1. Introduction
Paratuberculosis is a chronic and progressive enteritis affecting ruminants worldwide, also known as Johne’s disease (JD). It is caused by
Mycobacterium avium subsp.
paratuberculosis (MAP), and the affected animals show diarrhea, weight loss, and a decreased production performance, leading to substantial economic losses [
1]. Considering the direct effects of MAP on animal health, economic losses, and potential public health consequences, its control among domestic ruminants is critical. Particularly, control of MAP infection in dairy cattle is essential, as they represent the most common host of the organism. Although vaccination against MAP seems promising, currently available vaccines are not permitted for use in countries employing test-and-cull protocols for the eradication of bovine tuberculosis. These vaccines contain inactivated (heat-killed) MAP, which may compromise the accuracy of the tuberculin test by inducing cross-reactivity [
2]. Consequently, researchers have concentrated their efforts on subunit and DNA vaccines as potential solutions to this issue. These vaccine strategies can overcome cross-reactivity in diagnostic assays by targeting MAP-specific antigens. Furthermore, DNA vaccines confer better protection against MAP as an intracellular pathogen by inducing cell-mediated immune responses as well as humoral responses [
3,
4,
5]. However, the efficacy of DNA vaccines can be affected by the low delivery rate of DNA plasmids into the host cells. To solve this problem, researchers have proposed using facultative intracellular bacteria such as
Salmonella sp.,
Listeria monocytogenes, and
Shigella sp. as plasmid carriers that can specifically target host antigen-presenting cells [
6]. Bacterial carriers are easy to grow, modify, and scale up, making them a practical choice for vaccine development. This contributes to the cost-effectiveness and wide availability of bacterial carrier-based vaccines.
One of the key challenges in utilizing pathogenic bacteria as vaccine vectors lies in achieving an optimal balance between attenuation and immunogenicity; excessive attenuation can compromise the immune response. Compared to other bacterial vectors,
Salmonella stands out as a superior candidate for vaccine delivery, offering a favorable combination of safety and the ability to elicit strong and lasting immunity. Auxotrophic mutants of
Salmonella, particularly those with deletions in genes associated with the biosynthesis of aromatic amino acids, such as
aroA,
aroC, and
hisG, are unable to replicate within the host’s cells. Nevertheless, these bacteria are capable of invading the intestinal tract of the host and can persist long enough to elicit an immune response. A double mutant of
Salmonella typhimurium SL7207 has been engineered through the deletion of the
aroA and
hisG genes, which ensures the safety of this carrier [
7,
8]. Furthermore,
Salmonellae can act as a natural adjuvant while also facilitating the transfer of plasmids to host macrophages. They can also be administered orally, which makes them an attractive choice in veterinary medicine [
6,
9].
In addition to using appropriate vaccine technologies, selecting species-specific antigens with immunogenic potential is of great importance in the success of vaccine design against MAP, such as superoxide dismutase, antigen complex 85A (Ag85A), Ag85B, and Ag85C [
4]. When it comes to vaccine development, surface proteins that show both antigenic properties and virulence are usually regarded as appropriate targets.
M. paratuberculosis expresses a fibronectin attachment protein on its surface to target the host’s M cells [
10]. This protein, which is called FAP-P, has been shown to activate dendritic cells through toll-like receptor 4, leading to the induction of cell-mediated immune responses and a high level of IFNγ production [
11]. In addition, this antigen has species-specific B-cell epitopes, which have made it a candidate for MAP infection diagnosis [
12]. The combination of these traits, along with the possession of strong T-cell and B-cell epitopes makes FAP-P a promising candidate for the development of a vaccine targeting MAP infection.
This study builds upon our previous work, in which a DNA vaccine candidate encoding the FAP-P gene from MAP was constructed by cloning the gene (NCBI accession number: DQ241505.1) into the mammalian expression vector pcDNA3.1(+) [
13]. The recombinant plasmid was then electroporated into
Salmonella typhimurium strain SL7207 (Δ
hisG, Δ
aroA) to serve as a bacterial delivery vehicle. Antigen expression was confirmed in vitro using macrophages derived from the human monocytic THP-1 cell line [
14]. In the present study, the same vaccine construct and bacterial carrier are employed to evaluate the efficacy of oral immunization in a murine model. A Western blot analysis was performed to assess FAP-P-specific antibody responses, thereby investigating the immunogenic potential of the candidate vaccine in vivo.
