Abstract
Carao (Cassia grandis) contains numerous bioactive substances that contribute to gastrointestinal well-being. The present study assessed the potential impacts of carao on the viability and performance of Streptococcus thermophilus and Lactobacillus bulgaricus under various adverse conditions. These conditions included bile, acid, gastric juice, and lysozyme exposure, simulating the digestive process from the mouth to the intestines. The activity of proteases from cultures was monitored to examine their proteolytic capabilities. To achieve this, the cultures were cultivated in a solution containing plant material, and the results were compared against a control sample after an incubation period. Subsequently, the total phenolic content, total carotenoid content, antioxidant activity, sugar profile, and acid profile of the plant materials were analyzed. These analyses were conducted to explore these compounds’ influence on cultures’ survival. Seeds contained the highest total phenols (766.87 ± 11.56 µg GAE/mL), total carotenoid content (7.43 ± 0.31 mg Q/mL), and antioxidant activity (40.76 ± 1.87%). Pulp contained the highest moisture (12.55 ± 0.44%), ash (6.45 ± 0.15%), lipid (0.66 ± 0.07%), protein (16.56 ± 0.21%), sucrose (9.07 ± 0.78 g/100 g), and fructose (3.76 ± 0.06 g/ 100 g). The crust had the highest content of ash (85.14 ± 0.27%) and succinic acid (2.01 ± 0.06 g/100 g). Results indicated that seeds negatively affected cultures’ survival in the bile tolerance test and had positive effects on Lactobacillus bulgaricus in the protease activity test. Otherwise, the other carao tissues could not change the results significantly (p > 0.05) compared to the control in different tests. The carao crust positively affected cultures’ against protease activity, especially in Lactobacillus bulgaricus, and had a negative effect on the growth of S. thermophilus in the lysozyme and gastric acid resistance test.
1. Introduction
The FAO/WHO defines probiotics as “live microorganisms that, when administered in sufficient quantities, provide a positive impact on the well-being of the host.” Various health effects have been linked to lactic acid bacteria, and these effects have differing levels of supporting evidence. The positive effect of probiotics depends on the bacterial strain and the quantity used, and each individual probiotic must validate its benefits through laboratory tests, animal trials (when applicable), and human research as stipulated by the FAO/WHO. Concurrently, the appropriate dosage of probiotics is reliant on the strain and product to ensure survival and colonization in the intestine [1].
Owing to the increasing public awareness regarding the benefits of lactic acid bacteria for health, maintaining intestinal microbial balance, and reinforcing mucosal defenses against pathogens, there is a rapid surge in demand for functional foods containing microorganisms possessing lactic acid bacteria attributes. Lactic acid bacteria encompass beneficial bacteria that offer therapeutic advantages to the host organisms ingesting them. Probiotic products comprise live microorganisms at levels surpassing 106 to 107 CFU/mL, aiming to elicit a favorable influence on human health, and demonstrate resilience in the demanding conditions within the human digestive tract [2].
Many lactic acid bacteria can provide immune boosts, inflammation alleviation, alleviation of gastrointestinal distress, and prevention of diarrhea. These outcomes are achieved through a range of mechanisms, such as generating antimicrobial substances like organic acids and bacteriocins, regulating the immune response through the secretion of immunoglobulin A (IgA) to counter potential pathogens, reducing the likelihood of allergy development, strengthening the function of the intestinal mucosal barrier, aiding in the restoration of disrupted commensal microflora, adjusting the expression of host genes, and releasing functional proteins [3].
Dairy products represent the probiotic foods that are most widely accessible in the market. This success is attributed to the fact that milk is a natural and integral element of a well-rounded diet. Most foods containing probiotic microorganisms are dairy products that have undergone fermentation. This category encompasses yogurt, fermented milk and whey beverages, kefir, fermented sour cream, buttermilk, and various types of cheeses. These food products are well suited for incorporating probiotics due to their existing favorable consumer perception and their compatibility with current production methods, requiring minimal adjustments. Fruits and other plant-derived products have the potential to serve as effective substrates for probiotics, given their content of vitamins, minerals, fiber, carbohydrates, and antioxidant elements [4]. Recently, the probiotic properties of lactic acid bacteria were examined concerning new food sources such as weevil (Rhynchophorus palmarum), nipple fruit (Solanum mammosum), teosinte (Dioon mejiae), and Caesar mushroom (Amanita caesarea) [5,6,7,8,9]. Carao has been used in yogurt and it shows great antioxidant and hypoglycemic potential [10]. Furthermore, these food items do not contain common allergens found in milk-based products, which could help avoid intake limitations. Within this context, fruit-based probiotic products present an intriguing area for research, particularly due to their ability to endure acidic conditions. It is also essential for probiotics to be administered through food matrices that are acceptable to consumers [9].
Carao is a large, bushy tree with dark green foliage and pink flowers in bunches and is very showy. The fruit has a cylindrical shape measuring more than half a yard long and is full of crushed seeds [10]. Carao (Cassia grandis) contains numerous bioactive substances that contribute to gastrointestinal well-being. The flesh contains abundant carotenoid levels, while the seeds exhibit a significant presence of phenolic compounds and strong antioxidant properties. Among the trace minerals, magnesium, calcium, iron, and manganese are found in substantial quantities. There is a proposal for the utilization of carao in nutritional, pharmaceutical, and medicinal contexts [10,11,12].
L. bulgaricus and S. thermophilus are commonly employed together as starter cultures in the production of diverse dairy products. Their cooperative interaction involves metabolite exchange, known as protocooperation, which fosters the growth of both strains. L. bulgaricus produces PrtB, an external protease that decomposes milk proteins, serving as a nitrogen source for itself and S. thermophilus. Conversely, S. thermophilus contributes acids like formic acid and folic acid, alongside carbon dioxide, to benefit L. bulgaricus. Furthermore, S. thermophilus manufactures specific amino acids and expresses a cell envelope proteinase (PrtS). This symbiotic relationship enhances the acidification of milk and the overall proliferation of microorganisms [13,14].
Prebiotics are generally found in different food sources, such as plant materials. As a result, the primary objective of this study is to examine how carao enhances Streptococcus thermophilus and Lactobacillus bulgaricus characteristics throughout the digestive tract, spanning from the mouth to the intestines. As carao has significant amounts of bioactive compounds, it was worth examining carao’s prebiotic properties in yogurt starter culture. Various tests were conducted to assess the impact of carao peel, seeds, and pulp on the cultures’ growth.
2. Materials and Methods
2.1. Plant Material
Between May and June 2021, the carao fruit was collected within the Guapinol Biological Reserve, located in the Marcovia Municipality of the Choluteca Department in Honduras. The carao peel, seeds, and pulp were manually separated and subsequently combined with water (10% w/w). The mixture was then frozen and stored at a temperature of −80 °C until the plant material was subjected to lyophilization using the LIOTOP model L 101. The solution obtained was subjected to lyophilization under conditions of −73 to −76 °C and 0.1–0.3 Pa for a duration of 48 h. The resulting lyophilized pulp was then processed into powder form using a commercial mill (LABOR model SP31). Finally, the powdered pulp was vacuum-sealed in plastic bags [7].
