Abstract
Angular leaf spot of cashew tree in Brazil has been attributed to pigmented and nonpigmented strains of Xanthomonas citri pv. anacardii. Due to the possibility of dissemination of the disease by propagating material, it is necessary to understand the survival mechanisms of the causal agent. Thus, the present study aimed to characterize the behavior of viable but non-cultivable cells (VBNC) in two pigmented strains (CCRMTAQ13 and CCRMTAQ18) and one nonpigmented strain (IBSBF2579) of X. citri pv. anacardii, integrating in silico, in vitro, and in vivo studies. Thirteen genes associated with the VBNC phase were identified in the genomes of these strains. The log phase was observed at 24, 48, and 120 h for CCRMTAQ13, CCRMTAQ18, and IBSBF2579 strains, respectively. The death phase was observed at 96 h for both pigmented strains and 168 h for the nonpigmented strain. Using qPCR analyses, it was possible to characterize the occurrence of VBNC for the three strains. When inoculated, the strains showed 100% incidence during the VBNC phase, with the IBSBF2579 strain having the longest incubation period (IP). The strains did not differ concerning final severity (FS) in the VBNC phase. To our knowledge, this is the first report of the occurrence of the VBNC mechanism in X. citri pv. anacardii strains. Furthermore, it has been demonstrated that X. citri pv. anacardii in the VBNC state is potentially infective when they meet their host’s apoplast, which points to the need to use integrated practices to detect this bacterium in cashew seedlings.
1. Introduction
The cashew tree (Anacardium occidentale L.) is a tropical plant from Brazil, and it is dispersed throughout almost the entire national territory [1], where it stands out for its domestication and high economic use [2]. The cashew nuts are the main product of this crop, and the Northeast is the main producing region of the country, with more than 137 thousand tons produced in 2019 [3]. Among the several plant pathogens which can infect cashew trees, the bacterium Xanthomonas citri pv. anacardii stands out due to its capacity to cause angular spots and dark lesions surrounding the veins of the leaves, dieback, branches’ death, and water-soaked lesions in fruits [4,5]. From these lesions, pigmented and nonpigmented strains have been obtained, which are equally aggressive to cashew trees [6]. In addition, X. citri pv. anacardii appears to be a bacterium spread only within Brazil, where it has spread between states, possibly through contaminated seedlings [4].
So far, there are four genomes of X. citri pv. anacardii available from Genbank/NCBI [7], which contain important information about genes involved in bacterial metabolism and can help the study of the survival of this bacterium, such as genes related to the mechanism of viable but non-cultivable cells (VBNC) [8,9]. The term VBNC has been used to refer to cells unable to grow in a culture medium, but which remain alive and able to resume metabolic activities under favorable conditions [10], such as nutritionally rich environments [11] or in case of contact with components extracted from the host [12].
VBNC have been reported in several phytobacteria [9,10,13,14,15,16,17,18,19]. In X. campestris pv. campestris [8] and X. citri subsp. citri [9], the VBNC status has been induced by contacting the bacterial suspension with copper sulfate (CuSO4). In some studies, cell viability has been associated with genes related to motility [20], responsible for the biosynthesis of metabolites [11], involved in protection against oxidative stress [21], biofilm formation [22], adaptation to environmental stresses, energy generation, and cell wall composition [16].
Regarding the ability to reestablish an active metabolism from the state of VBNC, these cells have been characterized as potential inoculum sources for new infections [23]. Thus, given that bacteria in the VBNC state do not produce cultivable colonies, it may be necessary to combine molecular techniques with traditional microbiological isolation methods for more accurate detection of the pathogen [10], which could prevent the introduction of X. citri pv. anacardii into disease-free areas and avoid disease spread. Therefore, the present study aimed to verify the occurrence of VBNC using quantitative polymerase chain reaction (qPCR) in X. citri pv. anacardii and assess the capacity of these cells to initiate an infectious process in a leaf of cashew seedlings.
2. Results
2.1. In Vitro Growth Phases
The three strains of X. citri pv. anacardii showed the apex of the exponential phase at 24, 48, and 120 h, reaching concentrations of 1.15, 0.36, and 0.95 × 108 CFU/mL, for the strains CCRMTAQ13, CCRMTAQ18, and IBSBF2579, respectively (Figure 1a). For the death phase, the absence of bacterial growth was observed at 96, 96, and 168 h for strains CCRMTAQ13, CCRMTAQ18, and IBSBF2579, respectively. In turn, the growth curves obtained from the absorbance of the suspension of the three strains showed progressive increases up to 168 h (Figure 1b).
