Abstract
Aegopodium podagraria (A. podagraria) L. is a perennial herb valued for its medicinal properties but exhibits poor germination and inconsistent growth under conventional cultivation. To overcome these limitations and enhance its functional potential, this study investigated the effects of various LED light spectra on the plant’s physiological and antioxidant responses under controlled indoor conditions. Six light treatments were applied, consisting of different red (R) and blue (B) light ratios (R100, R80:B20, R60:B40, R40:B60, and B100), along with a white-light control. Red-dominant treatments, particularly R80:B20, not only improved germination traits but also significantly promoted shoot growth and biomass accumulation. In contrast, higher proportions of blue light generally inhibited germination performance and reduced growth-related parameters compared to the white-light control. Antioxidant activity was also modulated by light quality: R80:B20 induced the highest levels of total phenolics, ferric reducing antioxidant power, and vitamin C, whereas R40:B60 maximized flavonoid content and DPPH radical scavenging activity. These results suggest that optimizing the red-to-blue light ratio can effectively enhance both the cultivation performance and biofunctional quality of A. podagraria in controlled environments.
1. Introduction
Aegopodium podagraria (A. podagraria) L., commonly known as ground elder, is a perennial herbaceous species of the Apiaceae family, native to Europe and northern Asia. It exhibits remarkable adaptability to a range of ecological conditions, particularly favoring shaded and moist environments, where it often dominates the understory through vigorous vegetative propagation and dense ground cover formation [1,2]. While its invasive growth habit poses management challenges in both natural and cultivated settings, the species is also valued for its long-standing use in traditional medicine. Historically, dried leaves of A. podagraria have been used in herbal infusions to relieve ailments such as kidney stones, gout, hemorrhoids, and urinary tract inflammation [3]. Phytochemical analyses have revealed a rich array of bioactive constituents in A. podagraria, including polyacetylenes, essential oils, mono- and sesquiterpenes, phenolic compounds, and various vitamins [4]. These compounds collectively confer significant antioxidant capacity, making the plant a promising candidate for functional food and nutraceutical applications [5]. In recent years, growing interest in its health-promoting properties has driven research efforts aimed at optimizing extraction techniques and processing methods to maximize its pharmacological efficacy and commercial potential [6,7].
Despite its numerous applications, the cultivation of A. podagraria faces significant challenges, primarily due to environmental variability, including fluctuations in temperature, light, and rainfall. Seeds collected from wild populations often display poor and delayed germination, posing additional difficulties in establishing reliable cultivation systems [8]. These constraints necessitate innovative approaches to ensure consistent productivity and quality. LEDs offer precise control over spectral quality and intensity, which allows optimization of plant growth and development. Compared to traditional lighting technologies, LEDs are energy-efficient, durable, and capable of emitting specific wavelengths, such as red and blue light, which are known to regulate key physiological processes in plants, including photosynthesis and chlorophyll synthesis [9,10]. Red (R) and blue (B) light wavelengths also play significant roles in regulating the biosynthesis of secondary metabolites by modulating the expression of genes associated with key metabolic pathways, such as phenylpropanoid, flavonoid, and terpenoid biosynthesis [11]. Synergistic effects are also often observed under combined red and blue light treatments, which are presumed to arise from the coordinated activation of different photoreceptors and their downstream transcription factors [12]. However, plant responses to specific light spectra can vary considerably among species [13], and illumination with suboptimal wavelength combinations may fail to trigger sufficient metabolic activity or promote optimal growth and development. Therefore, species-specific optimization of light quality is essential for maximizing the desired physiological and biochemical responses in controlled environment agriculture. Hence, mixed light treatments are increasingly explored to balance multiple physiological responses. Despite these advances, little is known about how specific LED spectra affect the production of secondary metabolites in A. podagraria, an aromatic medicinal herb with growing commercial and nutraceutical importance in Asia. To date, no comprehensive studies have examined the relationship between spectral quality and key phytochemical traits A. podagraria.
