Probiotic Regulation to Modulate Aging Gut and Brain Health: A Concise Review
Abstract
1. Introduction
2. Mechanism of Actions of Probiotics
3. Gut Health Modulation by Probiotics
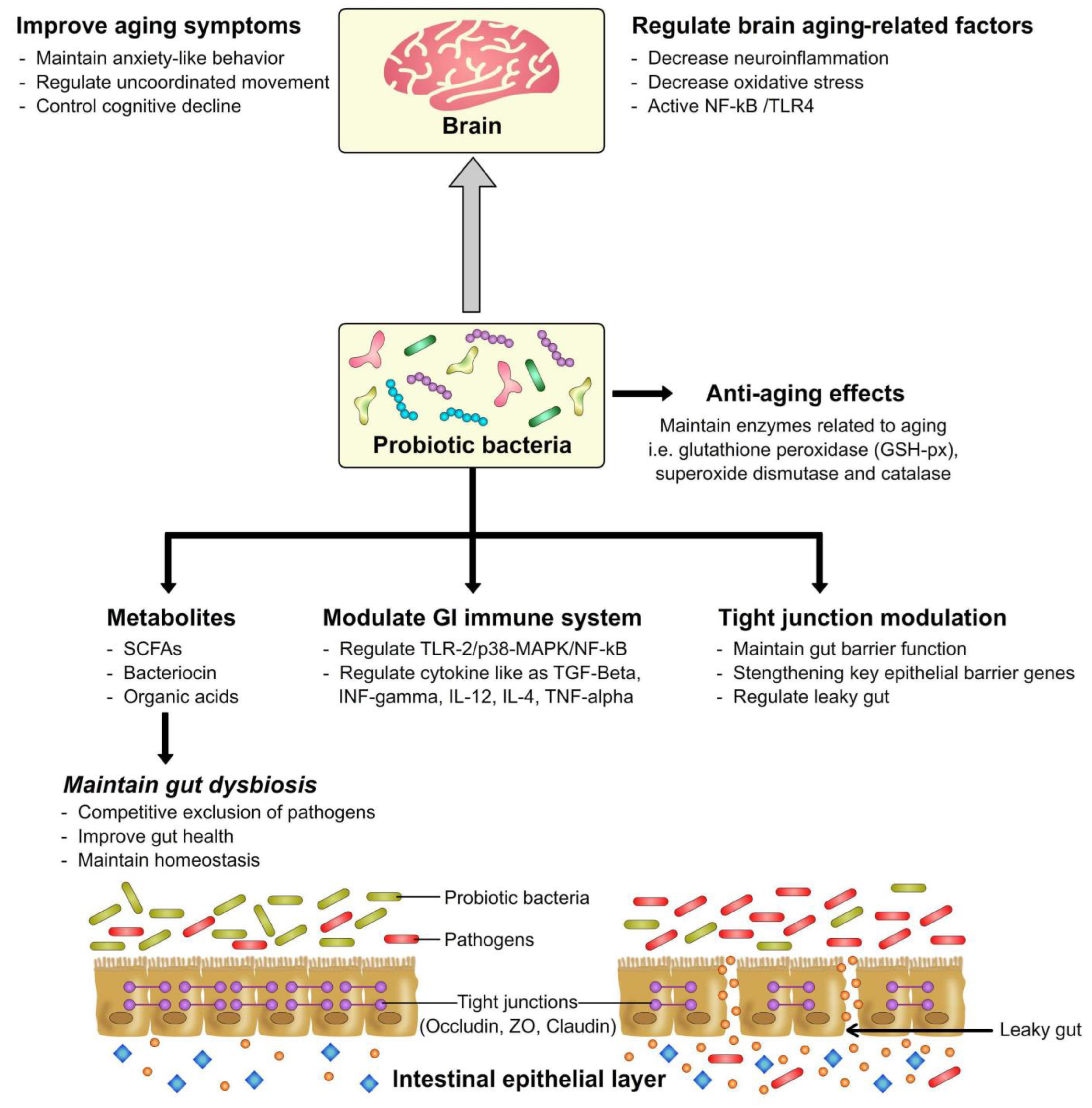
4. Probiotics as Anti-Aging Agents in Relation to the Gut-Brain Axis
5. Prospects and Developments
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Hedia, R.H.; Omara, S.T.; Mahmoud, M.A.; Kandil, M.M.; Bakry, M.A. Interspecies comparison of probiotics isolated from different animals. Vet. World 2018, 11, 227. [Google Scholar] [CrossRef]
- Jeong, J.J.; Kim, K.A.; Hwang, Y.J.; Han, M.J.; Kim, D.H. Anti-inflammaging effects of Lactobacillus brevis OW38 in aged mice. Benef. Microbes 2016, 7, 707–718. [Google Scholar] [CrossRef]
- Kawase, M.; He, F.; Miyazawa, K.; Kubota, A.; Yoda, K.; Hiramatsu, M. Orally administered heat-killed Lactobacillus gasseri TMC0356 can upregulate cell-mediated immunity in senescence-accelerated mice. FEMS Microbiol. Lett. 2012, 326, 125–130. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Probiotic bacteria as modulators of cellular senescence: Emerging concepts and opportunities. Gut Microbes 2020, 11, 335–349. [Google Scholar] [CrossRef]
- Arai, Y.; Martin-Ruiz, C.M.; Takayama, M.; Abe, Y.; Takebayashi, T.; Koyasu, S.; Suematsu, M.; Hirose, N.; von Zglinicki, T. Inflammation, but not telomere length, predicts successful ageing at extreme old age: A longitudinal study of semi-supercentenarians. EBioMedicine 2015, 2, 1549–1558. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Liu, J.; Geletka, L.; Delaney, C.; Delproposto, J.; Desai, A.; Oatmen, K.; Martinez-Santibanez, G.; Julius, A.; Garg, S.; et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J. Immunol. 2011, 187, 6208–6216. [Google Scholar] [CrossRef]
- Buford, T.W. (Dis) Trust your gut: The gut microbiome in age-related inflammation, health, and disease. Microbiome 2017, 5, 80. [Google Scholar] [CrossRef]
- Xia, C.; Cao, X.; Cui, L.; Liu, H.; Wang, S.; Chen, T. Anti-aging effect of the combination of Bifidobacterium longum and B. animalis in a d-galactose-treated mice. J. Funct. Foods 2020, 69, 103938. [Google Scholar] [CrossRef]
- Landete, J.M.; Gaya, P.; Rodríguez, E.; Langa, S.; Peirotén, Á.; Medina, M.; Arqués, J.L. Probiotic bacteria for healthier aging: Immunomodulation and metabolism of phytoestrogens. BioMed Res. Int. 2017, 2017, 5939818. [Google Scholar] [CrossRef] [PubMed]
- WHO. Aging and Health. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 12 September 2020).
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 178. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Matar, C.; Ismail, N. Adolescence and aging: Impact of adolescence inflammatory stress and microbiota alterations on brain development, aging, and neurodegeneration. J. Gerontol. Ser. A 2020, 75, 1251–1257. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10 (Suppl. 1), S49–S66. [Google Scholar] [CrossRef]
- Szajewska, H. What are the indications for using probiotics in children? Arch. Dis. Child 2016, 101, 398–403. [Google Scholar] [CrossRef][Green Version]
