Abstract
Guilandina bonduc L. is a pantropical coastal shrub with varied fruits and seeds, capable of germinating under saline stress. This study aimed to morphologically characterize the fruits and seeds of the species, correlate these characteristics, and evaluate the tolerance of seedlings to salt according to seed mass. Physical variables (length, width, thickness, and weight) were analyzed, and Spearman’s correlation was applied. Germination was tested with light seeds (<1.55 g) and heavy seeds (≥1.55 g) under five levels of salt stress, in a 2 × 5 factorial design. G. bonduc can produce seeds with variations in mass and size that are not necessarily related to fruit size. The reduction in osmotic potential resulted in lower seed germination and vigor; even so, the species demonstrated tolerance to salt stress, maintaining germination rates above 50% even under conditions of −1.0 MPa, regardless of seed mass. Lighter seeds germinate more quickly and uniformly, while heavier seeds produce more vigorous seedlings, especially in the absence of salinity, and are therefore more suitable for seedling production. These results indicate that G. bonduc has potential for revegetation of saline areas, being useful in adaptation to climate change due to its tolerance to saline stress and the relationship between seed mass and seedling vigor.
1. Introduction
The increase in demand for native seeds for ecological restoration and rehabilitation has stood out in the large global industry of seed supply and sale [1]. Therefore, research with native forest seeds is essential to obtain seedlings with good quality and in quantities that supply the segments of the forest sector [2]. Since seed propagation is the main form of disseminating for many species, studying seed technology and production becomes of great relevance in the process of management, conservation and genetic improvement [3].
Guilandina bonduc L. (=Caesalpinia bonduc (L.) Roxb.) is a thorny shrub belonging to the Fabaceae family that has a pantropical distribution and is common in coastal areas [4]. Due to its wide geographic distribution and large populations, the risk of extinction is very low for this species, and therefore it is assessed as of least concern (LC) on the IUCN Red List [5]. In Brazil, this species is part of the Atlantic Forest ecosystem, more specifically distributed in the different forest formations of galleries, mangroves, and Restingas in the Northeast and Southeast regions of the country [6]. Since ancient times, this plant has been traditionally used by indigenous tribes and peoples in different parts of the world to treat various health complications [7], including respiratory infections caused by the coronavirus [4]. G. bonduc seeds, when ripe, exhibit dormancy due to seed coat impermeability to water [8]. Although G. bonduc has a wide distribution in tropical countries, there is a lack of information on the biometric characterization of diaspores and on the ecophysiology of germination of seeds of this valuable species.
Studies on biometric traits of fruits and seeds, which allow quantitative evaluation of the relevance of one trait in relation to the other [9], are of paramount importance in the identification of species and can better assist in the interpretation of the seed germination test, as well as in decision making [10], during the collection of fruits and, consequently, in the production and establishment of seedlings of native tropical forest species [11]. Thus, the analysis of different traits of fruits and seeds, including their morphology, size, weight, and viability, is essential to assess physiological quality and to develop strategies for the conservation of forest species [12]. Seed size and mass are important physical indicators of physiological quality of seeds that influence several aspects, such as germination percentage and seedling vigor [13].
Most genetic differences in seed size manifest as differences in the total number of cells and their growth rate, with large seeds having more cells and higher growth rate than small seeds [14]. Seed size influences the mobilization and use of reserves [15], and in large seeds there is usually a higher content of reserves (e.g., carbohydrates, lipids, proteins), so that they produce seedlings that are more tolerant to some unfavorable conditions, such as water deficit, ensuring higher percentages of germination and vigorous seedlings, as observed in Luetzelburgia auriculata (German) Ducke [16]. However, other studies show opposite results, such as that of Farhoudi et al. [17], who observed that smaller seeds of Carthamus tinctorius L. germinated more quickly and showed greater seedling vigor under severe salt stress compared to larger seeds.
Water restriction caused by salinity is a limiting factor for seed germination, and seedling vigor is directly related to the osmotic potential of water in the soil [18]. Excess salts cause cytotoxicity, dehydration, and reduce metabolic activity and synthesis of new tissues in the seed, due to reduced water availability, resulting in lower germination speed and, in the most severe cases, loss of germination capacity [19,20]. However, the negative effects of drought and salinity on seed germination and plant establishment can be mitigated or enhanced by using seeds of different sizes [21]. Knowledge about the tolerance of seeds to water deficit caused by excess salts is extremely important, as it allows us to understand the establishment of plant populations, as well as how to exploit them [22,23].
In general, studies indicate that seed size significantly influences the physiological quality and adaptability of seedlings to adverse environmental conditions, such as salt stress. This relationship has important practical implications for ecological restoration programs, recovery of salinized areas, and seedling production in nurseries, where the selection of high-quality seeds can optimize resource use and increase project success rates.
Thus, the objective of this study was to characterize and morphologically correlate the fruits and seeds of Guilandina bonduc and to determine whether seed mass modulates seedling tolerance to salt stress.
2. Materials and Methods
2.1. Diaspore Collection Site
The fruits and seeds of Guilandina bonduc used in this study were collected from mother plants located at Praia da Barra do Abiaí (7°26′35.4″ S 34°48′39.0″ W) in the municipality of Pitimbu, Paraíba, Brazil, in January 2024. The climate of the region, according to the Köppen–Geiger classification, is of the Am type (tropical monsoon) [24], with annual rainfall and temperature averages of 2100 mm and 25.2 °C, respectively [25]. The vegetation of the diaspore collection area is typical of Restinga areas. The Restinga consists of a transition zone (ecotone) that borders the biomes and covers the coastal plains. Its vegetation is geologically recent and originated from other ecosystems, showing phenotypic variations for adaptation to different environmental conditions, which makes its diversity pattern widely heterogeneous throughout its geographic distribution [26].
2.2. Biometric Characterization of Guilandina bonduc Diaspores
After collection, the fruits were placed in plastic bags and transported to the Seed Analysis Laboratory (Laboratório de Análise de Sementes—LAS) of the Center for Agrarian Sciences of the Federal University of Paraíba, Campus II, Areia, PB (CCA/UFPB). Biometric determinations of fruits and seeds were performed, using 100 fruits and 100 seeds. Length, width, thickness and mass of fruits and seeds were measured, the first three with a digital caliper with 0.01 mm precision (Carbon Fiber Composite Digital Caliper 150 mm, NYBC—S. Prochownik Commercial Ltd.a., New York, NY, USA) and the last one with a precision analytical scale (0.001 g). Length was considered the region between the base and the apex, while width was measured in the intermediate part of fruits and seeds. Seeds were extracted manually and classified by eliminating those that were deteriorated and damaged, which allowed obtaining a uniform lot. Seeds were considered mature when they were blue-gray in color and present in dry pods with partial or total dehiscence.
2.3. Experiment Establishment and Conduction
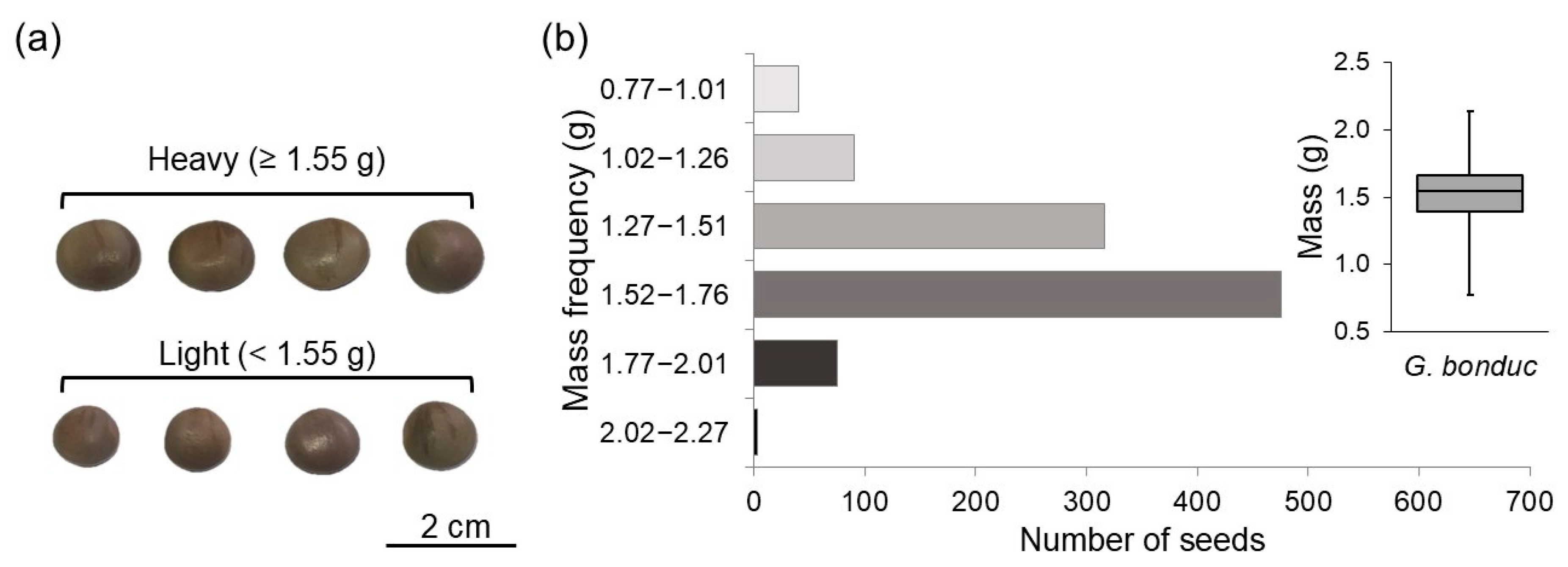
G. bonduc seeds were homogenized to form a single seed lot to ensure that the samples taken were representative of the whole. Subsequently, were weighed individually on an analytical scale to divide the sample into two classes of seeds according to their specific mass, defined as light (<1.55 g) and heavy (≥1.55 g) (Figure 1a). To define the classes, one thousand seeds were randomly weighed, the frequency histogram was obtained, and the classes were defined based on the boxplot (Figure 1b). The light class was represented by seeds with mass below the median, and the heavy class was represented by seeds with mass equal to or greater than the median.