2.2. Experimental Design
Four gentle stressors (including acid exposure, bile exposure, lysozyme exposure, and exposure to gastric juices) were assessed in cultures of Streptococcus thermophilus ST-M5 and Lactobacillus bulgaricus LB-12 (obtained from Chr. Hansen, Milwaukee, WI, USA) individually. Carao seeds, carao pulp, and carao crust were studied at a concentration of 2% [7]. Each assessment was subjected to a series of stressors: exposure to low pH (pH 2), exposure to oxgall salt (0.3%), exposure to lysozyme (100 mg/L), and exposure to gastric juice containing pepsin (0.32%) and NaCl (0.2%). A control group was also included without the ingredient being tested. The tests encompassed viability evaluation, lysozyme resistance assessment, and tolerance to gastric juices. These aspects were quantified by performing plate counts. The enumeration of test counts was carried out on MRS agar (for Lactobacillus bulgaricus) and M17 agar (for Streptococcus thermophilus) at different time intervals. The experimentation was replicated thrice, with duplicate readings obtained each time. Additionally, carao seeds, crust, and pulp were subjected to further analysis, encompassing antioxidant capacity, total phenolic content, total carotenoid content, total sugar content, and organic acid content.
2.3. Proximate Analysis of Plant Material
Moisture content (method 935.29), fat (method 954.02), ash (method 923.03), and proteins (method 991.20) were determined according to the AOAC. The total carbohydrate content was calculated by difference and the calorie content was calculated by the Atwater coefficient. Analyses were repeated twice, and each analysis was undertaken in triplicate [15].
2.4. Radical-Scavenging Activity Assay and Total Polyphenol Content
Freshly harvested plant tissues (including carao seeds, crust, and pulp) were freeze-dried at a temperature of −65 °C for 72 h. The resulting freeze-dried plant materials were finely crushed using a mortar and pestle. Approximately 100 mg of the finely powdered substance, with a precision level of ± 0.5 mg, was accurately measured and placed into a 15 mL centrifuge tube. For the extraction procedure, a mixture of 80% methanol (10 mL) was used as the solvent. The mixture was vigorously mixed for one minute using a vortex mixer and then subjected to sonication for fifteen min, pausing vortexing for one minute after every five minutes of sonication. Following this, the mixture was centrifuged for 5 min at 1230 g force. The resulting liquid supernatant was collected and utilized for the determination of total phenolics and total antioxidant content. The TPC (total phenolic content) was measured using a customized version of the Folin–Ciocalteu (FC) method [16]. Precisely 0.5 mL of the liquid supernatant was carefully added to a 25 mL test tube and combined with 8 mL of ultrapure water from the Barnstead MP-12A source in Haverhill, MA. Following this step, 0.5 mL of the Folin–Ciocalteu reagent was introduced into the mixture. After a 3 min interval, 1 mL of a 1 N solution of sodium bicarbonate (Na2CO3) was incorporated, and the resulting solution was allowed to remain undisturbed for a duration of 2 h at room temperature, approximately 22 °C. The absorbance of the resultant solution was assessed at 750 nm employing a Lambda 35 UV/Vis spectrophotometer (Perkin Elmer Instruments, Norwalk, CT). Gallic acid was utilized as the reference standard [16]. A calibration curve was constructed using gallic acid within a concentration range of 50 to 300 µg/mL. The total phenolic content was quantified and reported as milligrams of gallic acid equivalent per gram of dry weight (mg GAE/g). The antioxidant activity was measured according to the method developed by Brand-Williams et al. (1995) [17], with slight modifications. DPPH (1, 1-diphenyl-2-picrylhydazyl) was used as the source of free radicals [18]. The absorbance of free radicals at 517 nm disappears upon their reduction by an antioxidant. In this study, Trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid) was used as the standard antioxidant compound. Exactly 0.1 mL of extract was diluted with an additional 0.4 mL of 80% methanol and added to a 1.5 mL amber-colored centrifuge tube containing 0.5 mL of freshly prepared 80% methanol solution of DPPH (0.01577 g/100 mL). The resultant mixture was shaken in the dark for 2 min and then incubated for 30 min at room temperature in darkness. The percent inhibition of DPPH was calculated from the decrease in absorbance using Equation (1).
I% = [(Blank Absorbance − sample absorbance V)/(Blank Absorbance) × 100
The decrease in DPPH absorbance was measured at 517 nm using a Lambda 35 UV/Vis spectrophotometer (Perkin Elmer Instruments, Norwalk, CT, USA). The extract was replaced with 80% methanol in the control sample. A solution of 80% methanol without DPPH was used as the blank. The antioxidant activity was calculated from a standard curve made with known concentrations of Trolox [17].
2.5. Total Carotenoid Content
Conventional extraction of carotenoids and their esters from the freeze-dried carao tissues (seeds, pulp, and peel) was performed according to the procedure reported by Cano et al. (2019) [19] with some modifications. First, 1 g of the freeze-dried sample was mixed with 0.5 g magnesium carbonate and 50 μL of (all-E)-β-apo-8′-carotenal (0.40 mg/mL), as an internal standard. Then, 20 mL of tetrahydrofuran (THF) stabilized with 0.1% (w/v) of butylated hydroxytoluene (BHT) was added for the extraction. The sample was homogenized in an Omnimixer (OMNI Macro S®, OMNI International, Kennesaw, GA, USA) at 3000 g for 3 min and placed in an ultrasonic water bath (3000514 model, 50/60 Hz, 360 W, J.P. Selecta S.A., Barcelona, Spain) for 30 min. The extract was centrifuged at 15,000× g for 10 min at 4 °C and the supernatant was collected. Subsequently, 20 mL of acetone was added to the pellet and the sample was extracted again. Re-extraction of the recovered solids was carried out 3 times until a colorless residue was obtained. Supernatants were combined in the same separation funnel, where 20 mL of diethyl ether was added; when an emulsion was formed, 20 mL of saturated water with 30% (w/v) NaCl was added. The organic phase was collected and dried with 2.5 g of anhydrous sodium sulfate over 10 min at room temperature. The sample was filtered through Whatman No. 1 filter paper and the filtrate obtained was transferred to a round amber flask and vacuum-dried in a rotatory evaporator at 30 °C. Finally, the extract was dissolved to 2 mL with MeOH/MTBE/H2O (45.5:52.5:2, v/v/v) and filtered through a 0.45 μm filter. All procedures were carried out at room temperature and in dim light to avoid carotenoid isomerization and degradation. Total carotenoid content was determined spectrophotometrically at 450 nm using a UV–Vis spectrophotometer (Specord 210 Plus, Analytik Jena). After the carotenoid extraction procedure, the volume of the carotenoid oil extracts was noted, and the absorbance was measured against absolute hexane used as a blank. Hexane solutions were analyzed under diminished light. Total carotenoid content (μg carotenoids/g vegetable oil) was calculated according to Equation (2).
where A is the absorbance at 450 nm, V is the total volume of dissolution (mL), E1%1 cm is the extinction coefficient of the mixture of carotenoids in hexane (ξ = 2500 dL/g cm, according to Melendez-Martinez et al., 2007 [20]), and P is the plant tissue extract weight (g).
