Priming ‘Santa Isabel’ Pea (Pisum sativum L.) Seeds with NaCl and H2O2 as a Strategy to Promote Germination
Abstract
1. Introduction
2. Materials and Methods
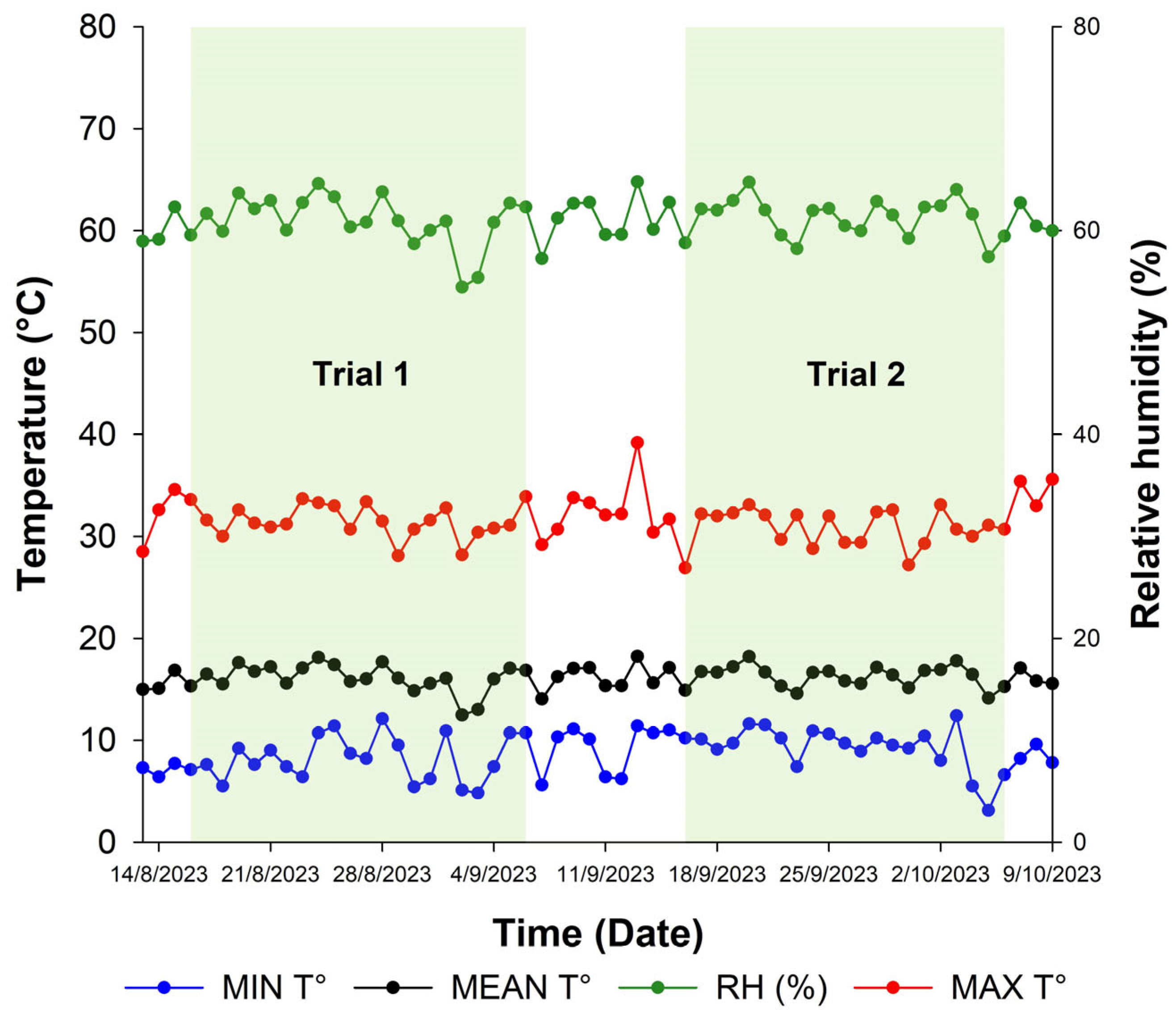
2.1. Location
2.2. Plant Material
2.3. Experimental Design and Field Establishment
2.4. Response Variables
2.5. Statistical Analysis
3. Results and Discussion
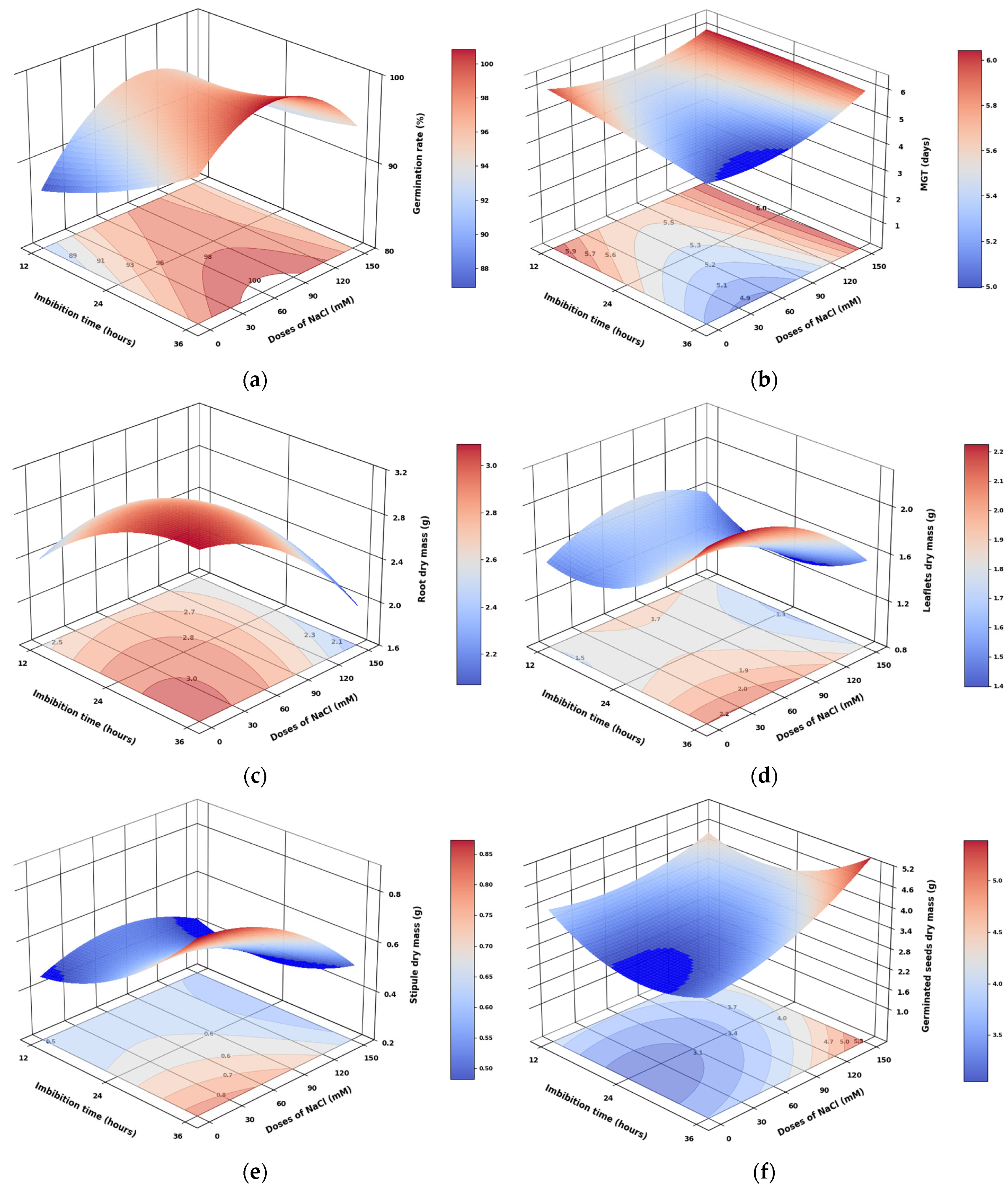
3.1. Effect of Imbibition Time and NaCl Doses on Germination Parameters in Pea Seeds
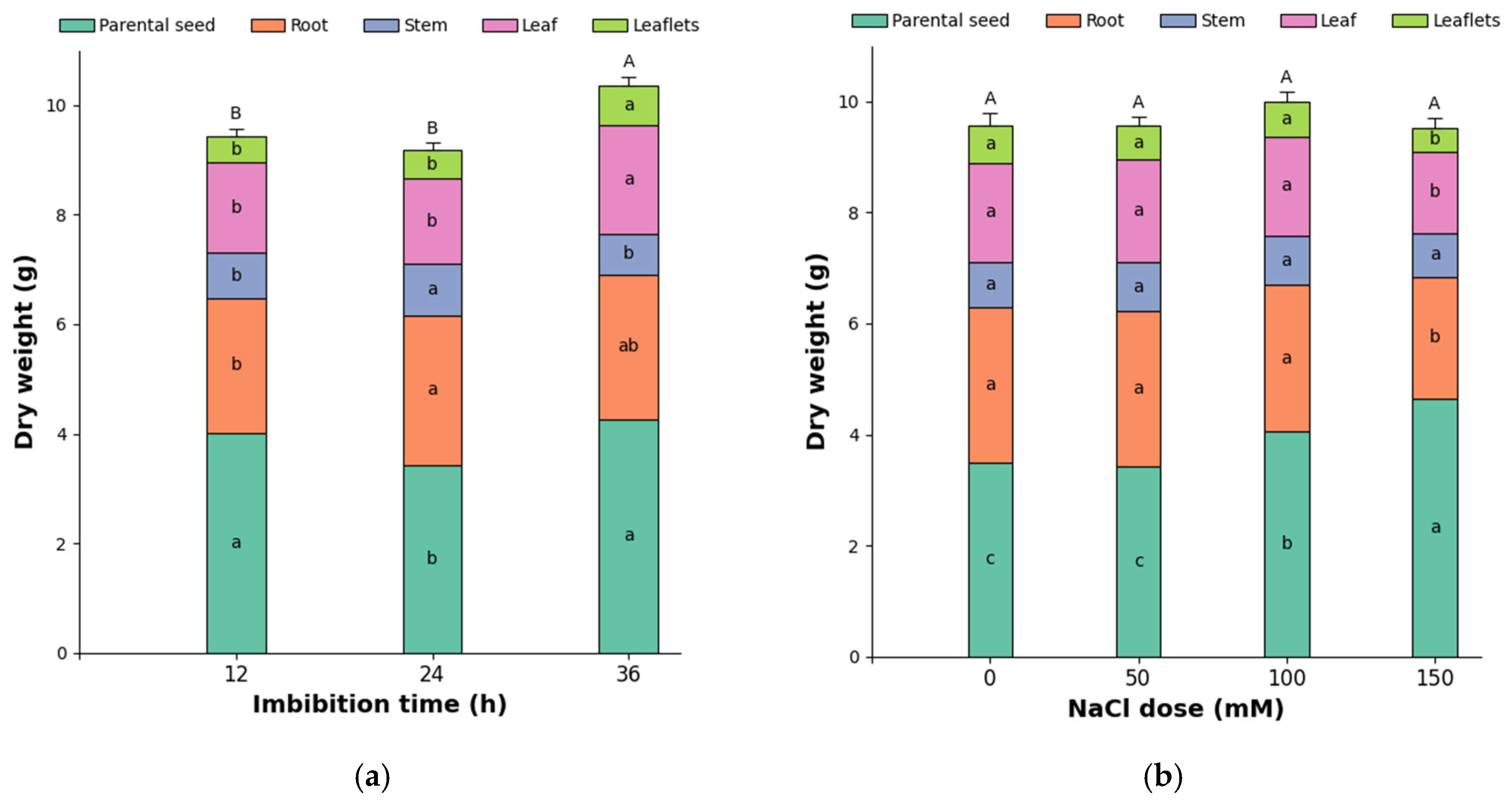
3.2. Effect of Imbibition Time and NaCl Doses on the Dry Mass Distribution of Different Organs in Pea Seedlings
3.3. Effect of Imbibition Time and NaCl Doses on the Leaf Area of Pea Seedlings
3.4. Correlation Analysis Between the Parameters Evaluated for the NaCl Applications
3.5. Effect of Imbibition Time and H2O2 Doses on Germination Parameters in Pea Seeds
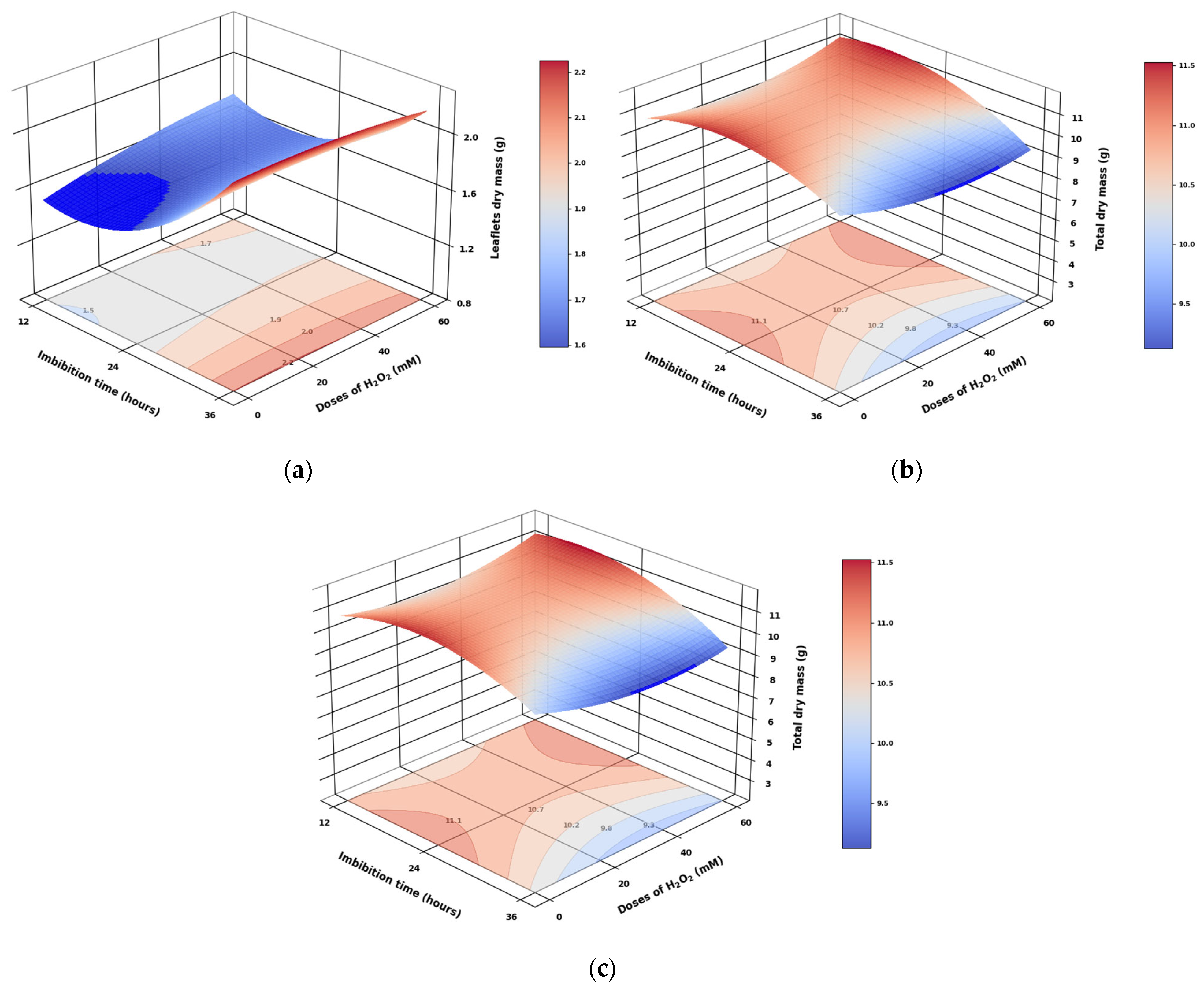
3.6. Effect of Imbibition Time and H2O2 Doses on the Dry Mass Distribution of Different Organs in Pea Seedlings
3.7. Effect of Imbibition Time and H2O2 Doses on the Leaf Area of Pea Seedlings
3.8. Analysis of the Response Surface Models Obtained for Pea Seed Germination Parameters and Seedling Biomass
3.9. Correlation Analysis Between the Parameters Evaluated for the H2O2 Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parihar, A.K.; Dixit, G.P.; Singh, U.; Singh, A.K.; Kumar, N.; Gupta, S. Potential of field pea as a nutritionally rich food legume crop. In Breeding for Enhanced Nutrition and Bio-Active Compounds in Food Legumes, 1st ed.; Gupta, D.S., Gupta, S., Kumar, J., Eds.; Springer: Cham, Switzerland, 2021; pp. 47–82. [Google Scholar] [CrossRef]
- Checa, O.; Rodríguez, D.; Ruiz, M.; Muriel, J. La Arveja-Investigación y Tecnología en el Sur de Colombia; Editorial Universidad de Nariño: San Juan de Pasto, Colombia, 2022. [Google Scholar]