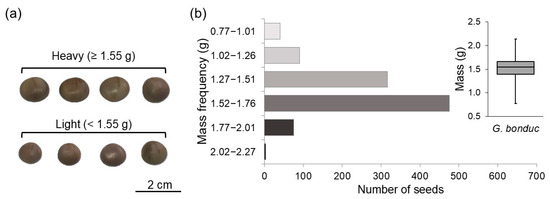
Figure 1.
(a) Representation of the distribution of light (<1.55 g) and heavy (≥1.55 g); (b) seed classes and mass frequency in histogram with boxplot distribution for a sample of one thousand seeds of Guilandina bonduc L. (Fabaceae).
The water content of the seeds of each class was determined using four replicates with approximately 10 g of seeds packed in aluminum capsules, previously identified, placed in an oven at 105 ± 3 °C and kept for 24 h [27], with the results expressed as percentage.
Prior to the test, due to seed coat dormancy, the seeds were scarified on the opposite side of the hilum using 120 grit iron sandpaper, followed by asepsis in a 5% sodium hypochlorite solution for five minutes. Effects of salt stress on germination and initial growth of seedlings were simulated using sodium chloride (NaCl) saline solutions at the following osmotic potentials: 0.0 (control), −0.25, −0.50, −0.75 and −1.0 MPa, according to the Van ’t Hoff equation [28]. The choice of these osmotic potentials, up to −1.0 MPa, is due to the fact that some species adapted to xeric and saline environments are able to germinate under these conditions, or even at even more negative potentials [18,22].
Treatments were arranged in a completely randomized design (CRD), in a 2 × 5 factorial scheme: two classes of seed mass (light and heavy) and five levels of osmotic potential (0.0, −0.25, −0.50, −0.75 and −1.0 MPa), with four replicates of 25 seeds each. To conduct the germination test, first the G. bonduc seeds of each mass class were evenly distributed among sheets of Germitest® paper (J. Prolab Industria e Comércio de Produtos para Laboratorio Ltd.a., Paraná, Brazil) with two at the base and one on the seeds, moistened with distilled water (control) and NaCl solutions, in the proportion of three times the mass of the dry substrate. The leaves were then made into rolls, which were packed in plastic bags to prevent evaporation and keep the relative humidity close to 100%. The germination test was performed in a Biochemical Oxygen Demand (B.O.D) germination chamber at a temperature of 25 °C and a photoperiod of 12/12 h (light/dark).
Due to the emergence of endogenous fungi, it was necessary to change the substrate 10 days after setting up the test.
2.4. Parameters Evaluated in the Germination and Seedling Vigor Test of Guilandina bonduc
The number of germinated seeds was counted daily for 21 days after sowing, with root protrusion of at least 1 cm being the criterion adopted to consider the seed as germinated. The variables evaluated were: water content (%); germination percentage (%); germination speed index—calculated according to the equation proposed by Maguire [29]; mean germination time—calculated using the formula proposed by Labouriau [30]; percentage of normal seedlings, considering normal seedlings to be those with well-developed and healthy essential structures, without signs of injuries or deformities; shoot length (cm) and root system length (cm). After 21 days, to obtain the dry mass of the seedlings, roots and shoots were separated with the aid of scissors and packed in Kraft paper bags, previously identified, taken to the forced air circulation oven at 65 °C, kept for 72 h and, subsequently, weighed on an analytical scale (0.001 g) to determine shoot dry mass (g seedling−1) and root dry mass (g seedling−1).
2.5. Statistical Analysis
Quantitative biometric data of fruits and seeds were analyzed through descriptive statistics. Next, Spearman’s correlation (rs) was performed for all combinations between the variables, and the significance of the rs values was determined by the t-test (p ≤ 0.05). The adjectives to describe the magnitude of the correlations were defined according to Davis’s [31] methodology, as follows: rs = 0.01 to 0.09—insignificant; rs = 0.10 to 0.29—low; rs = 0.30 to 0.49—moderate; rs = 0.50 to 0.69—substantial; rs = 0.70 to 0.99—very high; and rs = 1.0—perfect.
The Shapiro–Wilk test was used to verify the normality of the data obtained in the germination test. Next, the data were submitted to analysis of variance (ANOVA), considering the 2 × 5 factorial arrangement (seed class x osmotic potential levels), and the F test was applied to verify the significance of the main effects and the interaction between the factors (p ≤ 0.05). When significant interaction was identified, the comparison of means between seed classes was performed using the Tukey test (p ≤ 0.05). For quantitative data with significant effects, polynomial regression analysis was applied (p ≤ 0.05). The analyses were processed using R v4.2.1 [32] and SISVAR v4.3 [33] software.
3. Results and Discussion
3.1. Morphological Characterization of Guilandina bonduc L. (Fabaceae) Diaspores
According to the results of the descriptive statistics regarding the biometry of G. bonduc fruits and seeds (Table 1), fruits had an average length of 54.82 mm, width of 33.86 mm, thickness of 16.16 mm and mass of around 4.17 g. For the seeds, the average length, diameter and thickness were 16.20, 15.60 and 11.51 mm, respectively. The average seed mass was 1.51 g. Fruit mass and seed mass were the characteristics that had the highest relative dispersion, as denoted by their coefficients of variation (Table 1).

Table 1.
Descriptive analysis of the physical data of 100 fruits and seeds units of Guilandina bonduc L.
For amplitude (maximum and minimum values) of the biometric characteristics of G. bonduc fruits, it was possible to observe a considerable variation (Table 1). In relation to length, there was a variation from 32.88 to 64.23 mm, and the minimum and maximum values observed for width and thickness were 28.75 and 37.64 mm and 11.45 and 19.03 mm, respectively. For fruit mass, the maximum and minimum values observed were 7.33 g and 2.35 g, respectively, with 1 or 2 seeds per fruit. Seed length was the trait that had the lowest coefficient of variation (2.87%), and its minimum and maximum values ranged between 14.95 and 17.39 mm. For diameter and thickness, the minimum and maximum values were 13.68 and 17.85 mm and 9.75 and 13.10, respectively, and the mass ranged from 0.78 to 2.14 g (Table 1).
The highest coefficients of variation were observed for fruit mass (20.65%) and seeds (14.44%), indicating considerable phenotypic diversity within the population, which may reflect an adaptive strategy of the species to cope with different environmental conditions. This heterogeneity may favor survival and reproductive success in variable habitats, promoting greater ecological plasticity and resilience in the face of environmental changes.
As observed, it is possible to identify the presence of variation from the biometric data of fruits and seeds of G. bonduc in this study, since, as it is a wild shrub in its natural habitat, it is common for fruits and seeds to be uneven, which is generally not verified in populations of cultivated species, in which environmental variations are smaller and individuals are more similar to each other [34]. In tropical forest species, there is variability in relation to the size of the diaspores, and variation in the physical characteristics of fruits and seeds can occur within the same species or even in the same individual, due to biotic and abiotic factors throughout development, influenced by genetic variability [35,36].