Total carotenoid content (μg/g) = [(A × V)/(E1%1cm × P ×100)] × 106
2.6. HPLC Determination of Sugars and Organic Acids
For plant tissue extract preparation, 10 g of plant tissue powder was homogenized in 80% ethanol for 1 min at high speed using a Virtis 45 homogenizer [21]. The resulting slurry was immediately boiled for 15 min, cooled, and filtered through Whatman #4 paper; the residue and original container were washed with additional 80% ethanol and made up to a final volume of 100 mL. About 5 mL was filtered through a 0.45~ filter membrane before injecting into the HPLC. For sugar standard preparation, a 1% standard of any sugar (fructose, maltose, glucose, and sucrose) was prepared as 1 g of analytical-grade sugar (Sigma Chemical Co., St. Louis, MO, USA) was homogenized in 80% ethanol, boiled, filtered, made up to 100 mL with 80% ethanol and filtered before injecting into the HPLC. For the determination of sugars, a Beckman series 340 liquid chromatograph equipped with a model 112 pump, a model 210 injector fitted with a 20 /IL sample loop, and a model 156 refractive index detector were used. The detector signal was electronically integrated by a Varian 401 integrator in the external standard mode using an attenuation of 16 and a chart speed of 0.5 cm/min. Sugars were separated with a 300 mm × 7.8 mm id. Column packed with Aminex HPX-87C resin (Bio-Rad Labs, Richmond, CA, USA) heated to 75 °C. The following were plumbed between the injector and the analytical column, respectively: a 2~ Rhea-dyne 7302 column inlet filter; a 40 × 4.6 mm ion exclusion guard cartridge packed with Aminex HPX-85H resin (Bio-Rad Labs, Richmond, CA, USA); and a 40 × 4.6 mm anion/OH guard cartridge packed with Aminex A-25 resin (Bio-Rad Labs, Richmond, CA, USA). The mobile phase was degassed HPLC-grade Hz0 at a flow rate of 1.2 mL/min [21].
For organic acid standard preparation, a 0.5% standard of any organic acid (citric, tartaric, L-Malic, quinic, and succinic acids) was used. A 0.500 g amount of analytical-grade organic acid (Sigma Chemical Co., St. Louis, MO, USA) was homogenized in 80% ethanol, boiled, filtered, made up to 100 mL with 80% ethanol, and ultrafiltered before injecting into the HPLC. Organic acids were not affected by heating, as checked against unboiled standards dissolved in 80% ethanol. For organic acid determination, a Beckman series 340 liquid chromatograph (Beckman Instruments, Inc., Berkely, CA, USA) equipped with a model 112 pump, model 210 injector fitted with a 20 L sample loop, and a model 160 ultraviolet detector at a fixed wavelength was used after 214 nm. The detector was set at 0.100 AU and the peak area of the signal was electronically integrated by a Vista 401 integrator (Varían Assoc., Sunnyvale, CA, USA) in the external standard mode by using an attenuation of 4 and a chart speed of 0.5 cm/min. Organic acids were separated with a 300 mm × 7.8 mm i.d. Aminex HPX-87H column (BioRad Laboratories, Richmond, CA, USA). The column temperature was maintained at 75 °C with an SSI column heater (Scientific Systems, State College, PA, USA). Plumbed between the injector and the analytical column were, respectively, a 2-pm filter Rheodyne Model 7302 column inlet (Rheodyne, Cotati, CA, USA) and a 40 × 4.6 mm ion exclusion guard cartridge packed with Aminex HPX-85H resin (Bio-Rad Laboratories). The mobile phase was degassed 0.0008 N H2S04 made by diluting reagent-grade concentrated sulfuric acid in HPLC-grade water. The flow rate was 0.8 mL/ min [22].
Exactly 10.00 g (sufficient for adequate resolution of organic acids with the HPLC system described below) of randomly selected tissue was homogenized in 80% ethanol to ensure rapid enzyme denaturation for 1 min at high speed with a Virtis 45 homogenizer. The resulting slurry was immediately boiled for 15 min, cooled, and filtered through Whatman #4 paper; the residue and original container were washed with additional 80% ethanol and made up to a final volume of 100 mL [22].
2.7. Bile Tolerance Test
The bile tolerance of S. thermophilus and L. bulgaricus was determined by the method of Perei and Gibson (2002) [19] with some modifications. Yogurt’s stater cultures were evaluated for their ability to grow in MRS broth (Criterion™, Hardy Diagnostics, Santa Maria, CA, USA) and M17 broth (Criterion™, Hardy Diagnostics, Santa Maria, CA, USA), respectively, supplemented with 0.2% (wt/v) of sodium thioglycolate (Acros Organics, Fair Lawn, NJ, USA) and bile salt oxagall (0.3 wt/vol). Sodium thioglycolate was only used in MRS broth as an oxygen scavenger to achieve microaerophilic conditions. Starter cultures were inoculated (10% [v/v]) into MRS broth or M17 broth with 0.3% (wt/v) oxgall (bovine bile) (US Biological, Swampscott, MA, USA) and incubated under anaerobic conditions at 37 °C and anaerobically at 43 °C for 8 h (measured hourly) [9,23].
2.8. Acid Tolerance Test
The acid tolerance of S. thermophilus and L. bulgaricus was determined by the method of Perei and Gibson (2002) [23] with some modifications. Starter cultures were inoculated (10% [v/v]) into acidified MRS broth (Criterion™, Hardy Diagnostics, Santa Maria, CA, USA) earlier adjusted to pH 2.0 with 1N HCl. The acidified MRS broth with cultures was incubated in a water bath at 37 °C for 15 min. One-milliliter samples were taken at various times (0, 30, 60, and 120 min), serially 10-fold diluted in peptone water, and plated in duplicate onto MRS agar (Difco, Detroit, MI, USA) and M17 Agar (Difco, Detroit, MI, USA), respectively.
2.9. Tolerance to Simulated Gastric Juice
Tolerance of S. thermophilus and L. bulgaricus with the functional ingredients to simulated gastric juice (SGJ) was assessed according to García-Ruiz et al. (2014) [24] and Zhang et al. (2019) [25] with slight modifications. Aseptically, the SGJ was formulated with H2O, pepsin (Sigma-Aldrich, St. Louis, MO, USA) (0.32%), and NaCl (0.2%), and NaOH and HCl were utilized to adjust the pH. The simulated gastric juice was set to five concentration gradients (pH 7.0, 5.0, 4.0, 3.0, and 2.0) with 1 M HCl and 1 M NaOH. Cultures of S. thermophilus and L. bulgaricus were inoculated (5% [v/v]), individually, into SGJ and incubated under anaerobic conditions at 37 °C (S. thermophilus) and anaerobically at 43 °C (L. bulgaricus) for 30 min. The counts of viable bacteria were enumerated by plate counting at 0 and 30 min of incubation.