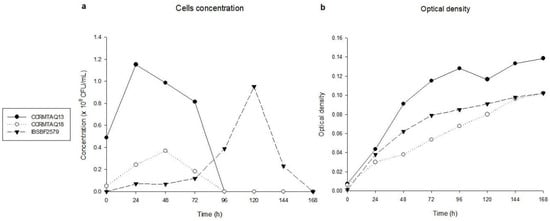
Figure 1.
In vitro growth of the pigmented (CCRMTAQ13 and CCRMTAQ18) and nonpigmented (IBSBF2579) strains of Xanthomonas citri pv. anacardii. (a) bacterial cells concentration. (b) bacterial optical density over time.
2.2. Genomic Annotation, Comparative Analysis, and Primer Construction for VBNC Status Assessment
Four thousand five hundred ninety-six and 4917 coding sequences were annotated, genomic content of 5,084,712 and 5,183,683 base pairs, 64.4% and 64.1% of GC for CCRMTAQ13 and CCRMTAQ18 strains, respectively.
In the literature, 60 genes were found upregulated during the state of VBNC in bacteria. From the in silico genomic analyses, 13 different genes were present in a single copy in the bacterial genome of the three strains of X. citri pv. anacardii (Table 1). Among them, the relA gene was detected in silico in the three genomes analyzed, and it was selected for the primer design.

Table 1.
Genes differentially expressed during the stage of viable but not cultivable cells (VBNC) reported in bacteria and found in genomes of the strains pigmented (CRMTAQ13 and CCRMTAQ18) and nonpigmented (IBSBF2579) of Xanthomonas citri pv. anacardii.
2.3. VBNC State Evaluation
The data obtained through qPCR show that the FXCA and RXCA primers constructed based on the relA gene efficiently amplified all the samples used, demonstrating specificity in the melting curve and uniformity of peaks for all samples (Figure 2).
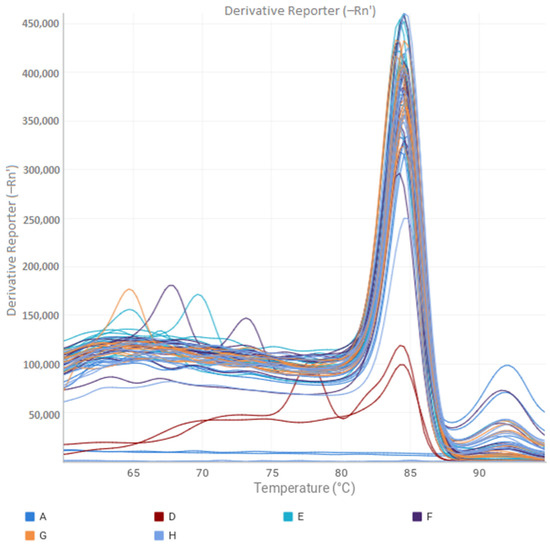
Figure 2.
Melting curve of qPCR obtained from samples of strains CCRMTAQ13, CCRMTAQ18, and IBSBF2579 strains of Xanthomonas citri pv. anacardii for the relA gene. A1–A12—serially diluted samples for standard curve. D11–D12—blank test. E11–F4—Diluted samples of isolated CCRMTAQ13. F5–G8—Diluted samples of CCRMTAQ18. G9–H12—Diluted samples of IBSBF 2579.
Considering the amplification of the relA gene, no significant differences were observed between the exponential and death phases, which presented 1.10 and 1.68, 0.47 and 0.33, 1.17 and 4.32 × 1014 copies/mL for CCRMTAQ13, CCRMTAQ18, and IBSBF2579 strains, respectively (Figure 3a). Considering the bacterial concentration in these phases, significant differences were observed between the exponential and death phases of all strains, with concentrations of 1.15, 0.36, and 0.95 × 108 CFU/mL for CCRMTAQ13, CCRMTAQ18, and IBSBF2579 (Figure 3b) in the exponential phase, while in the death phase growth was not observed.
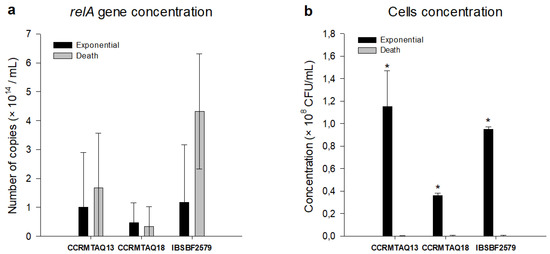
Figure 3.
