Abstract
Using Enterococcus faecium strains as probiotics raises several controversies related to their antibiotic resistance (AR). In the current study, we examined isolates of E. faecium obtained from human breast milk. Catalase-negative and γ-haemolytic isolates were identified by analyzing the sequences of 16S rRNA gene and their phenotypic resistance to antibiotics was investigated. We examined the expression of genes that were found on plasmids. The majority of isolates tested were resistant to erythromycin (96%), followed by trimethoprim (67%), tetracycline (57%), and gentamicin (55%). Ninety-seven percent of E. faecium isolates were resistant to at least two antibiotics. We detected the presence of the following genes on plasmids: ErmB (erythromycin), dfrA17 (trimethoprim), tetO, tetK (tetracycline), Aph(3′)-IIIa (neomycin), and marA (rifampicin). TetO was not expressed in all cases, dfrA14 was not expressed in CDCP1449, while tetK was only expressed in CDCP1128 and CDCP1331 isolates. In the majority of isolates, AR genes were located on chromosomes since they were not detected on plasmids. Our study shows that due to the spread of AR, human milk could be one of the first sources of the bacteria resistant to antimicrobials to infants.
1. Introduction
According to the European Food Safety Authority (EFSA), all bacterial strains used as food or animal feed additives must be tested against the resistance to relevant human or veterinary antimicrobial agents. Such examination ought to be conducted in accordance with international standards such as ISO 10932:2010 [1]. There are dozens of antibiotics mentioned in the paper published by EFSA [2] that need to be considered: ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline, chloramphenicol, and, in specific cases, tylosine, apramycin, nalidixic acid, sulfonamide, and trimethoprim. To distinguish strains resistant from susceptible ones, the cutoff values of antimicrobial concentrations were established. Strains which growth is inhibited at minimum inhibitory concentration (MIC) or below it are considered susceptible. MIC values are strictly defined for particular groups, genera, or species of microorganisms [2]. Strains that are considered resistant can be further investigated with molecular biology techniques to determine the nature of antimicrobial resistance. It is necessary to distinguish between strains that show acquired or intrinsic resistance. Strains that demonstrate intrinsic antibiotic resistance are generally considered as safe for human or animal consumption, whereas strains with acquired resistance must not be added to food or animal feed [2].
Even though probiotic strains are mostly known among Lactobacillus and Bifidobacterium genera, there are some representatives of the Enterococcus genus that have already received probiotic status, e.g., E. faecium SF68 [3]. There are more Enterococcus strains that are being assessed for their potential use as probiotics for human and animals. Some of them have been shown to be safe for use in various fish species [4,5] or piglets at different growth stages [6,7]. Moreover, others may reduce carryover infections with chlamydia in sows and piglets [8]. It has also been demonstrated that E. faecium C2 strain isolated from human breast milk could be a potential novel probiotic [9]. Therefore, the search for putative probiotics among that species in human breast milk seems justified.
On the other hand, it has been demonstrated that E. faecium might carry antibiotic resistance genes on plasmids. Moreover, the presence of those genes might be correlated with the presence of some virulence genes such as hyl, which encodes hyaluronodisase [10]. It has also been shown that antibiotic resistance could be transferred through breast milk [11]. Considering the above, it seems justified to search for antibiotic resistance genes on plasmids.
Originally, the project in which we obtained all tested isolates was focused on searching putative probiotics in human milk microbiota, but since antibiotic resistance was shown to be very common among tested bacteria, we decided to observe that phenomenon more closely. In the current study, we selected various E. faecium isolates that could be considered as potential probiotics, tested their susceptibility to antibiotics, determined the presence of resistance genes on plasmids, and then verified if those genes were truly responsible for antibiotic resistance by determining their expression.
2. Results
2.1. Identification of Bacterial Isolates
We originally collected 2000 isolates (20 from each donor) and 310 among them were classified as catalase negative and gamma-haemolytic. For those isolates, we carried out antibiotic susceptibility tests and Sanger sequencing of 16S rRNA gene. We established that 51 of them were Enterococcus faecium (Table 1). We also found many isolates that were Enterococcus faecalis (73 isolates) and few isolates that were identified as Enterococcus casseliflavus (1), Enterococcus durans (5), and Enterococcus hirae (2). The other 130 isolates were identified only to the genus level (data not shown). Since the study was focusing on the potential probiotics among E. faecium species, we continued further analysis using only isolates that were identified as such.

Table 1.
Determination of phenotypic antibiotic resistance of Enterococcus faecium isolates obtained from human breastmilk.
Based on the phylogenetic analysis, it could be stated that the vast majority of isolates created a common cluster (Figure 1). Among them, isolates CDCP523 and CDCP497 or isolates CDCP531 and CDCP976 formed subclusters, with the similarity of 71% and 79%, respectively. Another cluster was formed by isolates CDCP968 and CDCP1512 with the similarity of 74%.
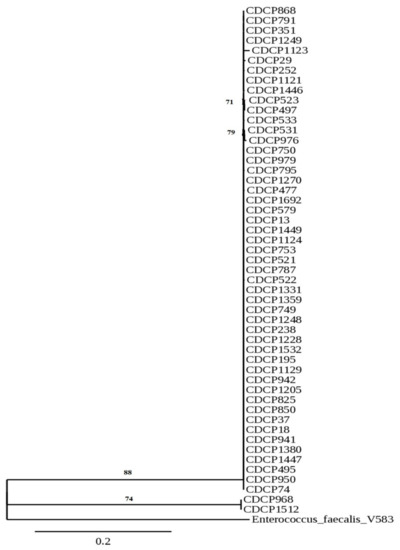
Figure 1.