Given the critical roles of germination, growth, and phytochemical composition in determining the agricultural and medicinal potential of A. podagraria, this study investigates the effects of LED spectral compositions on these parameters. By elucidating the optimal light spectrum for enhancing germination rates, shoot growth, and antioxidant properties, this research aims to provide a framework for maximizing the productivity and functional quality of A. podagraria in controlled environments. The findings will offer strategic insights for leveraging LED-based systems to overcome existing cultivation challenges and improve the agricultural and medicinal value of this versatile species.
2. Materials and Methods
Seeds of A. podagraria were utilized in this investigation and were procured from Aram Seed (Uijeongbu, Republic of Korea). The experiment was conducted using a metal shelving unit with six independent tiers, each assigned to one of the six LED light treatments (R100, B100, R80:B20, R60:B40, R40:B60, and a white-light control). Each tier was equipped with a dedicated LED panel, and five pots (13 cm × 17 cm), each containing 25 seeds, were placed directly beneath the corresponding light source to ensure consistent exposure to the assigned spectral condition. The seedlings were cultivated under controlled conditions at a temperature of 23 ± 1 °C and a relative humidity of 60 ± 10%, following a 12/12 h (light/dark) photoperiod. The cultivation trays were positioned 30 cm below the LED panels, with a maintained light intensity of 120 μmol·m−2·s−1. To minimize positional effects within each treatment, the pots were manually rotated every three days. Irrigation was also performed at each rotation interval using a plastic beaker, with 250 mL of water applied per pot. This spatial separation of treatments effectively prevented spectral overlap and ensured the independent application of each light regime.
Germination parameters, including germination percentage, energy, and T50, were assessed by monitoring seed germination from the initial day up to the 20th day post sowing. Germination percentage (GP%) was determined by dividing the number of germinated seeds by the total number of seeds, as outlined by Kim et al. [14]. Germination energy (GE%) was calculated by dividing the total number of germinated seeds on the 10th day by the total number of seeds.
The T50 value, representing the median germination time, was computed using the described below.
Here, in the equation, N represents the final number of germinated seeds, while Ni and Nj denote the total number of seeds germinated on consecutive counts at times Ti and Tj, respectively, where Ni < N/2 < Nj.
The growth characteristics of the ground elder were evaluated, encompassing shoot length, fresh shoot weight, and leaf count. Representative plants were selected based on visual assessment of growth performance, and the three most well-developed plants were collected from each pot. A total of five replicate pots were evaluated per treatment. The fresh shoot length of each plant was measured using a ruler, and the fresh weight was determined using an electronic balance (WBA-220; Witeg Labortechnik GmbH, Wertheim, Germany), and the number of leaves was recorded.
For the analysis of α-amylase activity and chlorophyll content, leaves were randomly collected from plants across the five pots within each treatment, and three composite samples were prepared to serve as biological replicates. To measure α-amylase activity, leaves of A. podagraria (0.5 g) subjected to different light treatments were pulverized using a mortar and pestle with liquid nitrogen. The resultant powder was homogenized in 4 mL of 0.02 M Tris–HCl buffer (pH 6.5), transferred to a 15 mL falcon tube, and centrifuged at 12,000 rpm for 30 min at 4 °C. The supernatant was collected and stored at −20 °C for subsequent analyses. α-Amylase activity was quantified using the dinitro salicylic acid (DNS) method. The assay included 1 mL of 1% starch solution and 1 mL of enzyme extract. Following a 3 min equilibration at 20 °C, 1 mL of color reagent (5.3 M potassium sodium tartrate, 96 mg DNS, in ultrapure water) was added post incubation to stop the reaction. The mixture was heated in a boiling water bath for 15 min, cooled, and diluted with 9 mL of ultrapure water. Absorbance was measured at 540 nm, and enzyme activity was expressed as units per milliliter using a maltose standard. Chlorophyll was spectrophotometrically quantified from fresh A. podagraria leaf samples. Leaves (1 g) were macerated in 80% acetone, and the suspension was centrifuged at 1320 rpm for 3 min. The clear green supernatant was decanted, the volume adjusted to 10 mL with 80% acetone, and further centrifuged at 10,000 rpm for 10 min. Absorbance at 665 nm and 649 nm was recorded. The samples were taken in triplicates and the results expressed as µg/mL were calculated by the following formulae:
Chlorophyll ‘a’ content (µg/mL) = 11.63 × A665 − 2.39 × A649
Chlorophyll ‘b’ content (µg/mL) = 20.11 × A649 − 5.18 × A665
Total chlorophyll content (µg/mL) = Chlorophyll ‘a’ + Chlorophyll ‘b’
To investigate the antioxidant properties of A. podagraria, all remaining leaves from the five pots—after completing the growth assessment, α-amylase activity, and chlorophyll content measurements—were thoroughly and randomly divided into three tubes to create three biological replicates. The leaf samples were then dried at 60 °C for three days and subsequently ground into a fine powder for further analysis. In total, 0.1 g of this powdered material was extracted in 10 mL of 100% methanol. The mixture was then subjected to a 30 min heat treatment at 60 °C in a Brasnocic bath (CPX3800H-E, Emerson, St. Louis, MO, USA) and centrifuged at 4000 rpm for 20 min. This extraction process was repeated three times, each in triplicate. Total phenolic content, total flavonoid content, and DPPH radical scavenging activity were quantified using the Folin–Ciocalteu method described by Geleta and Heo [15]. Briefly, 25 µL of extract was mixed with diluted Folin–Ciocalteu reagent, sodium bicarbonate, and water. After incubation in the dark for one hour, absorbance was measured at 765 nm using a microplate reader (Mobi, MicroDigital, Seongnam, Republic of Korea), and results were expressed as milligrams of gallic acid equivalent per gram of dry weight. Total flavonoid content was measured using the aluminum chloride colorimetric method. The sample was combined with methanol, aluminum chloride, and sodium acetate, incubated for 30 min in darkness, and the absorbance recorded at 415 nm. Results were reported as milligrams of quercetin equivalents per gram of dry weight. DPPH radical scavenging activity was assessed by mixing the extract with DPPH solution and measuring the decrease in absorbance at 517 nm. The percentage inhibition was calculated to quantify antioxidant capacity. The ferric reducing antioxidant power assay, based on the protocol by Benzie and Strain [16], involved reacting the sample with a FRAP solution and measuring the absorbance at 593 nm. Results were expressed in µM Trolox equivalents per gram of dry weight. Vitamin C content was determined using a modified version of the method by Desai and Desai [17]. The A. podagraria extract was treated with metaphosphoric acid, bromine, thiourea, and 2,4-Dinitrophenol, incubated at 37 °C, and the absorbance measured at 521 nm. Results were expressed as milligrams of ascorbic acid per gram of dry weight. The collected data were subjected to one-way ANOVA to determine the effect of LEDs treatment on the A. podagraria. Duncan’s multiple-separation test was used to determine mean separation, and analyses were performed by SPSS using version 28 (IBM, New York, NY, USA).
3. Results
The germination performance of A. podagraria exhibited substantial variation in response to different LED light spectra, with certain traits displaying marked sensitivity to spectral composition. Mean germination percentages ranged from 60.00% to 75.20%, indicating moderate variability across treatments. Germination initiated on day 6 and was completed by day 13 in all light conditions, suggesting a consistent temporal window for germination irrespective of spectral quality. Treatments containing 60% or more red light showed germination rates that were comparable to or higher than those of the control, whereas treatments with a high proportion of blue light resulted in reduced germination percentages. Similarly, germination energy was significantly affected by light quality (p ≤ 0.05), with the highest value (60.80%) observed under the R80:B20 treatment, which provided a red-dominant spectral environment. In contrast, the lowest germination energy (42.40%) was recorded under the B100 treatment, indicating that monochromatic blue light strongly suppresses early germination vigor. Furthermore, the R40:B60 treatment, which also had a high blue light proportion, led to reductions of approximately 15% in final germination percentage and 18% in germination energy compared to red-dominant or white light treatments, reinforcing the inhibitory effect of blue-enriched light on germination performance.