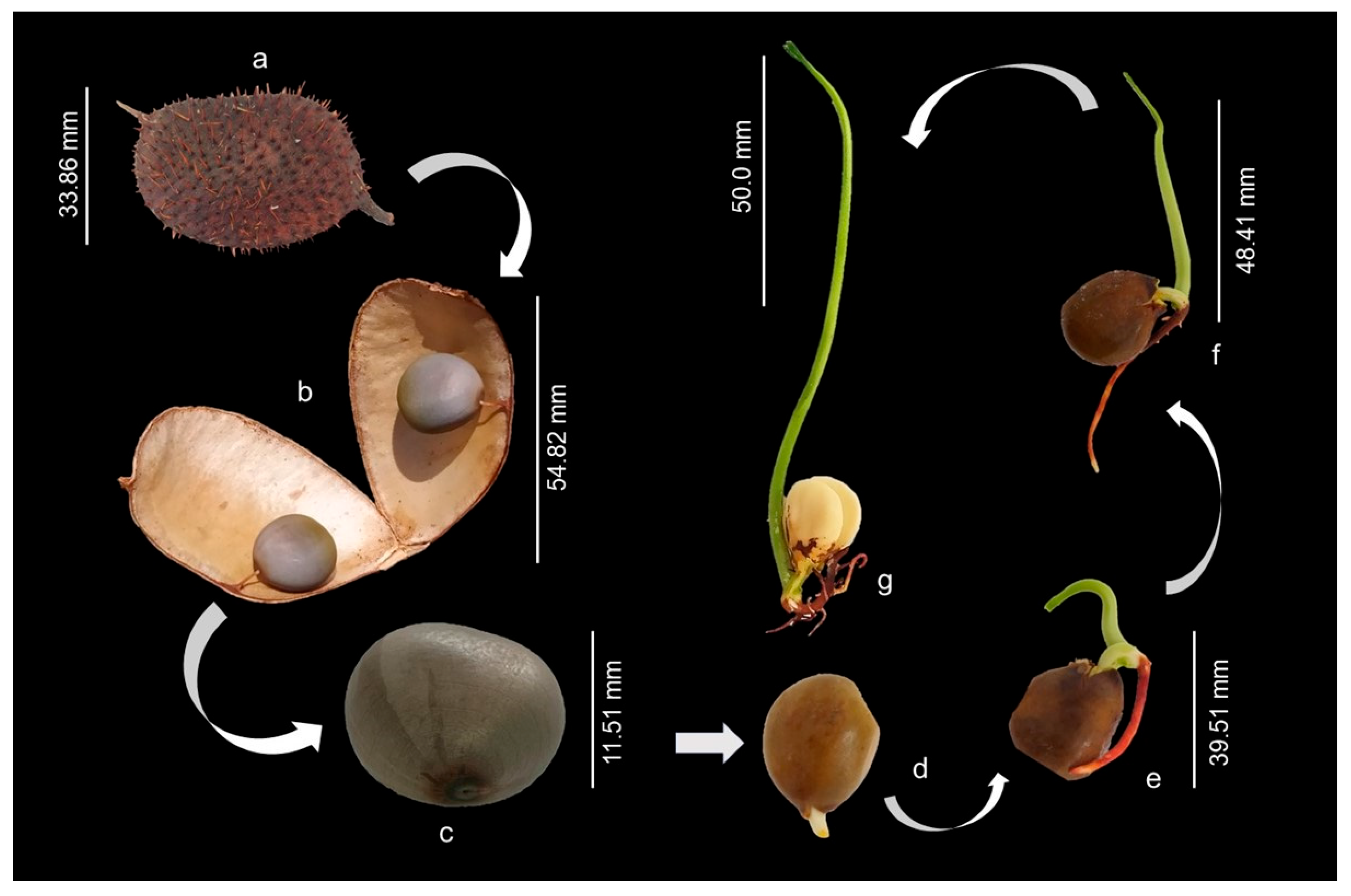
The fruits are oblong pods, dehiscent when ripe, covered with prickles, containing one or two seeds per pod (Figure 2a,b). The seeds are globular, hard, bluish-gray in color and have a smooth and shiny surface (Figure 2c). The seeds, when swollen, assume a brownish color, and the protrusion of the primary root was observed after 120 h of imbibition (Figure 2d). Subsequently, it was possible to observe the development of the epicotyl, which was approximately 30 mm long at 11 days after sowing (Figure 2e). At 16 days after sowing, epicotyl elevation and root system development were observed, along with the appearance of secondary or lateral roots (Figure 2f). After 21 days from sowing, the epicotyl was completely erect, about 120 mm long (Figure 2g). At this stage it was possible to observe the emergence of the primary leaves. Germination is hypogeal and cryptocotylar, requiring manual removal of the seed coat to visualize the cotyledons.
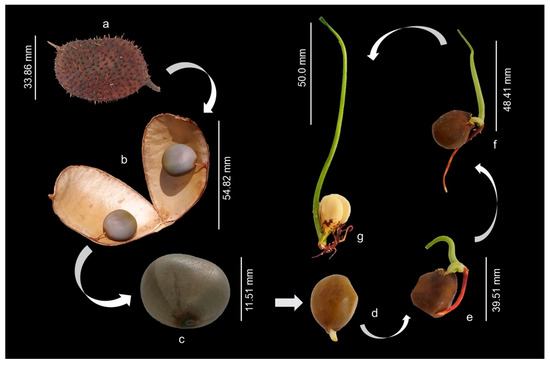
Figure 2.
Morphological aspects of the fruit, seed and seedling of Guilandina bonduc at different growth stages up to 21 days after sowing. Legend: (a) Fruit: oblong pods covered with prickles; (b) oblong pods containing two seeds; (c) seeds of bluish-gray color and smooth and shiny surface; (d) protrusion of the primary root; (e) epicotyl approximately 30 mm long; (f) development of the root system; (g) epicotyl completely erect, about 120 mm long.
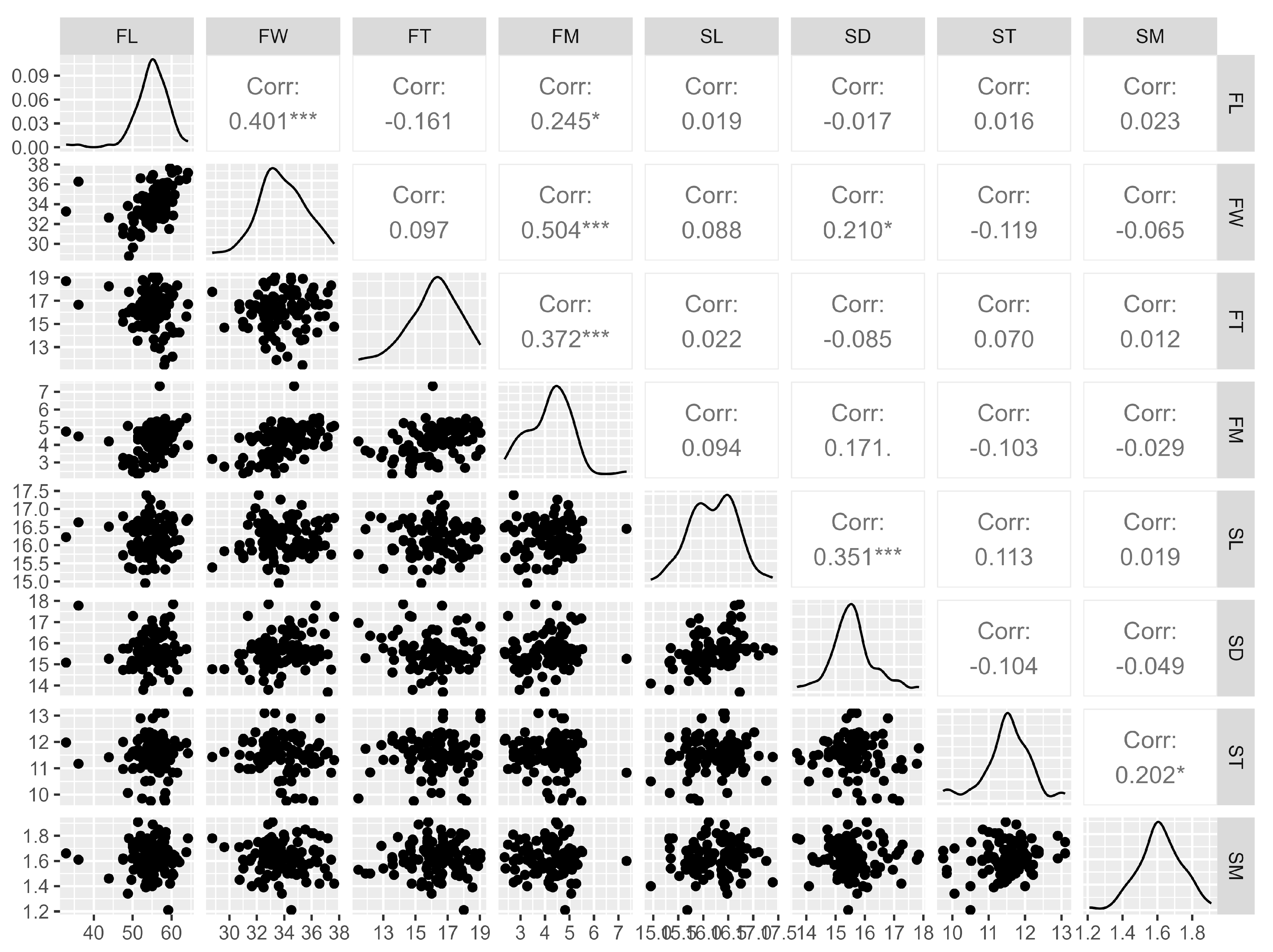
Knowledge of the correlations between fruit and seed characteristics allows us to know the behavior of one variable through the analysis of another. In this case, Spearman’s correlation (Figure 3) was verified for the evaluated physical characteristics of G. bonduc fruits and seeds, which indicated a significant and positive association between fruit length and fruit width (0.4). Fruit mass (FM) was significantly correlated with fruit length (0.2), width (0.5), and thickness (0.4). Seed diameter showed a positive and significant correlation with seed length (0.4) and fruit width (0.2). Seed mass also had a significant correlation with seed thickness (0.2). The correlation between most characteristics is considered moderate, low or insignificant. This indicates that G. bonduc is capable of producing seeds of varying mass and size, which are not necessarily linked to the mass and size of the fruits.