2. Materials and Methods
2.1. The Bacteria Used in the Present Study
All the bacteria used in this study were constructed in previously published work [
14]. The following are the names of the bacteria mentioned in this study, along with a brief description of each.
Sal-FAP: An attenuated strain of Salmonella typhimurium strain SL7207 that harbored the pre-constructed pcDNA 3.1(+) containing the FAP-P coding gene.
Sal-PC: An attenuated strain of Salmonella typhimurium strain SL7207 that harbored pcDNA 3.1(+), a mammalian expression vector.
Sal-GFP: The attenuated strain of Salmonella typhimurium that harbored the mammalian expression vector containing a green fluorescent protein coding gene
2.2. In Vitro Experiments
2.2.1. Construction of Salmonella SL7207 as a Vector-Harboring FAP Gene
The Salmonella containing pcDNA 3.1(+) harboring the FAP-P coding gene that was used in the present study, had been constructed during a previous investigation. Briefly, the FAP-P coding sequence (NCBI accession number: DQ241505.1), followed by a six-His tag-encoding sequence at its downstream end, was cloned into the pcDNA3.1(+) mammalian expression vector. The 5′ and 3′ ends of the sequence were flanked by
KpnI and
XhoI restriction sites, respectively. The electroporation procedure was used to transfer the constructed plasmid into the
S. Typhimurium strain SL7207 (Δ
hisG, Δ
aroA), kindly provided by Dr. Siegfried Weiss (German Research Centre for Biotechnology, Braunschweig). The isolate is referred to as Sal-FAP. The empty vector was also electroporated into
S. Typhimurium to serve as a control in subsequent assays (Sal-pc). The effective transfection of the constructed plasmid into macrophages, along with the expression of FAP-P, was also verified in macrophages derived from THP-1 cells [
14].
The isolate referred to as Sal-FAP was used in the present study for the immunization of mice. To evaluate innate, nonspecific responses elicited by the DNA plasmid and bacterial carrier,
Salmonella containing an empty pcDNA 3.1(+) vector (Sal-pc) was used as a control. Transformed
Salmonella, which harbored a mammalian expression vector encoding a green fluorescent protein (Sal-GFP), was also utilized to show the effective introduction of the plasmid into the immune system of mice [
14].
2.2.2. Verifying the Presence of the Pre-Constructed Plasmid in Salmonella
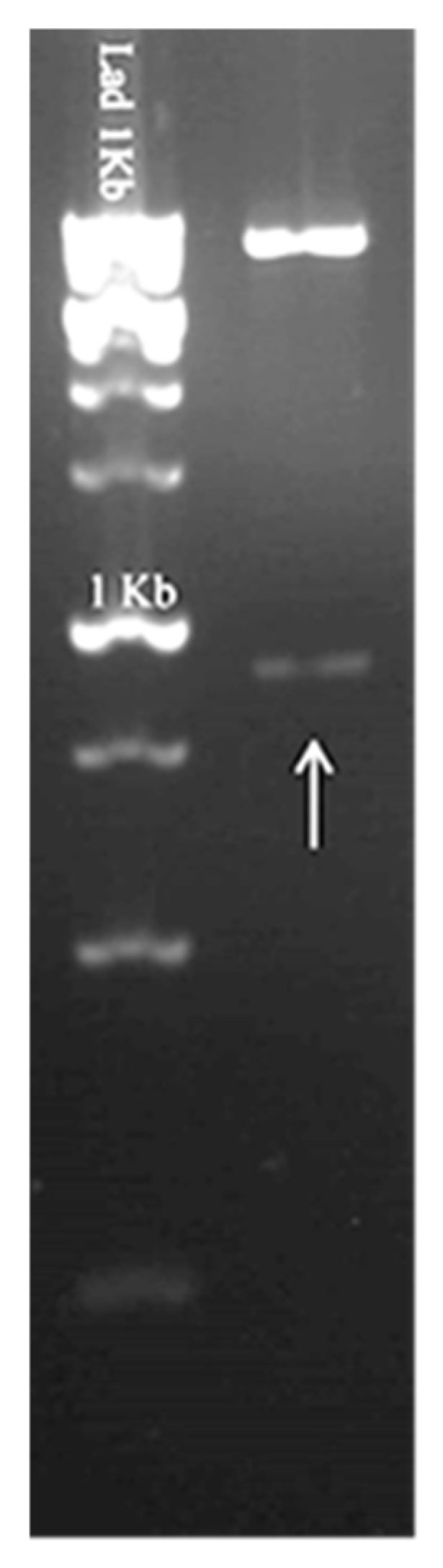
To confirm the presence of the pcDNA 3.1(+) plasmid containing the FAP-P coding gene in Salmonella, bacteria preserved in glycerol stock were cultured on Luria–Bertani (LB) agar supplemented with ampicillin (100 μg/mL). A single colony was then selected and inoculated into LB broth containing ampicillin (100 μg/mL) for overnight incubation. Following plasmid extraction, a double digestion was performed using the restriction enzymes KpnI and XhoI (Roche, Mannheim, Germany). The resulting fragments were visualized via agarose gel electrophoresis to confirm successful digestion and the presence of the target insert.