- Ramos, H.; Riascos, M.; Luna, L. Dinámica comercial y comportamiento del precio de arveja en el departamento de Nariño (Colombia). Rev. Investig. Agrar. Ambient. 2023, 14, 31–49. [Google Scholar] [CrossRef]
- Jaime-Guerrero, M.; Álvarez-Herrera, J.G.; Torres-Piña, D.S. Obtención de plántulas a partir de diferentes tratamientos pregerminativos en semillas de arveja. Entramado 2025, 21, e-11944. [Google Scholar] [CrossRef]
- Wagan, M.A.; Shar, H.A.; Miano, T.F.; Chandio, S.R.; Suthar, M.; Wagan, M.K.; Kumar, A.; Wagan, F.A.; Magsi, H.A. Influence of seed priming on germination of pea (Pisum sativum). Int. J. Agric. Stud. 2022, 1, 31–37. [Google Scholar] [CrossRef]
- Mahawar, M.K.; Samuel, D.V.K.; Sinha, J.P.; Jalgaonkar, K. Optimization of pea (Pisum sativum) seeds hydropriming by application of response surface methodology. Acta Physiol. Plantarum. 2016, 38, 212. [Google Scholar] [CrossRef]
- Bradford, K.J. Manipulation of seed water relations via osmotic priming to improve germination under stress. HortScience 1986, 21, 1105–1112. [Google Scholar] [CrossRef]
- Lal, S.K.; Kumar, S.; Sheri, V.; Mehta, S.; Varakumar, P.; Ram, B.; Borphukan, B.; James, D.; Fartyal, D.; Reddy, M.K. Seed priming: An emerging technology to impart abiotic stress tolerance in crop plants. In Advances in Seed Priming, 1st ed.; Rakshit, A., Singh, H.B., Eds.; Springer: Singapore, 2018; pp. 41–50. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef]
- Kiran, K.R.; Deepika, V.B.; Swathy, P.S.; Prasad, K.; Kabekkodu, S.P.; Murali, T.S.; Satyamoorthy, K.; Muthusamy, A. ROS-dependent DNA damage and repair during germination of NaCl primed seeds. J. Photochem. Photobiol. B Biol. 2020, 213, 112050. [Google Scholar] [CrossRef]
- Pandolfi, C.; Mancuso, S.; Shabala, S. Physiology of acclimation to salinity stress in pea (Pisum sativum). Environ. Exp. Bot. 2012, 84, 44–51. [Google Scholar] [CrossRef]