2.10. Lysozyme Tolerance Test
Resistance of S. thermophilus and L. bulgaricus to lysozyme was determined by the method described by Zago et al. (2011) [26] with modifications. Cultures were evaluated for their capacity to survive in a filter sterile electrolyte solution (0.22 g/L CaCl2, 6.2 g/L NaCl, 2.2 g/L KCl, 1.2 g/L NaHCO3) in the presence of 100 mg/L of lysozyme (SigmaeAldrich). Starter cultures were inoculated (10% [v/v]) into the electrolyte solution and incubated under anaerobic conditions at 37 °C (S. thermophilus) and anaerobically at 43 °C (L. bulgaricus). The counts of viable bacteria were enumerated by plate counting at 0, 30, and 120 min of incubation.
2.11. Protease Activity
The extracellular protease activity of S. thermophilus and L. bulgaricus was determined by the o-phthaldialdehyde (OPA) spectrophotometric assay according to the method described by Oberg et al. (1991) [27]. S. thermophilus and L. bulgaricus were inoculated (1% [v/v]) into sterile skim milk (autoclaved at 12 °C for 15 min) and incubated at 40 °C for 0, 12, and 24 h. After incubation, 2.5 mL from each sample was mixed with 1 mL distilled water and transferred into test tubes containing 5 mL of 0.75N trichloroacetic acid (TCA) (Fisher Scientific), and the test tubes were vortexed at the same time. After setting at room temperature for 10 min, the acidified samples were filtered through a Whatman Number 2 filter paper (Clifton, NJ, USA). Non-inoculated sterile skim milk was prepared similarly to be used as a reference in a duplicate aliquot from each TCA filtrate and was analyzed by the o-phthaldialdehyde (OPA) spectrophotometric assay with a UV-Vis spectrophotometer (Nicolet Evolution 100, Thermo Scientific; Madison, WI, USA). The o-phthaldialdehyde final solution was prepared by combining the following reagents and diluting to a final volume of 50 mL with distilled water: 25 mL of 100 mM sodium borate (Fisher Scientific); 2.5 mL 20% (wt/wt) SDS (Fisher Scientific); 40 mg of o-phthaldialdehyde reagent (Alfa Aesar, Ward Hill, MA, USA) dissolved in 1 mL methanol (Sigma); and 100 µL of β-mercaptoe. A total of 150 µL of each TCA filtrate was mixed with 3 mL of ophthaldialdehyde final solution in a 3 mL cuvette, and the absorbance at 340 nm was read. The absorbance of the o-phthaldialdehyde final solution with the non-inoculated sterile skim milk (reference) was subtracted from each sample reading. The o-phthaldialdehyde reagent solution (sigma) was used as a blank and the milk with or without the ingredient was used as the control [27]. The protease activity (PA) was calculated by Equation (3).
where SA is the absorbance of the sample and BA and CA are the absorbance from the blank and control, respectively.
PA (%) = (100 − ((SA − BA)/CA)) × 100
2.12. Enumeration of S. thermophilus
Agar for Streptococcus thermophilus was prepared using the following method: To 1 L of distilled water, the following ingredients were added using individual plastic weighing boats: 10 g of sucrose (Amresco, Solon, OH, USA), 2 g of K2HPO4 (Fisher Scientific, Fair Lawn, NJ, USA), 5 g of Bacto yeast extract, and 10 g of Bacto Tryptone (Becton, Dickinson and Co., Sparks, MD, USA). Distilled water was transferred from the graduated cylinder to a 2 L Erlenmeyer flask. The mix was stirred to dissolve the ingredients. To reduce the pH to 6.8, 1 N HCl was added. Then, 12 g of agar (Fisher Scientific, Fair Lawn, NJ, USA) was added to the medium and 6 mL of 0.5% bromocresol purple was added (Fisher Scientific, Fair Lawn, NJ, USA). The media was heated to boiling, and it was autoclaved at 121 °C for 15 min. Samples were diluted with 99 mL of sterilized MgCl2 KOH. Then, 1 mL of each diluted sample was pipetted into Petri dishes and then the media was aseptically poured into the Petri dish. Petri dishes were aerobically incubated at 37 °C for 24 h. To enumerate the colonies, a Quebec Darkfield Colony Counter (Leica Inc., Buffalo, NY, USA) was used.
2.13. Enumeration of L. bulgaricus
Lactobacilli MRS agar was prepared using 1 L of distilled water, 15 g of agar (Fisher Scientific, Fair Lawn, NJ, USA), and 55 g of Lactobacilli MRS broth powder (Becton, Dickinson and Co., Sparks, MD, USA). The pH was adjusted to 5.2 using 1 N HCl. The medium was heated to boiling with agitation. Then, the medium was autoclaved at 121 °C for 15 min. The sample was diluted in serial dilutions with 99 mL of sterilized MgCl2 KOH. A 1 mL volume of each diluted sample was pipetted into Petri dishes and then the medium was aseptically poured into the Petri dish. Petri dishes were placed in a BBL GasPaks (BBL, Becton, Dickinson and Co., Cockeysville, MD, USA) and then they were incubated anaerobically at 43 °C for 72 h. To enumerate the colonies, a Quebec Darkfield Colony Counter (Leica Inc., Buffalo, NY, USA) was used.
2.14. Statistical Analysis
Data were analyzed using the General Linear Model (PROC GLM) of the Statistical Analysis Systems (SAS). Differences of least square means were used to determine significant differences at p < 0.05 for main effects (ingredients vs. control). Data are presented as mean ± standard deviation. Significant differences were determined at α = 0.05. For chemical analysis, analysis of variance (ANOVA) was applied. Tukey’s test examined the statistical differences (p < 0.05) among the treatments and the main and interaction effects of the General Linear Model.
3. Results and Discussion
3.1. Chemical Compositions and Antioxidant Activity of Different Food Sources
Table 1 indicates the results of the proximal composition of flours of the carao tissue. Seeds, pulp, and crust have significantly different (p < 0.05) amounts of ash, lipids, proteins, and carbohydrate. The pulp of the carao has the highest moisture (12.55 ± 0.44%), ash (6.45 ± 0.15%), lipids (0.66 ± 0.07%), and proteins (16.56 ± 0.21%). The crust of the carao has the lowest moisture (10.23 ± 0.38%) and protein (2.41 ± 0.45%). Seeds of the carao have the lowest lipids (0.15 ± 0.09%) and ash (1.97 ± 0.34%).

Table 1.
Proximal composition of carao flours.