2.2.3. Bacteria Preparation
An overnight culture was prepared from all the bacteria in Luria–Bertani (LB) broth containing ampicillin (100 μg/mL). For immunization, 1000 μL of each overnight culture was inoculated in 20 mL of LB medium containing ampicillin and incubated in a shaker (150 rpm) at 37 °C until they reached the logarithmic phase of growth. Then, the bacteria were harvested by centrifugation at 13,000× g for 5 min at room temperature and washed twice in cold PBS. The pelleted cells were ultimately resuspended in 100 μL of 5% sodium bicarbonate, achieving a concentration of 1 × 109 CFU.
2.2.4. FAP-P Expression in E. coli
A pre-constructed pET-26b (+), containing the high antigenic region of the FAP-P coding gene, was used in this study. This plasmid had been transformed into
E. coli BL21 (DE3) and successfully expressed. The protein expression procedure was performed using the previously described conditions [
15]. Briefly, 1 ml of the overnight culture was inoculated into 100 mL of fresh LB medium containing the antibiotic and incubated at 37 °C, with shaking at 150 rpm until the optical density at 600 nm (OD600) reached 0.6. Then, IPTG was added to a final concentration of 1 mM, and the incubation period was extended for an additional 4 h. The bacterial pellet was obtained by centrifugation, resuspended in 50 μL 2× sample buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue, and 0.125 M Tris HCl, pH approx. 6.8), and heated at 90 °C for 10 min.
2.2.5. Evaluation of FAP-P Expression Using SDS-PAGE and Western Blot Analysis
The E. coli lysate from the previous step was separated using 12% SDS-PAGE and then transferred to a PVDF membrane with a semi-dry transfer apparatus (Bio-Rad, Mainland, Beijing, China). The PVDF membrane was blocked overnight in TBS (Tris-Buffered Saline) containing 5% low-fat dried milk. After blocking, the His-tagged expressed protein was detected using a 1:1000 dilution of anti-His tag HRP-conjugated antibody. The expected bands were visualized by treating the membrane with DAB (3,3′-diaminobenzidine).
2.3. In Vivo Experiments
2.3.1. Animals and Housing
A total of 15 female BALB/c mice aged 4 weeks were used. The animals were housed under standard conditions (temperature 22 ± 2 °C and humidity 50 ± 15%), and the light–dark cycle was 12:12. All the animals had free access to food and water. The animals were given a week to rest before the experiment began. This break was meant to help them adjust to their new surroundings and the procedures that would be used.
2.3.2. Experimental Groups
All the animals were divided into three groups, with each group containing five mice. One group received Sal-FAP (test group), another received Sal-pc (control-negative group), and the other received Sal-GFP.
2.3.3. Immunization Schedule
For the purpose of immunization, a total volume of 100 μL of a 10% sodium bicarbonate solution, which contained 1 × 109 CFU of bacteria, was delivered through intragastric gavage at ten-day intervals and repeated three times. The procedure was performed using a steel ball-tipped curved needle. Two groups of mice were used in the immunization procedure: (1) The test group, which received Sal-FAP to evaluate the immunogenic potential of the candidate vaccine, and (2) the negative control group, which received Sal-pc to assess any nonspecific reactions.
2.3.4. Blood Collection Procedure
Blood collection from the orbital sinus was performed ten days following the final immunization. The animals were anesthetized with ether prior to sample collection procedures. Capillary tubes were used to collect the blood into microcentrifuge tubes, and the animals were euthanized immediately after blood collection.