- Senturk, B.; Sivritepe, O.H. NaCl priming alleviates the inhibiting effect of salinity during seeding growth of peas (Pisum sativum L.). Fresen. Environ. Bull. 2016, 25, 4202–4208. [Google Scholar]
- Ellouzi, H.; Oueslati, S.; Hessini, K.; Rabhi, M.; Abdelly, C. Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci. Hortic. 2021, 288, 110360. [Google Scholar] [CrossRef]
- Moussa, H.R.; Mohamed, M.A.E.H. Role of nitric acid or H2O2 in antioxidant defense system of Pisum sativum L. under drought stress. Nat. Sci. 2011, 9, 211–216. [Google Scholar]
- Cadena-Guerrero, M.M.; Yepes-Chamorro, D.B.; Romero, J.V. Estabilidad fenotípica de arveja (Pisum sativum L.) en la zona productora de Nariño, Colombia. Agron. Mesoam. 2021, 32, 841–853. [Google Scholar] [CrossRef]
- Checa, O.; Rodríguez, M.; Wu, X.; Blair, M. Introgression of the Afila gene into climbing garden pea (Pisum sativum L.). Agronomy 2020, 10, 1537. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodríguez-Páez, L.A. Imbibition and germination of seeds with economic and ecological interest: Physical and biochemical factors involved. Sustainability 2023, 15, 5394. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, L.; Cheng, B.; Dong, Y.; Wei, J.; Tian, X.; Peng, Y.; Li, Z. Pretreatment with NaCl promotes the seed germination of white clover by affecting endogenous phytohormones, metabolic regulation, and dehydrin-encoded genes expression under water stress. Int. J. Mol. Sci. 2018, 19, 3570. [Google Scholar] [CrossRef]
- Kyari, B.A.; Lawan, Z.A.; Waziri, M.S.; Ajiri, H.M.; Apagu, B.; Mari, H.; Ibrahim, M.A. Effect of imbibition time on hormonal changes of germinating Tamarindus indica and Prosopis juliflora. Indones. Indones. J. Agric. Res. 2022, 5, 219–230. [Google Scholar] [CrossRef]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Gupta, N.K.; Prathibha, M.D.; Mehta, B.K.; Kumar, P.; Pandey, S.; et al. Drought stress responses and inducing tolerance by seed priming approach in plants. Plant Stress 2022, 4, 100066. [Google Scholar] [CrossRef]