Comparative qPCR amplification and cell formation units of the strains pigmented (CCRMTAQ13 and CCRMTAQ18) and nonpigmented (IBSBF2579) of Xanthomonas citri pv. anacardii in exponential and death phases. (a), Numbers of copy of the relA gene. (b), cell formation units. Asterisk indicates statistical difference between exponential and death phases.
The VBNC status was induced by the time of cultivation during 96, 96, and 168 h for the strains CCRMTAQ13, CCRMTAQ18, and IBSBF2579, which presented a high number of copies/mL of the relA gene and bacterial concentration equal to zero.
2.4. VBNC State Cell Infectivity
An incidence of 100% was observed for the three strains evaluated in the exponential and VBNC phases. For IP and FS, a significant interaction (p ≤ 0.05) was observed between strains and bacterial growth phases (Table 2). The IP of the strains IBSBF2579 and CCRMTAQ13 were lower than the strain CCRMTAQ18 in the exponential phase, while the strains CCRMTAQ18 and CCRMTAQ13 showed an IP lower in the VBNC state. The strains IBSBF2579 and CCRMTAQ18 showed a difference between the IP of the exponential phase and the state of VBNC. Regarding FS, the three strains showed differences among themselves in the exponential phase, while in the state of VBNC no differences were observed among the strains. Higher values of FS were observed in the exponential phase of strains CCRMTAQ13 and IBSBF2579 than in the state of VBNC, while in strain CCRMTAQ18 the values between these phases did not differ.

Table 2.
Infectivity of the pigmented (CCRMTAQ13 and CCRMTAQ18) and nonpigmented (IBSBF2579) strains of Xanthomonas citri pv. anacardii in cashew seedlings inoculated with cells in the exponential growth phase and on VBNC (viable but nonculturable cells) state.
3. Materials and Methods
3.1. Bacterial Strains and Growth Conditions
We used two pigmented strains (CCRMTAQ13 and CRMTAQ18) and one nonpigmented strain (IBSBF2579) of X. citri pv. anacardii. The genomes of these strains were sequenced in other studies [6,27] and deposited in the database of the GenBank/NCBI (https://www.ncbi.nlm.nih.gov/genome, accessed on 17 May 2021), with the assemblies: GCA_002898475.1 (CCRMTAQ13), GCA_002898415.1 (CRMTAQ18), and GCA_002837255.1 (IBSBF2579). The strains CCRMTAQ13 and CCRMTAQ18 are deposited in Rosa Mariano Culture Collection from the Phytobacteriology Laboratory (LAFIBAC) of Universidade Federal Rural de Pernambuco (UFRPE), and the strain IBSBF2579 is deposited in the Phytobacteria Culture Collection from the Instituto Biológico of the São Paulo state. In the different assays carried out in this study, the strains were cultivated in Petri dishes containing NYDA medium (20 g L−1 agar, 10 g L−1 dextrose, 5 g L−1 yeast extract, 3 g L−1 meat extract, and 5 g L−1 peptone) at 29 °C for 36 h.
3.2. Determination of Exponential and Death Phases In Vitro
From the growth of each strain, a suspension in sterilized distilled water (SDW) was prepared to adjust the concentration to A570 = 0.06, corresponding to 108 colony-forming units (CFU) mL−1. Subsequently, 1.0 mL of bacterial suspension was added to test tubes containing 9.0 mL of NYD liquid medium (NYDA, without agar), with subsequent incubation at 29 °C. Every 24 h, for seven days, the suspension concentration in the tubes was determined by removing 5 mL aliquots of the suspension to read the absorbance using a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 570 nm. Simultaneously, serial dilutions were performed by removing 1.0 mL aliquots of the suspension, diluting in SDW up to 10−7, and plating 0.1 mL of the last three dilutions (10−5, 10−6, and 10−7) in Petri dishes containing NYDA medium, spreading the suspension with the aid of a Drigalski loop. The colonies formed were counted 48 h after plating to determine the concentration of the bacterial suspension using the formula CFU mL−1 = average number of colonies x sample dilution factor x correction factor, where the dilution factor corresponds to the potency of base 10 in which the dilution was plated, and the correction factor corresponds to 10, which refers to the fact that only 0.1 mL of suspension was plated. After counting the colonies, concentrations were standardized to 108 CFU mL−1 to make easy comparisons among the curves built for the three strains.
The experiment was performed in a completely randomized design, with eight replicates per strain, and each replicate was characterized by a tube. For each day of absorbance reading, a tube has been randomly selected. From each test tube, bacterial concentration quantifications were performed by plating on six Petri dishes for each quantified dilution. The data were used to build growth curves to determine the exponential and death phases. The experiment was carried out twice.