- Stewart, L.; Crumley, B.; Walton, K. Effects of probiotic VSL# 3 on cytokine and tight junction protein expression in intestinal epithelial cells. FASEB J. 2015, 29, 1010–1014. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics importance and their immunomodulatory properties. J. Cell Physiol. 2019, 234, 8008–8018. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Bhat, M.I.; Gupta, T.; Kapila, S.; Kapila, R. Safety assessment of potential probiotic Lactobacillus fermentum MTCC-5898 in murine model after repetitive dose for 28 days (Sub-Acute Exposure). Probiot. Antimicrob. Proteins 2020, 12, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Immune-mediated mechanisms of action of probiotics and synbiotics in treating pediatric intestinal diseases. Nutrients 2018, 10, 42. [Google Scholar] [CrossRef]
- Wan, L.Y.M.; Chen, Z.J.; Shah, N.P.; El-Nezami, H. Modulation of intestinal epithelial defense responses by probiotic bacteria. Crit. Rev. Food. Sci. Nutr. 2016, 56, 2628–2641. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.K.; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: A review. Front. Microbiol. 2017, 8, 563. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Szabóová, R. Optimal Criteria for the Selection of Probiotics, Based on their Mode of Action. Folia Vet. 2019, 63, 60–69. [Google Scholar] [CrossRef]
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S.P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D.A.; Kritchevsky, S.B.; et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. Geroscience 2020, 42, 333–352. [Google Scholar] [CrossRef]
- Wu, Q.; Cheung, C.K.; Shah, N.P. Towards galactose accumulation in dairy foods fermented by conventional starter cultures: Challenges and strategies. Trends Food Sci. Technol. 2015, 41, 24–36. [Google Scholar] [CrossRef]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef]
- Nothaft, H.; Perez-Muñoz, M.E.; Gouveia, G.J.; Duar, R.M.; Wanford, J.J.; Lango-Scholey, L.; Panagos, C.G.; Srithayakumar, V.; Plastow, G.S.; Coros, C.; et al. Coadministration of the Campylobacter jejuni N-glycan-based vaccine with probiotics improves vaccine performance in broiler chickens. Appl. Environ. Microbiol. 2017, 83, e01523-17. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Shah, N.P. High γ-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit. Rev. Food Sci. Nutr. 2017, 57, 3661–3672. [Google Scholar] [CrossRef]
- Jacobson, A.; Lam, L.; Rajendram, M.; Tamburini, F.; Honeycutt, J.; Pham, T.; Van Treuren, W.; Pruss, K.; Stabler, S.R.; Lugo, K.; et al. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe 2018, 24, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.J.; Frutos, M.J. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Huang, R.; Tao, X.; Wan, C.; Li, S.; Xu, H.; Xu, F.; Shah, N.P.; Wei, H. In vitro probiotic characteristics of Lactobacillus plantarum ZDY 2013 and its modulatory effect on gut microbiota of mice. J. Dairy Sci. 2015, 98, 5850–5861. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.H.; Yang, J.C.; Yang, G.Y.; Zhou, D.; Wang, J.F. A selected Lactobacillus rhamnosus strain promotes EGFR-independent Akt activation in an enterotoxigenic Escherichia coli K88-infected IPEC-J2 cell model. PLoS ONE 2015, 10, e0125717. [Google Scholar] [CrossRef]
- Mujagic, Z.; De Vos, P.; Boekschoten, M.V.; Govers, C.; Pieters, H.J.H.; De Wit, N.J.; Bron, P.A.; Masclee, A.A.; Troost, F.J. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci. Rep. 2017, 7, 40128. [Google Scholar] [CrossRef]