- Snoussi, H.; Askri, H.; Nacouzi, D.; Ouerghui, I.; Ananga, A.; Najar, A.; El Kayal, W. Comparative transcriptome profiling of salinity-induced genes in citrus rootstocks with contrasted salt tolerance. Agriculture 2022, 12, 350. [Google Scholar] [CrossRef]
- Naz, F.; Gul, H.; Hamayun, M.; Sayyed, A.; Khan, H.; Sherwani, S. Effect of NaCl stress on Pisum sativum germination and seedling growth with the influence of seed priming with potassium (KCL and KOH). Am.-Eurasian J. Agric. Environ. Sci. 2014, 14, 1304–1311. [Google Scholar]
- Souza, L.M.D.; Conceição, E.M.D.; Barbosa, M.R.; Palhares, L.; Santos, A.M.M.D.; Souza, R.A.D.; Houllou, L.M. Effect of seed priming with NaCl on the induction of salinity tolerance in Myracrodruon urundeuva Allemão in vitro. Ciência Florest. 2022, 32, 2199–2218. [Google Scholar] [CrossRef]
- Ehtaiwwesh, A.F.; Emsahel, M.J. Impact of salinity stress on germination and growth of pea (Pisum sativum L.) plants. Al-Mukhtar J. Sci. 2020, 35, 146–159. [Google Scholar]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef] [PubMed]
- Smolko, A.; Bauer, N.; Pavlović, I.; Pěnčík, A.; Novák, O.; Salopek-Sondi, B. Altered root growth, auxin metabolism and distribution in Arabidopsis thaliana exposed to salt and osmotic stress. Int. J. Mol. Sci. 2021, 22, 7993. [Google Scholar] [CrossRef]
- Colin, L.; Ruhnow, F.; Zhu, J.K.; Zhao, C.; Zhao, Y.; Persson, S. The cell biology of primary cell walls during salt stress. Plant Cell 2023, 35, 201–217. [Google Scholar] [CrossRef]
- Jaime-Guerrero, M.; Álvarez-Herrera, J.G.; Camacho-Tamayo, J. Germinación y crecimiento de semillas de arveja var. ‘Santa Isabel’ sometidas a diferentes dosis de giberelinas. Rev. Investig. Agrar. Ambient. 2023, 14, 91–112. [Google Scholar] [CrossRef]
- Hernández, J.A. Seed science research: Global trends in seed biology and technology. Seeds 2022, 1, 1–4. [Google Scholar] [CrossRef]
