Abstract
Tularemia is a severe infectious disease caused by the Gram-negative bacteria Francisella tularensis. F. tularensis is currently divided into three subspecies, holarctica, tularensis, and mediasiatica, which differ in their virulence and geographic distribution. Subspecies mediasiatica is the least studied because of its very low documented virulence for humans and limited geographic distribution. It was discovered in sparsely populated regions of Central Asia. Since 2011, a new subsp. mediasiatica lineage was identified in Altai (Russia). In 2021, we isolated one subsp. mediasiatica strain in Krasnoyarsk Territory. In spite of its geographic origin, 500 km east from Altai, this strain belongs to the Altai lineage and contributes surprisingly little genetic diversity to previous knowledge.
1. Introduction
Francisella tularensis is a small Gram-negative aerobic coccobacillus. It is a facultative intracellular parasite capable of infecting a wide range of animals and causing a plague-like disease called tularemia. The pathogenic for human F. tularensis is divided into two subspecies. Whereas F. tularensis subsp. holarctica is present across the northern hemisphere, F. tularensis subsp. tularensis is restricted to North America [1]. F. tularensis subspecies mediasiatica is the third of the three currently known subspecies within F. tularensis [2] (the appropriate nomenclature for F. novicida remains controversial and non-standardized, and F. novicida is often considered not as a separate species, but as a subspecies of F. tularensis [3]). The virulence for humans of subspecies mediasiatica is unknown and it remains the least studied and understood subspecies. Mediasiatica was discovered in Central Asia, in some sparsely populated regions of Kazakhstan, Uzbekistan, and Turkmenistan. Since 2011, a number of strains of this subspecies were isolated in the Altai region (Russia) [4]. Multiple Locus VNTR (Variable Number of Tandem Repeats) Analysis (MLVA) [5] allowed researchers to distinguish the Altaic population of F. tularensis subsp. mediasiatica from the classical Central Asian population. According to MLVA data, we proposed to divide the subspecies into three subgroups: M.I—classical Central Asian strains, M.II.—Altaic strains, and M.III—represented by a single strain isolated in the Republic of Karakalpakstan (Uzbekistan) [4].
In 2021, we discovered subsp. mediasiatica strain K-334 in the Krasnoyarsk Territory, about 500 km in a straight line (and over rather rugged terrain) from the Altai focus (Figure S1) and more than 1500 km from the Central Asian focus. We report the phylogenetic analysis based on the results of whole genome sequencing of K-334 and of a panel of Altai and Central Asian strains, including the unique M.III representative.
2. Results
2.1. K-334 Strain Isolation and Subspecies Determination
In 2021, in the federal healthcare institution, the Center for Hygiene and Epidemiology in the Krasnoyarsk Territory, one F. tularensis strain was isolated from a homogenized mix of Haemaphysalis concinna and Ixodes persulcatus ticks (Table 1). To confirm the species and determine its subspecies, the strain was subsequently transferred to the Federal Budget Institution of Science’s State Research Center for Applied Microbiology and Biotechnology (SRCAMB), where it was given the name K-334 (K—Krasnoyarsk). After species confirmation, the subspecies was determined using two independent PCR assays, the first one allowing researchers to distinguish the three subspecies, as well as F. novicida (Figure 1), and the second one targeting a SNP position, allowing researchers to distinguish subspecies mediasiatica from the two others (Figure 2).

Table 1.
List of subsp. mediasiatica strains used in this work.
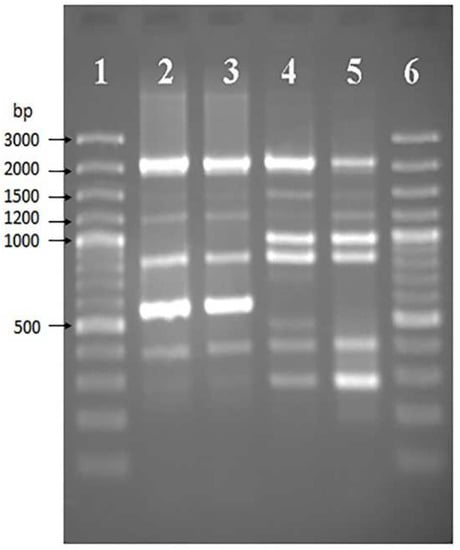
Figure 1.
