Matrigel Tunes H9 Stem Cell-Derived Human Cerebral Organoid Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. hESC Cell Lines
2.2. Solubilized Matrigel
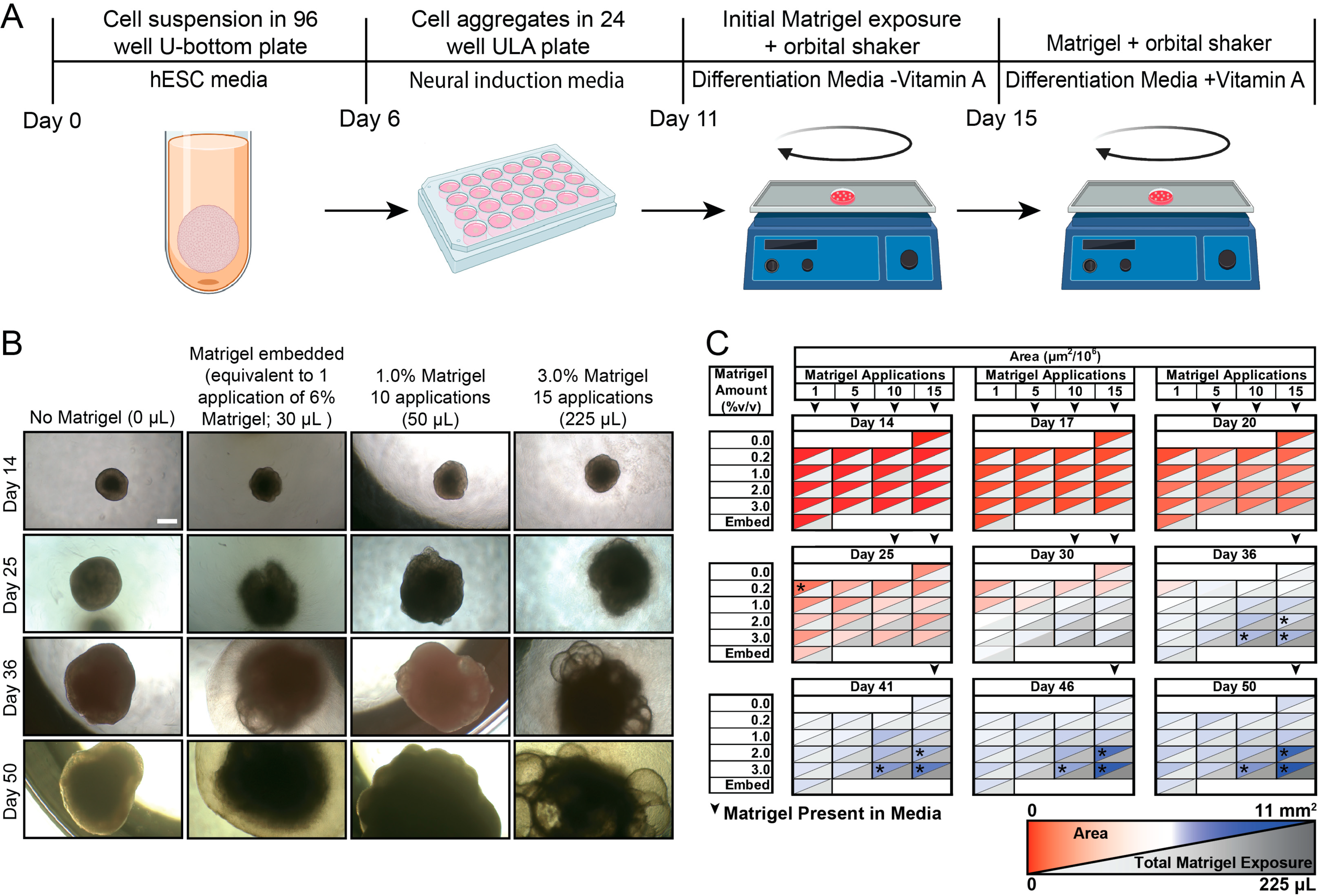
2.3. Cerebral Organoid Generation
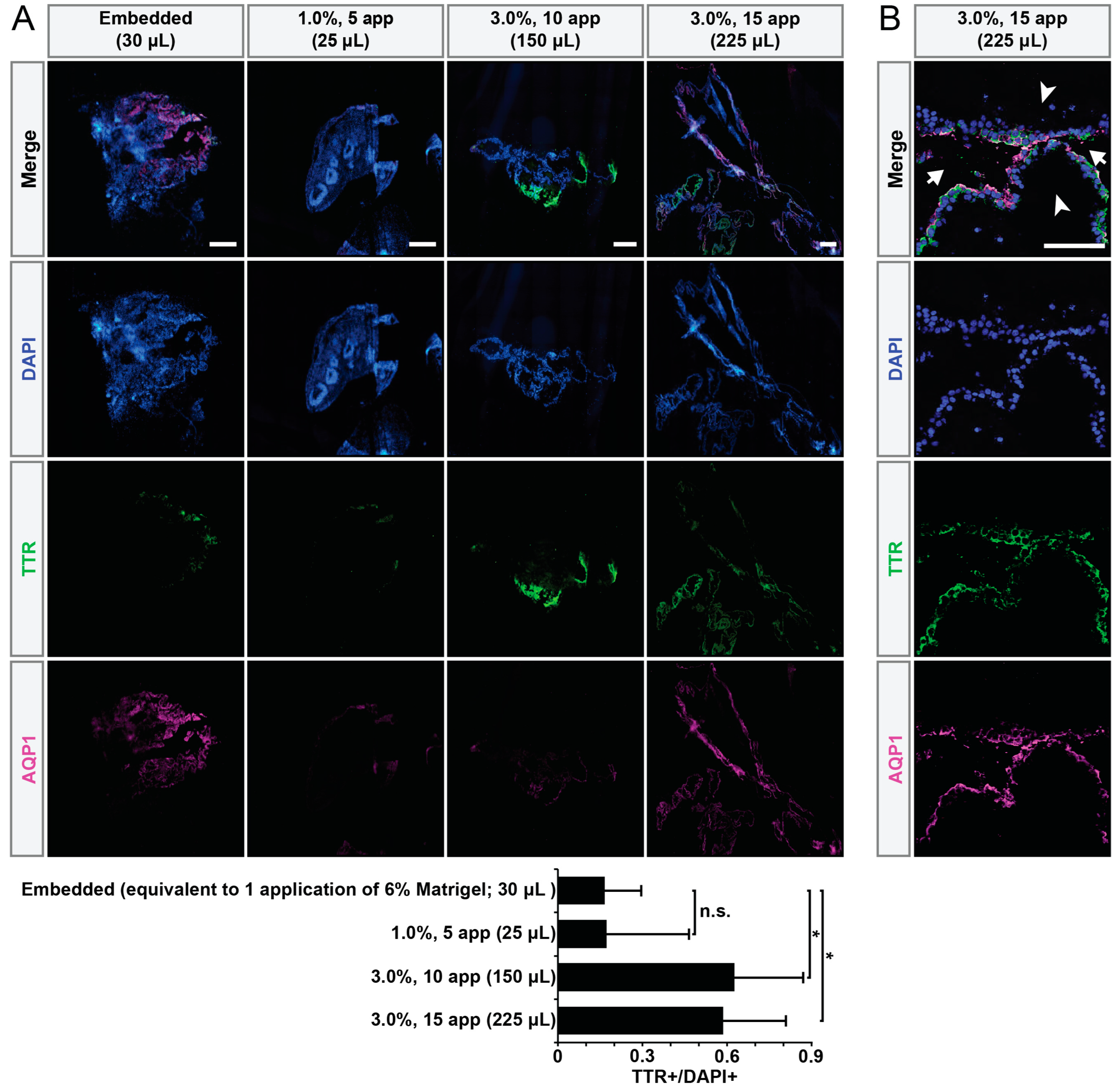
2.4. Cryosectioning and Immunohistochemistry
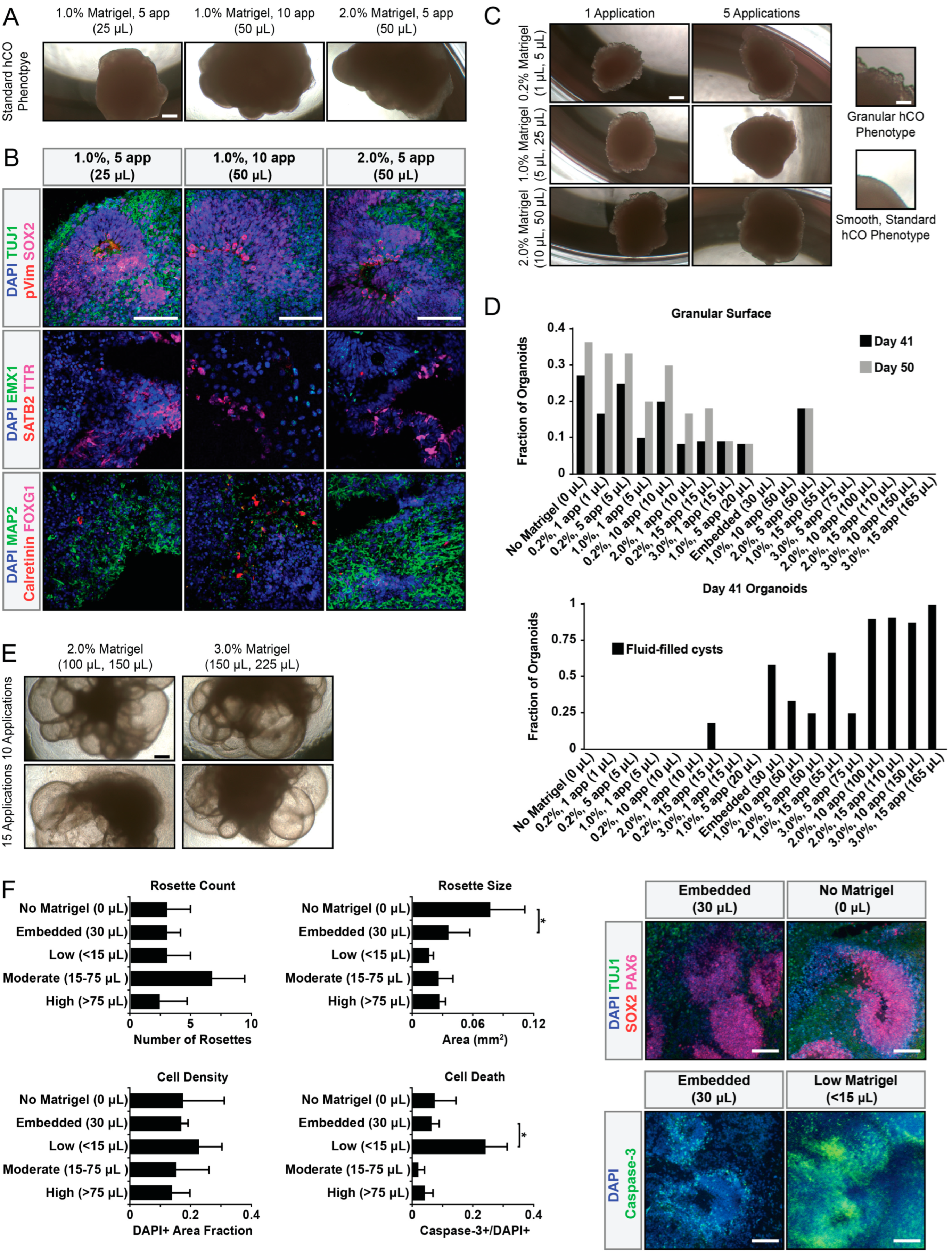
2.5. Granular Surface Phenotype Quantification
2.6. Inhibitor Treatment Experiments
2.7. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Bonfio, C.; Chadwick, J.; Begum, F.; Skehel, M.; Lancaster, M.A. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 2020, 369, eaaz5626. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Simonini, M.V.; Palejev, D.; Tomasini, L.; Coppola, G.; Szekely, A.M.; Horvath, T.L.; Vaccarino, F.M. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 12770–12775. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Valiulahi, P.; Vidyawan, V.; Puspita, L.; Oh, Y.; Juwono, V.B.; Sittipo, P.; Friedlander, G.; Yahalomi, D.; Sohn, J.-W.; Lee, Y.K.; et al. Generation of caudal-type serotonin neurons and hindbrain-fate organoids from hPSCs. Stem Cell Rep. 2021, 16, 1938–1952. [Google Scholar] [CrossRef]
- Eura, N.; Matsui, T.K.; Luginbühl, J.; Matsubayashi, M.; Nanaura, H.; Shiota, T.; Kinugawa, K.; Iguchi, N.; Kiriyama, T.; Zheng, C.; et al. Brainstem Organoids From Human Pluripotent Stem Cells. Front. Neurosci. 2020, 14, 538. [Google Scholar] [CrossRef]
- Cederquist, G.Y.; Asciolla, J.J.; Tchieu, J.; Walsh, R.M.; Cornacchia, D.; Resh, M.D.; Studer, L. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 2019, 37, 436–444. [Google Scholar] [CrossRef]
- Delepine, C.; Pham, V.A.; Tsang, H.W.S.; Sur, M. GSK3ß inhibitor CHIR 99021 modulates cerebral organoid development through dose-dependent regulation of apoptosis, proliferation, differentiation and migration. PLoS ONE 2021, 16, e0251173. [Google Scholar] [CrossRef]
