1. Introduction
One of the new FAO Strategies on Climate Change 2022–2031 is to transform agri-food systems to be more efficient, more inclusive, more resilient, and more sustainable, accelerating the achievement of the 2030 Agenda for Sustainable Development [
1]. Yet, soils around the world are suffering. For example, it has been estimated that about 60 to 70% of soils in the EU are not healthy [
2]. In addition, Latin America is responsible for 14% of all degraded land in the world [
3]. Moreover, while 65% of African soils are on the border of degradation and 45% are already affected by desertification [
4], around 31% of Indian soils are very degraded. Soil quality is not limited to agricultural land but also includes how soil function can be maintained from an ecological standpoint [
5]. Soil microbiota plays a crucial role in the health of agricultural ecosystems and the global climate. In fact, it is well known that soil health and productivity are influenced by soil microbial activity, nutrient cycling, and land-use management. Factors such as tillage practices, organic farming, and external environmental elements vary regionally, affecting sustainability and agricultural practices globally [
5,
6,
7]. Therefore, soil improvement is an important concern for farmers, and not all types of soil are suitable for fertile farming, so a good and efficient land management system is needed. Nowadays, global agricultural management is increasingly oriented towards organic farming systems. The main principle is to minimize chemical input, which negatively affects terrestrial and aquatic ecosystems, requires large amounts of natural resources, and generates nitrous oxide emissions with a greenhouse effect approximately 300 times stronger than that of CO
2. Similarly, this pollution also adds to climate change, destroying soils and biodiversity and changing food production. In the face of all these challenges, it is being increasingly realized that alternatives to the current agricultural system must be developed, and in the search for these alternatives, sustainable agriculture is emerging as the way to go. Applications of organic matter, biochar, compost, biofertilizer, and organic mulch, as well as conservation and integrated farming systems, are effective in dryland farming systems. Researchers in this field advocate for the use of biofertilizers and present microorganisms with a very important role from an agrobiotechnological perspective [
6,
8,
9]. These biofertilizers could contribute to crop productivity and help to reduce the heavy use of toxic chemical fertilizers. Biofertilizers can be produced by the application of microorganisms. They assist with the structuring of soil, the recovery of organic matter, the production of substances for plant nutrition and growth regulation, and the improvement in nutrient uptake by the plants. The use of microorganisms for biofertilizers is of paramount importance as it is a sustainable solution for greener agricultural practices and contributes to the recovery of soil structure, organic matter, and microorganisms, which are essential due to their metabolic processes in generating substances that help in nutrition and the promotion of plant growth to improve the uptake of nutrients by plants. Free-living bacteria or rhizosphere associates of plants (some known genera are
Acinetobacter,
Azotobacter,
Achromobacter,
Agrobacterium,
Alcaligenes,
Azospirillum,
Bacillus,
Burkholderia,
Enterobacter,
Pseudomonas,
Ralstonia,
Serratia,
Klebsiella,
Beijerinckia, and
Rhizobium) supply soil with nutrients by synthesizing growth-regulating substances, fixing nitrogen, solubilizing nutrients, producing siderophores, and inhibiting phytopathogens [
10]. In this way, the bioprospecting of bacteria in different terrestrial and aquatic ecosystems with specific bioactivity becomes a key point for the development of microbial biotechnology. In particular, marine environments, due to their biotic and abiotic conditions, have select microorganisms with functional metabolic activities that can be applied to industrial, pharmaceutical, environmental, and agricultural biotechnologies, where plant growth promoters stand out. In the scientific literature, many studies have reported on the selection of microorganisms from soil and plants with potential plant-promoting growth (PGPR) traits; however, marine ecosystems have been less explored. For this reason, in this work, we isolated several strains of
Pseudomonas spp. from the mangrove environment of Cartagena Bay in Colombia to study their bio-stimulant effect on the growth of lettuce (
Lactuca sativa). This was carried out to underline the fact that in Cartagena Bay, there are about 30 ha of mangroves; the most abundant species are
Avicennia germinans (67%),
Rhizophora mangle (30%),
Laguncularia racemosa, and
Conocarpus erectus, and they occur in fine unconsolidated sand and clay sediments rich in calcium carbonate. Two specific questions arise when attempting to understand the importance of our work: why isolate
Pseudomonas spp. and why isolate them from the marine environment?
Pseudomonas belongs to the group of plant growth-promoting rhizobacteria (PGPR) and is capable of not only surviving in the rhizosphere but also actively stimulating plant growth through nitrogen fixation, phosphate solubilization, and the production of indoleacetic acid, an essential hormone for the regulation of plant growth. Furthermore, siderophores and antimicrobial compounds protect plants from pathogenic microorganisms by chelating iron and making it available for plants. This robust ability to interact with roots for nutrient availability makes them a good candidate for biofertilization. Additionally, marine ecosystems, especially those like mangrove sediments, represent untapped resources for biofertilizer development. The marine environment is governed by highly peculiar abiotic stresses, including increased salinity, anaerobic conditions, and fluctuating nutrient availability, which eventually lead to the evolution of microorganisms with unique metabolic capabilities. Mangrove ecosystems are known for their significant biodiversity, in general, and their corresponding richness of well-adapted microbial communities living under harsh conditions.