3.3. Genomic Annotation and Design of Primers for Evaluation of VBNC State
The automatic annotation was performed using the Rapid Annotation using Subsystem Technology (RAST) Server [28]. Then, there was a manual curation of each of these genes of the VBNC state in the Artemis program [26].
A bibliographic survey was carried out in scientific articles contained in the database of the PubMed, Web of Science, and SCOPUS platforms, aiming to identify candidate genes previously reported as expressed during the state of VBNC in bacteria. The genomes of X. citri pv. anacardii were analyzed for the presence of these genes in a single copy using the Artemis program [29], and the detected genes were selected to design primers.
The alignment of genes expressed during the VBNC state was performed using the BLASTn database [30,31]. The primers FXCA F’ GATCGAAATCCAGATCCGTACC and RXCA R’ TGCCGCCTTCCTTGTATTT were designed based on the relA using the Primer-BLAST platform [32]. These primers were verified using the Geneious Prime 2021.1.1 program (https://www.geneoius.com, accessed on 25 June 2021). Subsequently, the primers were synthesized by IDT (Integrated DNA Technologies Inc., San Diego, CA, USA) for evaluation using qPCR.
3.4. Assessment of VBNC Status via Quantitative PCR (qPCR)
DNA extraction from the strains used in this study was performed using the MiniPrep kit for bacterial genomic DNA extraction (Axygen Biosciences, Union City, CA, USA) following the manufacturer’s recommendations. The genomic DNA was quantified as previously described [4]. Six samples were extracted from bacterial growth cultivated in NYD medium at 29 °C until the exponential and death phases, which corresponded to 24 and 96 h, 24 and 96 h, and 24 and 168 h, for the strains CCRMTAQ13, CCRMTAQ18, and IBSBF2579, respectively. Collections were carried out in triplicate.
Each qPCR reaction was composed of 1× GoTaq qPCR Master Mix (2×), 2.5 nM of each primer and 3 µL of DNA. For the qPCR reaction, QuantStudio 5 Real-Time PCR System from Thermo Fischer Scientific (Waltham, MA, USA) was used, starting the amplification with 2 min at 95 °C, and 45 cycles of 15 min at 98 °C, 1 min at 60 °C. For DNA quantification, a standard curve was developed based on a 10× serial dilution from 1000 ng/µL. Aiming to quantify the number of cells in the exponential and death phases in the samples, the calculation of the total number of copies/mL was determined from the ratio of the number of base pairs of the relA gene x number of base pairs in the genome and the DNA concentration in ng/µL per sample. The cycle limit was determined after manually adjusting the limit number of the linear part of the qPCR logarithmic amplification curves [33]. Procedures were performed in triplicate for all samples. Samples without the presence of DNA were used to verify the reliability of the data obtained. To determine the VBNC state, the strains were cultured in NYD medium at 29 °C until the exponential and death phases, and the concentrations in CFU/mL were estimated as described above. The VBNC status was determined by comparing the number of copies/mL of the relA gene in the exponential and death phases with the concentration of total cells in CFU/mL in the suspensions of the strains in the respective phases [33], and the values were standardized to 1014 to make possible comparisons among the concentrations obtained. The VBNC status was considered active when the number of copies of the relA gene/mL remained high, and the concentration of CFU/mL was equal to zero.
Student’s t test (p < 0.05) was used to assess significant differences between the exponential and death phases for the number of copies/mL of the relA gene and the bacterial concentration in CFU/mL using the STATISTIX software 9 (Tallahassee, FL, USA).
3.5. VBNC Cell Infectivity in Cashew Seedlings
The strains CCRMTAQ13, CCRMTAQ18, and IBSBF2579 were cultivated in 10 mL of NYD medium at 29 °C until reaching the exponential and death phases. Bacterial suspensions were obtained by centrifugation of NYD medium at 12,000 rpm, followed by removal of the supernatant. The concentration of the bacterial growth precipitated was adjusted in SDW to 108 CFU/mL. The strains were inoculated on the four apical leaves of CCP 76 cashew tree seedlings grown in a greenhouse using the infiltration method of 100 µL of suspension on the abaxial surface of the leaf blade with the aid of a needleless syringe. Leaves similarly treated with SDW were used as a control. After inoculations, the plants were incubated in a greenhouse (28 ± 2 °C, 65% RH) for 23 days, evaluating the disease incidence, the incubation period (IP), and the final disease severity (FS), as described by Gama et al. [4].