- Amin, N.D.; Kelley, K.W.; Hao, J.; Miura, Y.; Narazaki, G.; Li, T.; McQueen, P.; Kulkarni, S.; Pavlov, S.; Paşca, S.P. Generating human neural diversity with a multiplexed morphogen screen in organoids. bioRxiv 2023, 1–28. [Google Scholar] [CrossRef]
- Sen, D.; Voulgaropoulos, A.; Keung, A.J. Effects of early geometric confinement on the transcriptomic profile of human cerebral organoids. BMC Biotechnol. 2021, 21, 59. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Kohen, N.T.; Little, L.E.; Healy, K.E. Characterization of Matrigel interfaces during defined human embryonic stem cell culture. Biointerphases 2009, 4, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Yiangou, L.; Ross, A.D.; Goh, K.J.; Vallier, L. Human Pluripotent Stem Cell-Derived Endoderm for Modeling Development and Clinical Applications. Cell Stem Cell 2018, 22, 485–499. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. Adv. Sci. Drug Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 2021, 16, 579–602. [Google Scholar] [CrossRef]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Martins-Costa, C.; Pham, V.; Sidhaye, J.; Novatchkova, M.; Peer, A.; Möseneder, P.; Corsini, P.S.; Knoblich, P.A. Morphogenesis and development of human telencephalic organoids in the absence and presence of exogenous ECM. bioRxiv 2022, 1–28. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Su, Y.; Besner, G.E. Heparin-binding EGF-like growth factor (HB-EGF) promotes cell migration and adhesion via focal adhesion kinase. J. Surg. Res. 2014, 189, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Hyväri, L.; Ojansivu, M.; Juntunen, M.; Kartasalo, K.; Miettinen, S.; Vanhatupa, S. Focal adhesion kinase and ROCK signaling are switch-like regulators of human adipose stem cell differentiation towards osteogenic and adipogenic lineages. Stem Cells Int. 2018, 2018, 2190657. [Google Scholar] [CrossRef] [PubMed]