Pseudomonas strains isolated from marine environments are likely to exhibit increased stress tolerance, such as salinity and drought resistance, which can be directly exploited in agriculture, especially in regions where the salinization of soil and water is crucial in limiting crop productivity. This makes them valuable for biofertilizer development targeted at crops grown on coastal or degraded land. This study was an effort to harness the biotechnological potential of such bacteria in marine environments for sustainable agriculture. Thus, the main specific objectives of this research were (1) to isolate and identify
Pseudomonas strains from Cartagena Bay, Colombia; (2) to evaluate their PGPR traits, such as IAA production, phosphate solubilization, and salt tolerance; and (3) to establish their effectiveness in promoting
Lactuca sativa growth under saline conditions using in vitro and in vivo studies.
2. Materials and Methods
2.1. Sampling of Marine Sediments in Cartagena Bay
Marine sediment samples were taken from around the roots of mangroves on the small island of Maparadita in Cartagena Bay, Colombia (10°22′07.95″ N/75°30′40.94″ W) (
Figure 1). A total of ten marine sediment samples were taken from around the mangrove roots, approximately 30 cm from the surface, by digging with a shovel and taking 300 g samples in bags marked for each sampling point. There was a distance of 500 m between each sampling point. The samples were immediately placed in a portable cooler with dry ice and were taken to the laboratory and placed in a refrigerator at 4 °C until their respective analysis. The labeled samples were transported in airtight bags in a refrigerated cooler until processing.
2.2. Physicochemical Analysis of Mangrove Sediments
The physicochemical analyses were carried out immediately after collecting the samples. A multiparametric probe (Hanna Instruments, Woonsocket, RI, USA) was used to measure the parameters of turbidity, dissolved oxygen, conductivity, pH, and salinity, with data recorded digitally according to the instrument’s standard output format.
2.3. Isolation and Identification of Pseudomonas spp. from Mangrove Sediment
The growth of microorganisms from the environmental sample was promoted in multiple agar media under laboratory conditions. The media used were the following: Tryptic Soy Broth (TSA, Merck, Darmstadt, Germany), Marine Broth (Merck, Germany), and
Pseudomonas agar medium (Merck, Darmstadt, Germany). The plates were inoculated by spreading 50 µL of water over them and incubated at room temperature. Colony-forming units (CFUs) in the agar plates were monitored for one week. Morphologically distinct colonies were selected, purified on the same medium, and used for further studies. Bacterial isolates were tested for catalase and oxidase activity, Gram staining, and the production of fluorescent pigment on King B agar (Merck, Darmstadt, Germany) according to methods previously described [
11]. In our study, we isolated a total of 112 bacterial isolates from mangrove sediments in Cartagena Bay, Colombia. All isolates were purified and subjected to a preliminary test to evaluate their ability to promote germination by inoculating lettuce seedlings with potential PGPR, while only the strains that showed high PGPR traits were subjected to identification.
2.4. Molecular Identification
In this study, bacterial isolates were grown in BHI (Brain Heart Infusion Broth) medium for 24 h at 28 °C and genomic DNA was extracted using the DNA Extraction Kit (EuroClone, Milan, Italy). BHI medium was selected at this stage not for its selective properties, but because its nutrient-rich composition allows for consistent and rapid bacterial growth, ensuring sufficient biomass and high-quality genomic DNA for downstream extraction and PCR amplification. This differs from the selective and differential media used during the isolation step, where the aim was to recover and distinguish morphologically diverse colonies from mangrove sediments. Molecular identification was performed by amplification of the 16S rRNA gene with the universal primer pairs (F) 5′-AGTTTGATCCTGGCTCAG-3′ and (R) 5′-ATTACCGCGGCTGCTGG-3′. The PCR reaction was performed in a total volume of 50 µL of a solution containing 0.25 µL of 1× Wonder Taq Hot Start Reaction buffer (Euroclone), 1 µL of 100 µM Primer Forward, 1 µL of 100 µM Primer Reverse, 10 µL of DNA extract, and PCR water till reaching the final volume. Amplification was carried out in a thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA) as follows: 2 min at 95 °C, 25 cycles of 1 min at 95 °C, 1 min at 55 °C, 1 min at 72 °C and a final extension step of 7 min at 72 °C. Finally, the sequences obtained were aligned to other closely related bacterial species deposited in the NCBI database using the BLAST program (NCBI BLASTn, version 2.13.0) All primers used were purchased from Sigma Aldrich (Saint Louis, MO, USA).
2.5. Determination of Plant-Promoting Traits in Seeds of Lactuca sativa L. cv. Romana
2.5.1. Seed Material Preparation
The surfaces of lettuce seeds (Lactuga sativa L. cv. Romana, Seme nero.LT00022, Italian Sprout Srl, Cesena (FC), Italy) were preliminarily disinfected with 1% NaClO solution for 5 min and thoroughly rinsed with sterile distilled water; this step was independent of the salinity assay. For salt tolerance experiments, the control consisted of seeds germinated in medium without NaCl (0 mM), while salinity treatments were applied at 1%, 2.5%, 5%, and 10% NaCl.