Phylogenetic tree of Enterococcus faecium isolates originated from human breast milk based on their 16S rRNA gene sequences. The sequence of Enterococcus faecalis V583 was used as an outgroup.
2.2. Assessment of Antibiotic Resistance
In the next research step, we tested all 51 isolates against the resistance to antibiotics using ISO 10932:2010 norm. The majority of tested isolates were resistant to erythromycin (96%), followed by trimethoprim (67%), tetracycline (57%), and gentamicin (55%) (Table 1). There were three antibiotics that demonstrated particularly high efficacy and isolates were susceptible to them: linezolid (98%), then ampicillin (94%), and chloramphenicol (92%). It must be highlighted that cutoff values were not established for linezolid or many other antibiotics (neomycin, quinupristin/dalfopristin, trimethoprim, ciprofloxacin, and rifampicin), thus we decided to consider tested isolates as resistant to those antibiotics only when bacterial growth was noted in the whole tested concentration range, including trimethoprim.
There were no isolates susceptible to all tested antibiotics. Only three isolates (5.7%) were resistant to one antibiotic and it was erythromycin (Table 1). Eleven isolates (20.8%) were resistant to two antibiotics, 5 isolates to three (9.4%), 11 isolates to four (20.8%), 6 isolates to five (11.3%), 4 isolates to six (7.6%), 3 isolates to seven (5.7%), 1 isolate to eight (1.9%), 5 isolates to nine (9.4%), and 1 isolate to twelve (1.9%).
We also tested two other methods for the assessment of antibiotic resistance, such as disk diffusion method and E-test, but those were proven inaccurate because tested strains did not demonstrate even growth on solid media used for that analysis (brain heart infusion agar, data not shown).
2.3. Verifying the Presence of Transferable Genes
After examining phenotypes of antibiotic resistance for each isolate, we verified if those genes were located on plasmids. We found out that only one contained ant(6) gene, which encodes the resistance to streptomycin (Table 2). In the case of tetracycline, we detected three genes: tetM (2 isolates), tetO (7 isolates), and tetS (1 isolate). Ten isolates had ErmB gene, which provided the resistance to erythromycin (Table 2). Aph(3′)-IIIa gene encoding the resistance to neomycin was detected in four isolates while marA (rifampicin) was present in one isolate. The most frequently occurring gene was dfrA14. It encodes the resistance to trimethoprim, and it was found in 19 isolates (Table 2).

Table 2.
Isolates of Enterococcus faecium obtained from human breast milk in which antibiotic resistance genes were found on plasmids.
Isolate CDCP238 had four tested genes encoding antibiotic resistance: ant(6), tetM, tetO, and aph(3′)-IIIa; while CDCP351 had tetO, ErmB, and aph(3′)-IIIa (Table 2 and Table 3). There were few isolates for which we detected two genes: CDCP753 and CDCP787–ErmB and dfrA14; CDCP1228 and CDCP1449–tetO and dfrA14; CDCP1532 and CDCP1692–aph(3′)-IIIa and dfrA14; CDCP1331–tetO and marA. There were also other isolates that contained only one of considered genes: CDCP1205, CDCP1249, CDCP1447–tetK; CDCP477, CDCP495, CDCP521, CDCP523, CDCP531, CDCP533–ErmB; CDCP749, CDCP795, CDCP825, CDCP850, CDCP868, CDCP941, CDCP950, CDCP979, CDCP1248, CDCP1359, CDCP1380, CDCP1446–dfrA14 (Table 2 and Table 3).

Table 3.
Summary of the expression of antibiotic resistance genes located on plasmids of Enterococcus faecium isolates originating from human breast milk.
We also noted that if analyzed isolates originated from a common donor, they carried the same AMR genes: CDCP521, 531 and 533–ErmB1, CDCP750 and 753–dfrA14 gene; CDCP 787, 791 and 795–dfrA14 gene; and CDCP1446 and 1449–dfrA14 gene as well.
2.4. Gene Expression Analysis
For gene expression analysis, we selected isolates that carried at least two antibiotic resistance genes on plasmids (Table 2). Isolates CDCP238 and CDCP1512 were not included in those experiments because when we took them out of stock, they did not grow in any culture media that were used for their cultivation before banking. That left us with the following isolates: CDCP351 CDCP753, CDCP787, CDCP1228, CDCP1331, and CDCP 1449. There was no gene expression of the TetO gene, so we did not include those results. Data collected in that experiment for other genes (Table 3) showed very high relative ratios but with some exceptions: tetK was not expressed in CDCP351 and CDCP1449, while dfrA14 was not expressed in CDCP1449. It could also be stated that the most significant gene expression occurred in the following cases: tetK in CDCP1228 (1.49 105), ErmB in CDCP351 (7.69 × 104), and CDCP753 (4.72 × 104) or dfrA14 in CDCP753 (1.29 × 104) and CDCP787 (2.80 × 104).
3. Discussion
3.1. Identification of Bacterial Isolates
It is suspected that bacterial isolates in breastmilk are transferred from intestines with blood [12]. The majority of those microorganisms are nonculturable; however, Fernández and Rodríguez (2020) stated that if proper conditions are provided, most of the microorganisms might be cultured using traditional microbiology techniques. In our research, we applied three different media and culture conditions to support the isolation of the greatest diversity of potent probiotic microorganisms. Originally, we aimed at isolating putative probiotics from human breast milk among the representatives of Lactobacillus, Bifidobacterium, and Streptococcus genera; therefore, the media selected in the research were dedicated to lactic acid bacteria (LAB). The majority of isolates collected grew on MRS supplemented with L-cysteine. In general, the majority of probiotic isolates are microaerophilic [13], so it is not surprising that isolates in the current study demonstrated best growth at 5% of CO2 rather than under strictly anaerobic or aerobic conditions.