Electropherogram of amplicons obtained in single-primer PCR with F. tularensis DNA: (1,6)—GeneRulerTM 100 bp Plus DNA Ladder; (2,3)—strains 15 NIIEG and 1045 (subsp. holarctica); (4)—strain K-334; (5)—strain A-678 (subsp. mediasiatica).
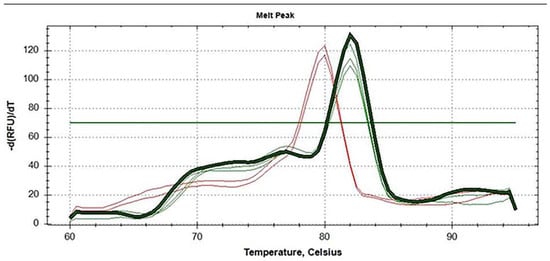
Figure 2.
Melt curve analysis of amplicons obtained in allele-specific PCR with a DNA template of: subsp. holarctica strains 1045 and 15 NIIEG (red lines) and subsp. mediasiatica strains A-678 (M.II lineage), 120, 117 (M.I lineage) (green lines), and strain K-334 (dark green bold line).
2.2. Whole-Genome Sequencing (WGS) and Phylogenetic Analysis
To confirm the subspecies assignment, we conducted WGS of the K-334 strain and compared its genome sequence with the genome sequences of 16 Altai (M.II) and three Central Asian strains (M.I and M.III) from our collection (Table 1). Twelve among the 16 Altai strains were previously analyzed by a multiple-locus variable number of tandem repeats (VNTR) analysis (MLVA) [4], while four were new (A-87, A-178, A-196, A-77). WGS data from three strains were previously published [4,6]. We also included five public WGS datasets derived from Central Asian strains (listed in Figure 3). Whole-genome SNP analysis shown in Figure 3 confirmed the presence of the three lineages previously suggested by MLVA [4]. The single M.III strain contributes the longest branch. The M.II group is split in two. Five Altaic strains originating from Mayminsky, Eltsovka, or Pervomayskoye are separated by ten up to 25 SNPs from the others. Krasnoyark region strain K-334 belongs to a very tight cluster comprising a progenitor genotype represented by three strains isolated in 2014–2015, and nine radiating branches with length of between one to nine SNPs. These nine branches are represented by eight Altai strains isolated in 2011–2014, in addition to K-334. Six of the branches, including the K-334 branch, have a length of one or two SNPs. This suggests that essentially identical subsp. mediasiatica strains are circulating over distances of a few hundred kilometers, which would constitute a single ecotype for F. tularensis subsp. mediasiatica (Figure S1). All manipulations with Altai and Central Asian strains were carried out in the period from 2017 to 2020. We began work with strain K-334 in the second half of 2021. In this period, no work with Francisella sp. was carried out in the BSL-3 block in which we worked with strain K-334, and disinfection measures were regularly carried out. K-334 was the only Francisella sp. strain sequenced in 2021 in SRCAMB. In addition, although very close to the other Altai strains, K-334 is unique, as it differs from all other Altai strains in our collection by a minimum of two SNPs (Figure 3). Therefore, intra-laboratory contamination would be a very unlikely explanation for this remarkable genetic similarity with the Altai strains.
