Characterization and Biological Activity of Magnesium Nanoparticles Synthesized from Escherichia coli Metabolites Against Multidrug-Resistant Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Phytochemical Analysis of BMs
2.2.1. Carbohydrates
2.2.2. Reducing Sugars
2.2.3. Glycosides
2.2.4. Terpenoids
2.2.5. Phenols
2.3. Total Phenolic Content
2.4. High-Performance Liquid Chromatography (HPLC)
2.5. Synthesis of MgNPs
2.6. Characterization of the Biosynthesized MgNPs
2.6.1. X-Ray Diffraction (XRD)
2.6.2. Transmission Electron Microscopy (TEM)
2.6.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.6.4. Photoluminescence (PL)
2.6.5. Ultraviolet–Visible Spectroscopy (UV–Vis)
2.7. Antibacterial Activity of the Biosynthesized MgNPs
2.7.1. Isolation, Identification, and Preparation of the Bacteria
2.7.2. Antibiogram Assay
2.7.3. Agar Well Diffusion Assay
2.7.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assay
2.7.5. Time–Kill Test
2.8. Antibiofilm Assays
2.9. DPPH Radical Scavenging Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Bacterial Metabolites (BMs)
3.1.1. BMs Produced by E. coli
3.1.2. Total Phenolic Content (TPC)
3.1.3. Characterization of E. coli Metabolites by HPLC
3.2. Synthesis and Characterization of the MgNPs
3.2.1. Synthesis
3.2.2. Characterization
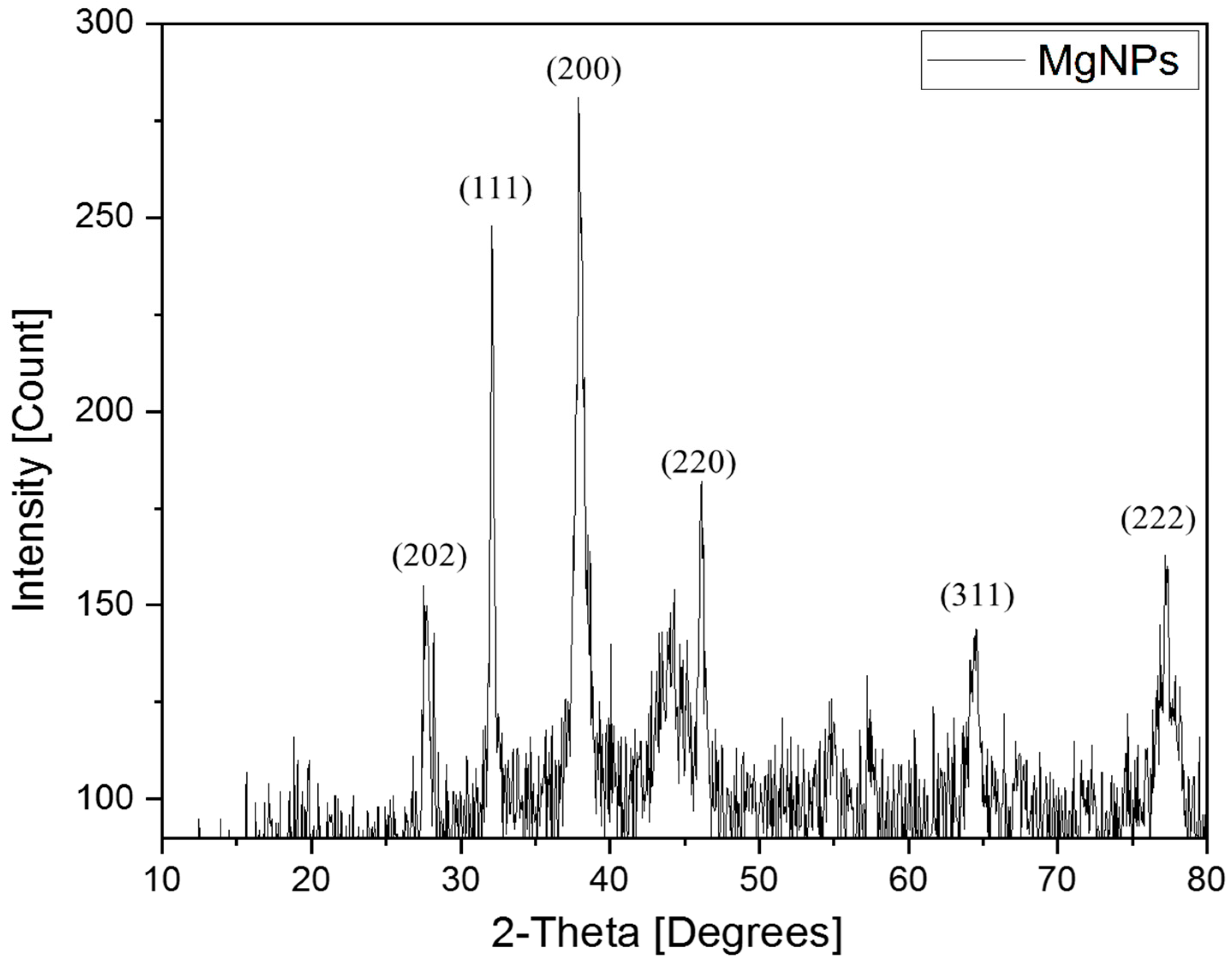
X-Ray Diffraction (XRD)
Transmission Electron Microscopy (TEM)
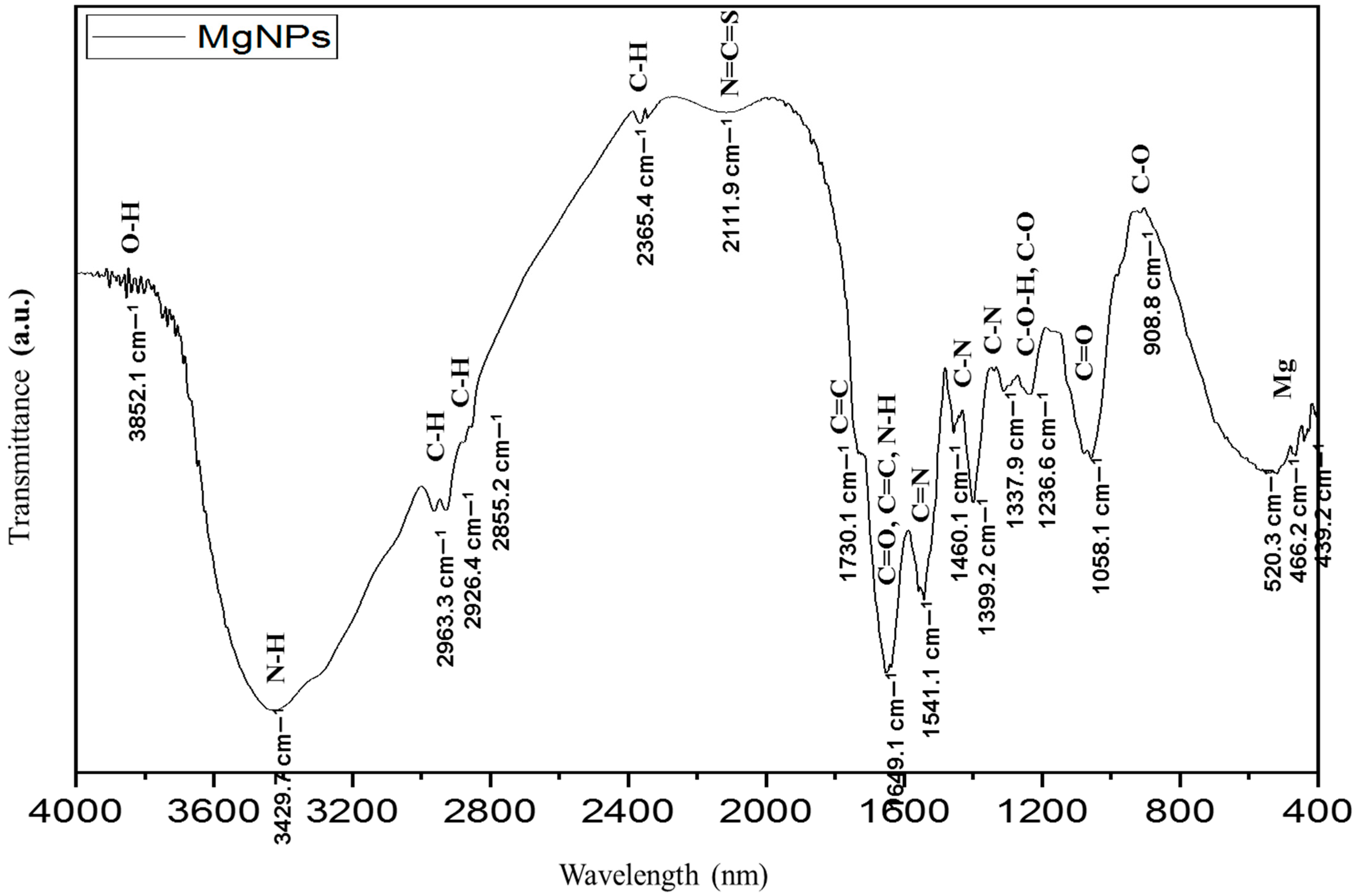
Fourier Transform Infrared Spectroscopy (FTIR)
Photoluminescence (PL)
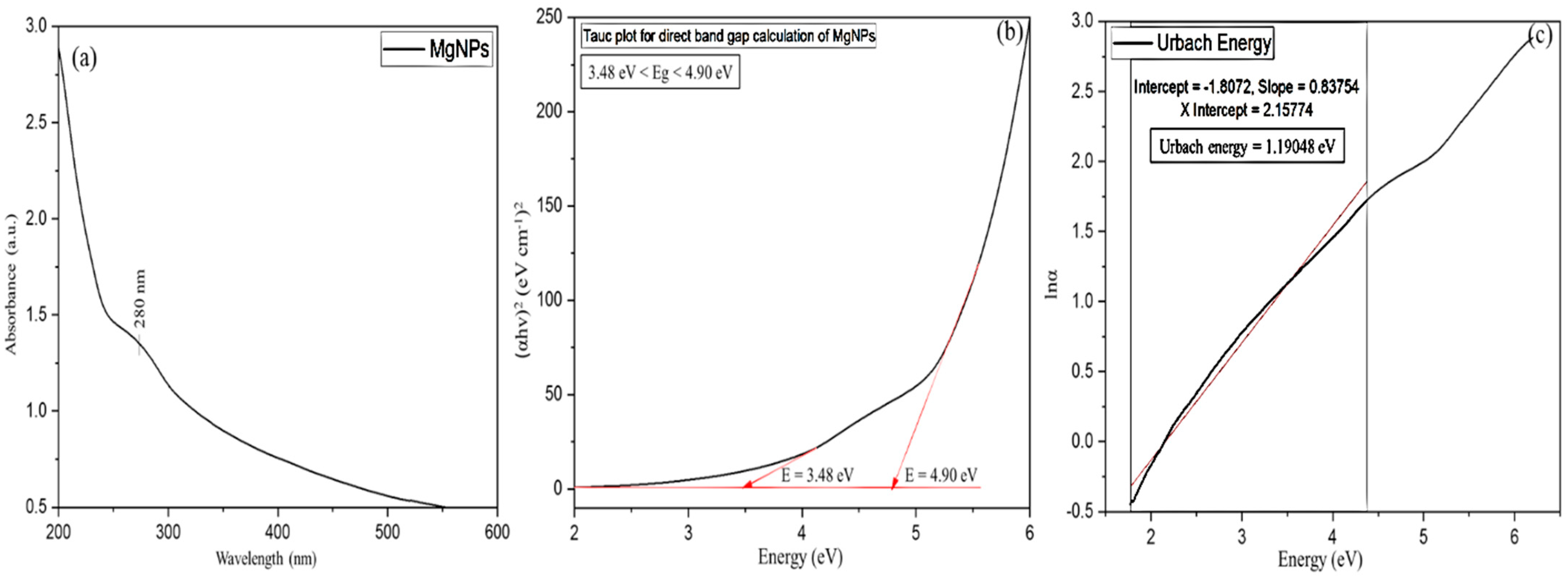
Ultraviolet–Visible Spectroscopy (UV–Vis)
3.3. Antibacterial Activity of the Biosynthesized MgNPs and BMs Against the Clinical Bacterial Isolates
3.3.1. Identification of the Clinical Bacteria Strains
3.3.2. Antibiogram Assay
3.3.3. Agar Well Diffusion
3.3.4. MIC and MBC of the Biosynthesized MgNPs and BMs
3.3.5. Time–Kill Results of the Biosynthesized MgNPs and BMs
3.4. Antibiofilm Results of the Biosynthesized MgNPs and BMs
3.5. DPPH Radical Scavenging of the Biosynthesized MgNPs and BMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMs | Bacterial metabolites |
| CFS | Cell-free supernatant |
| CLSI | Clinical and Laboratory Institute |
| CV | Crystal violet |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-diphenyl-1-picryhydrazyl |
| ELISA | Enzyme-linked immunosorbent assay |
| EPS | Extracellular polymeric substances |
| FTIR | Fourier transform infrared spectroscopy |
| GAE | Gallic acid equivalents |
| HHUMC | Hamoud Hospital University Medical Center |
| HPLC | High-performance liquid chromatography |
| MBC | Minimum bactericidal concentration |
| MgNPs | Magnesium nanoparticles |
| MgO-NPs | Magnesium oxide nanoparticles |
| MHA | Mueller–Hinton agar |
| MHB | Mueller–Hinton broth |
| MIC | Minimum inhibitory concentration |
| NA | Nutrient agar |
| NB | Nutrient broth |
| NPs | Nanoparticles |
| O.D. | Optical density |
| PL | Photoluminescence |
| SEM | Standard error of the mean |
| TEM | Transmission electron microscopy |
| TPC | Total phenolic content |
| UV–Vis | Ultraviolet–visible spectroscopy |
| XRD | X-ray diffraction |
| ZOIs | Zones of inhibition |
References
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Yoshikawa, T. You Oxidative Stress and Bio-Regulation. Int. J. Mol. Sci. 2024, 25, 3360. [Google Scholar] [CrossRef]
- Younis, I.Y.; El-Hawary, S.S.; Eldahshan, O.A.; Abdel-Aziz, M.M.; Ali, Z.Y. Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci. Rep. 2021, 11, 16868. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Hassan, S.E.D.; Fouda, A.; Saied, E.; Farag, M.M.; Eid, A.M.; Barghoth, M.G.; Awad, M.A.; Hamza, M.F.; Awad, M.F. Rhizopus oryzae-Mediated Green Synthesis of Magnesium Oxide Nanoparticles (MgO-NPs): A Promising Tool for Antimicrobial, Mosquitocidal Action, and Tanning Effluent Treatment. J. Fungi 2021, 7, 372. [Google Scholar] [CrossRef]