The representatives of Streptococcus and Staphylococcus genera occur most frequently in human breastmilk [12], but the presence of the Enterococcus genus is confirmed as well [14]. The majority of staphylococci are catalase-positive [15] while streptococci cause haemolysis [16], so the representatives of those genera were not considered for further investigation. Since various species of the Enterococcus genus are able to produce lactic acid and have similar metabolism to LAB [17], it is not surprising that they grew on the media dedicated for that group of bacteria. We did not obtain any Bifidobacterium isolates, and there were only 20 strains of Lactobacillus. It is not surprising since those genera constitute no more than 5% of milk microbiota [14]. We did not include the results obtained for those isolates because their application could be patented in the future after further studies.
Enterococcus faecalis is able to cause beta-haemolysis and those isolates were not considered for further analysis. Since in the current study we only considered catalase-negative and gamma-haemolytic Enterococcus faecium, the number of isolates considered was reduced to 51.
Phylogenetic analysis revealed a close relationship between analyzed isolates and the main reason for that observation could be that all donors came from the same region (50 km radius). Since Kielce is a relatively small city (less than 200,000 citizens), there is a chance that some of them had the same general practice doctors and obtained the same treatment for various diseases, especially regarding skin infections or medical care during pregnancy. Another possible route of acquiring strains is through food, especially meat. However, the questionnaire that was given to donors in the current study was insufficient to provide this type of information.
3.2. Identification of Antibiotic Resistance
Based on our results, it seems that almost all of the isolates tested were susceptible to linezolid (Table 1). The same findings were reported by Golob et al. (2019, Slovenia) [18], and Chakraborty et al. (2015, Northern India) [19] or in the metadata analysis study [20]. The resistance to linezolid among E. faecium isolates occurs quite sporadically in general [21]. In Poland, this antibiotic is recommended for use in lung infections caused by Gram positive bacteria, complicated skin infections, infections of soft tissues, or in the cases when infections are caused by vancomycin-resistant enterococci [22]. Enterococcal isolates identified in various hospitals in Poland seemed to be susceptible to linezolid [23], so it could also be related to the occurrence of lineages that are characteristic for the Polish population.
In our study, the isolates tested were also susceptible to ampicillin (Table 1), which contrasts with the results of metadata analysis from 2019 [20] showing that the prevalence of the resistance to that antibiotic is the greatest among E. faecium isolates collected from blood in Europe. On the other hand, Miller et al. (2014) [24] demonstrated that ampicillin is the most effective beta-lactam against enterococci. The reason for those differences could be that the application of ampicillin in different countries could vary so the exposure of women to that antimicrobial would vary as well. In Poland, ampicillin is mostly prescribed to patients who suffer from enterococcal infections [22] and, apparently, those occur more frequently as infections acquired in hospitals [23].
The vast majority of isolates considered in the current study were susceptible to chloramphenicol, which is also confirmed in studies considering E. faecium obtained from food products [25,26]; however, Hollenbeck and Rice (2012) stated that the resistance to that antibiotic is very common among enterococci. It is possible that isolates in our study were susceptible to chloramphenicol because that antibiotic is not present on the list of recommended antimicrobials in Poland [22]; it is rarely prescribed to patients.
Donors who were involved in our study were most likely not exposed to the antibiotics noted above; as a result, their microbiota did not have a chance to develop the resistance to that antimicrobial.
When isolates of E. faecium collected from various parts of human body in Slovenia were tested, they demonstrated the resistance to erythromycin, ampicillin and ciprofloxacin [18]. In our case, the vast majority of strains demonstrated the resistance to erythromycin as well (Table 1). This phenomenon could occur because that antibiotic is used in skin infections and considering the age of women that were donors of breast milk (25–35 years), they could have been exposed to that antibiotic since it is commonly used for acne treatment in Poland [27], but is also allowed for the treatment of some infections during pregnancy [22]. Therefore, microbiota of donors in the current study could have a chance to develop the resistance to that antibiotic.
More than half of the isolates tested in our study demonstrated the resistance to trimethoprim. Metadata analysis from Iran indicated that it occurred in 81% of the analyzed isolates [28]; however, the authors did not consider potential differences in methodologies used for assessing such phenomenon. Trimethoprim is prescribed to patients with urinary tract infections [22], which are quite common—up to 20% of infections treated in primary practice [29], so there are strong chances that donors involved in the current project were exposed to that antibiotic at some point of their life or even during pregnancy.
Our results showed that more than half of the isolates considered were resistant to tetracycline (Table 1). The same phenomenon was reported in other studies taking place in various parts of the world [20,28]. In Poland, that antibiotic is prescribed mostly in the cases of lower respiratory tract infections, inflammation of lesser pelvis, Lyme disease, or other zoonotic diseases [22]. The questionnaire given to donors along with the voluntary consent did not include questions regarding those diseases, but it is very unlikely that the majority of them could have suffered from at least one of them before donating a breast milk sample. Therefore, it is more probable that the resistance of the isolates present in human breast milk was acquired through a different route. One of the possibilities is that tetracycline-resistant strains were ingested with meat, especially that this antibiotic is still used as a growth promoter or in veterinary practices [30].