- Hemalatha, G.; Renugadevi, J.; Eevera, T. Studies on seed priming with hydrogen peroxide for mitigating salt stress in rice. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 691–695. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.; Nisar, F.; Rasheed, A.; Shah, S.Z. Seed priming as an effective technique for enhancing salinity tolerance in plants: Mechanistic insights and prospects for saline agriculture with a special emphasis on halophytes. Seeds 2025, 4, 14. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in pea seed germination. Plant Signal. Behav. 2012, 7, 193–195. [Google Scholar] [CrossRef]
- Rodrigues, L.; Nogales, A.; Nunes, J.; Rodrigues, L.; Hansen, L.D.; Cardoso, H. Germination of Pisum sativum L. seeds is associated with the alternative respiratory pathway. Biology 2023, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mu, Y.; Xuan, Y.; Wu, X.; Wang, W.; Zhang, H. Hydrogen peroxide signaling in the maintenance of plant root apical meristem activity. Antioxidants 2024, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Makhaye, G.; Mofokeng, M.M.; Tesfay, S.; Aremu, A.O.; Van Staden, J.; Amoo, S.O. Influence of plant biostimulant application on seed germination. In Biostimulants for Crops from Seed Germination to Plant Development, 1st ed.; Gupta, S., Staden, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 109–135. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cheng, J.; Yan, X.; Zhang, J.; Zhang, J. Germination of seeds subjected to temperature and water availability: Implications for ecological restoration. Forests 2022, 13, 1854. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]

| Variable | Equation | Units |
|---|---|---|
| Germination rate | % | |
| Germination potential | % | |
| Mean germination time | Days to germination | |
| Germination rate index | Seeds germinated by day |