- Rudibaugh, T.P.; Tam, R.W.; Estridge, R.C.; Keung, A.J. Single cell assessment of human stem cell derived mesolimbic models and their responses to substances of abuse. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Ding, L.; Mo, M.; Zou, J.; Lu, Z.; Li, H.; Wu, H.; Dai, Y.; Xu, P.; et al. Wnt1 Promotes EAAT2 Expression and Mediates the Protective Effects of Astrocytes on Dopaminergic Cells in Parkinson’s Disease. Neural Plast. 2019, 2019, 1247276. [Google Scholar] [CrossRef]
- Manfrin, A.; Tabata, Y.; Paquet, E.R.; Vuaridel, A.R.; Rivest, F.R.; Naef, F.; Lutolf, M.P. Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nat. Methods 2019, 16, 640–648. [Google Scholar] [CrossRef]
- Yang, X.; Niu, N.; Liang, C.; Wu, M.-M.; Tang, L.-L.; Wang, Q.-S.; Lou, J.; Song, B.-L.; Zheng, W.-W.; Ma, H.-P.; et al. Stimulation of Epithelial Sodium Channels in Endothelial Cells by Bone Morphogenetic Protein-4 Contributes to Salt-Sensitive Hypertension in Rats. Oxidative Med. Cell. Longev. 2020, 2020, 3921897. [Google Scholar] [CrossRef]
- La Rosa, I.; Camargo, L.S.; Pereira, M.M.; Fernandez-Martin, R.; Paz, D.A.; Salamone, D.F. Effects of bone morphogenic protein 4 (BMP4) and its inhibitor, Noggin, on in vitromaturation and culture of bovine preimplantation embryos. Reprod. Biol. Endocrinol. 2011, 9, 18. [Google Scholar] [CrossRef]
- Zhou, J.; Aponte-Santamaría, C.; Sturm, S.; Bullerjahn, J.T.; Bronowska, A.; Gräter, F. Mechanism of Focal Adhesion Kinase Mechanosensing. PLoS Comput. Biol. 2015, 11, e1004593. [Google Scholar] [CrossRef]
- Hébert, J.M.; Mishina, Y.; McConnell, S.K. BMP Signaling is required locally to pattern the dorsal telencephalic midline. Neuron 2002, 35, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kang, Y.-J.; Davies, L.M.; Meghpara, S.; Lau, K.; Chung, C.-Y.; Kathiriya, J.; Hadjantonakis, A.