2.5.2. Inoculum Preparation
From the initial 117 isolates, only 58 were selected, as these showed the most promising PGPR traits (e.g., phosphate solubilization, IAA production, salinity tolerance, or significant promotion of lettuce seed germination) during the preliminary screening. The remaining isolates were excluded due to low or inconsistent performance in these assays. Isolates were grown in BHI Agar medium (Becton Dickinson (BD), Franklin Lakes, NJ, USA) and then incubated for 24 h at 37 °C. A single colony was selected and transferred into a 2 mL Eppendorf tube containing Luria–Bertani Broth (LB) medium (Oxoid) and successively incubated at 37 °C for 24 h; after that, 1 mL of the pre-inoculum was inoculated in a separate Erlenmeyer flask containing the same media and incubated for 24 h at 30 °C in an orbital shaker (Centomat, BS-T, B.Braun, Milan, Italy). The cells of each strain were harvested during the exponential phase through centrifugation at 6.000 rpm and 4 °C for 10 min. Subsequently, they were resuspended in a sterile physiological solution (0.85%) and adjusted to achieve a final density of 108 colony-forming units per milliliter (CFU mL−1), serving as the stock inoculum for the experiment. Sterile distilled water was employed as a control.
2.5.3. Germination Assessment In Vitro
The experimental setup followed the methodology outlined in [
6,
8], with minor adjustments. Each treatment consisted of three Petri dishes (replicates) in a completely randomized design. A total of 30 lettuce seeds were spread on Petri dishes containing Whatman filter paper (No. 1). Bacterial suspensions were previously standardized spectrophotometrically at 600 nm (OD
600) to ensure comparable cell density across treatments. Subsequently, the filter paper and seeds were soaked in specific bacterial inoculum (5 mL). The dishes were closed with parafilm to prevent evaporation of the solution and incubated at 25 °C in a 12:12 h light–dark cycle for a total of 6 days, without the introduction of additional nutritional supplements during the experiment. Regular observations were conducted to record daily germination counts, identifying seeds with a 2 mm root length as germinated. The total germination percentage (GP, %) and the mean germination time (MGT, days) were computed using the formula established by Ellis and Roberts in 1986:
where
NG is the number of germinated grains and
NT is the total number of grains.
where
F is the number of germinated grains on day
x.
Root and shoot elongation was evaluated 7 days after germination. Ten seedlings per treatment were randomly selected, and root length (RL) and hypocotyl length (HL) were measured manually using a digital caliper. For biomass determination, seedlings were harvested and gently blotted to remove surface moisture. Fresh weight (FW) was recorded immediately after harvest, while dry weight (DW) was obtained after oven-drying the same seedlings at 70 °C for 48 h until constant weight.
2.6. PGPR Traits of Pseudomonas spp.
For biochemical analyses, lettuce seedlings were harvested 7 days after germination. For each treatment, fresh tissue (cotyledons and the first true leaves) from 10 seedlings was pooled to obtain approximately 0.2 g of material. Samples were immediately frozen in liquid nitrogen and subsequently processed for extraction. Pigment concentrations (chlorophyll a, chlorophyll b, and carotenoids) were expressed on a fresh weight basis, whereas antioxidant activity (FRAP, ABTS) and total phenolic content (TPC) were determined on a dry matter basis.
2.6.1. Determination of Indoleacetic Acid (IAA) Production
IAA production was evaluated using a modified procedure, as outlined in [
11]. Bacterial samples, each comprising 0.5 mL and adjusted to a concentration of 10
7 CFU/mL (OD620nm = 0.1), were mixed with 50 mL of Kings B broth (Condalab, Madrid, Spain) supplemented with 0.1% L-tryptophan (Sigma-Aldrich, Saint Louis, MO, USA). The cultures were then incubated for 72 h at 28 °C. Upon reaching full growth, the cultures were centrifuged at 12,000×
g for 10 min. The resulting supernatant (2 mL) was mixed with 4 mL of Salkowski reagent. OD530 in nm was determined using a spectrophotometer following a 20 min incubation period.
Azospirillum brasilense strain Sp 245 was used as a positive control, which consistently produced approximately 35 ppm of IAA, and a negative control represented by uninoculated soils.