| Imbibition Time (Hours) | Doses of NaCl (mM) | GR (%) | GP (%) | MGT (Days) | GRI (Number of Seeds Per Day) |
|---|---|---|---|---|---|
| 12 | 0 | 82.92 b | 34.17 ab | 6.21 a | 5.19 d |
| 50 | 98.33 a | 34.58 ab | 5.30 cd | 10.12 ab | |
| 100 | 94.17 ab | 35.00 ab | 5.62 abcd | 8.16 abcd | |
| 150 | 93.75 ab | 33.75 ab | 6.04 ab | 6.56 cd | |
| 24 | 0 | 92.08 ab | 37.92 ab | 5.44 bcd | 7.85 abcd |
| 50 | 98.75 a | 42.92 a | 5.17 d | 10.61 a | |
| 100 | 96.25 a | 32.50 ab | 5.73 abcd | 8.80 abc | |
| 150 | 89.58 ab | 24.58 b | 6.01 abc | 7.27 bcd | |
| 36 | 0 | 97.50 a | 41.67 a | 5.04 d | 9.73 abc |
| 50 | 97.08 a | 42.92 a | 5.07 d | 9.72 abc | |
| 100 | 98.75 a | 44.58 a | 5.07 d | 9.15 abc | |
| 150 | 97.50 a | 37.50 ab | 5.91 abc | 7.57 abcd | |
| Factor | Level | ||||
| Imbibición time (hours) | 12 | 92.29 b | 34.38 b | 5.79 a | 7.51 b |
| 24 | 94.17 ab | 34.48 b | 5.59 a | 8.63 ab | |
| 36 | 97.71 a | 41.67 a | 5.27 b | 9.04 a | |
| Doses of NaCl (mM) | 0 | 90.83 b | 37.92 ab | 5.56 b | 7.59 bc |
| 50 | 98.06 a | 40.14 a | 5.18 c | 10.15 a | |
| 100 | 96.39 a | 37.36 ab | 5.47 bc | 8.70 ab | |
| 150 | 93.61 ab | 31.94 b | 5.98 a | 7.13 c | |
| ANOVA | Significance | ||||
| I | ** | ** | ** | ** | |
| D | ** | * | ** | ** | |
| I × D | * | ns | ** | * | |
| Imbibition Time (Hours) | Doses of NaCl (mM) | SLA (cm2) | LA (cm2) | TLA (cm2) |
|---|---|---|---|---|
| 12 | 0 | 114.11 cd | 257.77 cd | 371.88 de |
| 50 | 83.15 d | 233.97 d | 317.12 de | |
| 100 | 123.12 cd | 263.42 cd | 386.54 de | |
| 150 | 90.91 d | 221.85 d | 312.75 de | |
| 24 | 0 | 183.19 abc | 288.23 cd | 471.41 bcde |
| 50 | 146.80 bcd | 290.03 cd | 436.84 cde | |
| 100 | 184.95 abc | 310.33 cd | 495.28 bcd | |
| 150 | 87.08 d | 199.38 d | 286.47 e | |
| 36 | 0 | 246.08 a | 470.16 a | 716.24 a |
| 50 | 214.57 ab | 447.60 ab | 662.16 ab | |
| 100 | 208.26 ab | 392.93 abc | 601.18 abc | |
| 150 | 154.95 bcd | 315.27 bcd | 470.22 bcde | |
| Factor | Level | |||