- Dolati, M.; Tafvizi, F.; Salehipour, M.; Movahed, T.K.; Jafari, P. Biogenic copper oxide nanoparticles from Bacillus coagulans induced reactive oxygen species generation and apoptotic and anti-metastatic activities in breast cancer cells. Sci. Rep. 2023, 13, 3256. [Google Scholar] [CrossRef]
- Patil, S.; Sastry, M.; Bharde, A. Size and Shape Directed Novel Green Synthesis of Plasmonic Nanoparticles Using Bacterial Metabolites and Their Anticancer Effects. Front. Microbiol. 2022, 13, 866849. [Google Scholar] [CrossRef]
- Saleh, M.N.; Khoman, S. Alwan Bio-synthesis of silver nanoparticles from bacteria Klebsiella pneumonia: Their characterization and antibacterial studies. J. Phys. Conf. Ser. 2020, 1664, 012115. [Google Scholar] [CrossRef]
- Abdel-Maksoud, G.; Abdel-Nasser, M.; Hassan, S.E.-D.; Eid, A.; Abdel-Nasser, A.; Fouda, A. Green synthesis of magnesium oxide nanoparticles using probiotic strain Lactobacillus gasseri and their activity against fungal strains isolated from historical manuscripts. Egypt. J. Chem. 2023, 66, 179–189. [Google Scholar] [CrossRef]
- Kumari, R.; Barsainya, M.; Singh, D.P. Biogenic synthesis of silver nanoparticle by using secondary metabolites from Pseudomonas aeruginosa DM1 and its anti-algal effect on Chlorella vulgaris and Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. 2017, 24, 4645–4654. [Google Scholar] [CrossRef]
- Altaee, N.; Kadhim, M.J.; Hameed, I.H. Characterization of Metabolites Produced by E. Coli and Analysis of Its Chemical Compounds Using GC-MS. Int. J. Curr. Pharm. Rev. Res. 2016, 7, 13–19. [Google Scholar]
- Kulkarni, D.; Sherkar, R.; Shirsathe, C.; Sonwane, R.; Varpe, N.; Shelke, S.; More, M.P.; Pardeshi, S.R.; Dhaneshwar, G.; Junnuthula, V.; et al. Biofabrication of nanoparticles: Sources, synthesis, and biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1159193. [Google Scholar] [CrossRef]
- Divya, K.; Kurian, L.C.; Vijayan, S.; Manakulam Shaikmoideen, J. Green synthesis of silver nanoparticles by Escherichia coli: Analysis of antibacterial activity. J. Water Environ. Nanotechnol. 2016, 1, 63–74. [Google Scholar]
- Yuan, Q.; Bomma, M.; Xiao, Z. Enhanced Silver Nanoparticle Synthesis by Escherichia Coli Transformed with Candida Albicans Metallothionein Gene. Materials 2019, 12, 4180. [Google Scholar] [CrossRef]
- Adnan, R.M.; Mezher, M.; Abdallah, A.M.; Awad, R.; Khalil, M.I. Synthesis, Characterization, and Antibacterial Activity of Mg-Doped CuO Nanoparticles. Molecules 2022, 28, 103. [Google Scholar] [CrossRef]
- Rotti, R.B. Green synthesis of MgO nanoparticles and its antibacterial properties. Front. Chem. 2023, 11, 1143614. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.-P.; Vega-Jiménez, A.L.; Vázquez-Olmos, A.R.; Ortega-Maldonado, M.; Ximenez-Fyvie, L.-A. Antibacterial Properties In Vitro of Magnesium Oxide Nanoparticles for Dental Applications. Nanomaterials 2023, 13, 502. [Google Scholar] [CrossRef]
- Amrulloh, H.; Fatiqin, A.; Simanjuntak, W.; Afriyani, H.; Annissa, A. Antioxidant and Antibacterial Activities of Magnesium Oxide Nanoparticles Prepared using Aqueous Extract of Moringa Oleifera Bark as Green Agents. J. Multidiscip. Appl. Nat. Sci. 2021, 1, 44–53. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Dhanjal, D.S.; Sharma, A.; Nepovimova, E.; Kalia, A.; Thakur, S.; Bhardwaj, S.; Chopra, C.; Singh, R.; Verma, R.; et al. Conifer-Derived Metallic Nanoparticles: Green Synthesis and Biological Applications. Int. J. Mol. Sci. 2020, 21, 9028. [Google Scholar] [CrossRef]