The experiment was carried out twice, using five and four repetitions per treatment in the first and second experiment, respectively. Each repetition consisted of a leaf containing four inoculation points along the leaf surface. The experiment was carried out in a completely randomized design in a factorial arrangement (3 strains × 2 growth phases). The means were submitted to analysis of variance (ANOVA) and compared by the LSD test (p < 0.05) using the STATISTIX 9 software (Analytical Software, Tallahassee, FL, USA).
4. Discussion
The in vitro growth curve performed with pigmented (CCRMTAQ13 and CCRMTAQ18) and nonpigmented (IBSBF2579) strains of X. citri pv. anacardii satisfactorily allowed the determination of the exponential and death phases (Figure 1a), which were used to assess the state of VBNC together with the values obtained by qPCR. The growth behavior of all strains was variable until reaching the apex of the exponential phase.
Regarding the duration of the stationary phase and, consequently, the beginning of the death phase, it was observed that the pigmented strains (CCRMTAQ13 and CCRMTAQ18) presented similar behavior to other X. citri strains [34], with no growth being observed at 96 h, while the nonpigmented strain (IBSBF2579) showed no growth only at 168 h. Although the bacterial concentration decreased and reached zero for all strains, the optical density curve of the bacterial suspensions kept growing until 168 h (Figure 1b), when the experience was completed. Thus, the VBNC was determined when the concentration of the suspension reached zero, and the optical density value continued to be high. These data indicated for the first time the occurrence of VBNC status in pigmented and nonpigmented strains of X. citri pv. anacardii and the ability of this bacterium to enter this state stimulated by the time of cultivation of its cells. A similar result was observed for X. campestris pv. campestris, which reached the VBNC status through liquid microcosms and sterile soil [8] and X. citri subsp. citri, with induction through low nutrient availability and copper stress [12].
Although they have been sequenced in another study [6], the genomes of pigmented strains of X. citri pv. anacardii (CCRMTAQ13 and CCRMTAQ18) had not yet been annotated. The annotations performed in this study showed a smaller number of base pairs (5,084,712 and 5,183,683 bp) and N50 (109,779 and 128,886 bp) and a higher number of coding sequences (4,761 and 4,917) and GC content (64.4 and 64.1%) for the strains CCRMTAQ13 and CCRMTAQ18, when compared to the number of base pairs (5,348,596 bp), N50 (418,068 bp), coding sequences (4,427) and GC content (63.84%) observed in the strain IBSBF2579 [27].
To prove the occurrence of the VBNC state in X. citri pv. anacardii, the genomes of the three strains used in the present study were analyzed for genes reported to be expressed during this state. Thirteen genes were detected in the genomes of X. citri pv. anacardii as upregulated in the VBNC state, which showed functions related to motility (fliG), hydrogen peroxide metabolism (katG), glutamate metabolism (gltB and gltD), bacterial conjugation pili (pilM), ribosomal metabolism (rps), transcriptional (soxR) and translational regulation (hfq), protection against oxidative stress (dps), peptidoglycan metabolism (murG), outer membrane composition (omp), molybdenum metabolism (mobA), and protection against amino acid deprivation (relA) (Table 1). This last gene was selected for the design of the primers because it is a gene previously successfully used to detect the VBNC phase in Vibrio cholerae strains [35], being responsible for both the synthesis and degradation of guanosine pentaphosphate synthetase ((p)ppGpp), as well as the regulation of this molecule, which is related to adaptation to environmental changes, such as nutritional deficit and low temperatures [36]. In addition, (p)ppGpp also promotes resistance to adverse conditions such as nutritional stress, antibiotics, and metabolic inhibitors [20,37].
All samples from the exponential and death phases were amplified with the FXCA and RXCA primers, used to assess the amplification of the relA gene (Figure 2). Analyses via qPCR showed a high number of copies/mL in the exponential and death phases, with no significant differences (p < 0.05) between these phases being observed for the three strains, demonstrating an adaptation in the stationary phase [38] concerning other phases of bacterial growth. Furthermore, the concentration of cells in the exponential phase ranged from 0.36 to 1.15 × 108 CFU/mL, while in the death phase the concentration was equal to zero. These results indicate an active cell multiplication even after the estimated time for cell death, demonstrating for the first time the occurrence of this mechanism in X. citri pv. anacardii. However, in the genus Xanthomonas, the occurrence of VBNC has been previously reported in X. citri subsp. citri [9,12] and in X. campestris pv. campestris [8], indicating that this survival mechanism may be consistently present in this genus.