Table 2 indicates the TPC, TCC, and antioxidant activity of the carao tissue to compare their antioxidation ability. Seeds of carao have the highest TPC (766.87 ± 11.56 μg GAE/mL), TCC (7.43 ± 0.31 mg Q/Ml), and antioxidant activity (40.76 ± 1.87%). The crust of carao has the lowest TPC (245.55 ± 10.48 μg GAE/mL)’ TCC (4.73 ± 0.33 mg Q/mL) and antioxidant activity (22.88 ± 2.84%). Pulp and crust indicated high TCC with significant differences (6.12 ± 0.45, 4.73 ± 0.33 mg Q/mL, respectively). Seeds, pulp, and crust have significantly different (p < 0.05) amounts of ash, lipids, proteins, and carbohydrate.

Table 2.
Total phenolic content, total carotenoid content, and antioxidant activity of carao tissues.
Table 3 and Table 4 show the important sugar and acid content (g/100 g) of seeds, pulp, and crust of carao. Pulp had the highest amount of sucrose and fructose (9.07 ± 0.78 and 3.76 ± 0.06 g/100 g, respectively) compared to seeds and crust. Also, maltose was not detected in seeds and crust significantly. Seeds and crust did not have significantly different (p > 0.05) amounts of sucrose. The only source in which maltose was detected was pulp (0.35 ± 0.05 g/100 g). Seeds had the highest significance (p < 0.05) amounts of citric acid (1.02 ± 0.19 g/100 g) and lowest amounts of succinic acid (0.27 ± 0.02 g/100 g) compared to other sources. Crust had the highest succinic acid (2.01 ± 0.06 g/100 g).

Table 3.
Sugar profile of different food sources.

Table 4.
Organic acid profile of different food sources.
Carao is a plant that is thought to have nutritional properties. The total phenolic compounds, minerals such as magnesium and calcium, and antioxidant activity evaluated by Marcia et al. (2020) [28] indicated higher amounts in the seeds compared to the crust and pulp. On the other hand, the total carotenoid content showed a higher concentration in the pulp, while microminerals such as manganese were more present in the crust [28]. The results of the chemical analysis implied that the rich content of sugar in the pulp can enhance the growth of lactic acid bacteria.
3.2. Bile Tolerance
Commonly, bile salts cause a leakage of hazardous particles by emulsifying the lipid content of the cell wall [29]. Figure 1 represents ST tolerance to bile and assesses if plant resources help cultures to be maintained longer in media. Pulp caused the highest stability in ST counts, and there was no significant difference (p < 0.05) among control, seed, and pulp at the start (0 h). After 4 h, a decrease in ST counts for seed treatment could be observed compared to the control, pulp, and crust, which had no significant difference in ST counts. Seed treatment continued to decrease till 8 h, and control, pulp, and crust treatments experienced a decrease, and they had no meaningful difference from each other.
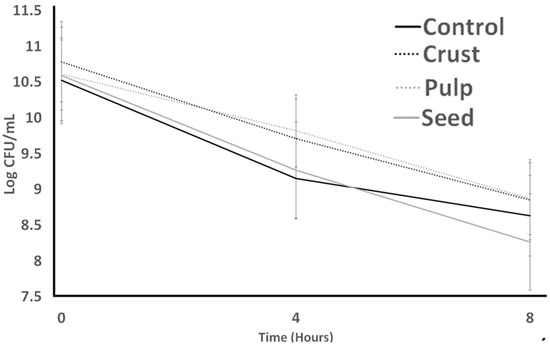
Figure 1.
Bile tolerance of S. thermophilus as influenced by treatments over 8 h. Average of three replicates. Error bars represent SE.
Figure 2 represents LB tolerance to bile and assesses if plant resources help cultures to be maintained longer in media. For LB counts, the pattern is different; pulp showed the highest counts significantly. The lowest bacterial counts resulted from the control treatment at 0, 4, and 8 h of the experiment. Although crust and pulp had a downward trend all the time, they were not significantly different from each other after 8 h. The highest LB count at the end of the experiment against bile resulted from pulp treatments. The results showed that ST was more stable than LB against bile.
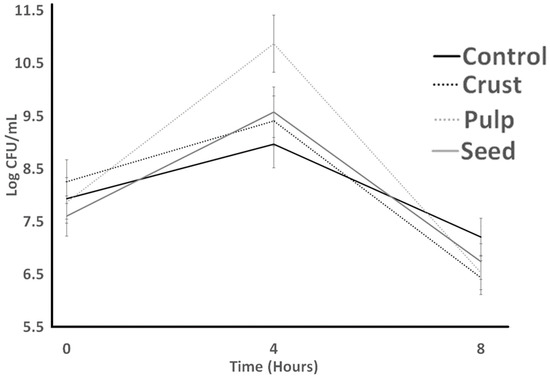
Figure 2.
Bile tolerance of L. bulgaricus as influenced by treatments over 8 h. Average of three replicates. Error bars represent SE.
Also, Vargas et al. [30] reported growth decays of S. thermophilus (in M17 broth) and L. bulgaricus (in MRS broth), respectively, in broth with oxgall (0.3%). Paz et al. (2022) [31] reported that carao pulp improved the bile tolerance of S. thermophilus (in M17 broth) and L. bulgaricus (in MRS broth). We hypothesize that carao could serve as an enzymatic inhibitor and act as a barrier between the lipid membrane and bile salts to shield these bacteria. Nevertheless, the hypothesis needs to be confirmed with further studies. The results of the bile tolerance test implied that the carao pulp could enhance the growth of lactic acid bacteria in digestion.
3.3. Acid Tolerance and Gastric Juice Resistance
Figure 3 (S. thermophilus; ST) and Figure 4 (L. bulgaricus; LB) illustrate the acid resistance of ST and LB. Figure 5 (ST) and Figure 6 (LB) illustrate the gastric juice resistance of ST and LB. Acid resistance was evaluated over a 60 min period, during which bacterial counts were monitored to investigate how plant sources influenced cultures’ activity and viability, simulating stomach conditions. In all treatments, ST counts displayed a noticeable reduction (p > 0.05) after 60 min. Pulp treatment had the highest resistance against acid, and seed treatment had the lowest survival after 60 min. All treatments indicated no significant difference from each other by the end of the experiment. LB counts for all treatments (control, seed, pulp, and crust) were different from ST results. Control experienced the lowest bacterial counts at all times. Seed treatments had no significant changes after 30 min, and crust treatment had the highest bacterial counts. At 0 h, the growth showed a significant decrease (p < 0.05) till 30 min. The tolerance to gastric juice had a similar trend to acid tolerance. The addition of the plant sources was ineffective on the acid tolerance of bacteria after 30 min, and LB was sensitive to acid that was not detected after 30 min and had a lower bacterial count than ST. The results of the acid tolerance test implied that the carao pulp could enhance the growth of lactic acid bacteria in gastric digestion.