- Girma, W.M.; Aragie, M.S.; Berehe, B.A.; Assen, A.H. Biogenic synthesis and characterization of MgO nanoparticles using Verbascum sinaiticum: Antibacterial, free radical, and reactive oxygen species scavenging activities. Mater. Adv. 2025, 6, 4003–4015. [Google Scholar] [CrossRef]
- Al-Harbi, L.M.; Ezzeldien, M.; Elhenawy, A.A.; Said, A.H. Assessment of the bioactivity of bioinspired magnesium oxide nanoparticles from the Azadirachta indica extract. Front. Bioeng. Biotechnol. 2024, 12, 1480694. [Google Scholar] [CrossRef]
- Mezher, M.; Hajj, R.E.; Khalil, M. Investigating the antimicrobial activity of essential oils against pathogens isolated from sewage sludge of southern Lebanese villages. Germs 2022, 12, 488. [Google Scholar] [CrossRef]
- Bassam, M.; Mezher, M.; Khalil, M. Optimization of the bio-synthesis of magnesium nanoparticles from staphylococcus haemolyticus: A pilot study. BAU J.—Sci. Technol. 2023, 5, 4. [Google Scholar] [CrossRef]
- Katoch, R. Carbohydrate Estimations. In Analytical Techniques in Biochemistry and Molecular Biology; Springer: New York, NY, USA, 2011; pp. 67–76. [Google Scholar] [CrossRef]
- Hernández-López, A.; Félix, D.A.S.; Sierra, Z.Z.; Bravo, I.G.; Dinkova, T.D.; Avila-Alejandre, A.X. Quantification of Reducing Sugars Based on the Qualitative Technique of Benedict. ACS Omega 2022, 5, 32403–32410. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Najda, A.; Michalak, I. Investigation of Chemical Constituents and Antioxidant Activity of Biologically Active Plant-Derived Natural Products. Molecules 2023, 28, 5572. [Google Scholar] [CrossRef]
- Das, B.K.; Al-Amin, M.M.; Russel, S.M.; Kabir, S.; Bhattacherjee, R.; Hannan, J.M.A. Phytochemical Screening and Evaluation of Analgesic Activity of Oroxylum indicum. Indian J. Pharm. Sci. 2014, 76, 571. [Google Scholar]
- Tongco, J.V.V.; Aguda, R.M.; Razal, R.A. Proximate analysis, phytochemical screening, and total phenolic and flavonoid content of Philippine bamboo Schizostachyum lumampao. J. Chem. Pharm. Res. 2014, 6, 709–713. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology 299; Academic Press: Cambridge, MA, USA, 1999; pp. 152–178. [Google Scholar] [CrossRef]
- Vizzotto, M.; Cisneros-Zevallos, L.; Byrne, D.H.; Ramming, D.W.; Large, W.R.O. Variation Found in the Phytochemical and Antioxidant Activity of Peach and Plum Germplasm. J. Am. Soc. Hortic. Sci. 2007, 132, 334–340. [Google Scholar] [CrossRef]
- Darwich, N.A.; Mezher, M.; Abdallah, A.M.; El-Sayed, A.F.; El Hajj, R.; Hamdalla, T.A.; Khalil, M.I. Green Synthesis of Yttrium Derivatives Nanoparticles Using Pine Needle Leaf Extract: Characterization, Docking, Antibacterial, and Antioxidant Potencies. Processes 2024, 12, 1713. [Google Scholar] [CrossRef]
- Available online: https://www.scribd.com/document/845816256/CLSI-2024-Compressed-1 (accessed on 4 September 2025).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Thompson, B.; Machas, M.; Abed, O.; Nielsen, D.R. Applying a ‘Metabolic Funnel’ for Phenol Production in Escherichia coli. Fermentation 2021, 7, 216. [Google Scholar] [CrossRef]
- Justice, S.S.; Hunstad, D.A.; Cegelski, L.; Hultgren, S.J. Morphological plasticity as a bacterial survival strategy. Nat. Rev. Microbiol. 2008, 6, 162–168. [Google Scholar] [CrossRef]
- Plamada, D.; Nemes, A.S.; Teleky, B.E.; Pascuta, M.S.; Odocheanu, R.; Mitrea, L.; Calinoiu, L.F.; Szabo, K.; Vodnar, D.C. Microbial Production of Aromatic Phenolic Compounds. In Microbial Production of Food Bioactive Compounds; Karaca, A.C., Jafari, S.M., Harzevili, F.D., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–24. [Google Scholar] [CrossRef]
- Saito, Y.; Sato, T.; Nomoto, K.; Tsuji, H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 2018, 94, fiy125. [Google Scholar] [CrossRef]
- Siddiqui, N.; Rauf, A.; Latif, A.; Mahmood, Z. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth). J. Taibah Univ. Med. Sci. 2017, 12, 360–363. [Google Scholar] [CrossRef]