2.6.2. Determination of Phosphate Solubilization
A qualitative assessment of phosphate solubilization was performed by inoculating bacterial cultures in Pikovskaya Agar Medium (Sigma-Aldrich). Following the method outlined in [
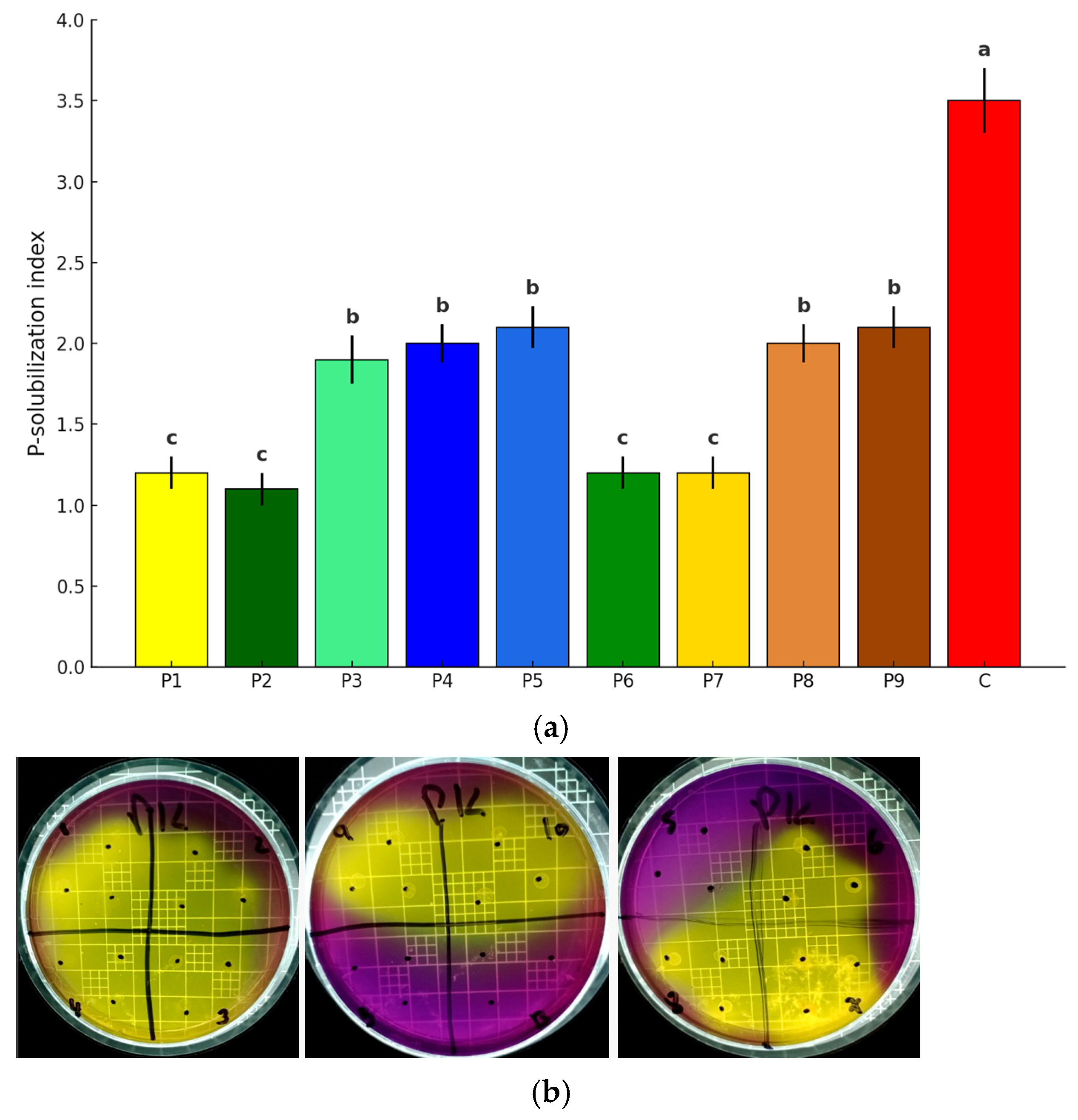
7], a positive indication of phosphate solubilization was observed after a 2-day incubation period. Bacteria that exhibited the largest clearance zones on the above agar surfaces were selected. The values were expressed according to the solubility index (P—solubilization index). Values < 2 correspond to low phosphate solubilization, values < 4 correspond to medium solubilization, and those >4 correspond to high phosphate solubilization.
Bacillus subtilis strain CNPMS B2084 was used as a positive control, and the negative control was represented by uninoculated soils. The solubilization of phosphorus is represented by the solubilization index (SI):
2.6.3. Production of Organic Acids
The analysis was carried out with a PerkinElmer Series 200 HPLC (PerkinElmer, Waltham, MA, USA) equipped with an autosampler and UV detector. The samples were filtered with 0.45 μm filters and 10 μL was injected into the chromatographic system with a Biorad organic acid ion-exchange column (300 × 8 mm 8 μm Biorad) at a temperature of 45 °C, a flow of 0.4 mL/min, a 4.5 mM sulfuric acid mobile phase, and a 210 nm detector. Data are expressed in mg/L.
2.6.4. Determination of Siderophore Production
For the detection of siderophores (siderophore units, %), the liquid method from [
12] with some modifications was followed. First, 500 mL of Luria–Bertani medium was prepared with the following components: 10 g L
−1 of Tryptone, 5 g L
−1 of yeast extract, 5 g L
−1 of NaCl, and 5 mM of L-Tryptophan. The medium was adjusted to pH 7.5 using NaOH and then autoclaved. The, 1 mL of the medium was transferred into sterile Eppendorf tubes, and microorganisms were inoculated using sterile loops. The samples were then placed in a shaking incubator. After 3 days, they were centrifuged for 2 min at 14,000 rpm. The supernatant was removed, and 100 µL was added to 12 multwell plates and incubated with CAS reagent for 4 h. A positive reaction was identified by a color change from blue to yellow in the wells.
Bacillus subtilis strain CAS15 was used as a positive control, and the negative control was represented by uninoculated soils.
Pseudomonas fluorescens CE-2 [
13] was used as a positive control, which consistently produced approximately 90–100% Siderophore units (SUs), and the negative control was represented by uninoculated soils.
2.6.5. PGPR Salt Tolerance In Vitro
The different strains were grown in LB for 24 h successively. The cultures were centrifuged and washed using phosphate buffer (pH = 7.2) and standardized to 0.1 of O.D at 620 nm. Ten microliters of each morphotype was spotted onto Plate Count Agar medium (PCA) containing different NaCl concentrations (0, 5, 10, and 15%). Plates were incubated for 96 h at 28 ± 2 °C. Growth was subsequently assessed for each treatment.