| Imbibición time (hours) | 12 | 102.82 c | 244.25 b | 347.07 c |
| 24 | 150.51 b | 271.99 b | 422.50 b | |
| 36 | 205.97 a | 406.49 a | 612.45 a | |
| Doses of NaCl (mM) | 0 | 181.13 a | 338.72 a | 519.85 a |
| 50 | 148.17 a | 323.87 a | 472.04 a | |
| 100 | 172.11 a | 322.22 a | 494.34 a | |
| 150 | 110.98 b | 245.50 b | 356.48 b | |
| ANOVA | Significance | |||
| I | ** | ** | ** | |
| D | ** | ** | ** | |
| I × D | ns | ns | ns | |
| Imbibition Time (Hours) | Doses of H2O2 (mM) | GR (%) | GP (%) | MGT (Days) | GRI (Number of Seeds Per Day) |
|---|---|---|---|---|---|
| 12 | 0 | 95.42 ab | 37.92 a | 5.68 ab | 8.31 cd |
| 20 | 97.50 ab | 37.08 a | 5.81 a | 8.30 cd | |
| 40 | 92.92 b | 41.25 a | 5.70 a | 8.06 d | |
| 60 | 95.00 ab | 40.42 a | 5.75 a | 8.14 d | |
| 24 | 0 | 99.17 a | 51.67 a | 5.06 c | 9.64 ab |
| 20 | 96.25 ab | 51.25 a | 5.05 c | 9.42 ab | |
| 40 | 99.58 a | 47.08 a | 5.02 c | 9.80 a | |
| 60 | 97.92 ab | 48.75 a | 5.18 bc | 9.30 abc | |
| 36 | 0 | 98.75 a | 42.08 a | 5.66 ab | 8.58 bcd |
| 20 | 98.33 ab | 47.08 a | 5.57 ab | 8.68 bcd | |
| 40 | 97.08 ab | 41.25 a | 5.45 abc | 8.84 abcd | |
| 60 | 97.08 ab | 40.00 a | 5.44 abc | 8.84 abcd | |
| Factor | Level | ||||
| Imbibición time (hours) | 12 | 95.21 b | 39.17 b | 5.74 a | 8.20 c |
| 24 | 98.23 a | 49.69 a | 5.08 c | 9.54 a | |
| 36 | 97.81 a | 42.60 b | 5.53 b | 8.74 b | |
| Doses of H2O2 (mM) | 0 | 97.78 a | 43.89 a | 5.47 a | 8.84 a |
| 20 | 97.36 a | 45.14 a | 5.48 a | 8.80 a | |
| 40 | 96.53 a | 43.19 a | 5.39 a | 8.90 a | |
| 60 | 96.67 a | 43.06 a | 5.46 a | 8.76 a | |
| ANOVA | Significance | ||||
| I | ** | ** | ** | ** | |
| D | ns | ns | ns | ns | |
| I × D | ns | ns | ns | ns | |
| Imbibition Time (Hours) | Doses of H2O2 (mM) | SLA (cm2) | LA (cm2) | TLA (cm2) |
|---|---|---|---|---|
| 12 | 0 | 247.15 bce | 542.38 ab | 789.53 abcd |
| 50 | 215.29 cde | 383.00 b | 598.29 cd | |
| 100 | 230.63 bcde | 433.59 b | 664.22 bcd | |