- Rani, R.; Arora, S.; Kaur, J.; Manhas, R.K. Phenolic compounds as antioxidants and chemopreventive drugs from Streptomyces cellulosae strain TES17 isolated from rhizosphere of Camellia sinensis. BMC Complement. Altern. Med. 2018, 18, 82. [Google Scholar] [CrossRef]
- Ispiryan, A.; Atkociuniene, V.; Makstutiene, N.; Sarkinas, A.; Salaseviciene, A.; Urbonaviciene, D.; Viskelis, J.; Pakeltiene, R.; Raudone, L. Correlation between Antimicrobial Activity Values and Total Phenolic Content/Antioxidant Activity in Rubus idaeus L. Plants 2024, 13, 504. [Google Scholar] [CrossRef]
- Sarıözlü, N.Y.; Kıvanç, M. Isolation of gallic acid-producing microorganisms and their use in the production of gallic acid from gall nuts and sumac. Afr. J. Biotechnol. 2009, 8, 1110–1115. [Google Scholar]
- Balderas-Hernández, V.E.; Treviño-Quintanilla, L.G.; Hernández-Chávez, G.; Martinez, A.; Bolívar, F.; Gosset, G. Catechol biosynthesis from glucose in Escherichia coli anthranilate-overproducer strains by heterologous expression of anthranilate 1,2-dioxygenase from Pseudomonas aeruginosa PAO1. Microb. Cell Fact. 2014, 13, 136. [Google Scholar] [CrossRef]
- Marhuenda-Muñoz, M.; Laveriano-Santos, E.P.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M.; Martínez-Huélamo, M.; Vallverdú-Queralt, A. Microbial Phenolic Metabolites: Which Molecules Actually Have an Effect on Human Health? Nutrients 2019, 11, 2725. [Google Scholar] [CrossRef]
- Alberto, M.R.; Gómez-Cordovés, C.; Manca de Nadra, M.C. Metabolism of Gallic Acid and Catechin by Lactobacillus hilgardii from Wine. J. Agric. Food Chem. 2004, 52, 6465–6469. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X.; Jiang, M.; Zhang, H.; Wang, Y.; Zhang, Y.; Seviour, R.; Kong, Y. In vitro co-metabolism of epigallocatechin-3-gallate (EGCG) by the mucin-degrading bacterium Akkermansia muciniphila. PLoS ONE 2021, 16, e0260757. [Google Scholar] [CrossRef]
- Nag, M.; Kar, A.; Chanda, J.; Mukherjee, P.K. RP-HPLC analysis of methanol extract of Viscum articulatum. J. Ayurveda Integr. Med. 2020, 11, 277–280. [Google Scholar] [CrossRef]
- Higgs, R.E.; Zahn, J.A.; Gygi, J.D.; Hilton, M.D. Rapid Method To Estimate the Presence of Secondary Metabolites in Microbial Extracts. Appl. Environ. Microbiol. 2001, 67, 371–376. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef]
- Saeed, S.; Iqbal, A.; Ashraf, M.A. Bacterial-mediated synthesis of silver nanoparticles and their significant effect against pathogens. Environ. Sci. Pollut. Res. 2020, 27, 37347–37356. [Google Scholar] [CrossRef]
- Sharma, R.; Bisen, D.P.; Shukla, U.; Sharma, B.G. X-ray diffraction: A powerful method of characterizing nanomaterials. Recent Res. Sci. Technol. 2012, 4, 77–79. [Google Scholar]
- Saberi, A.; Baltatu, M.S.; Vizureanu, P. Recent Advances in Magnesium–Magnesium Oxide Nanoparticle Composites for Biomedical Applications. Bioengineering 2024, 11, 508. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Singh, S.K.; Gautam, R.; Choudhary, K.; Maurino, V.G.; Saharan, V. MgO Nanoparticles Biosynthesis and Its Effect on Chlorophyll Contents in the Leaves of Clusterbean (Cyamopsis tetragonoloba L.). Adv. Sci. Eng. Med. 2014, 6, 538–545. [Google Scholar] [CrossRef]
- Lomonosov, V.; Yang, J.; Fan, Y.; Hofmann, S.; Ringe, E. Stability of Plasmonic Mg-MgO Core–Shell Nanoparticles in Gas-Phase Oxidative Environments. Nano Lett. 2024, 24, 7084–7090. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Elder, A.; Gelein, R.; Mercer, P.; Biswas, P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef]
- Anjum, D.H. Characterization of nanomaterials with transmission electron microscopy. IOP Conf. Ser. Mater. Sci. Eng. 2016, 146, 012001. [Google Scholar] [CrossRef]