Artificial inoculations in cashew seedlings with strains CCRMTAQ13, CCRMTAQ18, and IBSBF2579, both in the exponential phase and in the VBNC state, indicated a high capacity for infection, as demonstrated by the incidence of disease in 100% of inoculations. The significant interaction (p ≤ 0.05) among strains and growth states (exponential and VBNC) revealed that depending on the strain, lower IP values and higher FS values can be induced in each phase (Table 2). These results demonstrate a high pathogenic capacity of X. citri pv. anacardii in the VBNC state. The pathogenicity of phytopathogenic bacteria in the VBNC state has also been shown in X. citri subsp. citri, which causes symptoms typical of a citrus canker at this stage, similarly to the inoculation of exponential stage strains [9]. In this context, survival in the VBNC state and its high capacity to cause disease to suggest that infected organs can fully function as a source of inoculum [23]. Therefore, the application of conventional and molecular techniques for detection of X. citri pv. anacardii in propagation material is essential since detection accuracy is a highly relevant factor for the integrated management of diseases caused by Xanthomonas species and pathovars [39].
The high number of copies/mL of the relA gene in the death phase of the bacterial growth curve demonstrated the occurrence of the VBNC state in pigmented and non-pigmented strains of X. citri pv. anacardii. In this phase, the strains CCRMTAQ13, CCRMTAQ18, and IBSBF2579 showed high infectiousness in cashew leaves, indicating the possibility of dissemination of the pathogen in propagation materials. These results indicate that when used in isolation, traditional methods of diagnosis may underestimate the viability of the bacteria to be detected or even not detect the presence of the pathogen. Therefore, the results obtained in this study point to the need to integrate conventional and molecular approaches for more accurate detection of the presence of X. citri pv. anacardii in cashew seedlings.
Author Contributions
Conceptualization: all authors. Methodology: L.P.L., G.R.A., R.D.O.C. Formal analysis and investigation: L.P.L., F.F.A. and M.A.S.G. Writing: L.P.L., E.B.S., A.M.B.-I., F.F.A. and M.A.S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian National Research Council (CNPq) grant number 140052/2020-8.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This article does not contain any experiments on human participants or animals.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
L.P.L. acknowledges the Brazilian National Research Council (CNPq) for the scholarship awarded.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Barros, L.M. Botânica, origem e distribuição geográfica. In Cajucultura: Modernas Técnicas de Produção; Araújo, J.P.P., Silva, V.V., Eds.; EMBRAPA-CNPAT: Fortaleza, Brazil, 1995; pp. 55–71. [Google Scholar]
- Melo, Q.M.S.; Bleicher, E. Identification and management of the main cashew pests. In Cashew Plant Health; Melo, Q.M.S., Ed.; Embrapa Agroindústria Tropical: Brasília, Brazil, 2002; pp. 9–34. [Google Scholar]
- IBGE. Table 4—Area Destined for Harvest, Harvested Area, Quantity Produced, Average Yield and Value of Production in Brazil, the Major Regions and Federation Units, according to the Products of Permanent Crops; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2019. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9117-producao-agricola-municipal-culturas-temporarias-e-permanentes.html?edicao=29008&t=resultados (accessed on 20 May 2022).
- Gama Marco, A.S.; Mariano, R.L.R.; Viana, F.M.P.; Ferreira, M.A.S.V.; Souza, E.B. Polyphasic Characterization of Pigmented Strains of Xanthomonas Pathogenic to Cashew Trees. Plant Dis. 2011, 95, 793–802. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Viana, F.M.P.; Cardoso, J.E.; Saraiva, H.A.O.; Ferreira, M.A.S.V.; Mariano, R.L.R.; Trindade, L.C. First Report of a Bacterial Leaf and Fruit Spot of Cashew Nut (Anacardium occidentale) Caused by Xanthomonas campestris pv. mangiferaeindicae in Brazil. Plant Dis. 2007, 91, 1361. [Google Scholar] [CrossRef] [PubMed]
- Da Gama, M.A.S.; Mariano, R.D.L.R.; Da Silva Júnior, W.J.; De Farias, A.R.G.; Barbosa, M.A.G.; Ferreira, M.Á.D.S.V.; Costa Júnior, C.R.L.; Santos, L.A.; De Souza, E.B. Taxonomic Repositioning of Xanthomonas campestris pv. viticola (Nayudu 1972) Dye 1978 as Xanthomonas citri pv. viticola (Nayudu 1972) Dye 1978 comb. nov. and Emendation of the Description of Xanthomonas citri pv. anacardii to Include Pigmented Isolates Pathogenic to Cashew Plant. Phytopathology 2018, 108, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (NCBI). NCBI Genome. 2021. Available online: https://www.ncbi.nlm.nih.gov/genome/genomes/527? (accessed on 30 June 2021).