2.6.6. Polyphenol Extraction and Total Phenolic Content (TPC)
Determination of total phenolic content (TPC) present in the sprouts was conducted utilizing the Folin–Ciocalteu (FC) photometric assay. Polyphenol extraction was carried out as described in [
13], with slight modifications. Freeze-dried lettuce sprouts (1.5 gr) were homogenized and vortexed with 5 mL of 70%
v/
v methanol for 10 min. The solution was stirred for 10 min and then centrifuged for 10 min at 4000 rpm and 15 °C. The supernatant obtained was filtered with a 0.45 μm nylon filter and stored at −40 °C until analysis. For each sample, polyphenol extraction was performed in duplicate. In the initial step, 20 μL of a suitably diluted sample extract was combined with 20 μL of FC reagent and agitated for 3 min using an orbital shaker (VDRL 711/CT orbital shaker from Asal, Florence, Italy). Following this, 400 μL of 7.5% sodium carbonate was added, and subsequently, 550 μL of deionized water was introduced into the mixture. The solution was stirred for 60 min at room temperature and shielded from light. Afterward, the absorbance at 760 nm was measured and compared against the blank sample. Gallic acid was utilized as the reference standard for method calibration.
2.6.7. Evaluation of the Antioxidant Activity
The evaluation of antioxidant capacity (AOC) was carried out using the ABTS method. Initially, the ABTS+ radical reagent stock solution, according to the protocol outlined by [
14], was prepared and stored at −20 °C. The ABTS+ radical solution was promptly diluted in MeOH until it reached an absorbance value of 0.7 ± 0.05 (λ = 734 nm) and was directly employed for the assay. To summarize, 20 μL of a suitably diluted sample extract was mixed with 980 μL of ABTS+ and allowed to incubate for 5 min at room temperature in the absence of light. The final absorbance at 734 nm was measured in comparison to the control, where MeOH was added instead of the sample extract. Gallic acid was employed as the reference standard for method calibration.
2.6.8. Carotenoid and Chlorophyll Content Estimation
The measurement of carotenoids (Car) and chlorophylls (chlorophyll a, Chla; chlorophyll b, Chlb; Chla+Chlb) was carried out using spectrophotometry with a UV–VIS spectrophotometer (Lambda Bio 20, Perkin Elmer, Boston, MA, USA). Pigments were extracted from fresh lettuce leaves (cotyledons and the first true leaves) harvested 7 days after germination. Approximately 0.2 g of fresh tissue was homogenized in 80% acetone and centrifuged at 12,000× g for 10 min at 4 °C, and the supernatant was used for spectrophotometric determination. The results were expressed in units of µg g−1 FW (micrograms per gram of fresh weight).
2.7. Statistical Analysis
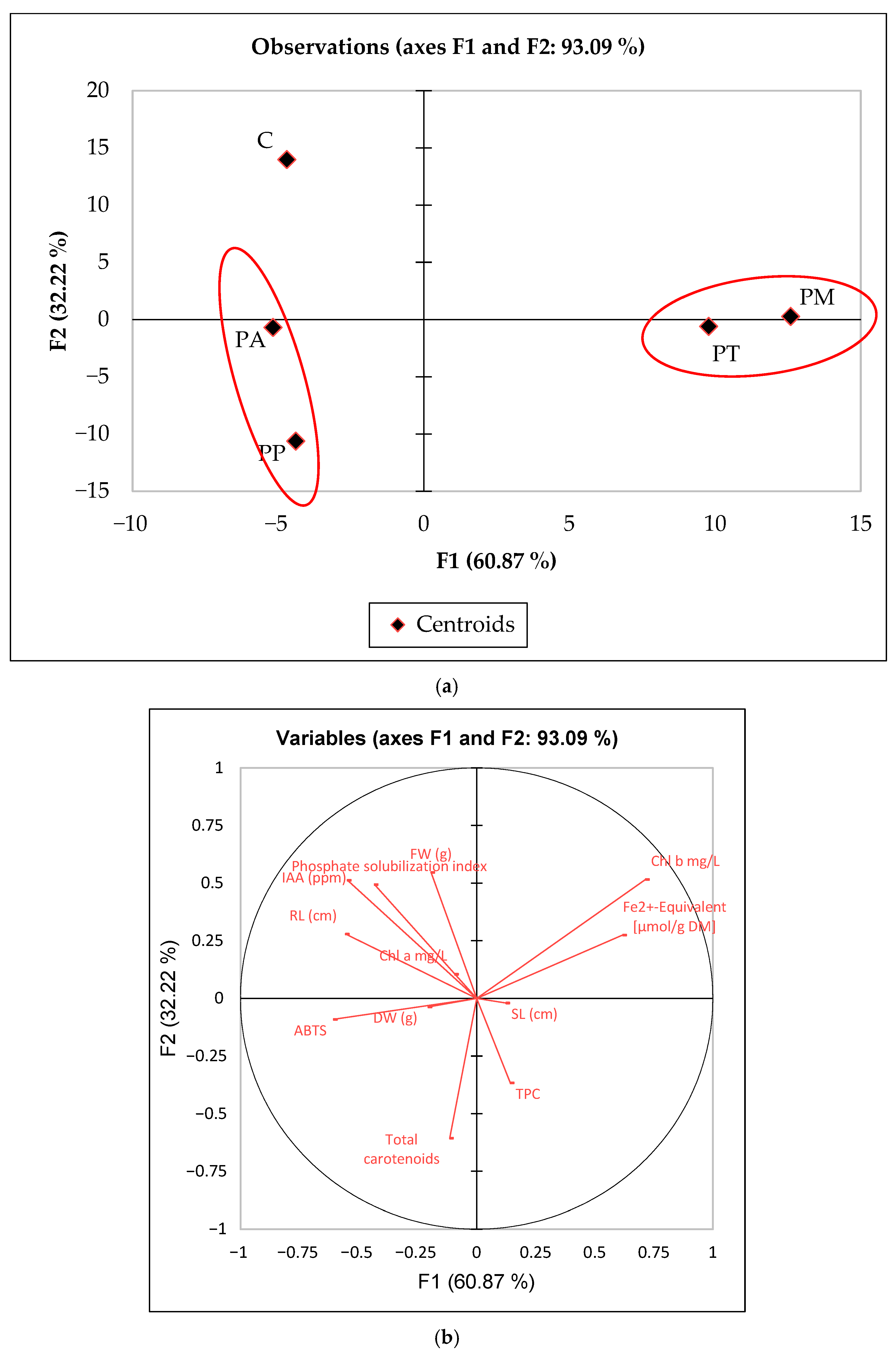
The statistical analyses of PGPR potential (i.e., IAA production, P solubilization index) were performed using a one-way analysis of variance (one-way ANOVA) to calculate the p-value (set at p < 0.05; n = 3 replicates, followed by mean separation performed using Tukey’s HSD test). The data collected, including physiological parameters (e.g., root and shoot length, biomass measurements, and germination rates) and biochemical traits (e.g., antioxidant activities, chlorophyll content, and phenolic compounds), were analyzed using linear discriminant analysis (LDA) to evaluate the classification of the different variants. The analysis was performed using the software XLSTAT version 2024.3 (Addinsoft, New York, NY, USA), nd the results were interpreted considering the F-score and the significance value (p-value). The confusion between the different classes was evaluated using a confusion matrix to determine the classification accuracy.
4. Discussion
Mangrove forests that are located at the interface between terrestrial and marine ecosystems are characterized by a rich biodiversity of plants, animals, and microorganisms. In this context, microorganisms are an essential and unique component in mangroves, not only because they play a very crucial role in the creation and maintenance of this biosphere but also because they can be studied to produce biotechnologically valuable products [
