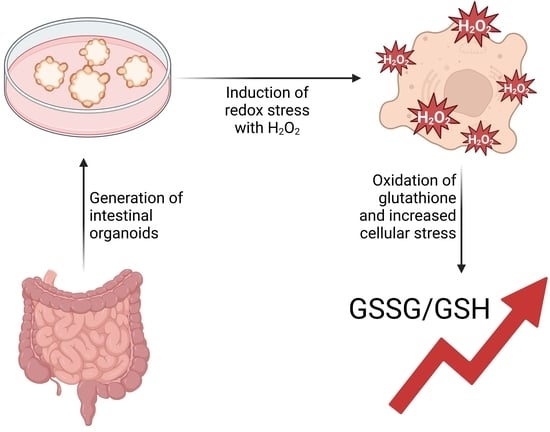
The Intricacies of Inflammatory Bowel Disease: A Preliminary Study of Redox Biology in Intestinal Organoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Intestinal Organoids
2.2. Immunofluorescent Analysis of Organoids
2.3. Production of Lentiviral Particles and Transduction of Organoids
2.4. Glutaredoxin-Based Redox Imaging
2.5. Glutathione Analysis
2.6. Mitochondrial Stress Analysis
2.7. Statistical Analysis
3. Results
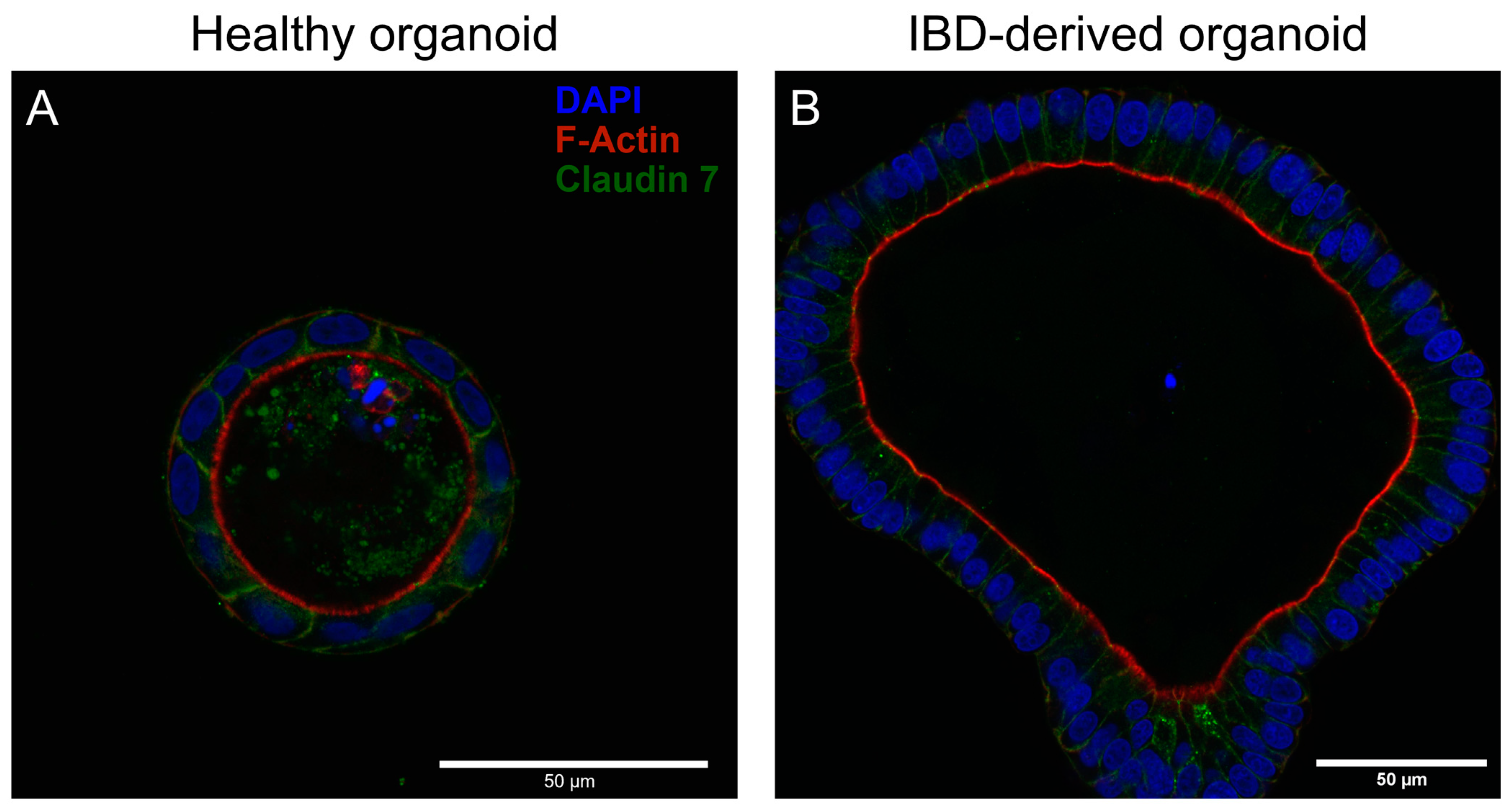
3.1. Immunofluorescent Analysis
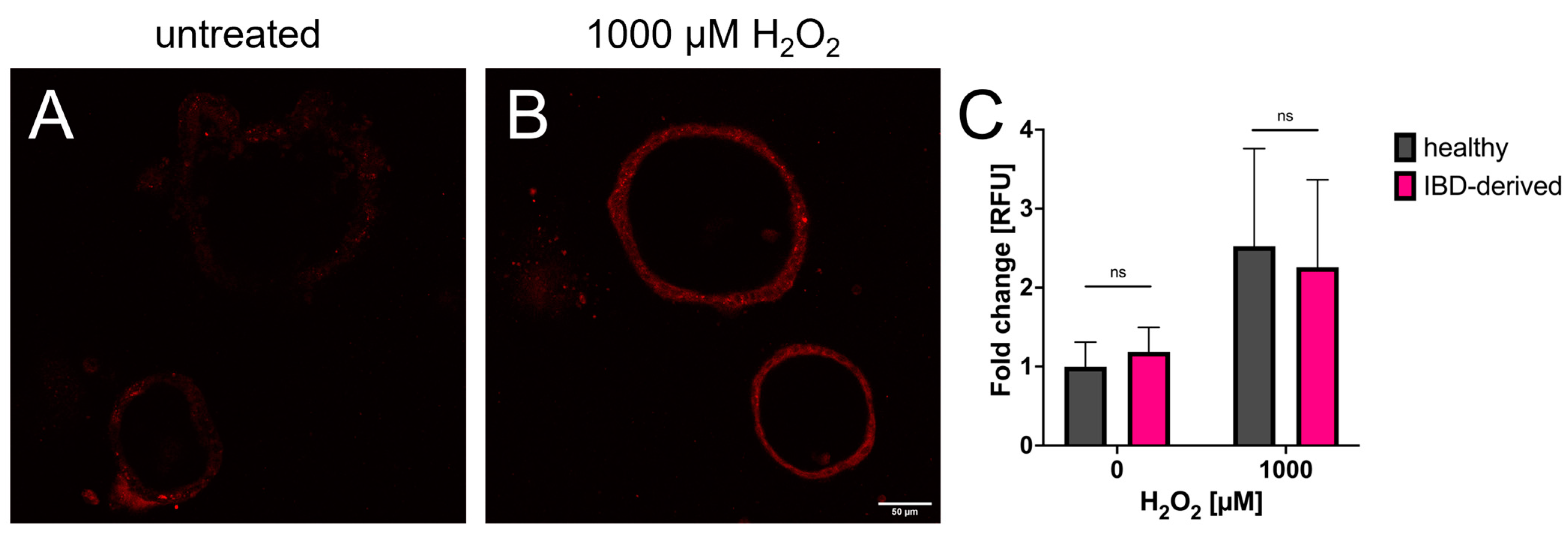
3.2. Redox Imaging
3.3. Glutathione Analysis
3.4. Mitochondrial Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Günther, C.; Winner, B.; Neurath, M.F.; Stappenbeck, T.S. Organoids in Gastrointestinal Diseases: From Experimental Models to Clinical Translation. Gut 2022, 71, 1892–1908. [Google Scholar] [CrossRef]
- Dutta, D.; Clevers, H. Organoid Culture Systems to Study Host–Pathogen Interactions. Curr. Opin. Immunol. 2017, 48, 15–22. [Google Scholar] [CrossRef]
- Csukovich, G.; Pratscher, B.; Burgener, I.A. The World of Organoids: Gastrointestinal Disease Modelling in the Age of 3R and One Health with Specific Relevance to Dogs and Cats. Animals 2022, 12, 2461. [Google Scholar] [CrossRef] [PubMed]
- Stange, E.F. Current and Future Aspects of IBD Research and Treatment: The 2022 Perspective. Front. Gastroenterol. 2022, 1, 914371. [Google Scholar] [CrossRef]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Webb, C.; Wennogle, S.; Dow, S. Humoral Immune Responses against Gut Bacteria in Dogs with Inflammatory Bowel Disease. PLoS ONE 2019, 14, e0220522. [Google Scholar] [CrossRef]