- Ghezzi, J.I.; Steck, T.R. Induction of the viable but non-culturable condition in Xanthomonas campestris pv. campestris in liquid microcosms and sterile soil. FEMS Microbiol. Ecol. 1999, 30, 203–208. [Google Scholar] [CrossRef]
- Campo, R.D.; Russi, P.; Mara, P.; Mara, H.; Peyrou, M.; León, I.P.D.; Gaggero, C. Xanthomonas axonopodis pv. citri enters the VBNC state after copper treatment and retains its virulence. FEMS Microbiol. Lett. 2009, 298, 143–148. [Google Scholar] [CrossRef]
- Oliver, J.D. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 2010, 34, 415–425. [Google Scholar] [CrossRef]
- Su, X.; Guo, L.; Ding, L.; Qu, K.; Shen, C. Induction of Viable but Nonculturable State in Rhodococcus and Transcriptome Analysis Using RNA-seq. PLoS ONE 2016, 11, e0147593. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Cubero, J.; López, M.M.; Llop, P. The viable but non-culturable state in Xanthomonas citri subsp. citri is a reversible state induced by low nutrient availability and copper stress conditions. J. Life Sci. 2013, 7, 1019–1029. [Google Scholar]
- Lee, J.H.; Zhao, Y. Integration Host Factor Is Required for RpoN-Dependent hrpL Gene Expression and Controls Motility by Positively Regulating rsmB sRNA in Erwinia Amylovora. Phytopathology 2015, 106, 29–36. [Google Scholar] [CrossRef]
- Santander, R.D.; Figàs-Segura, À.; Biosca, E.G. Erwinia amylovora catalases KatA and KatG are virulence factors and delay the starvation-induced viable but non-culturable (VBNC) response. Mol. Plant Pathol. 2018, 19, 922–934. [Google Scholar] [CrossRef]
- Jiang, N.; Lv, Q.Y.; Xu, X.; Cao, Y.S.; Walcott, R.R.; Li, J.Q.; Luo, L.X. Induction of the viable but nonculturable state in Clavibacter michiganensis subsp. michiganensis and in planta resuscitation of the cells on tomato seedlings. Plant Pathol. 2016, 65, 826–836. [Google Scholar] [CrossRef]
- Postnikova, O.A.; Shao, J.; Mock, N.M.; Baker, C.J.; Nemchinov, L.G. Gene Expression Profiling in Viable but Nonculturable (VBNC) Cells of Pseudomonas syringae pv. syringae. Front. Microbiol. 2015, 6, 1419. [Google Scholar] [CrossRef]
- Kan, Y.; Jiang, N.; Xu, X.; Lyu, Q.; Gopalakrishnan, V.; Walcott, R.; Burdman, S.; Li, J.; Luo, L. Induction and Resuscitation of the Viable but Non-culturable (VBNC) State in Acidovorax citrulli, the Causal Agent of Bacterial Fruit Blotch of Cucurbitaceous Crops. Front. Microbiol. 2019, 10, 1081. [Google Scholar] [CrossRef]
- Kong, H.G.; Bae, J.Y.; Lee, H.J.; Joo, H.J.; Jung, E.J.; Chung, E.; Lee, S.-W. Induction of the Viable but Nonculturable State of Ralstonia solanacearum by Low Temperature in the Soil Microcosm and Its Resuscitation by Catalase. PLoS ONE 2014, 9, e109792. [Google Scholar] [CrossRef]
- Palmieri, A.C.; Amaral, A.M.; Homem, R.A.; Machado, M.A. Differential expression of pathogenicity- and virulence-related genes of Xanthomonas axonopodis pv. citri under copper stress. Genet. Mol. Biol. 2010, 33, 348–353. [Google Scholar] [CrossRef]
- Asakura, H.; Ishiwa, A.; Arakawa, E.; Makino, S.; Okada, Y.; Yamamoto, S.; Igimi, S. Gene expression profile of Vibrio cholerae in the cold stress-induced viable but non-culturable state. Environ. Microbiol. 2007, 9, 869–879. [Google Scholar] [CrossRef]
- Um, H.Y.; Kong, H.G.; Lee, H.J.; Choi, H.K.; Park, E.J.; Kim, S.T.; Murugiyan, S.; Chung, E.; Kang, K.Y.; Lee, S. Altered Gene Expression and Intracellular Changes of the Viable But Nonculturable State in Ralstonia solanacearum by Copper Treatment. Plant Pathol. J. 2013, 29, 374–385. [Google Scholar] [CrossRef]
- Meng, L.; Alter, T.; Aho, T.; Huehn, S. Gene expression profiles of Vibrio parahaemolyticus in viable but non-culturable state. FEMS Microbiol. Ecol. 2015, 91, fiv035. [Google Scholar] [CrossRef]