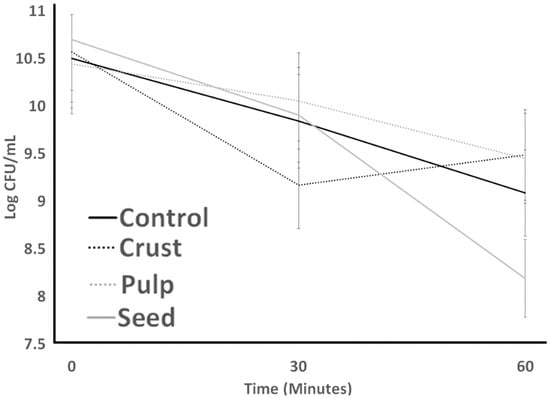
Figure 3.
Acid tolerance of S. thermophilus as influenced by treatments over 60 minutes. Average of three replicates. Error bars represent SE.
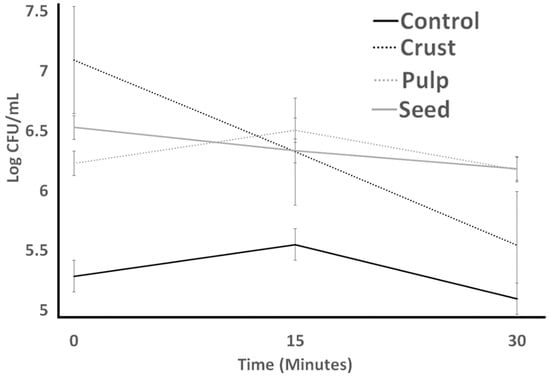
Figure 4.
Acid tolerance of L. bulgaricus as influenced by treatments over 60 min. Average of three replicates. Error bars represent SE.
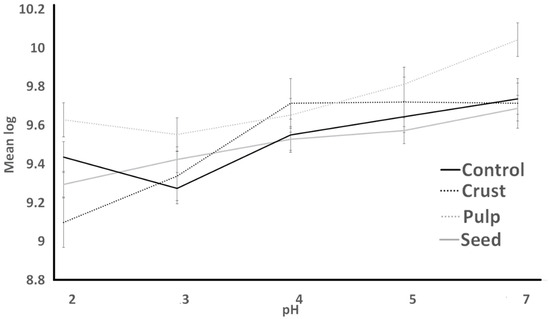
Figure 5.
Resistance to simulated gastric juice of S. thermophilus as influenced by treatments over different pH conditions.
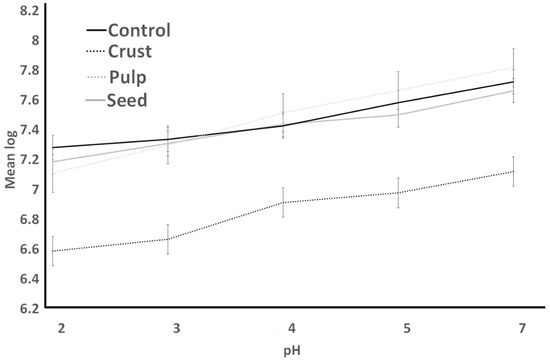
Figure 6.
Resistance to simulated gastric juice of L. bulgaricus as influenced by treatments over different pH conditions.
3.4. Resistance to Lysozyme
Resistance to lysozyme is indicated in Figure 7 for ST. The initial count of ST in the control had no significant difference from other treatments. The highest ST counts resulted from the seed sample, and the crust had the lowest ST counts at the end of the lysozyme resistance experiment (2 h). Seed treatments experienced an initial decrease after 1 h, and then an increase after 2 h. Crust experienced a slight increase after 1 h and then dramatically decreased after 2 h. Resistance to lysozyme is indicated in Figure 8. LB bacterial count experienced a decrease in all treatments. Treatments indicated dramatic differences at the initial count, and they decreased slightly after 1 h. The lowest LB counts resulted from the control treatment at the end, which was not significantly different (p > 0.05) from seed and crust treatments. Other treatments (pulp and crust) indicated the same LB counts at the end of the experiment.
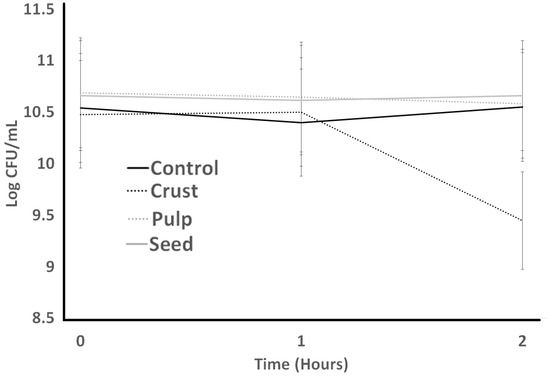
Figure 7.
Resistance to lysozyme of S. thermophilus as influenced by treatments during incubation time of 2 h.
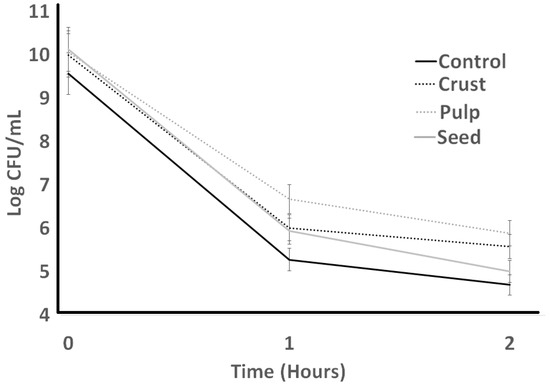
Figure 8.
Resistance to lysozyme of L. bulgaricus as influenced by treatments during incubation time of 2 h.
Marcia et al. (2023) [32] examined the lysozyme tolerance of L. acidophilus and concluded that carao pulp improved growth. Lysozyme is a natural enzyme that acts as a defense against probiotic bacteria [33]. This enzyme is found naturally in tears, saliva, mucus, and milk of human origin. Lysozyme works by attacking the peptidoglycans present in the cell walls of Gram-positive bacteria (while Gram-negative bacteria are less susceptible to attack due to the complexity of their cell walls) [34]. Lysozyme causes cleavage of the β(1-4) bond between N-acetylmuramic acid (NAM) and N-acetylglucosamine peptidoglycan (NAG) [35]. The results of the lysozyme tolerance test implied that the carao crust could inhibit the growth of lactic acid bacteria in the oral cavity.
3.5. Protease Activity
The protease activity of ST is indicated in Figure 9. The protease activity of ST showed an increase in 12 h and a slight decrease in 24 h for all treatments. The control had no significant difference (p > 0.05). Seeds had the lowest absorbance and no significant difference from crust, which had the highest absorbance at the end of the experiment. Control treatments had no significant difference from 0 to 24 h.