23]. As observed by our results, the Cartagena Bay mangroves showcase environmental heterogeneity that supports a rich and dynamic microbial ecosystem. Overall, the variations in D.O. and NTU parameters we observed confirm the environmental complexity of mangrove ecosystems. These parameters have critical implications for microbial communities and nutrient cycling. The low DO levels observed in samples such as M1 are most likely due to the high organic matter content and near-anaerobic conditions typical of mangrove sediments, as also reported in [
24]. Similarly, variations in turbidity can be explained by sediment resuspension, tidal forces, or freshwater runoff carrying suspended particles, consistent with observations in [
25,
26]. Furthermore, changes in conductivity and turbidity can influence the distribution of rhizobacteria in mangrove sediments [
27]. The observed results are consistent with previous studies, highlighting the unique biogeochemical characteristics of mangroves as vital coastal buffer zones and their implications for microbial bioprospecting and ecological research [
28]. Mangrove ecosystems, therefore, represent not only reservoirs of biodiversity but also a potential source of microorganisms highly adapted to stress, making them promising candidates for agricultural applications. Stress-adapted Pseudomonas strains isolated from saline environments can express multiple PGPR traits, such as hormone production, phosphate solubilization, and stress mitigation, which are directly relevant to climate-resilient agriculture. In the present study, we isolated 58 different bacteria from mangrove soil, but only nine belonging to the genus
Pseudomonas showed PGPR threats, as evidenced by the best performance in lettuce seed germination. They induced a significant increase in root length (up to 35%) and hypocotyl length (up to 97%) after 6 days of inoculation, with
P. putida P2 and
P. montelii P5 as the best performers. The ability of
Pseudomonas spp. to promote plant growth and increase the yield of various crops is well known. This effectiveness is due to their specific mechanisms of phytohormone production and phosphate solubilization, which gives them the ability to support plants even under adverse environmental conditions. It should be noted that the effect of IAA on germination is concentration-dependent and not strictly linear. High IAA levels, such as those produced by P. aeruginosa P7, may not further enhance—and can even limit—FGP and MGT due to auxin–ABA and auxin–ethylene interactions. This multifactorial regulation explains why only partial improvements were observed in RL and HL [
29]. Recently, researchers have focused their efforts on evaluating the effect of certain strains of
Pseudomonas spp. as PGPR on a variety of crops. For instance, the positive effect of
P. putida 1046 on seed germination was reported in [
30]; in this case, the in vitro treatment of corn seeds with this strain resulted in a 28.33% increase in the RL and 35% in the HL. On the other hand, ref. [
31] reported the stimulation of root growth parameters when seeds of
Eruca sativa Mill (rocket),
Lepidium sativum L. (garden cress), and
Raphanus sativus (radish) were treated with
P. montelii SKL10, which was attributed to the multiple plant growth-enhancing traits of this strain. In particular, auxin (IAA) plays a central role in breaking dormancy, promoting cell elongation, and coordinating root and shoot development during germination. The strong differences in IAA levels observed among our strains translated into clear phenotypic effects: high IAA producers such as
P. aeruginosa P7, P6, and P8 stimulated root elongation and hypocotyl growth, while low producers showed only modest effects. These results reinforce the link between microbial hormone biosynthesis and early seedling vigor, confirming previous reports that auxin-mediated priming enhances nutrient uptake and seedling establishment in crops [
32].