- Ordax, M.; Biosca, E.G.; Wimalajeewa, S.C.; López, M.M.; Marco-Noales, E. Survival of Erwinia amylovora in mature apple fruit calyces through the viable but nonculturable (VBNC) state. J. Appl. Microbiol. 2009, 107, 106–116. [Google Scholar] [CrossRef]
- Yaron, S.; Matthews, K.R. A reverse transcriptase-polymerase chain reaction assay for detection of viable Escherichia coli O157:H7: Investigation of specific target genes. J. Appl. Microbiol. 2002, 92, 633–640. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Wang, Y.; Zeng, J.; Ye, C.; Li, X.; Guo, L.; Zhang, S.; Yu, X. Induction of Escherichia coli into a VBNC state through chlorination/chloramination and differences in characteristics of the bacterium between states. Water Res. 2018, 142, 279–288. [Google Scholar] [CrossRef]
- Jameelah, M.; Dewanti-Hariyadi, R.; Nurjanah, S. Expression of rpoS, ompA and hfq genes of Cronobacter sakazakii strain Yrt2a during stress and viable but nonculturable state. Food Sci. Biotechnol. 2018, 27, 915–920. [Google Scholar] [CrossRef]
- Silva Junior, W.J.; Farias, A.R.; Lima, N.B.; Benko-Iseppon, A.M.; Aburjaile, F.; Balbino, V.Q.; Falcão, R.M.; Leitão Paiva Júnior, S.D.; Sousa-Paula, L.C.; Mariano, R.L.; et al. Complete Genome Sequence of Xanthomonas citri pv. anacardii Strain IBSBF2579 from Brazil. Genome Announc. 2018, 6, e01574-17. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Carver, T.; Harris, S.R.; Berriman, M.; Parkhill, J.; McQuillan, J.A. Artemis: An integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 2012, 28, 464–469. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Bairoch, A.; Apweiler, R.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gaisteger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. The Universal Protein Resource (UniProt). Nucleic Acids Res. 2005, 33 (Suppl. S1), D154–D159. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Aburjaile, F.F.; Madec, M.-N.; Parayre, S.; Miyoshi, A.; Azevedo, V.; Loir, Y.L.; Falentin, H. The long-term survival of Propionibacterium freudenreichii in a context of nutrient shortage. J. Appl. Microbiol. 2016, 120, 432–440. [Google Scholar] [CrossRef]
- Maximiano, M.R.; Oliveira-Neto, O.B.; Franco, O.L.; Mehta, A. Validation of an in vitro system for studies of pathogenicity mechanisms in Xanthomonas campestris. FEMS Microbiol. Lett. 2017, 364, fnx217. [Google Scholar] [CrossRef]
- González-Escalona, N.; Fey, A.; Höfle, M.G.; Espejo, R.T.; Guzmán, C.A. Quantitative reverse transcription polymerase chain reaction analysis of Vibrio cholerae cells entering the viable but non-culturable state and starvation in response to cold shock. Environ. Microbiol. 2006, 8, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Avarbock, D.; Salem, J.; Li, L.; Wang, Z.; Rubin, H. Cloning and characterization of a bifunctional RelA/SpoT homologue from Mycobacterium Tuberculosis. Gene 1999, 233, 261–269. [Google Scholar] [CrossRef]
- Potrykus, K.; Cashel, M. Preferential Cellular Accumulation of ppGpp or pppGpp in Escherichia coli. In Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; De Brujin, F.J., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 479–488. [Google Scholar] [CrossRef]
- Primm, T.P.; Andersen, S.J.; Mizrahi, V.; Avarbock, D.; Rubin, H.; Barry, C.E. The Stringent Response of Mycobacterium tuberculosis Is Required for Long-Term Survival. J. Bacteriol. 2000, 182, 4889–4898. [Google Scholar] [CrossRef] [PubMed]
- Catara, V.; Cubero, J.; Pothier, J.F.; Bosis, E.; Bragard, C.; Đermić, E.; Holeva, M.C.; Jacques, M.; Petter, F.; Pruvost, O.; et al. Trends in Molecular Diagnosis and Diversity Studies for Phytosanitary Regulated Xanthomonas. Microorganisms 2021, 9, 862. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).