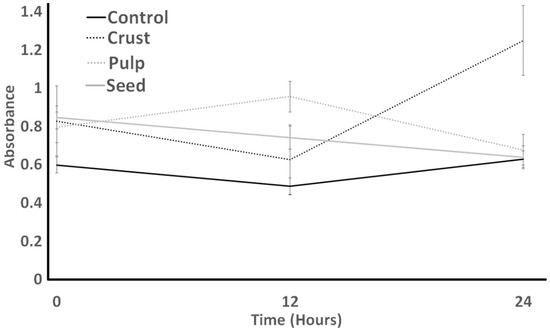
Figure 9.
Protease activity of S. thermophilus as influenced by different treatments over an incubation period of 24 h.
The protease activity of LB is indicated in Figure 10. LB protease activity was the lowest for the pulp treatment at the time 0 h, and it decreased over 24 h, with the lowest protease activity among others. Seed was the only treatment that was significantly highest at time 0. First, it decreased after 12 h and then increased slightly after 24 h. The lowest protease activity for LB was detected in the control treatment after 24 h. The protease activity of ST was higher than the protease activity of LB at the end of the experiment.
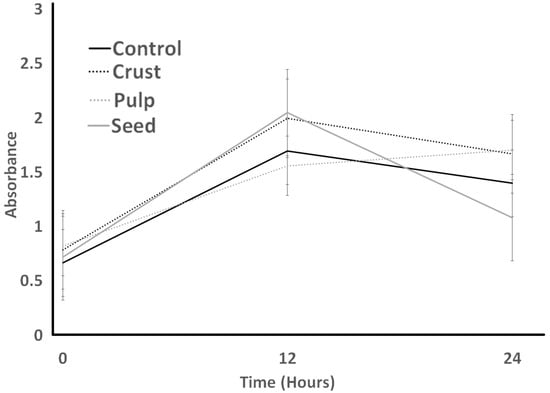
Figure 10.
Protease activity of L. bulgaricus as influenced by different treatments over an incubation period of 24 h.
Paz et al. (2022) [31] examined the protease activity of L. bulgaricus and concluded that carao pulp improved protease activity. Proteolytic bacteria, which are mainly housed in the large intestine, represent less than 0.001% of the microbiota and include Biovare coli, Clostridium, Proteus, Pseudomonas, Enterobacter, Citrobacter, and Klebsiella, among others [36]. The proteolytic activity is carried out by heat-resistant proteases, naturally present in milk or produced by bacteria, particularly from the psychrotrophic group, which break down κ-casein [37]. Proteolysis in fermented milk is essential for several aspects: it can determine the survival of the starter cultures, contribute to undesired flavor and odor, grant technological characteristics, and allow the formation of bioactive peptides [38,39].
4. Conclusions
Carao components such as seeds, pulp, and crust were examined to determine how they influence the growth and behavior of ST and LB under various challenging conditions, such as exposure to acid, lysozyme, bile, and gastric juice. While the seed and crust of carao did not exhibit beneficial effects on the viable counts of ST and LB, the pulp had adverse effects on bile tolerance and increased protease activity within the cultures. The carao’s pulp can be used in fermented dairy products when applied for fortification purposes to enhance nutritional content.
Author Contributions
Conceptualization, M.M.T., R.S.A. and A.K.; methodology, R.S.A., A.A. and D.A.; software, R.S.A.; formal analysis, M.M.T., (most of the research) R.S.A., A.C., A.Y., R.C., A.A. and D.A.; resources, R.S.A., J.M., A.K. and D.P.; data curation, R.S.A.; writing—original draft preparation, R.S.A.; writing—review and editing, R.S.A., S.K., L.K.P. and A.K.; project administration, R.S.A. and A.K.; funding acquisition, R.S.A. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hatch fund at Universidad National de Agriculture (UNAG) with registration # Hn-Subvención 003-2023. This research was also funded by with the International Development Research Center of Canada (IDRC), the General Secretariat of the Council Central American University Superior (CSUCA) (Ref. C-DSIP-008-2023-UNAG), and USDA Hatch funds LAB94511.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We wish to thank the Food Sciences, Louisiana State University Agricultural Center, and the Faculty of Technological Sciences, Universidad Nacional de Agricultura Road to Dulce, Catacamas, Olancho, Honduras.
Conflicts of Interest
The authors state that they have no conflicts of interest.
References
- Drago, L.; Rodighiero, V.; Celeste, T.; Rovetto, L.; De Vecchi, E. Microbiological evaluation of commercial probiotic products available in the USA in 2009. J. Chemother. 2010, 22, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Raza, A.; Ye, L.; Yu, Z. Viability and Composition Validation of Commercial Probiotic Products by Selective Culturing Combined with Next-Generation Sequencing. Microorganisms 2019, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Küçükgöz, K.; Trząskowska, M. Nondairy Probiotic Products: Functional Foods That Require More Attention. Nutrients 2022, 14, 753. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Avila, D.; Avila, A.; Marcia, J.; Picha, D.; Aryana, K.; Montero-Fernández, I. Effects of Weevil (Rhynchophorus palmarum), Teosinte (Dioon mejiae) and Caesar’s Mushroom (Amanita caesarea) on the Properties of Lactobacillus acidophilus LA-K. Fermentation 2023, 9, 852. [Google Scholar] [CrossRef]
- Lim, T.K. Solanum mammosum. In Edible Medicinal and Non-Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2013; Volume 6, pp. 364–369. [Google Scholar]
- Bastias-Montes, J.-M.; Flores-Varela, L.-E.; Reyes-Calderón, O.-A.; Vidal-San-Martín, C.; Muñoz-Fariña, O.; Quevedo-León, R.; Acuña-Nelson, S.-M. Teosinte (Dioon mejiae) Flour: Nutritional and Physicochemical Characterization of the Seed Flour of the Living Fossil in Honduras. Agronomy 2020, 10, 481. [Google Scholar] [CrossRef]
- Niemeyer, K.; Bell, I.; Koithan, M. Traditional knowledge of Western herbal medicine and complex systems science. J. Herb. Med. 2013, 3, 112–119. [Google Scholar] [CrossRef]
- Aleman, R.S.; Paz, D.; Cedillos, R.; Tabora, M.; Olson, D.W.; Aryana, K. Attributes of Culture Bacteria as Influenced by Ingredients That Help Treat Leaky Gut. Microorganisms 2023, 11, 893. [Google Scholar] [CrossRef]
- Fuentes, J.A.M.; López-Salas, L.; Borrás-Linares, I.; Navarro-Alarcón, M.; Segura-Carretero, A.; Lozano-Sánchez, J. Development of an innovative pressurized liquid extraction procedure by response surface methodology to recover bioactive compounds from Carao Tree Seeds. Foods 2021, 10, 398. [Google Scholar] [CrossRef]
- Marcía-Fuentes, J.; Santos-Alemán, R.; Borrás-Linares, I.; Sánchez, J.L. The Carao (Cassia grandis L.): Its Potential Usage in Pharmacological, Nutritional and Medicinal Applications. In Innovations in Biotechnology for a Sustainable Future; Springer: Cham, Switzerland, 2021; pp. 403–427. [Google Scholar]
- Aleman, R.S.; Marcia, J.; Page, R.; Kazemzadeh Pournaki, S.; Martín-Vertedor, D.; Manrique-Fernández, V.; Montero-Fernández, I.; Aryana, K. Effects of Yogurt with Carao (Cassia grandis) on Intestinal Barrier Dysfunction, α-glycosidase Activity, Lipase Activity, Hypoglycemic Effect, and Antioxidant Activity. Fermentation 2023, 9, 566. [Google Scholar] [CrossRef]
- Delorme, C.; Bartholini, C.; Bolotin, A.; Ehrlich, S.D.; Renault, P. Emergence of a cell wall protease in the Streptococcus thermophilus population. Appl. Environ. Microbiol. 2010, 76, 451–460. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, G.M.; García-Garibay, M.; Cruz-Guerrero, A.E.; Gómez-Ruiz, L.; Ayala-Nino, A.; Castaneda-Ovando, A.; Gonzalez-Olivares, L.G. Proteolytic System of Streptococcus thermophilus. J. Microbiol. Biotechnol. 2018, 28, 1581–1588. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists International. Official Methods, 20th ed.; AOAC: Rockville, MD, USA, 2019; Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 29 July 2020).