The time to 50% germination (T50) was markedly delayed under the B100 treatment, which exhibited the highest T50 value among all treatments. This result indicates that monochromatic blue light not only suppresses early germination vigor but also slows the overall germination process (Table 1). Significant variation in α-amylase activity was observed among the different LED light treatments, with enzymatic activity ranging from 75.44 to 95.21 U/mL. The highest activity was recorded under the R60:B40 treatment, while the lowest was observed under the B100 condition (Figure 1). This trend suggests a stimulatory effect of red-enriched spectra on α-amylase synthesis or activation. Correlation analysis revealed that α-amylase activity was significantly and positively associated with all measured germination parameters, except for T50. Although the correlation with T50 was also positive, it was not statistically significant. These findings underscore the pivotal role of α-amylase in seed germination, particularly in mobilizing starch reserves to support early seedling development (Table 2).

Table 1.
Effect of LEDs light quality on the germination characteristics of A. podagraria.
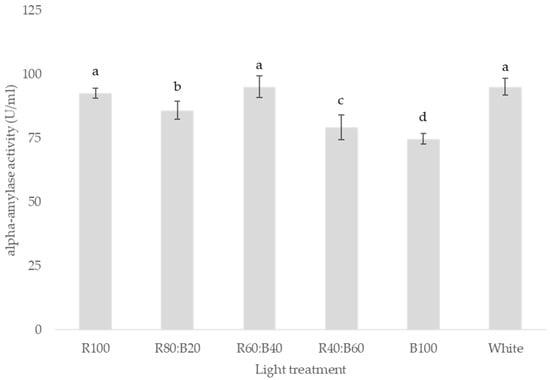
Figure 1.
Effect of LED light quality on α-amylase activity in Aegopodium podagraria. Bars labeled with different letters indicate significant differences according to Duncan’s multiple range test (p ≤ 0.05). Experiments were conducted with three replicates per treatment.

Table 2.
Pearson correlation coefficients between α-amylase activity and germination traits of A. podagraria.
Significant differences in growth characteristics were observed among the various LED light treatments, with the exception of leaf number, which remained statistically unchanged across all conditions. Treatments enriched in red light, particularly R100 and R80:B20, resulted in the greatest shoot length and fresh weight, indicating their promotive effect on vegetative development. Conversely, treatments with a higher proportion of blue light, such as R40:B60 and B100, significantly reduced shoot elongation and biomass accumulation compared to the white-light control (Table 3).

Table 3.
Effect of LED light quality on growth characteristics and chlorophyll content of A. podagraria.
Chlorophyll content also varied markedly depending on light quality. The R80:B20 treatment induced the highest levels of chlorophyll a and total chlorophyll, likely due to enhanced photosynthetic pigment synthesis under red-dominant conditions. In contrast, the lowest total chlorophyll content was recorded under R40:B60, indicating a suppressive effect of excessive blue light on pigment accumulation.
Correlation analysis further revealed strong positive associations between total chlorophyll content and key growth parameters, including shoot length and fresh shoot weight, suggesting that improved pigment levels contribute to enhanced biomass production under favorable spectral conditions (Table 4). Collectively, these results indicate that a higher red-to-blue light ratio significantly enhances A. podagraria germination, α-amylase activity, vegetative growth, and chlorophyll accumulation. In contrast, blue-light dominant treatments exert inhibitory effects on these physiological and developmental processes.

Table 4.
Pearson correlation coefficients between chlorophyll content and growth characteristics of Aegopodium podagraria.
The antioxidant properties of A. podagraria varied significantly among the different LED light treatments, reflecting a clear influence of light spectral composition on secondary metabolite accumulation. Total phenolic content ranged from 3.59 mg GAE/g under the R60:B40 treatment to 5.27 mg GAE/g under R80:B20, indicating that red-dominant light conditions, particularly R80:B20, are optimal for phenolic biosynthesis. In contrast, total flavonoid content was highest under the white-light control (3.93 mg QE/g) and the B100 treatment (3.88 mg QE/g), suggesting that either full-spectrum white or blue-enriched light environments favor flavonoid accumulation (Table 5). DPPH radical scavenging activity, a key indicator of antioxidant potential, peaked under the R40:B60 treatment, highlighting the possible stimulatory role of elevated blue light ratios in enhancing free radical scavenging capacity. In comparison, the R100 treatment, consisting of only red light, resulted in the lowest levels of total phenolics, flavonoids, and DPPH activity, implying a limited role of monochromatic red light in antioxidant induction in this species.