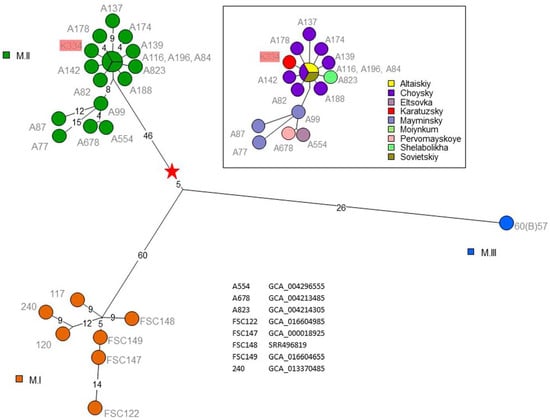
Figure 3.
Francisella tularensis subsp. mediasiatica maximum parsimony tree based on core genome SNPs. Three hundred and sixty-nine SNPs were called by mapping on genome accession GCA_000018925 (mediasiatica M.I strain FSC147). The size of the resulting tree is 369 (no homoplasia). Branch lengths of more than two SNPs are indicated. Nodes are labelled with strain IDs and colored according to clade M.I, M.II, or M.III, as indicated. Node coloring in the inset reflects geographic origin of the M.II strains. The red star indicates the root of the M.I, M.II, and M.III mediasiatica lineages (branching point towards the rest of F. tularensis). Correspondence between strain ID and sequence data accession numbers is indicated for previously published data.
3. Discussion
In this report, we first confirmed by sequencing 17 F. tularensis subsp. mediasiatica strains that the subspecies displays remarkably low genetic diversity. Currently available subsp. mediasiatica strains from the M.II lineage have been collected throughout a time range of ten years (2011–2021) over distances of up to approximately 500 km. The maximum genetic distance observed between two strains is 34 SNPs. Remarkably, the strain recovered from the Krasnoyarsk region is only two SNPs away from strains recovered from the Altai area, indicating that the geographic distribution of the M.II lineage is not restricted to Altai. Determining the geographic distribution of such bacteria is difficult because of the extremely low population density in the region, the resulting underdeveloped infrastructure outside the main cities, the mountainous landscape, and the absence of human infections. The vast majority of M.II strains were isolated from ticks. A single strain was isolated from a dead rodent out of the many rodents collected over a decade, and none were isolated from water or aquatic invertebrates. In addition, most of the local laboratories are not licensed to handle pathogens. Therefore, when the presence of a pathogen is suspected, this fact is often simply recorded, after which the clinical or field sample is usually eliminated, given that there are no opportunities for its storage and detailed study. The Altaic mediasiatica strains were isolated owing to the direct collaboration between the Altai Anti-Plague Station and SRCAMB, where subspecies were identified by genotyping. Since then, targeted collection of strains and shipment to reference centers for detailed study led to the identification of several strains belonging to subsp. mediasiatica. This illustrates the importance for future investigations to implement first-line genotyping assays that might be applied in local laboratories. Such enhanced local capacities might allow researchers to more precisely delineate the presence of mediasiatica in Siberia. The single PCR assay used here is a good candidate for such a first-line assay as it also discriminates F. tularensis from its nearest neighbor, F. novicida.
The distance between the Altai focus and the place of isolation of the almost identical K-334 strain exceeds 500 km over very rough terrain. A previous investigation pointed to migratory birds as potential long-distance carriers for the spreading of F. tularensis [7]. Almost all strains of subsp. mediasiatica M.II were isolated from ixodid (hard) ticks, H. concinna and D. silvarum, in agreement with the known area of distribution of these two species of ticks [8,9]. Ixodid ticks, including H. concinna and D. silvarum, can parasitize birds, which could spread both the ticks themselves and the infectious agents, including F. tularensis [8,9,10,11]. H. concinna is the second most abundant tick species collected from birds [8]. Both H. concinna and D. silvarum ticks prefer boreal climate conditions with precipitation all year round or with dry winters [8,11]. Data from satellite monitoring of migration of birds tagged in southern Siberia showed that regions where subsp. mediasiatica strains have been found can be linked by bird migration routes (Figure S2).