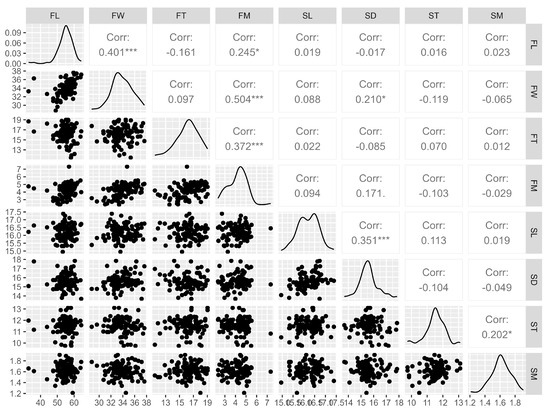
Figure 3.
Spearman’s correlation between the physical characteristics of the fruits and seeds of Guilandina bonduc L. * p < 0.05 and *** p < 0.001. Legend: fruit length (FL), fruit width (FW), fruit thickness (FT), fruit mass (FM), seed length (SL), seed diameter (SD), seed thickness (ST), seed mass (SM).
3.2. Seed Germination Under Salt Stress as a Function of G. bonduc Seed Mass
This study presents the first report of the effect of variation in the osmotic potential induced by salt stress on the physiological quality of G. bonduc seeds under different classes of mass. In both classes of seeds (light < 1.55 g and heavy ≥ 1.55 g), the water content was 5.8%. According to Marcos–Filho [20], the water content of seeds is a factor that directly impacts their weight and may vary according to harvest conditions, age and maturation. Thousand-seed weight was 1.67 kg for the heavy seeds and 1.34 kg for the light seeds.
Analysis of variance revealed a significant effect for the interaction between the factors osmotic potential levels (P) and seed mass classes (M) for all physiological characteristics evaluated, except for germination speed index (GSI), shoot length (SHL) and shoot dry mass (SDM) (Table 2). Thus, the results obtained from the morphological characteristics of light (<1.55 g) and heavy (≥1.55 g) seeds evaluated demonstrate that the application of salt stress under different combinations of P and M can exert a direct influence on the physiological behavior of seedlings. In addition, there was significant individual effect of the P factor for all the variables evaluated in the present study (Table 2), as well as an individual effect of the M factor (Table 2) for percentage of normal seedlings (NS), mean germination time (MGT), shoot length (SHL), shoot dry mass (SDM) and root dry mass (RDM).

Table 2.
Summary of the analysis of variance (ANOVA) for the physiological and morphological characteristics of light (<1.55 g) and heavy (≥1.55 g) seeds of Guilandina bonduc subjected to different levels of osmotic potential induced by salt stress.
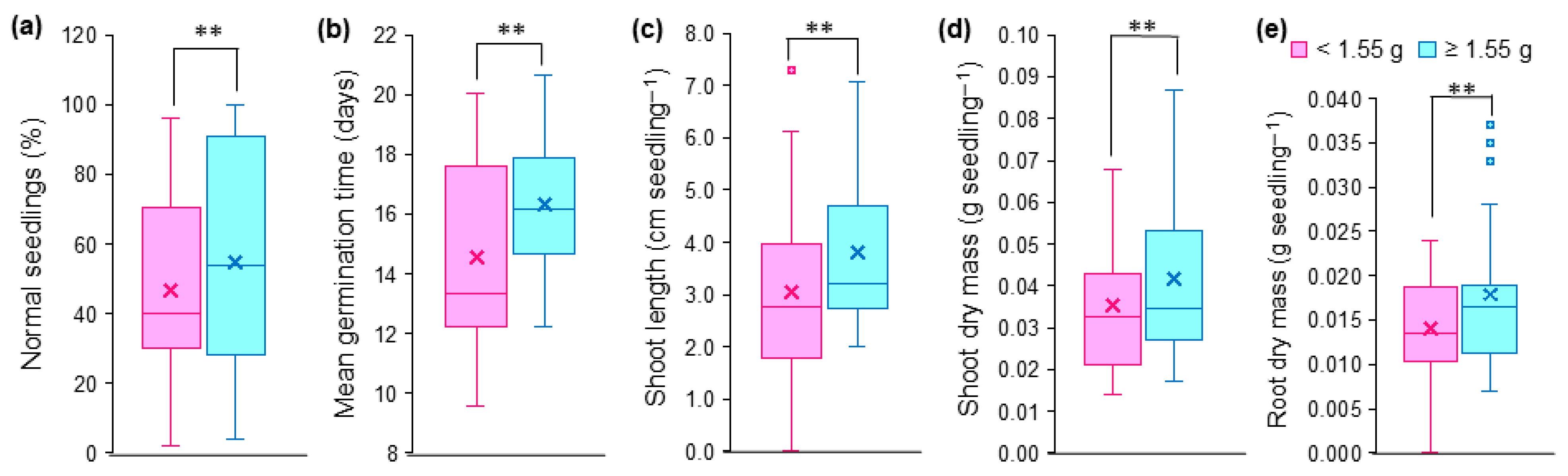
Seed mass influenced five of the eight variables evaluated, except for germination percentage, germination speed index and root system length (Table 2; Figure 4). Heavy seeds (≥1.55 g) promoted increases of 24.90, 17.51 and 26.95% for shoot length, shoot dry mass and root dry mass compared to light seeds (<1.55 g), respectively (Figure 4b–d), indicating that heavy seeds are more tolerant to salt stress. This same trend was observed for percentage of normal seedlings (Figure 4a) and mean germination time (Figure 4e).
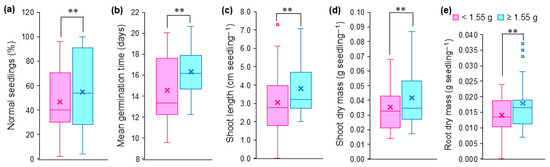
Figure 4.
Physiological and morphological characteristics of Guilandina bonduc seedlings grown from light (<1.55 g) and heavy (≥1.55 g) seeds subjected to different levels of osmotic potentials induced by salt stress. (a) percentage of normal seedlings; (b) mean germination time; (c) shoot length; (d) shoot dry mass; (e) root dry mass. ** indicates a significant difference at p < 0.01 by the F test of the analysis of variance. × corresponds to the mean of each characteristic and ▪ corresponds to outliers.