-K.; Monuki, E.S. BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors. J. Neurosci. 2012, 32, 15934–15945. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.-H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.-G.; et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L.; et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019, 10, 5658. [Google Scholar] [CrossRef]
- Orlando, G.; Booth, C.; Wang, Z.; Totonelli, G.; Ross, C.L.; Moran, E.; Salvatori, M.; Maghsoudlou, P.; Turmaine, M.; Delario, G.; et al. Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials 2013, 34, 5915–5925. [Google Scholar] [CrossRef]
- Zachos, N.C.; Kovbasnjuk, O.; Foulke-Abel, J.; In, J.; Blutt, S.E.; de Jonge, H.R.; Estes, M.K.; Donowitz, M. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J. Biol. Chem. 2016, 291, 3759–3766. [Google Scholar] [CrossRef]
- Gilpin, S.E.; Guyette, J.P.; Gonzalez, G.; Ren, X.; Asara, J.M.; Mathisen, D.J.; Vacanti, J.P.; Ott, H.C. Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale. J. Heart Lung Transplant. 2014, 33, 298–308. [Google Scholar] [CrossRef]
- Capeling, M.M.; Czerwinski, M.; Huang, S.; Tsai, Y.-H.; Wu, A.; Nagy, M.S.; Juliar, B.; Sundaram, N.; Song, Y.; Han, W.M.; et al. Nonadhesive Alginate Hydrogels Support Growth of Pluripotent Stem Cell-Derived Intestinal Organoids. Stem Cell Rep. 2019, 12, 381–394. [Google Scholar] [CrossRef]
- Baptista, P.M.; Siddiqui, M.M.; Lozier, G.; Rodriguez, S.R.; Atala, A.; Soker, S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011, 53, 604–617. [Google Scholar] [CrossRef]
- Saheli, M.; Sepantafar, M.; Pournasr, B.; Farzaneh, Z.; Vosough, M.; Piryaei, A.; Baharvand, H. Three-dimensional liver-derived extracellular matrix hydrogel promotes liver organoids function. J. Cell. Biochem. 2018, 119, 4320–4333. [Google Scholar] [CrossRef]