Table 5.
Effect of LED light quality on antioxidant properties of A. podagraria.
Additional antioxidant metrics, including ferric reducing antioxidant power (FRAP) and vitamin C content, also exhibited considerable variation across treatments. FRAP values ranged from 7.34 to 11.54 µM Trolox/g, while vitamin C content varied between 1.08 and 1.68 mg AA/g. Both parameters were maximized under the R80:B20 treatment, further supporting the efficacy of red-enriched but balanced light in promoting antioxidant metabolism. The lowest values were recorded under the control for FRAP and under the R40:B60 treatment for vitamin C, indicating that either full-spectrum light or a blue-dominant light with a lower proportion of red may not be sufficient to optimally enhance these traits. Collectively, these findings demonstrate that LED light quality exerts a profound influence on the antioxidant profile of A. podagraria. While red light alone appears insufficient to stimulate antioxidant synthesis, its combination with blue light—particularly in the R80:B20 ratio—proves effective in enhancing total phenolics, vitamin C, and FRAP activity. These results suggest that optimizing the red-to-blue light ratio is a promising strategy to improve the medicinal and nutritional value of A. podagraria cultivated under controlled environments.
4. Discussion
The results of this study demonstrate that A. podagraria exhibits distinct physiological and biochemical responses to different light spectra, highlighting the regulatory role of light quality in plant development, as reported in other species [18,19]. Specifically, seed germination, chlorophyll accumulation, and antioxidant activity were modulated by red and blue light treatments, reflecting the differential activation of photoreceptor-mediated signaling pathways. Seed germination was significantly enhanced under red-light-enriched conditions, whereas blue light generally suppressed germination performance. These observations are consistent with previous studies reporting that red light promotes germination via phytochrome-mediated pathways [20], while blue light may inhibit germination by interfering with gibberellin biosynthesis or disrupting hormonal processes essential for endosperm weakening and radicle emergence [21,22]. Furthermore, α-amylase activity—an important indicator of starch degradation and energy mobilization during early seedling growth [23]—was notably elevated under red-rich light, suggesting that red light may facilitate germination by enhancing key enzymatic processes. This pattern aligns with prior observations in Leonurus japonicus (motherwort) [5]. Light spectral quality also significantly influenced chlorophyll content, an indicator of photosynthetic potential. Among treatments, R100 produced the greatest shoot growth, while R80:B20 resulted in the highest chlorophyll accumulation, implying that red-dominant conditions enhance chloroplast development and light-harvesting efficiency. These findings are in line with previous studies on Momordica charantia L. and lettuce, in which red light promoted vegetative growth under controlled environmental conditions [24,25]. Red light has been reported to promote the development of adventitious roots, thereby enhancing early seedling growth. A similar effect is presumed to have occurred in A. podagraria as well [26]. As both red and blue wavelengths influence chlorophyll biosynthesis and chloroplast architecture, optimizing the red-to-blue ratio may serve as an effective strategy for improving biomass production in A. podagraria. In addition to growth, light treatments had a marked effect on antioxidant profiles. Total phenolic content and ferric reducing antioxidant power (FRAP) were highest under red-enriched spectral combinations, while blue-dominant treatments led to increased flavonoid accumulation and enhanced DPPH radical scavenging activity. Although gene expression analysis was not performed in this study, these patterns correspond to the established knowledge of light-regulated secondary metabolism. Red light has been associated with increased activity of key enzymes in the phenylpropanoid pathway, including phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H), and 4-coumarate:CoA ligase (4CL) [27]. In contrast, blue light is known to promote flavonoid biosynthesis through the activation of enzymes such as chalcone synthase (CHS), flavanone 3-hydroxylase (F3H), and dihydroflavonol 4-reductase (DFR), potentially via cryptochrome signaling and reactive oxygen species (ROS)-responsive pathways [28,29].