The vast majority of subsp. mediasiatica M.II strains were isolated from ticks, in spite of our previous report showing that subsp. mediasiatica, including M.II, is virulent and capable to effectively overcome post-vaccinal immunity in laboratory rodents [12]. Thus, subsp. mediasiatica strains are highly virulent in laboratory experiments, but do not appear to cause significant morbidity in natural conditions. One explanation of this phenomenon might be that mediasiatica is not efficiently transferred from ticks to warm-blooded animals and instead circulates as an endosymbiont via both horizontal and vertical transmission among ticks [9,13,14].
In summary, our limited current knowledge of subsp. mediasiatica may suggest that it represents a transition towards the two pathogenic for human subspecies, F. tularensis holarctica and tularensis.
4. Materials and Methods
4.1. Strains
F. tularensis strains used in this study are listed in Table 1. All strains were isolated as part of routine anti-epidemic work carried out in the USSR (strains isolated in Central Asia) and Russia (strains from Altai and Siberia). Sixteen strains were isolated from ticks and from one dead rodent within the Republic of Altai and Altai Territory in 2011–2015 by the Federal Healthcare Service Center for Hygiene and Epidemiology in the Altai region and by the Altai anti-plague station. Strain K-334 was isolated in the Krasnoyarsk region in 2021 from ticks. Two collection strains from Kazakhstan and one from Karakalpakstan in Uzbekistan were also included.
4.2. Bacterial Cultures
Strains were grown at 37 °C on solid (FT-agar) and liquid (FT-broth) nutrient media (SRCAMB, Obolensk, Russia). The composition of the FT-agar was 3.8% erythritol-agar, 1% dried bovine blood, 1% glucose, 0.05% cysteine, and 0.0025% thiamine chloride at pH 7.2. The composition of the FT-broth was 2% casein enzymatic hydrolysate, 1% yeast extract, 1.2% KH2PO4, 1% glucose, 0.001% cysteine, and 0.001% FeCl2 at pH 7.2. FT-broth has been used in some cases to increase the yield of biomass when the strain planted after storage did not grow well on solid nutrient medium. For the same purpose, the growth time was increased to 2–3 days.
4.3. DNA Preparation and PCR Analysis of Species and Subspecies
DNA from bacterial cultures was isolated using GenElute Bacterial Genomic DNA Kit (Sigma-Aldrich, Moscow, Russia). PCR amplifications were run on the CFX96 ™ Real-Time PCR Detection System (Bio-Rad, Moscow, Russia).
Species determination was performed using «MULTI-FLU» Real-Time PCR-test kit (SRCAMB, Obolensk, Russia) and «OM-screen-tularemia-RT» (Syntol, Moscow, Russia). Subspecies determination was performed by single-primer genotyping with primer Chif1 (5′-CTAGGGCTGGTGGG-3′), as previously described [4], and by typing of single nucleotide polymorphism (SNP) position 630822 in F. tularensis strain SCHU S4 genome accession GCA_000008985.1. SNP typing was performed by melt curve analysis of the PCR products, as previously described [15], using 2.5× PCRmix with SYBR-GreenI (Syntol, Moscow, Russia) with minor modifications. We used primers Bla2SNPTulF1 (5′-AATAAATCAAGATGATATTGGTAAAGCCG-3′), Bla2SNPMedF1 (5′-CGGGGCGGGGCGGGGCGGGCAATAAATCAAGATGATATTGGTAAAGGCA-3′), Bla2SNPRev3 (5′-ATCTTTAGTAATAGCCTTTGGAGGTG-3′). Two allele-specific (by 3′ ultimate nucleotide) forward primers were designed to detect the two SNP alleles, one of them was labeled with GC-clamp on the 5′ end. These primers worked in concert with a third common reverse primer to generate a PCR amplicon. This reverse primer had a 3′ ultimate nucleotide specific for subsp. tularensis and subsp. mediasiatica. Depending on the template, only one of the forward primers generated the amplicon in concert with the common primer. The ability to differentiate amplicon derived from each allele-specific primer was accomplished by the melt–curve analysis (the amplicon with the GC-clamp has a higher melting temperature). To increase the specificity of this method, artificial mismatches were added in -3 and -4 position (from the 3′ end) to the sequence of both forward primers and in -3 position to the sequence of reverse primer, according to the principle described in [15].