- Jiminez, J.A.; Uwiera, T.C.; Inglis, G.D.; Uwiera, R.R.E. Animal Models to Study Acute and Chronic Intestinal Inflammation in Mammals. Gut. Pathog 2015, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Craven, M.; Simpson, J.W.; Ridyard, A.E.; Chandler, M.L. Canine Inflammatory Bowel Disease: Retrospective Analysis of Diagnosis and Outcome in 80 Cases (1995–2002). J. Small Anim. Pract. 2004, 45, 336–342. [Google Scholar] [CrossRef]
- Washabau, R.J.; Day, M.J.; Willard, M.D.; Hall, E.J.; Jergens, A.E.; Mansell, J.; Minami, T.; Bilzer, T.W. Endoscopic, Biopsy, and Histopathologic Guidelines for the Evaluation of Gastrointestinal Inflammation in Companion Animals. J. Vet. Intern. Med. 2010, 24, 10–26. [Google Scholar] [CrossRef]
- Singh, V.; Ahlawat, S.; Mohan, H.; Gill, S.S.; Sharma, K.K. Balancing Reactive Oxygen Species Generation by Rebooting Gut Microbiota. J. Appl. Microbiol. 2022, 132, 4112–4129. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Cao, Z.; Lindsay, J.G.; Isaacs, N.W. Mitochondrial Peroxiredoxins: Structure and Function. Subcell. Biochem. 2007, 44, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS Promote Mitochondrial Dysfunction and Inflammation in Ischemic Acute Kidney Injury by Disrupting TFAM-Mediated MtDNA Maintenance. Theranostics 2021, 11, 1845. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hatori, Y.; Kubo, T.; Sato, Y.; Inouye, S.; Akagi, R.; Seyama, T. Visualization of the Redox Status of Cytosolic Glutathione Using the Organelle-and Cytoskeleton-Targeted Redox Sensors. Antioxidants 2020, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Pratscher, B.; Meneses, A.M.C.; Tschulenk, W.; Walter, I.; Swoboda, A.; Kruitwagen, H.S.; Schneeberger, K.; Penning, L.C.; Spee, B.; et al. Generation of Differentiating and Long-Living Intestinal Organoids Reflecting the Cellular Diversity of Canine Intestine. Cells 2020, 9, 822. [Google Scholar] [CrossRef]