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ibragic, S.; Barbini, S.; Oberlerchner, J.T.; Potthast, A.; Rosenau, T.; Böhmdorfer, S. Antioxidant Properties and Qualitative Analysis of Phenolic Constituents in Ephedra spp. by HPTLC Together with Injection Port Derivatization GC–MS. J. Chromatogr. B 2021, 1180, 122877. [Google Scholar] [CrossRef]
- Cano, M.P.; Gómez-Maqueo, A.; Fernández-López, R.; Welti-Chanes, J.; García-Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Picha, D.H. HPLC determination of sugars in raw and baked sweet potatoes. J. Food Sci. 1985, 50, 1189–1190. [Google Scholar] [CrossRef]
- Zeppa, G.; Conterno, L.; Gerbi, V. Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 2722–2726. [Google Scholar] [CrossRef]
- Pereira, D.I.A.; Gibson, G.R. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef]
- García-Ruiz, A.; González de Llano, D.; Esteban-Fernández, A.; Requena, T.; Bartolomé, B.; Moreno-Arribas, M.B. Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol. 2014, 44, 220–225. [Google Scholar] [CrossRef]
- Liao, N.; Luo, B.; Gao, J.; Li, X.; Zhao, Z.; Zhang, Y.; Ni, Y.; Tian, F. Oligosaccharides as co-encapsulating agents: Effect on oral Lactobacillus fermentum survival in a simulated gastrointestinal tract. Biotechnol. Lett. 2019, 41, 263–272. [Google Scholar] [CrossRef]
- Zago, M.; Fornasari, M.E.; Carminati, D.; Burns, P.; Suàrez, V.; Vinderola, G.; Reinheimer, J.; Giraffa, G. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 2011, 28, 1033–1040. [Google Scholar] [CrossRef]
- Oberg, C.J.; Weimer, B.C.; Moyes, L.V.; Brown, R.J.; Richardson, G.H. Proteolytic Characterization of Lactobacillus delbrueckii ssp. bulgaricus Strains by the o-Phthaldialdehyde Test and Amino Acid Analysis. J. Dairy Sci. 1991, 74, 398–403. [Google Scholar] [CrossRef]
- Fuentes, J.A.M.; Fernández, I.M.; Fernández, H.Z.; Sánchez, J.L.; Alemán, R.S.; Navarro-Alarcón, M.; Borrás-Linares, I.; Maldonado, S.A.S. Quantification of Bioactive Molecules, Minerals and Bromatological Analysis in Carao (Cassia grandis). J. Agric. Sci. 2020, 12, 88. [Google Scholar] [CrossRef]
- Theegala, M.; Arévalo, R.A.C.; Viana, V.; Olson, D.; Aryana, K. Effect of Flaxseed on Bile Tolerances of Lactobacillus acidophilus, Lactobacillus bulgaricus, and Streptococcus thermophilus. Food Nutr. Sci. 2021, 12, 670–680. [Google Scholar]
- Vargas, L.; Olson, D.; Aryana, K. Whey protein isolate improves acid and bile tolerances of Streptococcus thermophilus ST-M5 and Lactobacillus delbrueckii ssp. bulgaricus LB-12. J. Dairy Sci. 2015, 98, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Paz, D.; Aleman, R.S.; Cedillos, R.; Olson, D.W.; Aryana, K.; Marcia, J.; Boeneke, C. Probiotic characteristics of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus as influenced by carao (Cassia grandis). Fermentation 2022, 8, 499. [Google Scholar] [CrossRef]
- Marcia, J.; Aleman, R.S.; Montero-Fernández, I.; Martín-Vertedor, D.; Manrique-Fernández, V.; Moncada, M.; Kayanush, A. Attributes of Lactobacillus acidophilus as Effected by Carao (Cassia grandis) Pulp Powder. Fermentation 2023, 9, 408. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Koushki, M.R.; Sohrabvandi, S.; Yousefi, M. An overview of antimicrobial activity of lysozyme and its functionality in cheese. Front. Nutr. 2022, 9, 833618. [Google Scholar] [CrossRef]
- Jana, M.; Ghosh, A.; Santra, A.; Kar, R.K.; Misra, A.K.; Bhunia, A. Synthesis of novel muramic acid derivatives and their interaction with lysozyme: Action of lysozyme revisited. J. Colloid Interface Sci. 2017, 498, 395–404. [Google Scholar] [CrossRef]
- Salton, M.R.J. The properties of lysozyme and its action on microorganisms. Bacteriol. Rev. 1957, 21, 82–100. [Google Scholar] [CrossRef]
- Guentzel, M.N. Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 26. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8035/ (accessed on 2 March 2023).
- Yuan, L.; Sadiq, F.A.; Burmølle, M.; Wang, N.; He, G. Insights into Psychrotrophic Bacteria in Raw Milk: A Review. J. Food Prot 2019, 82, 1148–1159. [Google Scholar] [CrossRef]
- Capodifoglio, E.; Vidal, A.M.C.; Lima, J.A.S.; Bartoletto, F.; D’Abreu, L.F.; Gonçalves, A.C.S.; Vaz, A.C.N.; de Carvalho Balieiro, J.C.; Netto, A.S. Lipolytic and proteolytic activity of Pseudomonas spp. isolated during milking and storage of refrigerated raw milk. J. Dairy. Sci. 2016, 99, 5214–5223. [Google Scholar] [CrossRef]
- Li, S.; Tang, S.; He, Q.; Hu, J.; Zheng, J. Changes in Proteolysis in Fermented Milk Produced by Streptococcus thermophilus in Co-Culture with Lactobacillus plantarum or Bifidobacterium animalis subsp. lactis During Refrigerated Storage. Molecules 2019, 24, 3699. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).