- Paswan, S.K.; Kumari, S.; Kar, M.; Singh, A.; Pathak, H.; Borah, J.P.; Kumar, L. Optimization of structure-property relationships in nickel ferrite nanoparticles annealed at different temperature. J. Phys. Chem. Solids 2021, 151, 109928. [Google Scholar] [CrossRef]
- Abbas, Z.; Hassan, M.A.; Huang, W.; Yu, H.; Xu, M.; Chang, X.; Fang, X.; Liu, L. Influence of Magnesium Oxide (MgO) Nanoparticles on Maize (Zea mays L.). Agronomy 2024, 14, 617. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry, 1st ed.; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Nisa, F.Y.; Rahman, M.A.; Saha, S.; Ahmed, A.A.; Rafi, M.K.J.; Sultana, F.; Majid, M.; Choudhury, T.R. Unraveling Tamarindus indica Pulp-Derived Green Magnesium Oxide Nanoparticles for Cardioprotective Potential against Doxorubicin-Induced Cardiomyopathy: A Comprehensive Biochemical and Gene Expression Study. ACS Omega 2023, 8, 45626–45644. [Google Scholar] [CrossRef]
- Koley, R.; Mishra, D.; Mondal, N.K. Magnesium oxide nanoparticles alleviate arsenic toxicity, reduce oxidative stress and arsenic accumulation in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. Int. 2023, 30, 117932–117951. [Google Scholar] [CrossRef]
- Vega-Jiménez, A.L.; González-Alva, P.; Rodríguez-Hernández, A.P.; Vázquez-Olmos, A.R.; Paz-Díaz, B. Oxide nanoparticles based in magnesium as a potential dental tool to inhibit bacterial activity and promote osteoblast viability. Dent. Mater. J. 2023, 43, 11–19. [Google Scholar] [CrossRef]
- Naguib, G.H.; Abd El-Aziz, G.S.; Kayal, R.A.; Mira, A.I.; Hajjaj, M.S.; Mously, H.A.; Hamed, M.T. Cytotoxic effects of dose dependent inorganic magnesium oxide nanoparticles on the reproductive organs of rats. Ann. Med. 2023, 55, 2258917. [Google Scholar] [CrossRef]
- Verma, A.; Mehata, M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Rabaa, M.; Mezher, M.; Aridi, A.; Naoufal, D.; Khalil, M.I.; Awad, R.; Abdeen, W. Influence of Lanthanum Doping on the Photocatalytic and Antibacterial Capacities of Mg0.33Ni0.33Co0.33Fe2O4 Nanoparticles. Catalysts 2023, 13, 693. [Google Scholar] [CrossRef]
- Adnan, R.; Abdallah, A.M.; Mezher, M.; Noun, M.; Khalil, M.; Awad, R. Impact of Mg-doping on the structural, optical, and magnetic properties of CuO nanoparticles and their antibiofilm activity. Phys. Scr. 2023, 98, 55935. [Google Scholar] [CrossRef]
- Rabaa, M.; Mezher, M.; Aridi, A.; Naoufal, D.; Khalil, M.; Awad, R. Improved Photocatalytic and Antibacterial Activity of Mg0.33 Ni0.33Co0.33GdxFe2−xO4 Nanoparticles Synthesized via the Co-precipitation Method. ChemistrySelect 2023, 8, e202301951. [Google Scholar] [CrossRef]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Amer, M.S.; Ahamed, M. Facile Synthesis, Characterization, Photocatalytic Activity, and Cytotoxicity of Ag-Doped MgO Nanoparticles. Nanomaterials 2021, 11, 2915. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Sisubalan, N.; Sridevi, M.; Varaprasad, K.; Basha, M.H.G.; Shucai, W.; Sadiku, R. Biocidal chitosan-magnesium oxide nanoparticles via a green precipitation process. J. Hazard. Mater. 2021, 411, 124884. [Google Scholar] [CrossRef]
- Vasireddy, R.; Paul, R.; Mitra, A.K. Green Synthesis of Silver Nanoparticles and the Study of Optical Properties. Nanomater. Nanotechnol. 2012, 2, 8. [Google Scholar] [CrossRef]
- Ringe, E. Shapes, Plasmonic Properties, and Reactivity of Magnesium Nanoparticles. J. Phys. Chem. C 2020, 124, 15665–15679. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah, I.; Rehman, G.; Hamayun, M.; Ali, S.; Rahman, A.; Lee, I.J. Magnesium and Zinc Oxide Nanoparticles from Datura alba Improve Cognitive Impairment and Blood Brain Barrier Leakage. Molecules 2022, 27, 4753. [Google Scholar] [CrossRef]