- Cӑtoi, A.F.; Corina, A.; Katsiki, N.; Vodnar, D.C.; Andreicuț, A.D.; Stoian, A.P.; Rizzo, M.; Pérez-Martínez, P. Gut microbiota and aging-A focus on centenarians. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165765. [Google Scholar] [CrossRef]
- Metchnikoff, E. Optimistic Studies; Putman’s Sons: New York, NY, USA, 1908; pp. 161–183. [Google Scholar]
- Ayala, F.R.; Bauman, C.; Cogliati, S.; Leñini, C.; Bartolini, M.; Grau, R. Microbial flora, probiotics, Bacillus subtilis and the search for a long and healthy human longevity. Microb. Cell 2017, 4, 133. [Google Scholar] [CrossRef]
- Patel, P.J.; Singh, S.K.; Panaich, S.; Cardozo, L. The aging gut and the role of prebiotics, probiotics, and synbiotics: A review. J. Clin. Gerontol. Geriatr. 2014, 5, 3–6. [Google Scholar] [CrossRef]
- Lew, L.; Hor, Y.; Jaafar, M.; Lau, A.; Ong, J.; Chuah, L.; Yap, K.; Azzam, G.; Azlan, A.; Liong, M. Lactobacilli modulated AMPK activity and prevented telomere shortening in ageing rats. Benef. Microbes 2019, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.; Kim, S.A.; Nam, W.; Jeung, W.H.; Park, S.-D.; Lee, J.-L.; Sim, J.-H.; Jang, S.S. Lactobacillus plantarum HY7714 restores TNF-α induced defects on tight junctions. Prev. Nutr. Food Sci. 2019, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Joishy, T.; Das, S.; Kalita, M.C.; Mukherjee, A.K.; Khan, M.R. A potential probiotic Lactobacillus plantarum JBC5 improves longevity and healthy aging by modulating antioxidative, innate immunity and serotonin-signaling pathways in Caenorhabditis elegans. Antioxidants 2022, 11, 268. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Fern, L.A.; Hj, D.S.N.R.P.; Chaiyasut, C. The influence of probiotics on bile acids in diseases and aging. Biomed. Pharmacother. 2020, 128, 110310. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Fong, F.L.Y.; Shah, N.P.; Kirjavainen, P.; El-Nezami, H. Mechanism of action of probiotic bacteria on intestinal and systemic immunities and antigen-presenting cells. Int. Rev. Immunol. 2016, 35, 179–188. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Y.; Cai, R.; Chen, Y.; Gu, B. The impact of dietary fiber and probiotics in infectious diseases. Microb. Pathog. 2020, 140, 103931. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.W.; Cheng, M.Y.; Yang, X.; Lu, Y.Y.; Yin, H.D.; Zeng, Y.; Wang, R.Y.; Jiang, Y.L.; Yang, W.T.; Wang, J.Z.; et al. Probiotic Lactobacillus rhamnosus GG Promotes Mouse Gut Microbiota Diversity and T Cell Differentiation. Front. Microbiol. 2020, 11, 3216. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Nawaz, M.; Anjum, A.A.; Ahmad, M.U.D.; Mehmood, A.; Rabbani, M.; Mustafa, A.; Ali, M.A. Effect of Indigenous Probiotics on Gut Morphology and Intestinal Absorption Capacity in Broiler Chicken Challenged with Salmonella enteritidis. Pak. J. Zool. 2020, 52, 1825. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017, 23, 2696. [Google Scholar] [CrossRef] [PubMed]
- Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Lau, A.S.Y.; Ong, J.S.; Kato, T.; Nakanishi, Y.; Azzam, G.; Azlan, A.; Ohno, H.; et al. Lactobacillus sp. improved microbiota and metabolite profiles of aging rats. Pharmacol. Res. 2019, 146, 104312. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Le Nevé, B.; Quinquis, L.; Pichon, C.; Whorwell, P.J.; Guyonnet, D. Consumption of a fermented milk product containing Bifidobacterium lactis CNCM I-2494 in women complaining of minor digestive symptoms: Rapid response which is independent of dietary fibre intake or physical activity. Nutrients 2019, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef]