- van Ineveld, R.L.; Ariese, H.C.R.; Wehrens, E.J.; Dekkers, J.F.; Rios, A.C. Single-Cell Resolution Three-Dimensional Imaging of Intact Organoids. J. Vis. Exp. 2020, 2020, e60709. [Google Scholar] [CrossRef]
- Gutscher, M.; Pauleau, A.L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-Time Imaging of the Intracellular Glutathione Redox Potential. Nat. Methods 2008, 5, 553–559. [Google Scholar] [CrossRef] [PubMed]
- d’Aldebert, E.; Quaranta, M.; Sébert, M.; Bonnet, D.; Kirzin, S.; Portier, G.; Duffas, J.P.; Chabot, S.; Lluel, P.; Allart, S.; et al. Characterization of Human Colon Organoids from Inflammatory Bowel Disease Patients. Front. Cell Dev. Biol. 2020, 8, 363. [Google Scholar] [CrossRef]
- Dotti, I.; Mora-Buch, R.; Ferrer-Picón, E.; Planell, N.; Jung, P.; Masamunt, M.C.; Leal, R.F.; de Carpi, J.M.; Llach, J.; Ordás, I.; et al. Alterations in the Epithelial Stem Cell Compartment Could Contribute to Permanent Changes in the Mucosa of Patients with Ulcerative Colitis. Gut 2017, 66, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Noben, M.; Verstockt, B.; de Bruyn, M.; Hendriks, N.; Van Assche, G.; Vermeire, S.; Verfaillie, C.; Ferrante, M. Epithelial Organoid Cultures from Patients with Ulcerative Colitis and Crohns Disease: A Truly Long-Term Model to Study the Molecular Basis for Inflammatory Bowel Disease? Gut 2017, 66, 2193–2195. [Google Scholar] [CrossRef]
- Stenke, E.; Bourke, B.; Knaus, U.G. NAPDH Oxidases in Inflammatory Bowel Disease. In Methods in Molecular Biology; Springer Nature: Cham, Switzerland, 2019; Volume 1982. [Google Scholar]