- Pathania, D.; Kumar, S.; Thakur, P.; Chaudhary, V.; Kaushik, A.; Varma, R.S.; Furukawa, H.; Sharma, M.; Khosla, A. Essential oil-mediated biocompatible magnesium nanoparticles with enhanced antibacterial, antifungal, and photocatalytic efficacies. Sci. Rep. 2022, 12, 11431. [Google Scholar] [CrossRef]
- Malik, M.A.; Batterjee, M.G.; Kamli, M.R.; Alzahrani, K.A.; Danish, E.Y.; Nabi, A. Polyphenol-Capped Biogenic Synthesis of Noble Metallic Silver Nanoparticles for Antifungal Activity against Candida auris. J. Fungi 2022, 8, 639. [Google Scholar] [CrossRef]
- Ali, I.A.M.; Ahmed, A.B.; Al-Ahmed, H.I. Green synthesis and characterization of silver nanoparticles for reducing the damage to sperm parameters in diabetic compared to metformin. Sci. Rep. 2023, 13, 2256. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Kamat, P.V. Photophysical, Photochemical and Photocatalytic Aspects of Metal Nanoparticles. J. Phys. Chem. B 2022, 106, 7729–7744. [Google Scholar] [CrossRef]
- Ling, T.K.W.; Liu, Z.K.; Cheng, A.F.B. Evaluation of the VITEK 2 System for Rapid Direct Identification and Susceptibility Testing of Gram-Negative Bacilli from Positive Blood Cultures. J. Clin. Microbiol. 2003, 41, 4705–4707. [Google Scholar] [CrossRef]
- Wang, X.; Biswas, S.; Paudyal, N.; Pan, H.; Li, X.; Fang, W.; Yue, M. Antibiotic Resistance in Salmonella Typhimurium Isolates Recovered From the Food Chain Through National Antimicrobial Resistance Monitoring System Between 1996 and 2016. Front. Microbiol. 2019, 10, 985. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, S.; Zhang, L.; Wu, H.; Wu, H. Characteristics of antibiotic resistance mechanisms and genes of Klebsiella pneumoniae. Open Med. 2023, 18, 20230707. [Google Scholar] [CrossRef]
- Cillóniz, C.; Garcia-Vidal, C.; Ceccato, A.; Torres, A. Antimicrobial Resistance Among Streptococcus pneumoniae. In Antimicrobial Resistance in the 21st Century; Fong, I.W., Shlaes, D., Drlica, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 13–38. [Google Scholar] [CrossRef]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–569. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M.; et al. Antibiotic action and resistance: Updated review of mechanisms, spread, influencing factors, and alternative approaches for combating resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Darwich, N.A.; Mezher, M.; Abdallah, A.M.; El-Sayed, A.F.; El Hajj, R.; Hamdalla, T.A.; Khalil, M.I. Biosynthesis; Characterization; and Antibacterial, Antioxidant, and Docking Potentials of Doped Silver Nanoparticles Synthesized from Pine Needle Leaf Extract. Processes 2024, 12, 2590. [Google Scholar] [CrossRef]
- El-Khawaga, A.M.; Ayman, M.; Hafez, O.; Shalaby, R.E. Photocatalytic, antimicrobial and antibiofilm activities of MgFe2O4 magnetic nanoparticles. Sci. Rep. 2024, 14, 12877. [Google Scholar] [CrossRef]
- Tian, Q.; Wei, S.; Su, H.; Zheng, S.; Xu, S.; Liu, M.; Bo, R.; Li, J. Bactericidal activity of gallic acid against multi-drug resistance Escherichia coli. Microb. Pathog. 2022, 173, 105824. [Google Scholar] [CrossRef]
- Mita, S.R.; Muhtar, N.I.; Kusuma, S.A.; Sriwidodo, S.; Hendrawan, R.P. Catechins as Antimicrobial Agents and Their Contribution to Cosmetics. Cosmetics 2025, 12, 11. [Google Scholar] [CrossRef]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement. Altern. Med. 2019, 19, 141. [Google Scholar] [CrossRef]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Emam, T.M.; Elsherbiny, E.A. Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium Burkholderia rinojensis against Fusarium oxysporum. Mater. Sci. Eng. C 2020, 109, 110617. [Google Scholar] [CrossRef]