The metadata analysis [20] demonstrated that the second antibiotic that is ineffective against E. faecium is gentamicin. The resistance to that antimicrobial was also confirmed in studies involving patients suffering from nosocomial infections in Eastern India [19] or among E. faecium isolates from rectal swabs [10]. Our findings confirm the resistance to that antibiotic as well (Table 1). Considering such a wide geographical spread of the resistance to gentamicin, it could be speculated that E. faecium is able to develop intrinsic resistance.
In general, it can be stated that only one isolate met the criteria of EFSA and that was CDCP579. It could be considered as a putative probiotic strain, but then further analysis would be needed: whole genome sequence data analysis, assessment of strain cytotoxicity, survival in gastrointestinal tract, assessment of antimicrobial properties of the strain, etc.
3.3. Verifying the Presence of Transferable Genes
Despite the fact that almost all tested isolates were resistant to erythromycin, ErmB gene was detected only on plasmids of 10 of them (Table 2) and the expression of that gene was confirmed in the case of three (CDCP351, CDCP753, and CDCP787). In the case of other isolates, genes encoding the resistance to that antibiotic were either present on a chromosome or there were other genes present on plasmids that were responsible for that feature. Garrido et al. (2014) [31] stated that numerous genes could be involved in that phenomenon and many of them could be intrinsic. ErmB gene was detected in isolates of E. faecium obtained from diseased farm animals [32], but the authors were using genomic DNA so it is impossible to determine whether that gene was located on plasmids or chromosomes.
There is still relatively little known about the molecular background of the resistance of E. faecium to trimethoprim. Our study suggested that it could be dfrA14 gene located on plasmids while the study carried out with E. faecalis revealed that dfrF gene was involved in that process and it could be an acquired gene but located on a chromosome [33]. Our results confirmed that dfrA14 could also be involved in the resistance to that antibiotic because that gene was expressed in five isolates carrying multiple AMR genes.
In the case of tetracycline, it was already shown that genes encoding the resistance to that antibiotic that could be acquired by enterococci are tetK, tetL, tetO, tetS, and tetM [21]. Our study indicates that tetO gene occurred most frequently on plasmids but only among very few strains. We excluded the expression of that gene in tested isolates (Table 3). Since tetO, tetS, and tetM genes could also be present on chromosomes [24], it is possible in the case of isolates obtained in the current study that the resistance to that antibiotic was intrinsic, especially that resistance genes were present on plasmids of only nine strains (Table 2). On the other hand, the expression of tetK gene was confirmed in the case of two isolates. It was previously shown in a study carried out in Scandinavia and published in 2018 that breast milk could be the source of tetracycline resistance genes or transposases that could contribute to the relocation of those genes [11].
As for the gentamicin resistance, none of the tested isolates had aac(6′)-aph(2″) gene on plasmids. It is possible that this gene was present on chromosomes of those isolates since that gene was responsible for intrinsic resistance of enterococci in previous studies [24].
The presence of neomycin resistance gene in Enterococcus faecium has been previously confirmed [34]. In our study, we demonstrated the expression of aph(3′)-IIIa gene in the case of isolates in which that gene was discovered on plasmids. On the other hand, it seems that our study is the first to report the presence of marA gene on the plasmid of E. faecium.
It is safe to say that molecular mechanisms of the resistance to vancomycin are best known in the Enterococcus genus [35] and some of them, namely the clusters vanA, vanB, vanG, vanM, and vanT are transferable. Curiously, in the current study, only 21% of tested isolates were resistant to vancomycin; however, none contained tested resistance genes on plasmids (vanHa and ErmG, Table 2). We decided to choose those genes because after careful analysis of CARD and the search in BLAST we discovered that those two genes could be frequently distributed among Enterococcus spp.
It is possible then that the isolates tested held other genes that determined their resistance to vancomycin or that they developed intrinsic resistance to that antibiotic that has already been reported [21]. Another explanation is that acquired resistance genes could be located on the chromosome. The same conclusion applies to all the other antibiotics tested, in which case genes encoding resistance were not detected in plasmid DNA of the tested isolates.
We also noticed that if isolates originated from the same donor, then they carried the same AMR genes on plasmids. This suggests that either those isolates acquired those genes in the same environment and were then transferred to human body—the transfer of those genes between bacterial cells in human body is very easy and happens spontaneously—or that the human microbiome acquired the resistance in contact with the antibiotic that was taken incorrectly (the treatment interrupted before it was complete).