We used 10 pmol of each of the three primers per 25 µL of reaction mix. Thermocycling parameters were the following: (i) 50 °C for 2 min; (ii) 95 °C for 10 min; (iii) 25 cycles: 95 °C for 20 s, 60 °C for 30 s, 72 °C for 30 s, Plate Read (SYBR); (iv) 95 °C for 15 s; (v) Melt Curve Read (SYBR) from 60 °C to 95 °C, increment 0.5 °C, time interval 5 s. In this assay, subsp. mediasiatica strains had a melting curve peak two degrees higher than that of strains of other subspecies. PCR primers were synthesized by Syntol.
4.4. Sequencing
DNA libraries were prepared using the Nextera DNA Library Preparation Kit (Albiogen, Moscow, Russia). Whole-genome sequencing was performed using the MiSeq Illumina instrument and the corresponding reagent kit MiSeq Reagent Kit v3 (Albiogen, Moscow, Russia). WGS data from 17 newly published genomes are available at https://www.ncbi.nlm.nih.gov/sra/PRJNA870100 (accessed on 10 October 2022). Data for three previously described M.II genomes are available at the link indicated in [6].
4.5. WGS Analysis
Core whole-genome SNP analyses were performed essentially as previously described [7]. Assemblies and sequence reads archives (SRA) were downloaded from EBI ENA. Large-size SRAs (.gz files larger than 100 Mo) were assembled with SKESA 2.3.0 [16] and subsequently processed as assemblies. Smaller-size SRAs were directly imported in BioNumerics version 7.6.3 (Applied-Maths, Sint-Martens-Latem, Belgium). All assemblies were split in 50 bp-long artificial reads with 10× coverage. SNPs were called by mapping reads on genome GCA_000018925 (mediasiatica M.I strain FSC147) using BioNumerics default parameters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bacteria1040018/s1, Figure S1: Geographic origins of F. tularensis mediasiatica M.II strains; Figure S2: Bird migration tracks linking the regions in which subsp. mediasiatica was found.
Author Contributions
Conceptualization, G.V. (Gilles Vergnaud) and V.T.; methodology, G.V. (Gilles Vergnaud) and A.M.; validation, G.V. (Gilles Vergnaud); formal analysis, G.V. (Gilles Vergnaud) and V.T.; investigation, I.B., G.V. (Galina Vakhrameeva), E.G., R.Z., Y.A. and G.B.; resources, A.M., E.R. and E.G.; data curation, V.T. and G.V. (Gilles Vergnaud ); writing—original draft preparation, V.T. and G.V. (Gilles Vergnaud); writing—review and editing, G.V. (Gilles Vergnaud) and V.T.; visualization, G.V. (Gilles Vergnaud) and Y.A.; supervision, I.D.; project administration, A.M. and I.D.; funding acquisition, I.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Sectoral Scientific Program of the Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used for this study are available in the text of the article and in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Telford, S.R., 3rd; Goethert, H.K. Ecology of Francisella tularensis. Annu. Rev. Entomol. 2020, 65, 351–372. [Google Scholar] [CrossRef]
- Svensson, K.; Larsson, P.; Johansson, D.; Byström, M.; Forsman, M.; Johansson, A. Evolution of subspecies of Francisella tularensis. J. Bacteriol. 2005, 187, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Kingry, L.C.; Petersen, J.M. Comparative review of Francisella tularensis and Francisella novicida. Front. Cell. Infect. Microbiol. 2014, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, V.; Bakhteeva, I.; Titareva, G.; Kopylov, P.; Christiany, D.; Mokrievich, A.; Dyatlov, I.; Vergnaud, G. Russian isolates enlarge the known geographic diversity of Francisella tularensis subsp. mediasiatica. PLoS ONE 2017, 12, e0183714. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Farlow, J.; Larsson, P.; Dukerich, M.; Chambers, E.; Bystrom, M.; Fox, J.; Forsman, M.; Sjostedt, A.; Keim, P. Worldwide genetic relationships among Francisella tularensis isolates determined by multiple-locus variable-number tandem repeat analysis. J. Bacteriol. 2004, 186, 5808–5818. [Google Scholar] [CrossRef] [PubMed]