- Sagar, P.K.; Sharma, P.; Singh, R. Inhibition of Quorum Sensing Regulated Virulence Factors and Biofilm Formation by Eucalyptus globulus against Multidrug-Resistant Pseudomonas aeruginosa. J. Pharmacopunct. 2022, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, M.R.K.; Makhuvele, R.; Njobeh, P.B. Phytochemical screening, antioxidant activity of selected methanolic plant extracts and their detoxification capabilities against AFB1 toxicity. Heliyon 2024, 10, e24435. [Google Scholar] [CrossRef] [PubMed]




| Phytochemical Compound | Present/Absent |
|---|---|
| Carbohydrates | + 1 |
| Reducing sugars | + |
| Glycosides | − 2 |
| Terpenoids | − |
| Phenols | + |
| Bacterial Isolates | Level of Identification (%) | |
|---|---|---|
| Gram-positive | S. pneumonia | 97 (excellent) |
| E. faecium | 93 (very good) | |
| Gram-negative | K. pneumonia | 96 (excellent) |
| S. typhimurium | 95 (very good) | |
| Antibiotic | Bacterial Isolates and 1 ZOIs ± 2 SEM (mm) | |||
|---|---|---|---|---|
| S. pneumonia | E. faecium | K. pneumonia | S. typhimurium | |
| Cefamandole | 0.000 ± 0.000 5 (R) | 14.333 ± 0.272 4 (I) | 0.000 ± 0.000 (R) | 14.666 ± 0.720 (I) |
| Moxifloxacin | 33.000 ± 0.942 3 (S) | 22.666 ± 0.272 (S) | 22.000 ± 0.471 (S) | 20.333 ± 0.272 (S) |
| Ceftizoxime | 22.000 ± 1.247 (S) | 15.333 ± 0.544 (I) | 0.000 ± 0.000 (R) | 8.666 ± 0.272 (R) |
| Cefpodoxime | 0.000 ± 0.000 (R) | 8.666 ± 0.272 (R) | 11.66 ± 0.272 (I) | 14.666 ± 0.272 (I) |
| Flumequine | 29.666 ± 0.720 (S) | 16.000 ± 0.000 (I) | 24.666 ± 0.272 (S) | 12.666 ± 0.272 (I) |
| Neomycin | 3.333 ± 0.720 (R) | 17.333 ± 0.720 (I) | 16.333 ± 0.272 (I) | 0.000 ± 0.000 (R) |
| Cephazolin | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) |
| Chloramphenicol | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) | 12.333 ± 0.272 (I) | 0.000 ± 0.000 (R) |
| Cefradine | 0.000 ± 0.000 (R) | 10.666 ± 0.272 (I) | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) |
| Clindamycin | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) | 8.000 ± 0.000 (R) | 0.000 ± 0.000 (R) |
| Cefadroxil | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) |
| Lincomycin | 0.000 ± 0.000 (R) | 16.666 ± 0.272 (I) | 0.000 ± 0.000 (R) | 9.333 ± 0.272 (R) |
| Sulfamethoxazole | 32.333 ± 1.186 (S) | 0.000 ± 0.000 (R) | 22.333 ± 0.272 (S) | 22.000 ± 0.471 (S) |
| Ampicillin | 0.000 ± 0.000 (R) | 12.666 ± 0.272 (I) | 7.666 ± 0.272 (I) | 18.666 ± 0.272 (I) |
| Minocycline | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) | 0.000 ± 0.000 (R) |
| Sample | Concentration | 3 ZOI ± 4 SEM (mm) | |||
|---|---|---|---|---|---|
| S. pneumonia | E. faecium | K. pneumonia | S. typhimurium | ||
| 1 MgNPs (mg/mL) | 0.03125 p-value | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** |
| 0.0625 p-value | 0.000 ± 0.000 *** | 7.333 ± 0.072 *** | 0.000 ± 0.000 *** | 7.466 ± 0.072 *** | |
| 0.125 p-value | 7.200 ± 0.047 *** | 7.933 ± 0.027 *** | 0.000 ± 0.000 *** | 8.133 ± 0.027 *** | |
| 0.25 p-value | 7.466 ± 0.027 *** | 8.833 ± 0.027 *** | 7.133 ± 0.072 *** | 8.866 ± 0.027 *** | |
| 0.5 p-value | 7.666 ± 0.027 *** | 9.433 ± 0.054 *** | 7.733 ± 0.098 *** | 9.833 ± 0.054 *** | |
| 1 p-value | 8.166 ± 0.072 *** | 10.100 ± 0.047 *** | 8.633 ± 0.072 *** | 10.433 ± 0.098 *** | |
| 2 BMs (%) | 3.125 p-value | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** |
| 6.25 p-value | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | |
| 12.5 p-value | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | |
| 25 p-value | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | |
| 50 p-value | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | 0.000 ± 0.000 *** | |
| 100 p-value | 7.366 ± 0.072 *** | 7.233 ± 0.027 *** | 7.633 ± 0.072 *** | 8.033 ± 0.072 *** | |
| Sample | Concentration | Bacterial Isolates | |||
|---|---|---|---|---|---|
| S. pneumonia | E. faecium | K. pneumonia | S. typhimurium | ||