4. Materials and Methods
4.1. Chemicals
All dry culture media and their components or supplements used for their preparation were purchased from Biomaxima (Lublin, Poland), unless otherwise stated. L-cysteine hydrochloride, L-tryptophan, biotin, thiamine hydrochloride, adenine, guanine, xanthine, uracil, glucose, cyanocobalamin, lactose monohydrate, sucrose, soluble starch, gelatine, sodium chloride, disodium hydrogen phosphate, sodium acetate, zinc sulfate heptahydrate, manganese(II) chloride tetrahydrate, magnesium glycerophosphate monohydrate, calcium D-gluconate monohydrate, cobalt(II) sulfate monohydrate, copper(II) sulfate pentahydrate, and Gram staining kit were purchased from Pol-Aura (Dywity near Olsztyn, Poland). Menadione, pyridoxine, pantothenate, nicotinamide, and ascorbic acid were purchased from Sigma Aldrich (St. Louis, MO, USA). Peptone was purchased from A&A Biotechnology (Gdynia, Poland). Seventeen antibiotics were obtained for susceptibility testing: gentamycin, neomycin sulfate, tetracycline, chloramphenicol, vancomycin hydrochloride, tylosine phosphate (Pol-Aura, Dywity near Olsztyn, Poland), kanamycin sulfate, streptomycin sulfate salt, erythromycin, clindamycin hydrochloride, ampicillin, linezolid, trimethoprim, ciprofloxacin, rifampicin (Sigma Aldrich, St. Louis, MO, USA), and quinupristin–dalfopristin mesylate complex (Santa Cruz Biotechnology Inc., Dallas, TX, USA). All kits for the extraction of nucleic acids, purification of PCR products, and cDNA synthesis were purchased from A&A Biotechnology (Gdynia, Poland): Genomic Mini AX Bacteria+ Spin for the extraction of genomic DNA; Plasmid Mini AX and mutanolisine solution for the extraction of plasmid DNA; EPPiC for the purification of products obtained after PCR; Total RNA Mini for the extraction of RNA; TranScriba Kit for cDNA synthesis. Luna Universal qPCR Master Mix (New England Biotechnology, Ipswich, MA, USA) was used for the determination of gene expression. Primers were synthesized and purified by Future Synthesis sp. z o.o. (Poznań, Poland). Agarose, 10X TAE buffer, SimplySafe dye, Perfect Plus Molecular Weight Quantitative Ladder, Perfect Plus 50–500 bp DNA Ladder, and Color Taq PCR Master Mix (2x) were purchased from EURx sp. z o.o. (Gdańsk, Poland).
4.2. Isolation of Bacterial Isolates
Breastmilk samples from healthy women up to 4 days after giving birth were collected from July to October 2019 in Szpital Kielecki Św. Aleksandra (Kielce, Poland). Samples were collected with the breast pump Madela Swing (Madela Polska sp. z o.o., Warszawa, Poland) to 30 mL sterile capped containers after cleaning the breast with water. Samples were then transferred immediately to the laboratory, diluted with peptone (1 g/L) saline water (8.5 g/L), and plated on MRS agar supplemented with L-cysteine (0.3 g/L, MRS-Cys), bifidobacterium medium (BM), and M17 medium with lactose. Plates were incubated for 72 h under following conditions: MRS-Cys at 37 °C (5% of CO2), BM at 37 °C under anaerobic conditions in a Bactron 300 anaerobic chamber (Sheldon Manufacturing Inc., Cornelius, OR, USA), and M17 at 45 °C under aerobic conditions. After incubation we selected 10 random colonies from MRS-Cys, 5 from BM, 5 from M17, and we streaked them on plates with fresh medium. We then carried out catalase testing and Gram staining. We deposited pure cultures in Microbank vials (Biomaxima, Lublin, Poland) and stored them at −80 °C until further analysis.
4.3. Assessment of Haemolytic Properties and Catalase Activity Testing
Collected isolates were tested against their haemolytic properties. Prior to experiments, isolates were incubated overnight in fresh liquid medium. We then transferred bacterial cultures to plates with Colombia agar (g/L): casein peptone 10, beef extract 5, brain heart infusion 3, yeast extract 5, soluble starch 1, sodium chloride 5, and agar 13 supplemented with 5% of defibrinated sheep blood. Plates were incubated for 48 h at 37 °C and 5% CO2. When we inoculated isolates on Colombia agar, we tested in parallel their catalase activity by transferring a drop of overnight culture and a drop of 3% hydrogen peroxide solution on the glass slide. Only isolates that demonstrated gamma haemolysis (no halo or clearance zone around colonies) and were catalase negative were considered for further experiments.
4.4. Antibiotic Susceptibility Testing
Resistance to antibiotics was examined according to ISO 10932:2010 [1]. Firstly, we prepared sterile solutions of each antibiotic at concentrations required in the norm and 50 µL was transferred into a certain well of a 96-well sterile plate. In columns for negative control, we pipetted 50 µL of sterile water. Plates were stored at −20 °C until the day of analysis but no longer than one month. Twenty-four hours prior to experiments, we inoculated Elliker broth with bacterial culture and incubated at 37 °C and 5% CO2. We then adjusted the optical density of the suspensions to 1 MF (DEN-1B densitometer, Biosan Medical-Biological Research and Technologies, Riga, Latvia) and diluted it 500 fold with IST broth. The bacterial suspension obtained was added (50 µL) to wells containing antibiotic solutions and to the column for positive control. In the column designated for negative control, sterile IST broth (50 µL) was added. Lids were placed on the top of plates and the plates were incubated at 37 °C and 5% of CO2 for 48 h. Antibiotic concentration at which there was at least 80% visual growth reduction in comparison to positive control was indicated as MIC.