- Arboleya, S.; Ruas-Madiedo, P.; Margolles, A.; Solís, G.; Salminen, S.; Clara, G.; Gueimonde, M. Characterization and in vitro properties of potentially probiotic Bifidobacterium strains isolated from breast-milk. Int. J. Food Microbiol. 2011, 149, 28–36. [Google Scholar] [CrossRef]
- Miles, M.P. Probiotics and Gut Health in Athletes. Curr. Nutr. Rep. 2020, 9, 129–136. [Google Scholar] [CrossRef]
- Ku, S.; Park, M.S.; Ji, G.E.; You, H.J. Review on Bifidobacterium bifidum BGN4: Functionality and nutraceutical applications as a probiotic microorganism. Int. J. Mol. Sci. 2016, 17, 1544. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.W.; El-Nezami, H.; Shah, N.P. The protective effects of enriched citrulline fermented milk with Lactobacillus helveticus on the intestinal epithelium integrity against Escherichia coli infection. Sci. Rep. 2020, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lu, J.; Oliphant, K.; Gupta, N.; Claud, K.; Lu, L. Maternal administration of probiotics promotes gut development in mouse offsprings. PLoS ONE 2020, 15, e0237182. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Perspectives of the potential implications of polyphenols in influencing the interrelationship between oxi-inflammatory stress, cellular senescence and immunosenescence during aging. Trends Food Sci. Technol. 2020, 98, 41–52. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: The Conductor in the Orchestra of Immune-Neuroendocrine Communication. Clin. Ther. 2015, 37, 954–967. [Google Scholar] [CrossRef]
- Sun, Y.; Baptista, L.C.; Roberts, L.M.; Jumbo-Lucioni, P.; McMahon, L.L.; Buford, T.W.; Carter, C.S. The gut microbiome as a therapeutic target for cognitive impairment. J. Gerontol. A 2020, 75, 1242–1250. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J., Eds.; Springer: New York, NY, USA, 2014; pp. 115–133. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Lo Monaco, M.R.; Landi, F.; Bernabei, R.; Marzetti, E. Of microbes and minds: A narrative review on the second brain aging. Front. Med. 2018, 5, 53. [Google Scholar] [CrossRef]
- Sharma, R.; Kapila, R.; Kapasiya, M.; Saliganti, V.; Dass, G.; Kapila, S. Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr. Res. 2014, 34, 968–981. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Leggio, F.; Vacante, M.; Motta, M.; Giordano, M.; Biondi, A.; Basile, F.; Mastrojeni, S.; Mistretta, A.; Malaguarnera, M.; et al. Probiotics in the gastrointestinal diseases of the elderly. J. Nutr. Health Aging 2012, 16, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Kalam, A.; Sarker, M.; Li, T.; Yin, J. Probiotic species in the modulation of gut microbiota: An overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Simon, F.; Trollet, C.; Santibañez, J.F. Oxidative stress in disease and aging: Mechanisms and therapies 2016. Oxid. Med. Cell Longev. 2017, 2017, 4310469. [Google Scholar] [CrossRef] [PubMed]