2.3.5. Serum Preparation
The collected blood was placed into a sterile microcentrifuge tube. The microcentrifuge tubes were left undisturbed at room temperature to allow the blood to clot. After clotting, the tubes were centrifuged at 2000× g for 10 min to separate the serum. The serum was carefully transferred to new tubes. Then, the serum samples from all mice were pooled in equal proportions and stored at −20 °C for further analysis.
2.4. Evaluation of Anti-FAP-P Antibody Production Using Western Blot Analysis
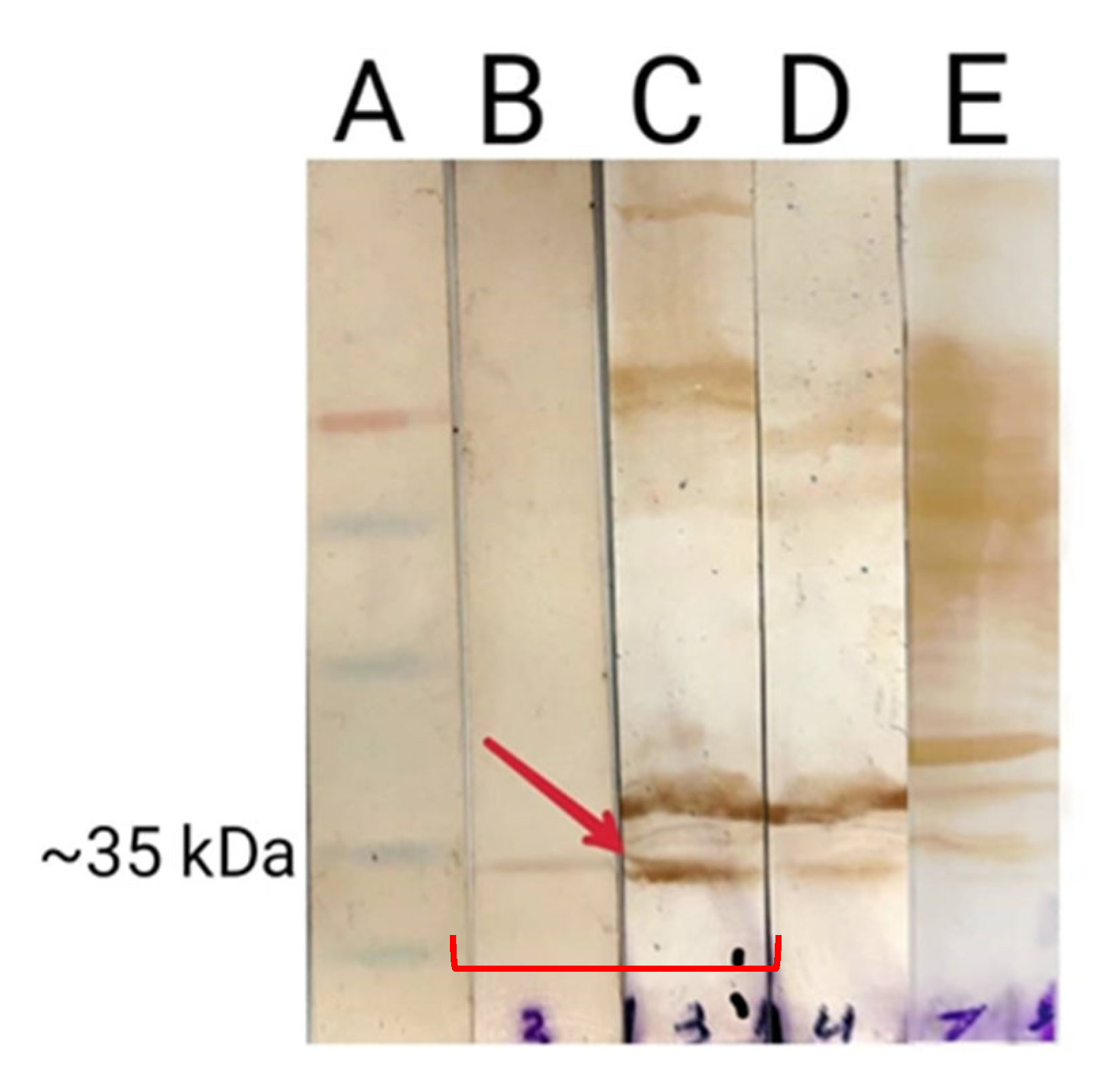
Bacterial lysates were separated using 12% SDS-PAGE, and protein bands were transferred onto a PVDF membrane using a semi-dry transfer system (Bio-Rad, Mainland, China). The membrane was then blocked overnight in TBS containing 5% low-fat dried milk. Following blocking, the membrane was washed three times with TBST (0.5 M NaCl, 0.02 M Tris [pH 7.5], and 0.05% Tween 20). It was subsequently incubated with mouse serum (1:20 dilution) as the primary antibody for four hours at room temperature. One lane contained E. coli lysate expressing FAP-P, another contained Salmonella lysate incubated with serum from the test group, and a third lane contained E. coli lysate probed with serum from the negative control group. After incubation with the primary antibody, the membrane was washed three times and then incubated with a 1:1000 dilution of monoclonal HRP-conjugated anti-mouse IgG (Sigma Lifesciences, Whitby, ON, Canada) for two hours. Finally, DAB (3,3′-diaminobenzidine) treatment (Sigma Aldrich, St Louis, MO, USA) was performed to visualize the immunoreactive bands.