Interestingly, monochromatic red-light treatment yielded relatively low levels of phenolics and antioxidant activity compared to mixed red–blue light treatments, a phenomenon similarly observed in Salvia miltiorrhiza [30]. This may be due to the absence of blue light, which appears critical for activating certain branches of secondary metabolism. The lack of spectral complexity likely limited synergistic interactions between phytochrome- and cryptochrome-mediated signaling, thereby constraining the biosynthesis of specific antioxidant compounds. Collectively, these findings underscore the functionally divergent yet partially complementary roles of red and blue light in regulating the physiological and antioxidant-related responses of A. podagraria. Red-enriched spectra were most effective in promoting vegetative growth and phenolic accumulation, while blue light more selectively enhanced flavonoid biosynthesis. Although the exact molecular mechanisms remain to be fully elucidated, our results suggest that manipulating light spectra represents a promising non-genetic strategy for optimizing specific physiological traits in this species.
Future research incorporating transcriptomic and metabolomic analyses will be essential to uncover the regulatory networks underpinning these light-responsive processes. Moreover, validating the consistency of these responses under commercial-scale cultivation systems, across developmental stages, and under diverse environmental conditions will be critical for applying light quality modulation as a tool to enhance the production of functional compounds and the medicinal value of A. podagraria in controlled-environment agriculture.
5. Conclusions
This study highlights the substantial impact of LED light spectra on the physiological development and secondary metabolism of A. podagraria, a species with emerging potential as a functional food. By demonstrating the distinct effects of red and blue wavelengths on germination, chlorophyll biosynthesis, and antioxidant properties, our findings offer valuable insights for designing precision lighting strategies that enhance the productivity and health-promoting qualities of this plant. These insights are particularly relevant in the context of sustainable agriculture and the development of value-added leafy vegetables in Korea and beyond.
Author Contributions
Conceptualization, B.T.G. and J.-Y.H.; methodology, B.T.G. and J.-Y.H.; software, B.T.G.; validation, B.T.G.; formal analysis, J.-Y.H.; investigation, B.T.G.; data curation, B.T.G.; writing—original draft preparation, B.T.G.; writing—review and editing, J.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, JYH, upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jakubczyk, K.; Janda, K.; Styburski, D.; Łukomska, A. Goutweed (Aegopodium podagraria L.)-botanical characteristics and prohealthy properties. Postępy Hig. Med. Dośw. 2020, 74, 28–35. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Łukomska, A.; Czaplicki, S.; Wajs-Bonikowska, A.; Gutowska, I.; Czapla, N.; Janda-Milczarek, K. Bioactive compounds in Aegopodium podagraria leaf extracts and their effects against fluoride-modulated oxidative stress in the THP-1 cell line. Pharmaceuticals 2021, 14, 1334. [Google Scholar] [CrossRef] [PubMed]
- Orav, A.; Viitak, A.; Vaher, M. Identification of bioactive compounds in the leaves and stems of Aegopodium podagraria by various analytical techniques. Procedia Chem. 2010, 2, 152–160. [Google Scholar] [CrossRef]
- Dębia, K.; Dzięcioł, M.; Wróblewska, A.; Janda-Milczarek, K. Goutweed (Aegopodium podagraria L.)—An edible weed with health-promoting properties. Molecules 2025, 30, 1603. [Google Scholar] [CrossRef]
- Lee, W.H.; Zebro, M.; Heo, J.Y. Light-emitting diode light quality influences germination and sprout characteristics of Motherwort. J. Food Qual. 2023, 2023, 4644078. [Google Scholar] [CrossRef]
- Wróblewska, A.; Janda, K.; Makuch, E.; Walasek, M.; Miądlicki, P.; Jakubczyk, K. Effect of extraction method on the antioxidative activity of ground elder (Aegopodium podagraria L.). Pol. J. Chem. Technol. 2019, 21, 13–18. [Google Scholar] [CrossRef]