- Mokrievich, A.N.; Kislichkina, A.A.; Kudryavtseva, T.Y.; Mironova, R.I.; Vakhrameeva, G.M.; Shishkova, N.A.; Timofeev, V.S.; Bogun, A.G.; Pavlov, V.M.; Dyatlov, I.A. Draft Genome Sequences of Three Francisella tularensis subsp. mediasiatica Strains Isolated in the Altai Territory, Russian Federation. Microbiol. Resour. Announc. 2020, 9, e01202-19. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, V.; Kairzhanova, A.; Shevtsov, A.; Shustov, A.; Kalendar, R.; Abdrakhmanov, S.; Lukhnova, L.; Izbanova, U.; Ramankulov, Y.; Vergnaud, G. Genetic diversity of Francisella tularensis subsp. holarctica in Kazakhstan. PLoS Negl. Trop. Dis. 2021, 15, e0009419. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Walter, M.; Vogelgesang, J.R.; Didyk, Y.M.; Fu, S.; Kahl, O. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick Borne Dis. 2018, 9, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 2013, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-B.; Shi, W.-Q.; Wang, Q.; Pan, Y.-S.; Chang, Q.-C.; Jiang, B.-G.; Cheng, J.-X.; Cui, X.-M.; Zhou, Y.-H.; Wei, J.-T.; et al. Distribution of Dermacentor silvarum and Associated Pathogens: Meta-Analysis of Global Published Data and a Field Survey in China. Int. J. Environ. Res. Public Health 2021, 18, 4430. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Brugger, K.; Belova, O.A.; Kholodilov, I.S.; Didyk, Y.M.; Kurzrock, L.; Garcia-Perez, A.L.; Kahl, O. Vectors of disease at the northern distribution limit of the genus Dermacentor in Eurasia: D. reticulatus and D. silvarum. Exp. Appl. Acarol. 2020, 82, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, V.; Titareva, G.; Bahtejeva, I.; Kombarova, T.; Kravchenko, T.; Mokrievich, A.; Dyatlov, I. The Comparative Virulence of Francisella tularensis Subsp. mediasiatica for Vaccinated Laboratory Animals. Microorganisms 2020, 8, 1403. [Google Scholar]
- Buczek, A.; Bartosik, K.; Buczek, W.; Buczek, A.M.; Kulina, D.; Kulisz, J.; Tomasiewicz, K. A unique phenomenon of oral-anal contact between ticks observed in two tick species Ixodes ricinus and Dermacentor reticulatus. Ann. Agric. Environ. Med. 2018, 25, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Buczek, A.; Bartosik, K.; Buczek, A.M.; Buczek, W.; Stanko, M. Conspecific hyperparasitism in the Hyalomma excavatum tick and considerations on the biological and epidemiological implications of this phenomenon. Ann. Agric. Environ. Med. 2019, 26, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Bakhteeva, I.V.; Kravchenko, T.B.; Ryabko, A.K.; Titareva, G.M.; Lev, I.O.; Mokrievich, A.N.; Timofeev, V.S. Features of beta-lactamase activity in Francisella tularensis subsp. mediasiatica. Russ. J. Infect. Immun. 2018, 8, 33–42. [Google Scholar] [CrossRef]
- Souvorov, A.; Agarwala, R.; Lipman, D.J. SKESA: Strategic k-mer extension for scrupulous assemblies. Genome Biol. 2018, 19, 153. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).