| 1 MgNPs (mg/mL) | 3 MIC | 0.125 | 0.0625 | 0.25 | 0.0625 |
| 4 MBC | 0.25 | 0.125 | 0.5 | 0.125 | |
| MBC/MIC | 2 | 2 | 2 | 2 | |
| 2 BMs (%) | MIC | 100 | 100 | 100 | 100 |
| MBC | 5 ND | ND | ND | ND | |
| MBC/MIC | ND | ND | ND | ND | |
| Sample | Bacterial Isolates and Time of Inhibition (h) | |||
|---|---|---|---|---|
| S. pneumonia | E. faecium | K. pneumonia | S. typhimurium | |
| 1 MgNPs | 3 | 1 | 2 | 3 |
| 2 BMs | 2 | 2 | 3 | 3 |
| Test | Bacterial Isolates | Concentration | |||||
|---|---|---|---|---|---|---|---|
| 1 MgNPs (mg/mL) | 2 BMs (%) | ||||||
| 0.25 p-Value | 0.5 p-Value | 1 p-Value | 2 p-Value | 4 p-Value | 100 p-Value | ||
| Percentage of inhibition (%) ± 3 SEM | S. pneumonia | 47.242 ± 4.495 * | 49.405 ± 5.254 * | 51.816 ± 4.922 * | 55.227 ± 5.385 * | 57.676 ± 5.766 * | 33.554 ± 0.271 *** |
| E. faecium | 16.942 ± 7.653 4 NS | 22.923 ± 6.944 NS | 27.403 ± 8.543 NS | 30.666 ± 7.890 NS | 38.149 ± 9.024 NS | 27.051 ± 4.515 0.039 * | |
| K. pneumonia | 44.691 ± 3.782 * | 53.942 ± 4.142 ** | 56.214 ± 4.045 ** | 60.433 ± 3.877 ** | 64.931 ± 5.548 * | 71.407 ± 2.671 ** | |
| S. typhimurium | 3.349 ± 6.537 * | 40.694 ± 3.333 ** | 45.760 ± 1.449 *** | 49.160 ± 2.546 ** | 53.497 ± 1.426 *** | 20.867 ± 4.757 NS | |
| Percentage of destruction (%) ± SEM | S. pneumonia | −7.779 ± 28.399 NS | 11.846 ± 15.696 NS | 25.484 ± 17.597 NS | 44.167 ± 8.716 NS | 48.667 ± 6.730 * | 37.730 ± 8.09 NS |
| E. faecium | −42.121 ± 17.503 NS | −34.060 ± 18.556 NS | −7.474 ± 3.309 NS | 7.651 ± 5.250 NS | 13.176 ± 2.205 * | −69.158 ± 11.656 * | |
| K. pneumonia | 25.264 ± 1.126 ** | 30.778 ± 2.832 * | 34.158 ± 2.590 ** | 45.740 ± 6.067 * | 48.493 ± 5.283 * | −13.443 ± 4.468 NS | |
| S. typhimurium | −32.495 ± 12.207 NS | −15.446 ± 4.458 NS | −14.069 ± 4.859 NS | −7.449 ± 5.482 NS | 7.074 ± 4.77 NS | 25.442 ± 3.231 ** | |
| Sample | Concentration | Percentage of 3 DPPH Scavenging ± 4 SEM (%) |
|---|---|---|
| 1 MgNPs (mg/mL) | 0 p-value | 0.000 ± 0.000 *** |
| 0.0625 p-value | 17.412 ± 0.125 *** | |
| 0.125 p-value | 21.183 ± 0.104 *** | |
| 0.25 p-value | 29.303 ± 0.243 *** | |
| 0.5 p-value | 32.821 ± 0.121 *** | |
| 1 p-value | 41.842 ± 0.460 *** | |
| Ascorbic acid (mg/mL) | 0 p-value | 0.000 ± 0.000 *** |
| 0.0625 p-value | 35.823 ± 0.132 *** | |
| 0.125 p-value | 40.467 ± 0.150 *** | |
| 0.25 p-value | 43.522 ± 0.055 *** | |
| 0.5 p-value | 43.994 ± 0.083 *** | |
| 1 p-value | 49.074 ± 0.145 *** | |
| 2 BMs (%) | 100 p-value | 29.680 ± 0.35 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezher, M.; Khazaal, S.; Khalil, M.I.; El Badan, D.; Hamdalla, T.A. Characterization and Biological Activity of Magnesium Nanoparticles Synthesized from Escherichia coli Metabolites Against Multidrug-Resistant Bacteria. Bacteria 2025, 4, 48. https://doi.org/10.3390/bacteria4030048
Mezher M, Khazaal S, Khalil MI, El Badan D, Hamdalla TA. Characterization and Biological Activity of Magnesium Nanoparticles Synthesized from Escherichia coli Metabolites Against Multidrug-Resistant Bacteria. Bacteria. 2025; 4(3):48. https://doi.org/10.3390/bacteria4030048
Chicago/Turabian StyleMezher, Malak, Salma Khazaal, Mahmoud I. Khalil, Dalia El Badan, and Taymour A. Hamdalla. 2025. "Characterization and Biological Activity of Magnesium Nanoparticles Synthesized from Escherichia coli Metabolites Against Multidrug-Resistant Bacteria" Bacteria 4, no. 3: 48. https://doi.org/10.3390/bacteria4030048
APA StyleMezher, M., Khazaal, S., Khalil, M. I., El Badan, D., & Hamdalla, T. A. (2025). Characterization and Biological Activity of Magnesium Nanoparticles Synthesized from Escherichia coli Metabolites Against Multidrug-Resistant Bacteria. Bacteria, 4(3), 48. https://doi.org/10.3390/bacteria4030048