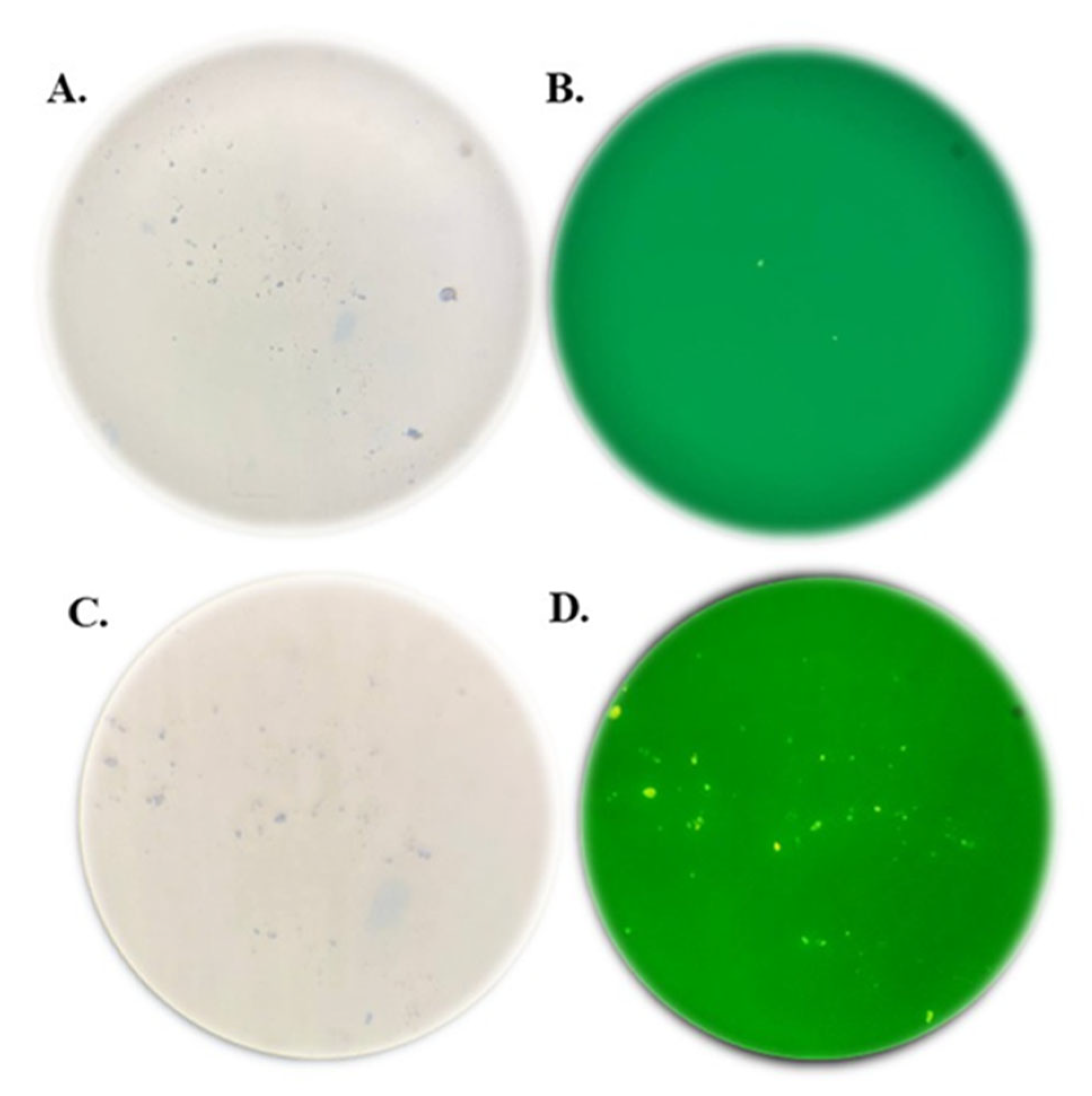
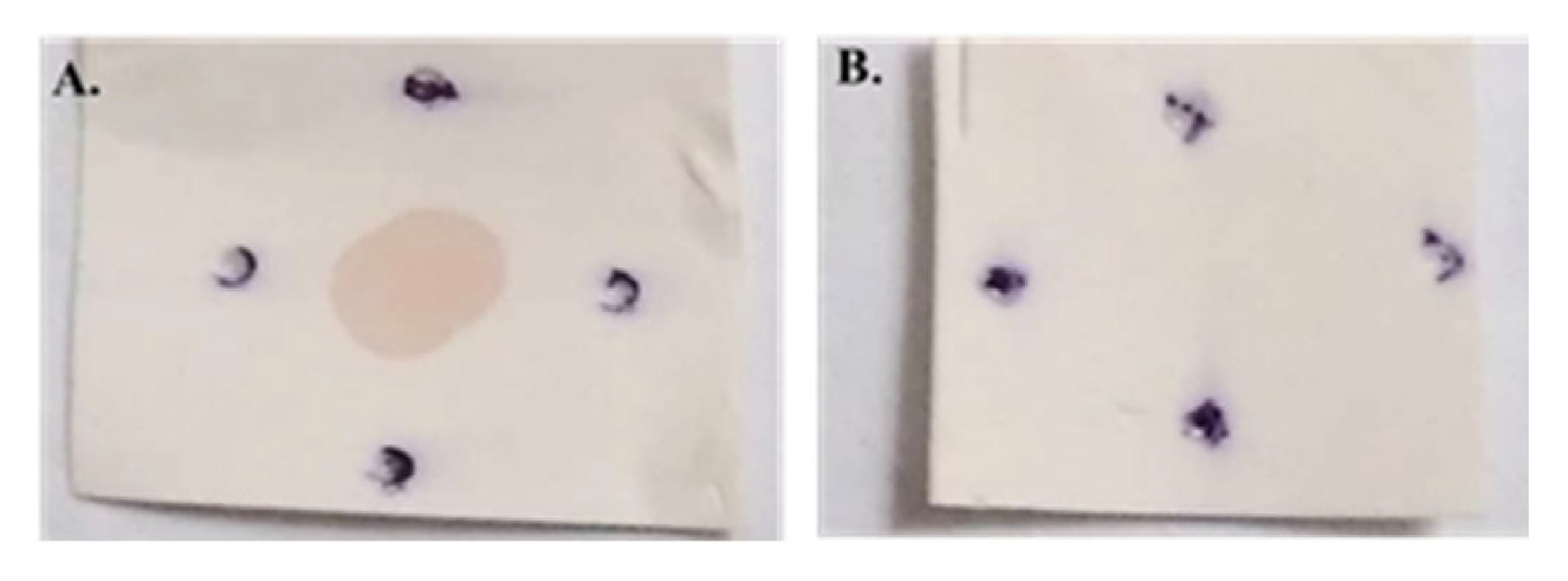
2.5. Assessment of Antibody Specificity Using Dot Blot Analysis
The dot blot assay was conducted by blotting purified protein derivatives of Johnin onto a nitrocellulose membrane. After blotting the PPD, the membrane was blocked in TBS containing 5% low-fat dried milk for one hour at room temperature. Following three washes with TBST, the membrane was incubated with the primary antibody for two hours. Serum samples from the test and negative control groups were used as the primary antibody at a 1:20 dilution. After washing, the membrane was incubated with a 1:1000 dilution of HRP-conjugated anti-mouse IgG (Sigma Lifesciences, Whitby, ON, Canada) as the secondary antibody for two hours at room temperature. Finally, DAB (Sigma Aldrich, St Louis, MO, USA) was applied to visualize the results.