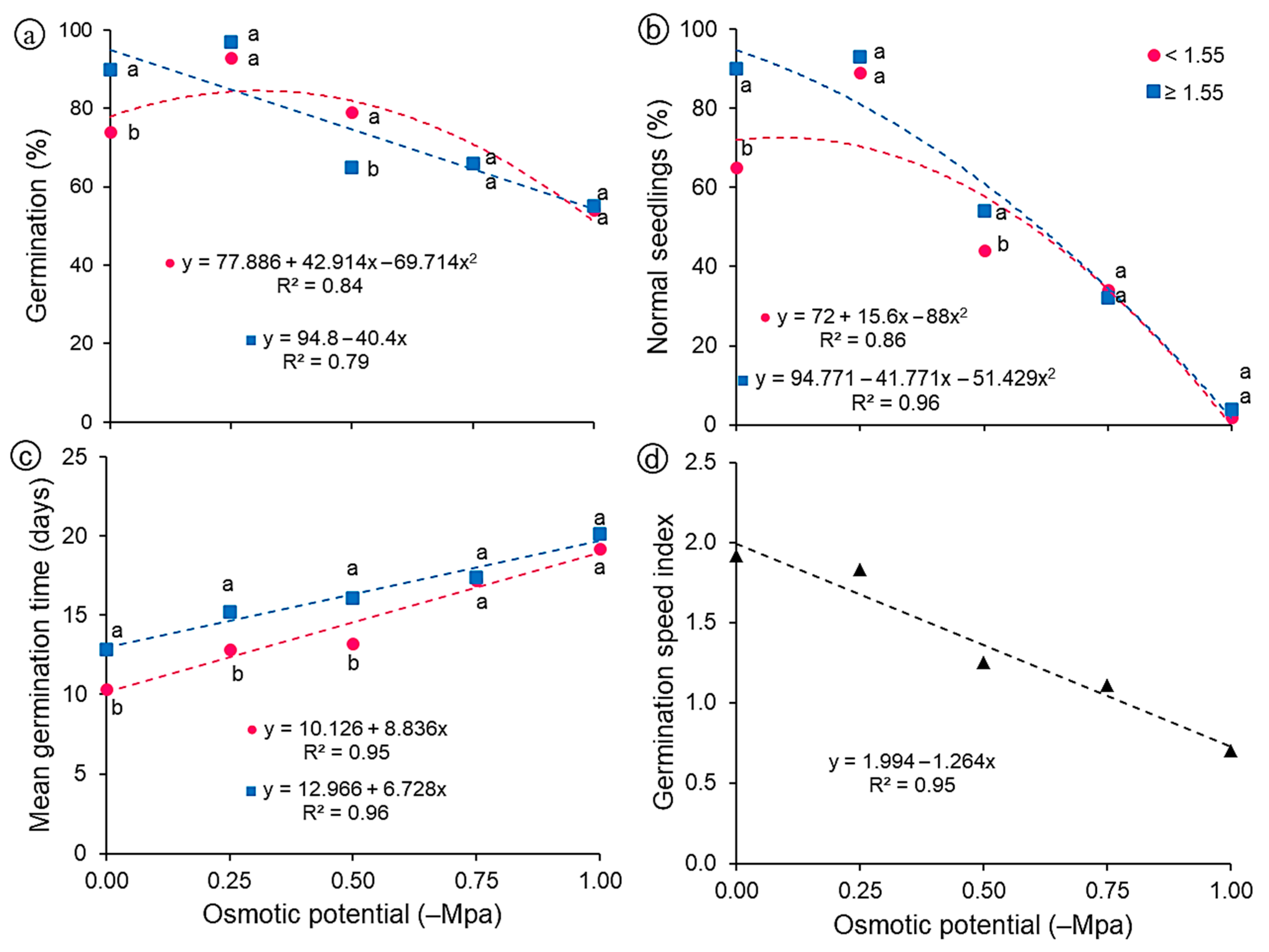
Regarding the germination percentage (Figure 5a), there was a reduction in this characteristic in both classes of seeds as the osmotic potentials became more negative, except for the potential of −0.25 MPa, whose percentage was higher than 90% in both classes. This potential (−0.25 MPa) may have favored the germination of this species, since in its natural habitat G. bonduc seeds are found in soils with high concentration of salts and, therefore, lower osmotic potential. G. bonduc seeds are tolerant to salt stress, as they can germinate under up to −1.0 MPa with satisfactory germination percentages (54%, light; 55%, heavy). However, few seeds give rise to normal seedlings under more negative potentials such as −0.75 and −1.0 MPa (Figure 5b). There were reductions of 33 and 40% in the percentage of normal seedlings at the potential of −0.50 compared to the control (0.0 MPa), for light and heavy seeds, respectively, and the data were described by a quadratic regression model for both classes of seeds.
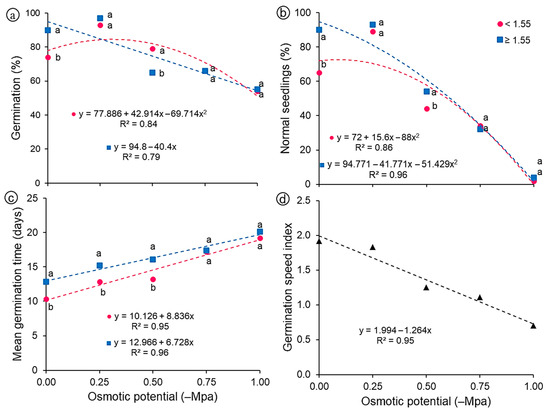
Figure 5.
Physiological variables of light (<1.55 g) and heavy (≥1.55 g) seeds of Guilandina bonduc subjected to different levels of osmotic potential. (a) germination percentage; (b) percentage of normal seedlings; (c) mean germination time—days; (d) germination speed index. Means with equal letters at the same osmotic potential did not differ statistically from each other by the Tukey test (p < 0.05).
Regarding the mean germination time, its values increased as the potentials became lower (Figure 5c). Light seeds germinated faster when compared to heavy seeds, especially at the osmotic potentials of 0.0, −0.25 and −0.50 MPa, whose mean germination time was 10, 12 and 13 days (light seeds) versus 13, 15 and 16 days (heavy seeds), respectively. For both classes of G. bonduc seeds, the data were described by the increasing linear model. Norden et al. [37] studied a large number of tropical tree species covering a wide range of seed sizes and taxa and revealed that smaller seeds germinated faster than larger seeds, contrary to what theoretical models predicted. For the germination speed index, there was no interaction (p > 0.05), but an effect of the osmotic potential was observed, with a linear model showing a peak of 1.92 in the control treatment (0.0 MPa) and gradually decreasing, culminating in 0.70 at −1.0 MPa (Figure 5d).
The variables associated with germination speed are parameters considered for the vigor of a seed lot, being important during plant establishment [20]; likewise, seeds with higher vigor lead to greater length in seedling performance tests [38]. In addition, seeds with higher mass are generally more vigorous, as they promote greater emergence and more developed seedlings [39]. Small seeds, however, have a higher surface/volume ratio than large ones, which makes it easier to obtain water to start the germination process, although they have a smaller amount of reserves [40].
The decrease in water potential causes osmotic stress that negatively affects seed germination, but stress tolerance depends on the intrinsic characteristics of each species and is generally related to adaptation to stressful habitats, such as deserts or mangroves [41]. The accumulation of salts inside cells can trigger ionic imbalances and interfere with fundamental metabolic processes, impairing the initial growth of the seedling. In extreme cases, excess salts can lead to toxicity, causing irreversible damage to seeds and completely inhibiting their germination [42].
When evaluating the influence of seed mass on the germination and initial growth of Amburana cearensis (Allemão) A.C. Smith under water stress, Almeida et al. [43] found that a decrease in osmotic potential impaired germination and initial growth of seedlings grown from medium and heavy seeds compared to light seeds, and from the potential of −0.6 MPa the condition is strictly limiting in the formation of normal seedlings of A. cearensis. According to these authors, light seeds are more likely to absorb water faster and, consequently, start the germination process more efficiently. These results are corroborated by those found in the present study with G. bonduc, since the seed vigor determined by the mean germination time demonstrated that the best values were obtained with light seeds, that is, in this case, seed mass became a defense strategy for survival in environments with water restrictions.