- Engelhardt, L.; Pöhnl, T.; Neugart, S. Edible wild vegetables Urtica dioica L. and Aegopodium podagraria L.–antioxidants affected by processing. Plants 2022, 11, 2710. [Google Scholar] [CrossRef]
- Phartyal, S.S.; Kondo, T.; Baskin, J.M.; Baskin, C.C. Temperature requirements differ for the two stages of seed dormancy break in Aegopodium podagraria (Apiaceae), a species with deep complex morphophysiological dormancy. Am. J. Bot. 2009, 96, 1086–1095. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Nájera, C.; del Mar Gea, M. Effect of the spectral quality and intensity of light-emitting diodes on several horticultural crops. HortScience 2016, 51, 268–271. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beyond vegetables: Effects of indoor LED light on specialized metabolite biosynthesis in medicinal and aromatic plants, edible flowers, and microgreens. J. Sci. Food Agric. 2022, 102, 472–487. [Google Scholar] [CrossRef]
- Stamford, J.D.; Stevens, J.; Mullineaux, P.M.; Lawson, T. LED lighting: A grower’s guide to light spectra. HortScience 2023, 58, 180–196. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, D.C.; Zebro, M.; Heo, J.Y. Effects of various combinations of red and blue LEDs on seed germination and growth in Indian spinach (Basella alba L.). Italus Hortus 2022, 29, 196–205. [Google Scholar] [CrossRef]
- Geleta, B.T.; Heo, J.Y. Variation of fruit characteristics and antioxidant properties among ten apple cultivars grown in two different high-latitude regions of South Korea. Erwerbs-Obstbau 2023, 65, 2165–2174. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.P.; Desai, S. UV spectroscopic method for determination of vitamin C (ascorbic acid) content in different fruits in South Gujarat region. Int. J. Environ. Sci. Nat. Resour. 2019, 22, 41–44. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The control of seed dormancy and germination by temperature, light and nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, S.; Yu, D. The role of light quality in regulating early seedling development. Plants 2023, 12, 2746. [Google Scholar] [CrossRef]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, X.; Foo, E.; Symons, G.M.; Lopez, J.; Bendehakkalu, K.T.; Xiang, J.; Weller, J.; Liu, X.; Reid, J.B.; et al. A study of gibberellin homeostasis and cryptochrome-mediated blue light inhibition of hypocotyl elongation. Plant Physiol. 2007, 145, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ma, W.; Shen, S.; Gu, A. Underlying biochemical and molecular mechanisms for seed germination. Int. J. Mol. Sci. 2022, 23, 8502. [Google Scholar] [CrossRef]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The rice alpha-amylase, conserved regulator of seed maturation and germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Fan, H.; Huang, P. Effects of light-emitting diode (LED) red and blue light on the growth and photosynthetic characteristics of Momordica charantia L. J. Environ. Chem. 2020, 10, 1–15. [Google Scholar]
- Solano, C.J.; Hernández, J.A.; Suardíaz, J.; Barba-Espín, G. Impacts of LEDs in the red spectrum on the germination, early seedling growth and antioxidant metabolism of pea (Pisum sativum L.) and melon (Cucumis melo L.). Agriculture 2020, 10, 204. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Fang, W.; Yang, R.; Yin, Y. Production of high-quality wheat sprouts of strong antioxidant capacity: Process optimization and regulation mechanism of red light treatment. Foods 2024, 13, 2703. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light induced regulation pathway of anthocyanin biosynthesis in plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.H.; Park, H.S.; Min, C.W.; Woo, J.H.; Choi, B.R.; Rahman, M.M.; Lee, K.W. Light quality plays a crucial role in regulating germination, photosynthetic efficiency, plant development, reactive oxygen species production, antioxidant enzyme activity, and nutrient acquisition in Alfalfa. Int. J. Mol. Sci. 2025, 26, 360. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Zou, H.; Zhang, L.; Li, S.; Wang, Y. The combination of blue and red LED light improves growth and phenolic acid contents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2020, 158, 112959. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).