2.6. The Plasmid Delivery to the Immune System
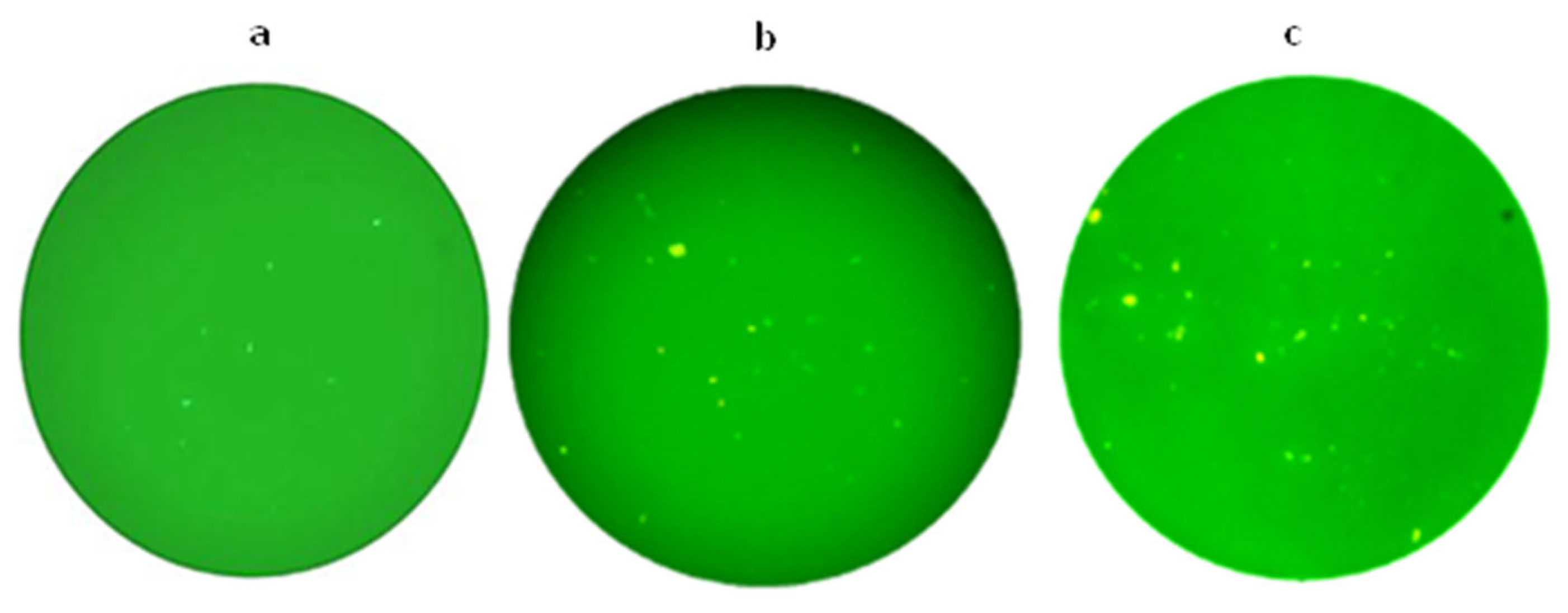
To verify the delivery of plasmids to the immune system, a group received Salmonella-GFP, and subsequent to this treatment, mice were euthanized at 24, 48, and 72 h post-administration. Spleens were removed and mashed through 70 μM cell strainers with the plunger end of a syringe. The strainer was washed with 5 mL cold PBS, and the red blood cells were lysed using RBC lysis buffer. Followed by washing the cells with cold PBS, the cell pellets were resuspended in PBS and studied with fluorescence microscopy.
4. Discussion
Mycobacterium avium subsp.
paratuberculosis, as the etiological agent of Johne’s disease, which causes chronic enteritis in ruminants, can cause serious economic losses in the dairy cattle industry. Additionally, it may also play a role in some human autoimmune diseases, such as Crohn’s disease, which has intensified the need to control paratuberculosis infection among domestic ruminants. Two vaccines that are presently available, Gudair
® and Silirum
®, are effective in preventing the advancement of clinical disease in the majority of vaccinated sheep and cattle, respectively. However, in addition to not providing complete protection against infection, they cannot be used in countries that use test-and-cull strategies to control bovine tuberculosis [
16]. As a result, researchers have focused on designing subunit- and DNA-based vaccines using MAP-specific antigens.