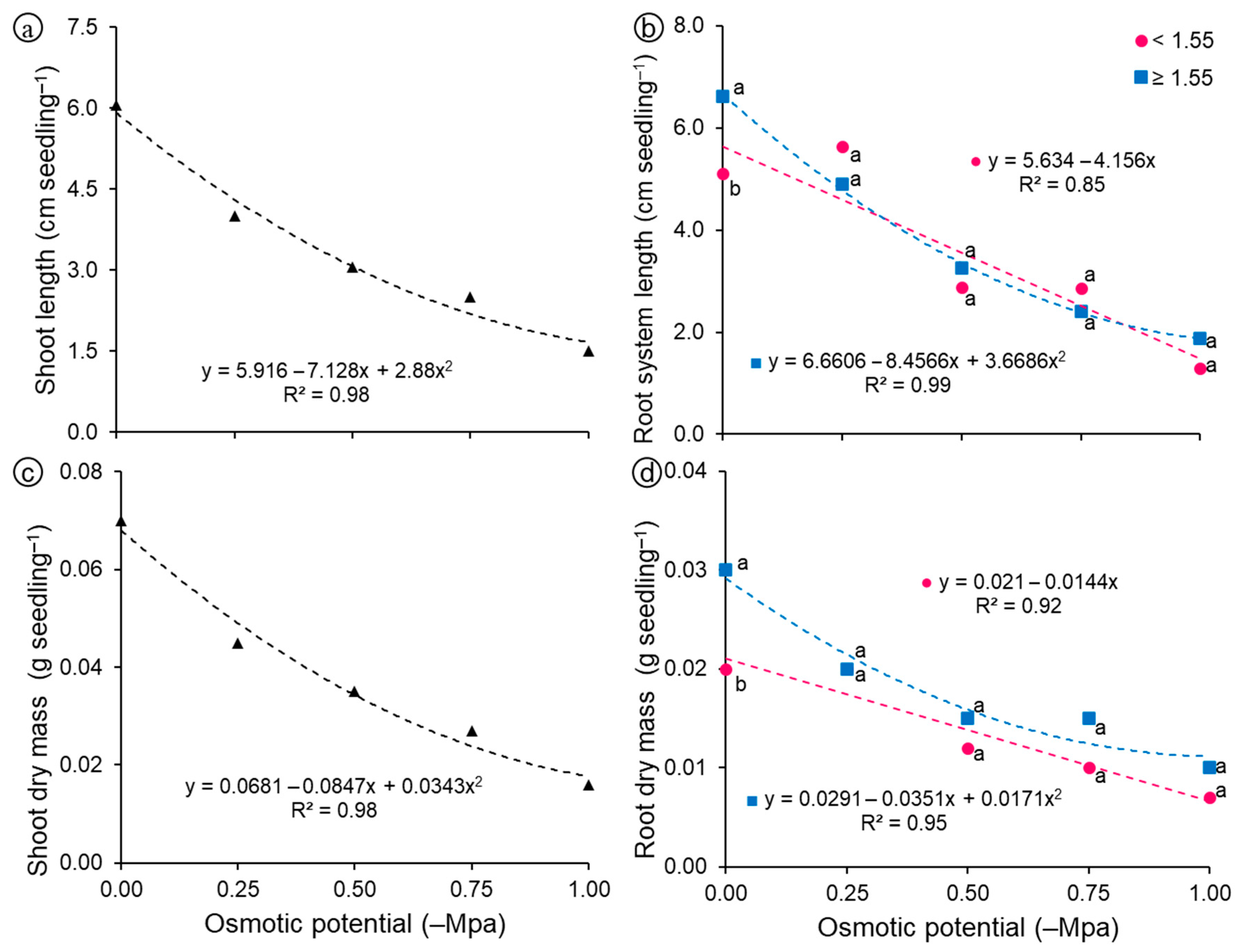
Regarding seedling shoot length (Figure 6a), a linear model explained most of the variation (R2 = 0.97), with values close to 6.0 cm in the control treatment (0.0 MPa), reaching shortest length at the most negative potential (−1.0 MPa), around 1.5 cm. Regarding root system length, linear and quadratic reductions were observed for light and heavy seeds, respectively (Figure 6b), and heavy seeds (≥1.55 g) obtained values ranging from 6.6 to 1.6 cm, with the decrease in osmotic potential, whereas in light seeds (<1.55 g) the root system length values ranged between 5.1 and 1.3 cm, depending on the osmotic potential.
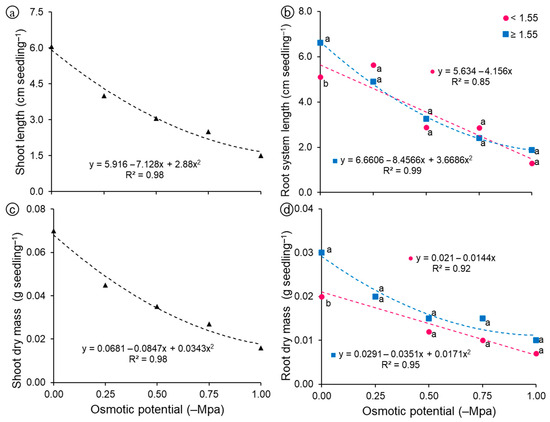
Figure 6.
Morphological variables of seedlings grown from light (<1.55 g) and heavy (≥1.55 g) seeds of Guilandina bonduc subjected to different levels of osmotic potential. (a) Shoot length; (b) Root system length; (c) Shoot dry mass; (d) Root dry mass. Means with equal letters at the same osmotic potential did not differ statistically from each other by the Tukey test (p < 0.05).
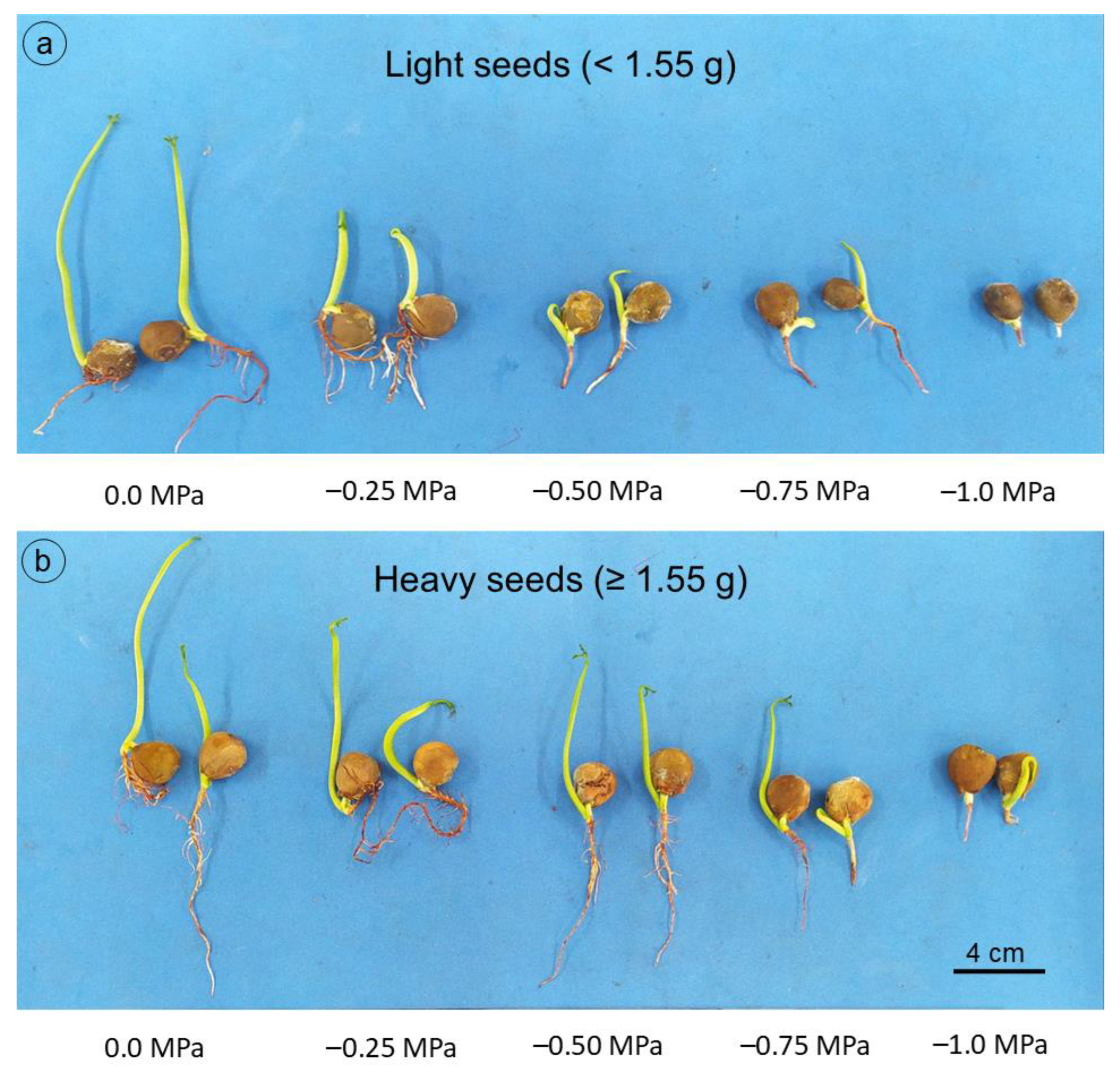
For shoot dry mass (Figure 6c), a linear model explained most of the variation (R2 = 0.98), with values close to 0.07 g seedling−1 in the control treatment (0.0 MPa), reaching the lowest mass at the most negative potential (−1.0 MPa), about 0.02 g seedling−1. Root dry mass gradually decreased as salt concentration increased, regardless of seed mass (Figure 6d). However, this reduction was even more pronounced for seeds with lower mass, whose means were described by the decreasing linear model. Even with the decrease in root system length and dry mass, due to the reduction in osmotic potential, seedlings grown from heavy seeds still stood out compared to those grown from light seeds (Figure 7), which can be explained by the greater amount of reserves available for embryonic axis development [21].