- Zhou, G.; Yu, L.; Fang, L.; Yang, W.; Yu, T.; Miao, Y.; Chen, M.; Wu, K.; Chen, F.; Cong, Y.; et al. CD177+ Neutrophils as Functionally Activated Neutrophils Negatively Regulate IBD. Gut 2018, 67, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Lim, J.B.; Langford, T.F.; Huang, B.K.; Deen, W.M.; Sikes, H.D. A Reaction-Diffusion Model of Cytosolic Hydrogen Peroxide. Free Radic. Biol. Med. 2016, 90, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.; Wang, N.; Sugimura, R. Give Them Vasculature and Immune Cells—How to Fill the Gap of Organoids. Cells Tissues Organs 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic Enteropathies in Dogs: Evaluation of Risk Factors for Negative Outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csukovich, G.; Huainig, J.; Troester, S.; Pratscher, B.; Burgener, I.A. The Intricacies of Inflammatory Bowel Disease: A Preliminary Study of Redox Biology in Intestinal Organoids. Organoids 2023, 2, 156-164. https://doi.org/10.3390/organoids2030012
Csukovich G, Huainig J, Troester S, Pratscher B, Burgener IA. The Intricacies of Inflammatory Bowel Disease: A Preliminary Study of Redox Biology in Intestinal Organoids. Organoids. 2023; 2(3):156-164. https://doi.org/10.3390/organoids2030012
Chicago/Turabian StyleCsukovich, Georg, Janina Huainig, Selina Troester, Barbara Pratscher, and Iwan Anton Burgener. 2023. "The Intricacies of Inflammatory Bowel Disease: A Preliminary Study of Redox Biology in Intestinal Organoids" Organoids 2, no. 3: 156-164. https://doi.org/10.3390/organoids2030012
APA StyleCsukovich, G., Huainig, J., Troester, S., Pratscher, B., & Burgener, I. A. (2023). The Intricacies of Inflammatory Bowel Disease: A Preliminary Study of Redox Biology in Intestinal Organoids. Organoids, 2(3), 156-164. https://doi.org/10.3390/organoids2030012