. The TickApp for Texas and the Southern Region. Available online: https://tickapp.tamu.edu/index.html (accessed on 15 July 2022).
- Centers for Disease Control and Prevention (CDC). Estimated distribution of blacklegged ticks (Ixodes scapularis). Available online: https://www.cdc.gov/ticks/surveillance/BlackleggedTick.html (accessed on 15 May 2022).
- Companion Animal Parasite Council. 2021. Available online: https://Capcvet.Org/Maps/#/2021/All-Year/Ehrlichiosis/Dog/United-States/Texas (accessed on 18 May 2022).
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).