One potential approach to counteracting plant stress, particularly the negative effects of soil salinization on glycophyte crops, is the application of PGPR [
33]. In this study, we evaluated the ability of the different strains to tolerate moderate/high concentrations of NaCl. As reported above, all the strains were able to tolerate up to 5% NaCl. The ability to combine salt tolerance with PGPR traits makes mangrove-derived isolates particularly interesting for applications in marginal soils. These results suggest that selected strains could be developed as biofertilizers for crops cultivated in saline-prone areas, reducing the need for chemical amendments and enhancing sustainability [
34]. These results could be relevant for bioremediation in saline environments, where salinity may limit the effectiveness of the degrading bacteria. In the literature, several strains of
Pseudomonas spp. that are resistant to salt stress have been reported. For example, the strain
Pseudomonas putida KT2440 improved the growth of corn and soybean in saline soils, tolerating up to 5%
w/
v NaCl [
35]. Furthermore, the strain
Pseudomonas sp. S3 increased the development of tomato plants in similar conditions [
36].
Phosphorus is one of the most important nutrients for plant growth. However, it is also a growth-limiting nutrient in soil, as it can be easily fixed and converted into insoluble complex forms with iron, aluminum, or calcium. The application of beneficial soil microbiota, either alone or combined with natural nutrient sources such as rock phosphate, represents a promising strategy for soils in which this element is a limiting factor for plant growth [
37]. In our study, the P solubilization assays of the nine screened halotolerant bacterial strains confirmed phosphorus solubilization through the formation of clear zones. The most promising results were observed by the strain
P. montelii P5 and the P.
aeruginosa strains P8 and P9, which achieved a PSI of 2.5. These results were consistent with early studies, in which two strains of
Pseudomonas showed a PSI of 2.7. On the other hand, the strains
P. aeruginosa P1 and
P. putida P2 appear to have limited solubilization efficiency, with PSI values around 1. This behavior is often associated with insufficient secretion of organic acid or low enzymatic activity, as reported in [
38]. Such strains may not be suitable for direct application unless they are integrated with other more efficient microorganisms in microbial consortium formulations. Although the strains
P. monteiilli P3 and
P. taiwanensis P4 have a higher capacity than
P. aeruginosa P1 and
P. putida P2, they fall into the category of moderate solubilizers. Organic acid profiling confirmed that the strains with the highest PSI values also secreted greater amounts of lactic and acetic acids, both of which are known to chelate cations and lower rhizospheric pH, thus increasing phosphate availability. This functional link between metabolite production and phosphate solubilization is consistent with other studies reporting that Pseudomonas spp. differ widely in their efficiency, depending on their organic acid secretion profile. This evidence further supports the potential of strains P5, P8, and P9 as effective phosphate solubilizers [
39]. These results are consistent with previous studies in which bacterial strains with different phosphate solubilization capacities were identified. A previous study in [
9] reported PGPR activities, including the phosphate-solubilizing capacity of 122 bacterial strains associated with
Rhizophagus intraradices spores. The results showed that 49.2% of the strains possessed this ability, with significant differences between the different isolates. In addition, studies on phosphate-solubilizing bacteria have shown that genera such as
Pseudomonas,
Agrobacterium, and
Bacillus can convert unavailable phosphorus compounds, such as dicalcium phosphate, tricalcium phosphate, and rock phosphate, into soluble forms that are easily assimilated by plants [
38]. This ability varies from strain to strain, which influences their effectiveness as biostimulants for plants [
40]. The efficiency of the phosphate solubilization of bacteria, as mentioned before, is often attributed to the secretion of organic acids, which play a key role in lowering local pH and improving phosphate availability in the soil. This mechanism is well documented, as organic acids such as lactic acid, citric acid, and oxalic acid are known to chelate cations and dissolve mineral phosphates, thus improving nutrient availability for plants [
36,
41]. In this context, the most promising strains,
P. montelii strain P5 and P.
aeruginosa strains P8 and P9, produced the highest concentrations of lactic acid and acetic acid. It is known that
P. aeruginosa is a very versatile bacterium that can produce secondary metabolites such as organic acids in variable amounts depending on environmental conditions [
42]. Ref. [
43] demonstrated that
P. aeruginosa is particularly efficient in producing lactic and acetic acid, which is also confirmed by our data. However, among the released organic acids, gluconic acid is the most common in fluorescent
Pseudomonas spp., but this acid was not reported in our strains.
It has been estimated that 80% of bacteria isolated from the rhizosphere can produce the plant growth regulator IAA [
44]. Our results highlight significant differences in IAA production among the analyzed strains.