4.5. Extraction of Genomic DNA, Sequencing and Identification
For the extraction of genomic DNA, we collected 1 mL of overnight culture of bacterial isolate, centrifuged it at 9800× g (Gusto high-speed mini centrifuge, Heathrow Scientific, Vernon Hills, IL, USA), discarded the supernatant, added 1 mL of sterile water, vortexed (V3, Elmi Skyline, Riga, Latvia), and repeated centrifugation. We then discarded the supernatant and carried out the extraction of genomic DNA with Genomic Mini AX Bacteria+ Spin kit per manufacturer’s instruction with slight modification—we extended the time of each incubation with enzymes by 5 min. We then measured DNA concentration and purity using a NanoDrop™ One/OneC Microvolume UV–Vis Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). DNA samples were stored at −20 °C for further analysis. PCR for 16S rRNA gene was carried out in a LightCycler 96 thermal cycler (Roche Polska, Warszawa, Poland). Reactions were carried out in 50 µL volume: 25 µL of Color Taq PCR Master Mix (2x); 1 µL of 10 µM solution of each primer—27F 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1495R 5′-CTACGGCTACCTTGTTACGA-3′ [36]; 5 µL of 25 mM MgCl2; 5 µL of DNA solution containing 30 ng of DNA; and 13 µL of nuclease-free water. Time/temperature profile was as follows: initial denaturation 94 °C/5 min; 30 cycles: denaturation 94 °C/30 s, annealing 58 °C/30 s, elongation 72 °C/2 min; final elongation 72 °C/7 min; and cooling at 37 °C [36]. Reaction products were visualized on 1.5% (w/v) agarose gel in TAE containing 5 µL of SimplySafe dye per 100 mL of gel against Perfect Plus Molecular Weight Quantitative Ladder. Electrophoresis was carried out for 60 min/100 V in Wide Mini-Sub Cell GT Cell (Bio-Rad Polska sp. z o.o., Warszawa, Poland) and gels were visualized in Gel Doc EZ System (Bio-Rad Polska sp. z o.o., Warszawa, Poland). After PCR, 10 µL of product obtained was combined with 2 µL of EPPiC and purification was carried out according to the manufacturer’s instructions (37 °C/15 min and 70 °C/15 min). We then adjusted the concentration of DNA to 50 ng/µL and added a primer (5 µM solution) and sent samples for Sanger sequencing to Macrogene Europe (Amsterdam, Netherlands). The sequences obtained were trimmed in Chromas (version 2.6.6; http://www.technelysium.com.au/chromas.html, accessed on 22 February 2022) to remove ambiguous sequences and subjected to analysis in BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome, accessed on 22 February 2022) for identification. Only results including identity above 98% and e-value lower than 1 × 10−6 were considered. After that process, we submitted sequences for assigning accession numbers. Additionally, we carried out the alignment of all collected sequences to exclude the possibility that the isolates obtained could be the same strains. We used the phylogeny.fr platform for that purpose. For the rest of the analysis and experiments, we selected isolates that were identified to the species level. Isolates that belonged to other species were not considered in the current study.
4.6. Phylogenetic Analysis
Phylogenetic relationships between tested isolates were examined on the phylogeny.fr platform [37] with the settings as described by Wajda et al. (2019) [38]. If support values were below 70%, then branches were dropped. We selected Enterococcus faecalis V583 (accession no. AE016830.1) as an outgroup.
4.7. Extraction of Plasmid DNA and Detection of Antibiotic Resistance Genes with PCR
The selection of reference genes was carried out in The Comprehensive Antibiotic Resistance Database (CARD, https://card.mcmaster.ca/, accessed on 22 February 2022) based on the distribution of those among species that could come into contact with E. faecium in the intestine [39] or on skin [40]. The extraction of plasmid DNA was carried out with Plasmid Mini AX according to manufacturer’s instructions; however, 5 µL mutanolisin (10 U/µL) was added in the first extraction step. PCR was carried out in a 20 µL volume: 10 µL of Color Taq PCR Master Mix (2x); 0,4 µL of each primer (10 µM) solution (Table 4); volume of 25 mM MgCl2 solution adjusted to the particular primer pair (Table 4); 1 µL of DNA solution containing 2 ng of plasmid DNA; and adjusted to 20 µL with nuclease free water. Time/temperature profile was as follows: initial denaturation 94 °C/5 min; 35 cycles: denaturation 94 °C/30 s, annealing temperature established for particular primer pair /30 s, elongation 72 °C/2 min; final elongation 72 °C/7 min; and cooling at 37 °C. PCR products were visualized as above; however, for products smaller than 500 bp, Perfect Plus 50–500 bp DNA Ladder and 2% agarose gel were used.

Table 4.
PCR and real-time PCR conditions for the detection of antibiotic resistance genes in Enterococcus faecium isolates obtained from human breast milk.
4.8. Determination of Gene Expression
We determined gene expression only for those isolates that held genes encoding resistance to at least two antibiotics. We prepared bacterial biomass as for antibiotic susceptibility testing. We then combined 0.5 mL of resulting suspension with 0.5 mL of the antibiotic solution that the isolate tested was resistant to and obtained final antibiotic concentration one fold lower than the MIC value determined previously. We used sterile water for control samples instead of the antibiotic solution. After incubation (37 °C/48 h), we isolated total RNA using Total RNA Mini and synthesized cDNA with the TranScriba Kit, and used it together with the Luna Universal qPCR Master Mix to verify if detected genes were involved in antibiotic resistance. The reaction mixture (20 µL) contained 10 µL of Luna Universal qPCR Master Mix, 0.5 µL of each primer (10 µM solution, Table 4), 2 µL cDNA (30 ng), and 7 µL of nuclease free water (NFW). In the case of the negative control of reverse transcription, we used NFW instead of TranScriba. For positive controls, we used primers designed for the 16S rRNA region. The thermal/time profile used in those reactions was: 95 °C/60 s, 95 °C/15 s, 40 cycles at 95 °C for 15 s and 60 °C for 30 s. Reactions were carried out in triplicate using a LightCycler 96 thermal cycler (Roche Polska, Warszawa, Poland).