- DiMarco, R.L.; Dewi, R.E.; Bernal, G.; Kuo, C.; Heilshorn, S.C. Protein-engineered scaffolds for in vitro 3D culture of primary adult intestinal organoids. Biomater. Sci. 2015, 3, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Wylie, R.G.; Ahsan, S.; Aizawa, Y.; Maxwell, K.L.; Morshead, C.M.; Shoichet, M.S. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat. Mater. 2011, 10, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Candiello, J.; Grandhi, T.S.P.; Goh, S.K.; Vaidya, V.; Lemmon-Kishi, M.; Eliato, K.R.; Ros, R.; Kumta, P.N.; Rege, K.; Banerjee, I. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials 2018, 177, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.; Saeb-Parsy, K.; Blackford, S.J.; Segal, J.M.; Serra, M.P.; Horcas-Lopez, M.; No, D.Y.; Mastoridis, S.; Jassem, W.; Frank, C.W.; et al. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials 2018, 182, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Acuña, R.; Quirós, M.; Farkas, E.A.; Dedhia, P.H.; Huang, S.; Siuda, D.; García-Hernández, V.; Miller, A.J.; Spence, J.R.; Nusrat, A.; et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 2017, 19, 1326–1335. [Google Scholar] [CrossRef]
- Cruz-Acuña, R.; Quirós, M.; Huang, S.; Siuda, D.; Spence, J.R.; Nusrat, A.; García, A.J. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat. Protoc. 2018, 13, 2102–2119. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Zheng, D.; Wang, J.; Yang, H.; Zhang, X. Transcriptome variations among human embryonic stem cell lines are associated with their differentiation propensity. PLoS ONE 2018, 13, e0192625. [Google Scholar] [CrossRef]
- Ortmann, D.; Brown, S.; Czechanski, A.; Aydin, S.; Muraro, D.; Huang, Y.; Tomaz, R.A.; Osnato, A.; Canu, G.; Wesley, B.T.; et al. Naive Pluripotent Stem Cells Exhibit Phenotypic Variability that Is Driven by Genetic Variation. Cell Stem Cell 2020, 27, 470–481.e6. [Google Scholar] [CrossRef]