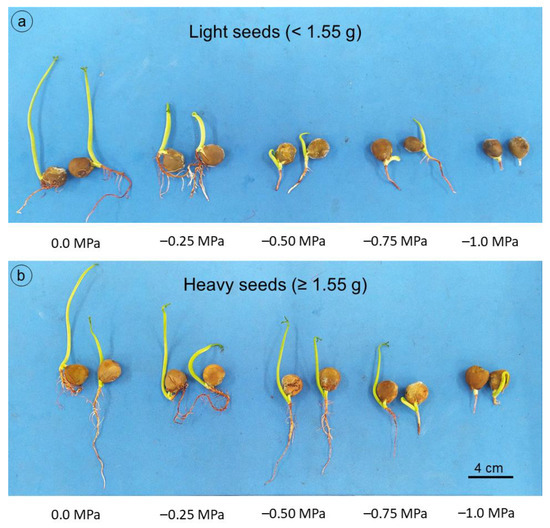
Figure 7.
Guilandina bonduc seedlings from light seeds (<1.55 g) (a) and heavy seeds (≥1.55 g) (b) at 21 days, subjected to different osmotic potentials with NaCl during germination and initial growth.
Similar results were found by Soares et al. [44] when investigating the effect of seed size on the germination and initial growth of soybean seedlings under conditions of water and salt stress. According to these authors, there was no effect of seed size on seedling germination and length; however, larger seeds resulted in seedlings with higher dry mass, even when subjected to these stress conditions. For seeds of Peltophorum dubium (Speng.) Taubert Padilha et al. [45] observed that those with smaller size had higher dormancy intensity, medium-sized seeds showed higher physiological quality, and large-sized seeds had greater potential for the formation of seedlings with higher dry mass.
The present study demonstrated the intrinsic differences within a lot of G. bonduc seeds, being possible to identify the differences in biometric characteristics, physiological quality, stress tolerance, and the relationships between these characteristics, helping in decision making during processing and future use in the field. However, further studies are needed to check whether, after prolonged salt stress, seeds have the ability to germinate when subjected to favorable conditions, indicating that the species has adaptive mechanisms to cope with abiotic stress and whether the response depends on seed mass.
4. Conclusions
Guilandina bonduc can produce seeds with variations in mass and size that are not necessarily related to the dimensions of the fruits. With the reduction in the osmotic potential, G. bonduc seeds exhibit decrease in germination and seedling vigor. G. bonduc seeds are considered tolerant to salt stress, as they can germinate at osmotic potentials of −1.0 MPa with satisfactory germination percentages (>50%), regardless of the mass. Light seeds of G. bonduc exhibit shorter germination time and uniformity in the stand. Heavy seeds produce more vigorous seedlings, especially under conditions without salt stress, and are thus recommended for seedling production.
In the context of climate change, which tends to intensify soil salinization in coastal and semi-arid regions, the results of this research indicate that G. bonduc has significant potential for use in revegetation and degraded ecosystem recovery programs, especially in saline environments. The identification of the species’ tolerance to salt stress and the relationship between seed mass and seedling vigor allows for the optimization of seed selection for the production of more resistant seedlings, contributing to strategies for adaptation and mitigation of extreme climate effects.
Author Contributions
Conceptualization, J.H.C.S.S. and E.U.A.; methodology, J.H.C.S.S.; validation, E.U.A.; formal analysis, J.H.C.S.S. and J.N.d.S.; investigation, J.H.C.S.S., L.G.A.d.A. and E.L.F.d.S.; data curation, J.H.C.S.S., L.G.A.d.A. and E.L.F.d.S.; writing—original draft preparation, J.H.C.S.S.; writing—review and editing, J.N.d.S., A.d.G.S. and E.U.A.; visualization, A.d.G.S. and E.U.A.; supervision, E.U.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) for the doctoral scholarship of the first author, and the National Council for Scientific and Technological Development (CNPq, Brazil) for the research productivity scholarships of the last author and the senior postdoctoral scholarship (process 1018672024-7) of the fifth author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ANOVA | Analysis of variance |
| BOD | Biochemical oxygen demand |
| CCA | Centro de Ciências Agrárias |
| CRD | Completely randomized design |
| CV | Coefficient of variation |
| DF | Degrees of freedom |
| FL | Fruit length |
| FM | Fruit thickness |
| FT | Fruit thickness |
| FW | Fruit width |
| G | Germination |
| GSI | Germination speed index |
| IUCN | International Union for Conservation of Nature |
| LAS | Laboratório de Análise de Sementes |
| LC | Least concern |
| MGT | Mean germination time |
| NS | Normal seedlings |
| RDM | Root dry mass |
| RSL | Root system length |
| SD | Seed diameter |
| SDM | Shoot dry mass |
| SHL | Shoot length |
| SL | Seed length |
| SM | Seed mass |
| ST | Seed thickness |
| UFPB | Universidade Federal da Paraíba |
References
- Smiderle, O.J.; Souza, A.G. Do scarification and seed soaking periods promote maximum vigor in seedlings of Hymenaea courbaril? J. Seed Sci. 2021, 43, e202143030. [Google Scholar] [CrossRef]
- Leão, N.V.M.; Araújo, E.A.A.; Shimizu, E.S.C.; Felipe, S.H.S. Características biométricas e massa de frutos e sementes de Lecythis pisonis Cambess. Enciclop. Biosf. 2016, 13, 167–175. [Google Scholar] [CrossRef]
- Menegatti, R.D.; Souza, A.G.; Bianchi, V.J. Estimating genetic divergence between peach rootstock cultivars using multivariate techniques based on characteristics associated with seeds. Genet. Mol. Res. 2019, 18, gmr18345. [Google Scholar] [CrossRef]
- Srinivasan, P.; Karunanithi, K.; Muniappan, A.; Singamoorthy, A.; Kadaikunnan, S.; Narayanan, S.P.; Thiruvengadam, M.; Nagamuthu, P. Botany, traditional usages, phytochemistry, pharmacology, and toxicology of Guilandina bonduc L.: A systematic review. Naunyn-Schmiedeberg’s Arch Pharmacol. 2024, 397, 2747–2775. [Google Scholar] [CrossRef] [PubMed]
- Bachman, S. Guilandina bonduc. In The IUCN Red List of Threatened Species 2018; International Union for Conservation of Nature: Fontainebleau, France, 2018. [Google Scholar] [CrossRef]
- Queiroz, R.T. Guilandina in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. 2024. Available online: https://floradobrasil.jbrj.gov.br/FB100887 (accessed on 26 June 2024).