- Kure, C.; Timmer, J.; Stough, C. The immunomodulatory effects of plant extracts and plant secondary metabolites on chronic neuroinflammation and cognitive aging: A mechanistic and empirical review. Front Pharmacol. 2017, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, H.; Dong, S.; Sha, H.; Wei, W.; Liu, C. High mobility group box-1 mediates hippocampal inflammation and contributes to cognitive deficits in high-fat high-fructose diet-induced obese rats. Brain Behav. Immun. 2019, 82, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Zhang, F.; Wang, H.; Yao, J.; Chen, R.; Zhou, Z.; Yang, K.; Xie, Y.; Wan, T.; Ding, H. Apigenin exhibits protective effects in a mouse model of d-galactose-induced aging via activating the Nrf2 pathway. Food Funct. 2017, 8, 2331–2340. [Google Scholar] [CrossRef]
- Yang, W.; Shi, L.; Chen, L.; Zhang, B.; Ma, K.; Liu, Y.; Qian, Y. Protective effects of perindopril on d-galactose and aluminum trichloride induced neurotoxicity via the apoptosis of mitochondria-mediated intrinsic pathway in the hippocampus of mice. Brain Res. Bull. 2014, 109, 46–53. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the microbiota-gut-brain axis: Focus on psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef]
- Sharma, R.; Kapila, R.; Dass, G.; Kapila, S. Improvement in Th1/Th2 immune homeostasis, antioxidative status and resistance to pathogenic E. coli on consumption of probiotic Lactobacillus rhamnosus fermented milk in aging mice. Age 2014, 36, 9686. [Google Scholar] [CrossRef]
- Schifano, E.; Zinno, P.; Guantario, B.; Roselli, M.; Marcoccia, S.; Devirgiliis, C.; Uccelletti, D. The foodborne strain Lactobacillus fermentum MBC2 triggers pept-1-dependent pro-longevity effects in Caenorhabditis elegans. Microorganisms 2019, 7, 45. [Google Scholar] [CrossRef]
- Yu, X.; Li, S.; Yang, D.; Qiu, L.; Wu, Y.; Wang, D.; Shah, N.P.; Xu, F.; Wei, H. A novel strain of Lactobacillus mucosae isolated from a Gaotian villager improves in vitro and in vivo antioxidant as well as biological properties in D-galactose-induced aging mice. J. Dairy Sci. 2016, 99, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xia, Y.; Wang, G.; Xiong, Z.; Zhang, H.; Lai, F.; Ai, L. Lactobacillus plantarum AR501 Alleviates the Oxidative Stress of D-Galactose-Induced Aging Mice Liver by Upregulation of Nrf2-Mediated Antioxidant Enzyme Expression. J. Food Sci. 2018, 83, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Chen, L.H.; Wang, M.F.; Hsu, C.C.; Chan, C.H.; Li, J.X.; Huang, H.Y. Lactobacillus paracasei PS23 delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (SAMP8) mice. Nutrients 2018, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Hor, Y.Y.; Ooi, C.H.; Lew, L.C.; Jaafar, M.H.; Lau, A.Y.; Lee, B.K.; Azlan, A.; Choi, S.B.; Azzam, G.; Liong, M.T. The molecular mechanisms of probiotic strains in improving ageing bone and muscle of d-galactose-induced ageing rats. J. Appl. Microbiol. 2021, 130, 1307–1322. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. Ser. A 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front Aging Neurosci. 2016, 10, 256. [Google Scholar] [CrossRef]
- Flanagan, E.; Lamport, D.; Brennan, L.; Burnet, P.; Calabrese, V.; Cunnane, S.C.; De Wilde, M.C.; Dye, L.; Farrimond, J.A.; Lombardo, N.E.; et al. Nutrition and the ageing brain: Moving towards clinical applications. Ageing Res. Rev. 2020, 62, 101079. [Google Scholar] [CrossRef]