- Volpato, V.; Webber, C. Addressing variability in iPSC-derived models of human disease: Guidelines to promote reproducibility. DMM Dis. Model. Mech. 2020, 13, dmm042317. [Google Scholar] [CrossRef]
- Yang, X.; Chen, D.; Sun, Q.; Wang, Y.; Xia, Y.; Yang, J.; Lin, C.; Dang, X.; Cen, Z.; Liang, D.; et al. A live-cell image-based machine learning strategy for reducing variability in PSC differentiation systems. Cell Discov. 2023, 9, 53. [Google Scholar] [CrossRef]
- Cassel de Camps, C.; Aslani, S.; Stylianesis, N.; Nami, H.; Mohamed, N.-V.; Durcan, T.M.; Moraes, C. Hydrogel Mechanics Influence the Growth and Development of Embedded Brain Organoids. ACS Appl. Bio Mater. 2021, 5, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Redzic, Z.B.; Segal, M.B. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv. Drug Deliv. Rev. 2004, 56, 1695–1716. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.K.; Bjornsson, C.S.; Dymecki, S.M.; Gilbertson, R.J.; Holtzman, D.M.; Monuki, E.S. The choroid plexus and cerebrospinal fluid: Emerging roles in development, disease, and therapy. J. Neurosci. 2013, 33, 17553–17559. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estridge, R.C.; O’Neill, J.E.; Keung, A.J. Matrigel Tunes H9 Stem Cell-Derived Human Cerebral Organoid Development. Organoids 2023, 2, 165-176. https://doi.org/10.3390/organoids2040013
Estridge RC, O’Neill JE, Keung AJ. Matrigel Tunes H9 Stem Cell-Derived Human Cerebral Organoid Development. Organoids. 2023; 2(4):165-176. https://doi.org/10.3390/organoids2040013
Chicago/Turabian StyleEstridge, R. Chris, Jennifer E. O’Neill, and Albert J. Keung. 2023. "Matrigel Tunes H9 Stem Cell-Derived Human Cerebral Organoid Development" Organoids 2, no. 4: 165-176. https://doi.org/10.3390/organoids2040013
APA StyleEstridge, R. C., O’Neill, J. E., & Keung, A. J. (2023). Matrigel Tunes H9 Stem Cell-Derived Human Cerebral Organoid Development. Organoids, 2(4), 165-176. https://doi.org/10.3390/organoids2040013