Relative ratios were automatically generated by the software according to Equation (1):
where E is reaction efficiency obtained for the target and reference gene, respectively, (E = 2); ΔCt is a value of the difference of Ct values calculated for the target gene and a reference gene.
5. Conclusions
Many bacterial specimens isolated from human breast milk, especially the representatives of the Enterococcus genus, are resistant to multiple antibiotics. The investigation of the molecular background of that phenomenon could be very complex and time consuming because sometimes acquired resistance genes can be present on chromosomes due to the presence of transposons. It also seems justified to sequence plasmid DNA for each isolate considered as a putative probiotic because that information could help verify the presence of transferable antibiotic genes. We demonstrated that some of the genes found on plasmids are expressed, especially dfrA14 that encodes the resistance to trimethoprim. However, it is possible to find potential candidates for probiotics in human breast milk because one of the tested isolates (CDCP539) did not show any antibiotic resistance.
Author Contributions
Conceptualization, Ł.W.; methodology, Ł.W.; software, A.O.; validation, Ł.W., A.O., E.B. and P.G.; formal analysis, Ł.W., A.O., E.B. and P.G.; investigation, A.O., E.B. and P.G.; resources, Ł.W.; data curation, Ł.W.; writing—original draft preparation, Ł.W. and A.O.; writing—review and editing, Ł.W.; visualization, P.G.; supervision, Ł.W.; project administration, Ł.W.; funding acquisition, Ł.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Funds for Regional Development in the competition 1.2 “R&D capacity of Swietokrzyskie companies” for The Regional Operational Programme for Świętokrzyskie 2014-2020, grant number RPSW.01.02.00-26-0003/18: Development of innovative preparations based on human breast milk.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of Swietokrzyski Medical Board (protocol code 11/2019-VII approved on 07.02.2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Informed consent has been obtained from the patient(s) to publish all papers based on collected data.
Data Availability Statement
Since the project was performed by a private company, data were not published in any publicly available databases.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- ISO. Milk and Milk Products—Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria (LAB); ISO: Geneva, Switzerland, 2012; Volume 9936. [Google Scholar]
- EFSA (European Food Safety Authority). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 1–10. [Google Scholar] [CrossRef]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The genus Enterococcus: Between probiotic potential and safety concerns-an update. Front. Microbiol. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.A.R.; Abe, H.A.; Sousa, N.C.; Silva, R.D.F.; Cordeiro, C.A.M.; Gomes, G.F.E.; Ready, J.S.; Mouriño, J.L.P.; Martins, M.L.; Carneiro, P.C.F.; et al. Enterococcus faecium as potential probiotic for ornamental neotropical cichlid fish, Pterophyllum scalare (Schultze, 1823). Aquac. Int. 2019, 27, 463–474. [Google Scholar] [CrossRef]
- Da Costa Sousa, N.; Silva do Couto, M.V.; Andrade Abe, H.; Guimarães Paixão, P.E.; Martins Cordeiro, C.A.; Monteiro Lopes, E.; Ready, J.S.; Fernandes Alves Jesus, G.; Laterça Martins, M.; Pereira Mouriño, J.L.; et al. Effects of an Enterococcus faecium -based probiotic on growth performance and health of Pirarucu, Arapaima gigas. Aquac. Res. 2019, 50, 1–9. [Google Scholar] [CrossRef]
- Hu, C.; Xing, W.; Liu, X.; Zhang, X.; Li, K.; Liu, J.; Deng, B.; Deng, J.; Li, Y.; Tan, C. Effects of dietary supplementation of probiotic Enterococcus faecium on growth performance and gut microbiota in weaned piglets. AMB Express 2019, 9, 1–2. [Google Scholar] [CrossRef]
- Chen, Y.J.; Min, B.J.; Cho, J.H.; Kwon, O.S.; Son, K.S.; Kim, I.H.; Kim, S.J. Effects of dietary Enterococcus faecium SF68 on growth performance, nutrient digestibility, blood characteristics and faecal noxious gas content in finishing pigs. Asian-Australas. J. Anim. Sci. 2006, 19, 406–411. [Google Scholar] [CrossRef]
- Pollmann, M.; Nordhoff, M.; Pospischil, A.; Tedin, K.; Wieler, L.H. Effects of a Probiotic Strain of Natural Chlamydia Infection in Swine. Infect. Immun. 2005, 73, 4346–4353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalkhali, S.; Mojgani, N. In vitro and in vivo safety analysis of Enterococcus faecium 2C isolated from human breast milk. Microb. Pathog. 2018, 116, 73–77. [Google Scholar] [CrossRef]
- Aşgın, N.; Taşkın, E. Is there any association between antibiotic resistance and virulence genes in Enterococcus isolates? Med. Sci. Discov. 2019, 6, 310–315. [Google Scholar] [CrossRef]
- Pärnänen, K.; Karkman, A.; Hultman, J.; Lyra, C.; Bengtsson-Palme, J.; Larsson, D.G.J.; Rautava, S.; Isolauri, E.; Salminen, S.; Kumar, H.; et al. Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fernández, L.; Rodríguez, J.M. Human Milk Microbiota: Origin and Potential Uses. Nestle Nutr. Inst. Workshop Ser. 2020, 94, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Breast milk microbiota: A complex microbiome with multiple impacts and conditioning factors. J. Infect. 2020, 81, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-Negative Staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [Green Version]