- Khalesi, S.; Vandelanotte, C.; Thwaite, T.; Russell, A.M.; Dawson, D.; Williams, S.L. Awareness and attitudes of gut health, probiotics and prebiotics in Australian adults. J. Diet. Suppl. 2021, 18, 418–432. [Google Scholar] [CrossRef]
- Guarino, A.; Guandalini, S.; Vecchio, A.L. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2015, 49, S37–S45. [Google Scholar] [CrossRef]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Mohanty, D.; Mohapatra, S. Applications of probiotics as a functional ingredient in food and gut health. J. Food Nutr. Res. 2019, 7, 213–223. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Pitchumoni, C.; Mishra, S.P.; Yadav, H. Gut Microbiota and Aging: A Broad Perspective. In Geriatric Gastroenterology; Spinger: Berlin/Heidelberg, Germany, 2020; pp. 1–21. [Google Scholar] [CrossRef]
- Kaku, N.; Matsumoto, N.; Sasaki, D.; Tsuda, K.; Kosai, K.; Uno, N.; Morinaga, Y.; Tagami, A.; Adachi, S.; Hasegawa, H.; et al. Effect of probiotics on gut microbiome in patients with administration of surgical antibiotic prophylaxis: A randomized controlled study. J. Infect. Chemother. 2020, 26, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Van Schoor, J. Probiotics and gut health. SA Pharm. Assist. 2020, 20, 32–33. [Google Scholar] [CrossRef]
- Bubnov, R.V.; Spivak, M.Y.; Lazarenko, L.M.; Bomba, A.; Boyko, N.V. Probiotics and immunity: Provisional role for personalized diets and disease prevention. EPMA J. 2015, 6, 14. [Google Scholar] [CrossRef]

| Probiotic(s) | Animal Model | Effects on Host | Results | References |
|---|---|---|---|---|
| Lactobacillus fermentum | Weanling Mice | Gut health modulation | Increased mRNA expression of claudin-1 and MUC-2 in intestinal epithelial cells. | [23] |
| Lactobacillus paracasei | Old mice | Gut health modulation | Improved mucin production, decreased leaky gut and inflammation. | [29] |
| Bifidobacterium longum and Lactobacillus rhamnosus | Healthy volunteers | Gut health modulation | Decreased Firmicutes abundance, the overall reduction of potentially harmful bacteria, and an increase in beneficial bacteria. | [56] |
| 5 Lactobacillus and 5 Enterococcus strains (Coaktail-Human originated) | Older mice | Gut health modulation | Increased the beneficial commensals in older HFD mice and decreased the expression of IL-6, TNF-α, and IL-1β, while increasing the anti-inflammatory markers, such as IL-10 and TGF-β expression in the colon tissues. Improved the mRNA expression of tight junction proteins such as Zo1 and Ocln in the intestinal tissues. | [59] |
| Lactobacillus fermentum, Lactobacillus fermentum, and Lactobacillus salivarius | Broiler chicken | Gut health modulation | Improved the villus height and villus-height-to-crypt-depth ratio improved the gut morphometric parameters and absorption capacity. | [55] |
| Lactobacilli strains | D-Galactose senescence-induced aging rats | Gut health modulation | Reduced Bacteroides, increased the ratio of Firmicutes/Bacteroidetes. | [57] |
| Lactobacillus acidophilus and Bifidobacterium infantis | Mouse pre-weaned pups | Gut health modulation | Supported intestinal epithelial-cell differentiation, reduced loss of mucin, protected the intestinal integrity and barrier function, and reduced serum levels of IL-1β, TNF-α, and IL-6. | [65] |
| Probiotic(s) | Animal Model | Effects on Host | Results | References |
|---|---|---|---|---|