In addition to effectively inducing cellular immune responses by producing endogenous antigens, some features of DNA vaccines, such as stability and ease of production, have made them a tempting choice for the rapid screening of candidate vaccine antigens. Consequently, many researchers have used this approach for the preliminary assessment of MAP antigens, recording those antigens that can trigger substantial amounts of INFγ production following immunization in mouse models [
17,
18]. However, the most significant obstacle to DNA vaccines is their low immunogenicity when used in large animals. To address this problem, researchers have tried many solutions to improve the delivery of DNA vaccines [
19]. Live attenuated strains of
Salmonella typhimurium have a prominent position among these solutions because they are very easy to produce and maintain on a large scale, making
Salmonella carrier vaccines more economically viable and accessible. Qi-long Wang et al. showed that using attenuated
Salmonella typhimurium aroA SL7207 harboring the
Mycobacterium tuberculosis H37Rv ESAT6-Ag85B fusion gene could improve protective responses and triggered antigen-specific mucosal responses [
20]. In the present study, observing green fluorescent splenocytes 24, 48, and 72 h after oral administration of 1 × 10
9 CFU
Salmonella containing a GFP expression plasmid confirmed the efficient delivery of plasmid using a
salmonella vector in mice.
Therefore, it seems that using eukaryotic expression plasmids harboring the gene coding for MAP-specific, immunodominant antigens in combination with a Salmonella carrier has great potential to develop protective vaccines against paratuberculosis.
FAP-P is one of the mycobacterial fibronectin attachment proteins expressed by MAP. This protein is able to stimulate cell-mediated immune responses, including high levels of IFNγ through DC activation [
11]. Donghee Cho et al. have reported that the B-cell epitopes around the C-terminal end of FAP-P have the most significant specificity for
M. paratuberculosis, which have turned this antigen to a candidate for MAP infection diagnosis [
12]. Based on the immunoinformatics analysis of FAP-P by Moezzi et al., this protein is immunogenic, non-allergenic, and non-toxic and possesses potent T-cell and B-cell epitopes [
21]. Eraghi et al. reported that goats immunized with a chimeric fusion protein, consisting of heparin-binding hemagglutinin adhesin and a high antigenic region of FAP-P, can induce a strong IFN-γ response [
15,
22]. In a previous study, we showed that
Salmonella typhimurium strain SL7207 (Δ
hisG, Δ
aroA) can successfully be used as a carrier for delivering pcDNA3.1+ containing
fap-P gene (pcDNA-
fap) to THP-1-derived macrophages, and FAP-P expression by these cells was approved using the Western blot analysis [
14].
In this study, the Western blot analysis indicated that the oral immunization of mice with Salmonella carrying the pcDNA-fap elicited a detectable humoral immune response against FAP-P. Nonetheless, the appearance of a faint band in the Western blot results of the negative control group raised concerns regarding the conclusiveness of this finding.
Since the lysate of E. coli expressing FAP-P had been used to detect anti- FAP anti-bodies, it was hypothesized that cross-reactivity with E. coli proteins might be the reason for this unexpected band. To test this, a dot blot assay was performed using the PPD of MAP, which contains the native form of FAP-P. The assay revealed a positive signal with serum from the immunized group, while no reaction was observed with serum from the negative control group. These results support the hypothesis that the weak band observed in the Western blot of the control group was likely due to cross-reactivity with E. coli proteins and also confirmed the specificity of antibodies produced following mice immunization using Salmonella carrying the pcDNA-fap.
5. Conclusions
In conclusion, Salmonella typhimurium strain SL7207 (ΔhisG, ΔaroA) can be used as an efficient carrier for designing DNA vaccine candidates against MAP. The ease of Salmonella carrier propagation can effectively reduce the time and labor required for the large-scale isolation of highly purified plasmids. This advantage simultaneously simplifies the initial screening of antigens and the mass production of vaccines. However, for optimal application of this carrier system, anatomical differences in the gastrointestinal tracts of various hosts must be carefully considered.
Moreover, the results suggest that FAP-P may be immunogenic in mice, although further studies are necessary to evaluate its protective efficacy and cellular immune responses in the target animal species.