- Patterson, M.J. Streptococcus. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 2020; pp. 1–25. [Google Scholar]
- Ramsey, M.; Hartke, A.; Huycke, M. The Physiology and Metabolism of Enterococci. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Clewell, D., Gilmore, M., Ike, Y., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014; pp. 1–55. [Google Scholar]
- Golob, M.; Pate, M.; Kušar, D.; Dermota, U.; Avberšek, J.; Papić, B.; Zdovc, I.; Bondi, M. Antimicrobial Resistance and Virulence Genes in Enterococcus faecium and Enterococcus faecalis from Humans and Retail Red Meat. Biomed Res. Int. 2019, 2019, 14–16. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Pal, N.K.; Sarkar, S.; Gupta, M. Sen Antibiotic resistance pattern of Enterococci isolates from nosocomial infections in a tertiary care hospital in Eastern India. J. Nat. Sci. Biol. Med. 2015, 6, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, S.M.; Shiadeh; Pormohammad, A.; Hashemi, A.; Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 2713–2725. [Google Scholar] [CrossRef] [Green Version]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Hryniewicz, W.; Ozorowski, T. Szpitalna Lista Antybiotyków Propozycja Kierowana do Szpitali; Narodowy Program Ochrony Antybiotyków: Warszawa, Poland, 2011. [Google Scholar]
- Gawryszewska, I.; Żabicka, D.; Bojarska, K.; Malinowska, K.; Hryniewicz, W.; Sadowy, E. Invasive enterococcal infections in Poland: The current epidemiological situation. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti. Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Trivedi, K.; Cupakova, S.; Karpiskova, R. Virulence factors and antibiotic resistance in enterococci isolated from food-stuffs. Vet. Med. 2011, 56, 352–357. [Google Scholar] [CrossRef]
- Sanlibaba, P.; Senturk, E. Prevalence, characterization and antibiotic resistance of enterococci from traditional cheeses in Turkey. Int. J. Food Prop. 2018, 21, 1955–1963. [Google Scholar] [CrossRef] [Green Version]
- Kamuś, M. Leczenie Trądziku Pospolitego—Charakterystyka Schorzenia, Przegląd Preparatów OTC Oraz Rx do Stosowania Miejscowego; Wielkopolska Okręgowa Izba Aptekarska: Poznań, Poland, 2021. [Google Scholar]
- Asadollahi, P.; Razavi, S.; Asadollahi, K.; Pourshafie, M.R.; Talebi, M. Rise of antibiotic resistance in clinical enterococcal isolates during 2001–2016 in Iran: A review. New Microbes New Infect. 2018, 26, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, E.; Suchocka, U.; Bosacka, K.; Hryniewicz, W. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1363–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granados-Chinchilla, F.; Rodríguez, C. Tetracyclines in Food and Feedingstuffs: From Regulation to Analytical Methods, Bacterial Resistance, and Environmental and Health Implications. J. Anal. Methods Chem. 2017, 2017, 1315497. [Google Scholar] [CrossRef]
- Marin Garrido, A.; Gálvez, A.; Pérez Pulido, R. Antimicrobial Resistance in Enterococci. J. Infect. Dis. Ther. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Šeputiene, V.; Bogdaite, A.; Ružauskas, M.; Sužiedeliene, E. Antibiotic resistance genes and virulence factors in Enterococcus faecium and Enterococcus faecalis from diseased farm animals: Pigs, cattle and poultry. Pol. J. Vet. Sci. 2012, 15, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Coque, T.M.; Singh, K.V.; Weinstock, G.M.; Murray, B.E. Characterization of dihydrofolate reductase genes from trimethoprim- susceptible and trimethoprim-resistant strains of Enterococcus faecalis. Antimicrob. Agents Chemother. 1999, 43, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Woegerbauer, M.; Zeinzinger, J.; Springer, B.; Hufnagl, P.; Indra, A.; Korschineck, I.; Hofrichter, J.; Kopacka, I.; Fuchs, R.; Steinwider, J.; et al. Prevalence of the aminoglycoside phosphotransferase genes aph(3’)-IIIa and aph(3’)-IIa in Escherichia coli, Enterococcus faecalis, Enterococcus faecium, Pseudomonas aeruginosa, Salmonella enterica subsp. Enterica and Staphylococcus aureus isolates in Aust. J. Med. Microbiol. 2014, 63, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Gao, W.; Qing, M.; Sun, Z.; Wang, W.; Liu, W.; Pan, L. Identification and characterization of lactic acid bacteria isolated from traditional pickles in Sichuan, China. J. Gen. Appl. Microbiol 2012, 58, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Wajda, Ł.; Wyderka, M.; Polak, Z.; Duda-Chodak, A.; Makarewicz, M. Examination of novel Aureobasidium pullulans isolates dominating apple microflora and assessing their potential for apple juice spoilage. World J. Microbiol. Biotechnol. 2018, 34, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Urbaniak, C.; Burton, J.P.; Reid, G. Breast, milk and microbes: A complex relationship that does not end with lactation. Women’s Health 2012, 8, 385–398. [Google Scholar] [CrossRef] [Green Version]
- Ouoba, L.I.I.; Lei, V.; Jensen, L.B. Resistance of potential probiotic lactic acid bacteria and bifidobacteria of African and European origin to antimicrobials: Determination and transferability of the resistance genes to other bacteria. Int. J. Food Microbiol. 2008, 121, 217–224. [Google Scholar] [CrossRef]
- Agersø, Y.; Jensen, L.B.; Givskov, M.; Roberts, M.C. The identification of a tetracycline resistance gene tet(M), on a Tn916-like transposon, in the Bacillus cereus group. FEMS Microbiol. Lett. 2002, 214, 251–256. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).