| Lactobacillus brevis | Aged mice | Anti-aging | Suppressed the expression of senescence markers p16, p53, and SAMHD1 and restored expression of brain-derived neurotrophic factor and doublecortin in aged mice. | [5] |
| Bifidobacterium longum and Bifidobacterium animalis | D-Galactose-treated mice | Anti-aging | Improved the anxiety-like behavior, uncoordinated movement, cognitive decline, and hippocampus senescence; ameliorate age-related cognitive degeneration by inhibiting NF-κB/TLR4-induced-neuroinflammation and oxidative stress. | [11] |
| Lactobacillus paracasei | Old mice | Anti-aging | Improved physical and cognitive functions, modulating the TLR-2/p38-MAPK/NF-kB pathway, which may reduce age-related leaky gut and inflammation. | [29] |
| Lactobacillus fermentum | C. elegans | Anti-aging | Improved pumping rate, lipofuscin accumulation, and body bending. | [84] |
| Lactobacillus plantarum | D-Galactose-treated mice | Anti-aging | Reduced abnormal activities of superoxide dismutase, glutathione peroxidase, and catalase. Reduced expressions of several antioxidant genes, such as glutathione reductase, glutathione S-transferase, glutamate–cysteine ligase catalytic subunit, glutamate–cysteine ligase modifier subunit, and NAD(P)H quinone oxidoreductase 1. | [86] |
| Lactobacillus paracasei | Senescence-accelerated mouse prone 8 (SAMP8) mice | Anti-aging | Reduced senescence and low-serious anxiety-like behaviors and memory impairment and enhanced the antioxidative enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx). | [87] |
| Lactobacillus fermentum | D-Galactose induced aging rats | Anti-aging | Improved expression of SOD in bone and muscle, increased AMPK-α2 expression, and reduced the expression of IL-6 and TRAP in tibia. | [88] |
| B. bifidum BGN4 and B. longum BORI | Older adults | Brain health (gut–brain axis) | Alleviated stress and improved mental flexibility in older adults, along with modulating gut microbiota. | [89] |
| L. acidophilus, L. casei, B. bifidum, and L. fermentum | Alzheimer’s disease AD (60–95 years age) | Brain health | Findings of the study suggested that the probiotic-treated group had shown considerable improvement in Mini-mental state examination score. Overall study concluded that probiotic supplementation for 12 weeks could improve the metabolic status and cognitive functioning in the AD patients. | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samtiya, M.; Puniya, A.K.; Puniya, M.; Shah, N.P.; Dhewa, T.; Vemuri, R. Probiotic Regulation to Modulate Aging Gut and Brain Health: A Concise Review. Bacteria 2022, 1, 250-265. https://doi.org/10.3390/bacteria1040019
Samtiya M, Puniya AK, Puniya M, Shah NP, Dhewa T, Vemuri R. Probiotic Regulation to Modulate Aging Gut and Brain Health: A Concise Review. Bacteria. 2022; 1(4):250-265. https://doi.org/10.3390/bacteria1040019
Chicago/Turabian StyleSamtiya, Mrinal, Anil Kumar Puniya, Monica Puniya, Nagendra P. Shah, Tejpal Dhewa, and Ravichandra Vemuri. 2022. "Probiotic Regulation to Modulate Aging Gut and Brain Health: A Concise Review" Bacteria 1, no. 4: 250-265. https://doi.org/10.3390/bacteria1040019
APA StyleSamtiya, M., Puniya, A. K., Puniya, M., Shah, N. P., Dhewa, T., & Vemuri, R. (2022). Probiotic Regulation to Modulate Aging Gut and Brain Health: A Concise Review. Bacteria, 1(4), 250-265. https://doi.org/10.3390/bacteria1040019