- Kandasamy, V.; Balasundaram, U. Caesalpinia bonduc (L.) Roxb. as a promising source of pharmacological compounds to treat poly cystic ovary syndrome (PCOS): A review. J. Ethnopharmacol. 2021, 279, 114375. [Google Scholar] [CrossRef]
- Rathi, J.J.; Sasirekha, R.; Kumar, R.R. Effect of physical and chemical treatments on breaking the seed dormancy of Caesalpinia bonduc (L.) Roxb. Plant Sci. Today 2021, 8, 572–577. [Google Scholar] [CrossRef]
- Souza, A.G.; Smiderle, O.J.; Spinelli, V.M.; Souza, R.O.; Bianchi, V.J. Correlation of biometrical characteristics of fruit and seed with twinning and vigor of Prunus persica rootstocks. J. Seed Sci. 2016, 38, 322–328. [Google Scholar] [CrossRef]
- Montenegro, R.A.; Smiderle, O.J.; Souza, A.G. Correlation of biometric characteristics of fruits and seeds with the vigor of Agonandra brasiliensis seedlings in northern Amazonia. Biosci. J. 2022, 38, e38011. [Google Scholar] [CrossRef]
- Silva, E.L.M.; Steiner, F.; Zuffo, A.L. Caracterização morfológica de frutos e sementes de guavira [Campomanesia adamantium (Cambess.) O. Berg.]. Rev. Agronegócio Meio. Ambient. 2023, 16, e10121. [Google Scholar] [CrossRef]
- Silva, M.J.; Alves, E.U.; Silva, J.N.; Silva, R.S.; Bernardo, M.K.F.; Rodrigues, C.M.; Pádua, G.V.G.; Silva, J.H.C.S.; Silva, M.C.L.; Souza, A.G.; et al. Biometric aspects of fruits and seeds and determination of the absorption curve of Hymenaea martiana Hayne seeds. Braz. J. Biol. 2024, 84, e285632. [Google Scholar] [CrossRef]
- Souza, O.M.; Smiderle, O.J.; Souza, A.G.; Chagas, E.A.; Chagas, P.C.; Bacelar-Lima, C.G.; Morais, B.S. Influência do tamanho da semente na germinação e vigor de plântulas de populações de camu-camu. Sci. Agropecu. 2017, 8, 119–125. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Padilha, M.S.; Siega, Y.P.; Coelho, C.M.M.; Ehrhardt-Brocardo, N.C.M. O tamanho de sementes de feijão afeta a utilização das reservas armazenadas durante a germinação. Rev. Ciênc. Agroveterinárias 2023, 22, 529–537. [Google Scholar] [CrossRef]
- Lopes, M.F.Q.; Lima, L.K.S.; Ferreira, J.T.A.; Silva, T.I.; Bruno, R.L.A. Seed mass modulates tolerance to water deficit in Luetzelburgia auriculata (Allemão) Ducke seedling. Sci. Forest. 2022, 50, e3773. [Google Scholar] [CrossRef]
- Farhoudi, R.; Motamedi, M. Effect of salt stress and seed size on germination and early seedling growth of safflower (Carthamus tinctorius L.). Seed Sci. Technol. 2010, 38, 73–78. [Google Scholar] [CrossRef]
- Silva, J.H.C.S.; Azerêdo, G.A. Germination of cactus seeds under saline stress. Rev. Caatinga 2022, 35, 79–86. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017. [Google Scholar]
- Marcos-Filho, J. Fisiologia de Sementes de Plantas Cultivadas, 2nd ed.; ABRATES: Londrina, Brazil, 2015. [Google Scholar]
- Steiner, F.; Zuffo, A.M.; Busch, A.; Sousa, T.O.; Zoz, T. Does seed size affect the germination rate and seedling growth of peanut under salinity and water stress? Pesq. Agropecuária Trop. 2019, 49, e54353. [Google Scholar]
- Silva, J.H.C.S.; Azerêdo, G.A.; Ferreira, W.M.; Souza, V.C. Water restriction in seeds of Cereus jamacaru DC. Rev. Bras. Cienc. Agrar. 2021, 16, e8431. [Google Scholar] [CrossRef]
- Silva, J.H.C.S.; Rodrigues, C.M.; Souza, A.G.; Nascimento, N.F.F.; Alves, E.U. Effects of temperature and salt stress on Cereus fernambucensis seed germination. Biology 2025, 14, 393. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Weather Spark. Climate and Average Weather Year-Round in Pitimbu, Paraíba, Brazil. 2024. Available online: https://weatherspark.com/ (accessed on 30 September 2024).
- Correia, B.E.F.; Almeida Jr, E.B.; Zanin, M. Key points about North and Northern Brazilian Restinga: A review of geomorphological characterization, phytophysiognomies classification, and studies’ tendencies. Bot. Rev. 2020, 3, 329–337. [Google Scholar] [CrossRef]
- Brasil. Regras para Análise de Sementes; Ministério da Agricultura, Pecuária e Abastecimento, Secretaria de Defesa Agropecuária, MAPA/ACS: Brasília, Brazil, 2009. [Google Scholar]
- Salisbury, F.B.; Ross, C.W. Plant Physiology, 4th ed.; Wadworth: Belmont, CA, USA, 1991. [Google Scholar]
- Maguire, J.O. Speed of germination and in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Labouriau, L.F.G. Germinação das Sementes; Secretaria da OEA: Washington, DC, USA, 1983. [Google Scholar]
- Davis, J.A. Elementary Survey Analysis; Prentice-Hall: Englewood, CO, USA, 1971. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 15 September 2024).
- Ferreira, D.F. Sisvar: A computer analysis system to fixed effects split plot type designs. Braz. J. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Silva, R.A.R.; Pinheiro, L.G.; Chagas, K.P.T.; Freire, A.S.M.; Santos, J.R.M.; Vieira, F.A. Características biométricas dos frutos e sementes da palmeira Copernicia prunifera (Arecaceae). Rev. Ciênc. Agroambientais 2018, 15, 144–149. [Google Scholar] [CrossRef]
- Piña-Rodrigues, F.C.M.; Figliolia, M.B.; Silva, A. Sementes Florestais Tropicais: Da Ecologia à Produção; ABRATES: Londrina, Brazil, 2015; p. 477. [Google Scholar]
- Silva, J.N.; Alves, E.U.; Medeiros, M.L.S.; Pádua, G.V.G.; Silva, M.J.; Rodrigues, M.H.B.S.; Bernardo, M.K.F.; Cruz, J.M.F.L.; Souza, A.G.; Araújo, L.D.A. Caracterização morfológica de frutos e sementes em uma população natural de Hymenaea martiana Hayne. Sci. Forest. 2022, 50, e3929. [Google Scholar] [CrossRef]
- Norden, N.; Daws, M.I.; Antoine, C.; Gonzalez, M.A.; Garwood, N.C.; Chave, J. The relationship between seed mass and mean time to germination for 1037 tree species across five tropical forests. Funct. Ecol. 2009, 23, 203–210. [Google Scholar] [CrossRef]
- Krzyzanowski, C.F.; Vieira, R.D.; França-Neto, J.B.; Marcos-Filho, J. Vigor De Sementes: Conceitos E Testes, 2nd ed.; ABRATES: Londrina, Brazil, 2020. [Google Scholar]
- Campos, K.M.; Herrera, R.C.; Prates, H.U.S.; Lima, L.O.; Debrito, I.I.C.; Cruz, C.F.; Garcia, M.G.; Brito, G.C. Desenvolvimento de Vouacapoua americana Aubl. (Fabaceae): Influência da biometria de sementes e diferentes níveis de luz. Rev. Bras. Ciênc. Amaz. 2024, 13, 31–47. [Google Scholar]
- Kopper, A.C.; Malavasi, M.M.; Malavasi, U.C. Influência da temperatura e do substrato na germinação de sementes de Cariniana estrellensis (Raddi) Kuntze. Rev. Bras. Sementes 2010, 32, 160–165. [Google Scholar] [CrossRef]
- Mircea, D.M.; Estrelles, E.; Al Hassan, M.; Soriano, P.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Effect of water deficit on germination, growth and biochemical responses of four potentially invasive ornamental grass species. Plants 2023, 12, 1260. [Google Scholar] [CrossRef]
- Stefanello, R.; Viana, B.B.; Goergen, P.C.H.; Neves, L.A.S.; Nunes, U.R. Germination of chia seeds submitted to saline stress. Braz. J. Biol. 2020, 80, 285–289. [Google Scholar] [CrossRef]
- Almeida, J.P.N.; Pinheiro, C.L.; Lessa, B.F.T.; Gomes, F.M.; Medeiros Filho, S. Estresse hídrico e massa de sementes na germinação e crescimento de plântulas de Amburana cearensis (Allemão) AC Smith. Rev. Ciênc. Agron. 2014, 45, 777–787. [Google Scholar] [CrossRef]
- Soares, M.M.; Santos-Junior, H.C.; Simões, M.G.; Pazzin, D.; Silva, L.J. Estresse hídrico e salino em sementes de soja classificadas em diferentes tamanhos. Pesq. Agropecuária Trop. 2015, 45, 370–378. [Google Scholar] [CrossRef]
- Padilha, M.S.; Donatto, N.M.; Sobral, L.S. Qualidade fisiológica de sementes de Peltophorum dubium (Sprengel.) Taubert classificadas pelo tamanho. BIOFIX Sci. J. 2021, 6, 20–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).