P. aeruginosa P7, P6, and P8 demonstrated the highest IAA concentrations, with values reaching approximately 29.9 ppm, 24.67 ppm, and 19.84 ppm, respectively. When comparing the IAA production of the
Pseudomonas strains used in this study with data in the literature, we found that
P. montelii,
P. putida,
P. taiwanensis, and some strains of
P. aeruginosa isolated from mangrove environments produced lower IAA concentrations compared to the strains isolated from other ecosystems. This variability underlines how the biosynthetic potential of IAA is strongly strain-dependent and influenced by environmental adaptation. While some strains match or exceed literature values, others remain below the reported ranges for rhizosphere-isolated Pseudomonas. Such differences highlight the importance of strain selection for inoculant formulation, ensuring consistent and predictable PGPR effects. For example, selected strains of
P. fluorescens reached more than 63 µg/mL in multiple isolates of rhizosphere soil [
45], and P. aeruginosa FG106 isolated from tomato plants was reported to produce 211 µg/mL of IAA [
40]. These discrepancies emphasize the considerable differences in the biosynthetic potential of IAA between
Pseudomonas species, which may be related to strain-specific metabolic pathways and environmental adaptations.
Regarding the effect of different PGPR on the quality characteristics and production of bioactive compounds (biomass, antioxidant activity, phenolic compounds, photosynthetic pigments) of the lettuce seedlings, it was possible to show that the application of different PGPR strains significantly influenced the performance of the lettuce seedlings. Linear discriminant analysis (LDA) highlighted significant differences among Pseudomonas species, confirming the effectiveness of physiological parameters in discriminating between bacterial inoculations (F = 78.28,
p < 0.0001).
P. aeruginosa and
P. putida stood out for their high carotenoid content and scavenging activity. On the contrary,
P. montellii and
P. taiwanensis were characterized by an increase in chlorophyll b concentration and a reduction in iron. The observed increases in chlorophylls and carotenoids are particularly relevant because they indicate enhanced photosynthetic efficiency and antioxidant protection, two traits that directly contribute to plant vigor under stress. The correlation between PGPR inoculation, pigment accumulation, and FRAP activity suggests that these strains not only support growth but also prepare plants to better cope with oxidative stress. This dual role is a hallmark of efficacy [
46]. The high classification accuracy (96.67%) confirms the robustness of the model, although the partial overlap of
P. aeruginosa suggests some shared metabolic responses. The control group, characterized by the highest values of indoleacetic acid and phosphate solubilization, maintained a greater fresh weight, although some
P. aeruginosa strains (P1 and P9) showed a similar effect. These findings align with previous studies highlighting the role of
Pseudomonas spp. in plant growth promotion and stress resilience. Further research should explore the molecular mechanisms underlying these bacteria–plant interactions, particularly the modulation of antioxidant pathways and phytohormone signaling [
47]. Finally, the integration of IAA production, phosphate solubilization, organic acid secretion, pigment enhancement, and antioxidant activity underscores the multifunctionality of mangrove-derived Pseudomonas strains. Such multifunctionality is essential for developing microbial inoculants with broad agronomic value. However, field trials, formulation studies, and safety assessments (especially for
P. aeruginosa) are required before application. This work therefore provides a basis for future translational research aimed at the sustainable use of stress-adapted PGPR in agriculture [
48].
5. Conclusions
The results of this study demonstrate the significant potential of Pseudomonas spp. strains isolated from marine sediments in Cartagena Bay. Our results confirm their suitability for use in coastal environments and degraded soils, as they stood out for their performance in terms of phosphate solubilization, organic acid, and phytohormone production, as well as their high salinity tolerance. These characteristics suggest their potential suitability for applications in coastal environments and degraded soils, although further studies are required to directly evaluate their effects on plant growth under such conditions. In particular, the strains P. montelii P5, P. aeruginosa P8, and P9 showed high efficiency in terms of germination parameters, with significant increases in biomass and phytochemical properties. Their PSI values (>2), which are fully comparable to those reported in the literature, highlight that these strains represent exceptional candidates for practical applications, often outperforming strains isolated from conventional soils. Isolation from mangroves represents a unique and advantageous strategy, considering that these areas naturally select microorganisms adapted to extreme conditions such as salinity, anaerobic fluctuations, and nutrient scarcity. This gives the strains resilience, making them potentially useful in addressing current issues related to the salinization of agricultural soils. Despite these promising results, some concerns remain. Attention must be paid to assessing the pathogenic potential of strains such as Pseudomonas aeruginosa, which requires careful monitoring before large-scale application. This aspect highlights the need for further studies to ensure the safety of such biofertilizers. As Pseudomonas aeruginosa is a well-documented opportunistic human pathogen, particularly known for its multidrug resistance and association with nosocomial infections, its agricultural use demands rigorous biosafety evaluation. Furthermore, the less well-performing strains, such as P. aeruginosa P1 and P. putida P2, while showing limited capabilities, could have a complementary role within the microbial consortia, contributing to improving resilience and agroecological stability. The results of the phytochemical analyses and germination parameters further strengthen the efficacy of these strains. Additionally, our results highlight significant differences in IAA production among the analyzed strains. P. aeruginosa P7, P6, and P8 demonstrated the highest IAA concentrations; these values remain lower than those reported in the literature for other Pseudomonas species. These findings reinforce the importance of strain-specific metabolic pathways in IAA biosynthesis and suggest that, although these strains contribute to phytohormone production, their efficiency in IAA biosynthesis may be strain-dependent and influenced by environmental factors. Finally, this study not only highlights and outlines the key role of mangroves as a reservoir of biotechnologically relevant microorganisms but also lays the foundation for the development of new eco-sustainable biofertilizers capable of replacing current synthetic products. This translates into improved soil health and the promotion of agriculture towards an increasingly sustainable path. Obviously, further field studies will be essential to test the efficacy of these strains in increasingly complex environments and to evaluate their impact on soil microbial biodiversity.