| 150 | 200.01 e | 329.52 b | 529.53 e | |
| 24 | 0 | 336.69 ab | 545.54 ab | 882.23 abc |
| 50 | 283.85 abcde | 432.84 b | 716.69 bcd | |
| 100 | 306.01 abcde | 528.34 ab | 834.35 abc | |
| 150 | 319.75 abc | 552.30 ab | 872.05 abc | |
| 36 | 0 | 310.93 abcd | 510.02 ab | 820.94 abcd |
| 50 | 291.74 abcde | 724.12 a | 1015.86 a | |
| 100 | 205.40 de | 544.28 ab | 749.68 abcd | |
| 150 | 357.51 a | 551.22 ab | 908.73 ab | |
| Factor | Level | |||
| Imbibition time (hours) | 12 | 223.27 b | 422.12 b | 645.39 b |
| 24 | 311.57 a | 514.75 a | 826.33 a | |
| 36 | 291.39 a | 582.41 a | 873.80 a | |
| Doses of H2O2 (mM) | 0 | 298.25 a | 532.65 a | 830.90 a |
| 20 | 263.63 ab | 513.32 a | 776.95 a | |
| 40 | 247.35 b | 502.07 a | 749.42 a | |
| 60 | 292.42 ab | 477.68 a | 770.10 a | |
| ANOVA | Significance | |||
| I | ** | ** | ** | |
| D | * | ns | ns | |
| I × D | ** | ** | ** | |
| Estimated | GR | MGT | RDM | LDM | SDM | DMGS |
|---|---|---|---|---|---|---|
| a | 84.583333 | 6.292854 | 1.474960 | 1.982127 | 0.497687 | 6.332820 |
| b | 0.114583 | −0.020423 | 0.092714 | −0.060040 | −0.010087 | −0.259080 |
| c | 0.216667 | −0.015844 | 0.009269 | 0.008608 | 0.003287 | −0.011612 |
| d | 0.005787 | −0.000392 | −0.001329 | 0.001854 | 0.000573 | 0.004968 |
| e | −0.002222 | 0.000234 | −0.000288 | −0.000204 | −0.000095650 | 0.000415 |
| f | −0.001000 | 0.000088895 | −0.000042440 | −0.000038553 | −0.000015473 | 0.000065800 |
| r2 | 0.3036 | 0.5580 | 0.4111 | 0.5164 | 0.6027 | 0.5706 |
| Estimated | LDM | TDM | SLA |
|---|---|---|---|
| a | 0.445370 | 8.197823 | 78.391033 |
| b | 0.115916 | 0.298814 | 19.797850 |
| c | 0.001729 | −0.023079 | −4.055098 |
| d | −0.001893 | −0.006829 | −0.376688 |
| e | −0.000316 | −0.000886 | 0.037388 |
| f | 0.000006875 | 0.000700 | 0.049815 |
| r2 | 0.3469 | 0.4763 | 0.4126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Herrera, J.G.; Lozano, J.S.; Alvarado-Sanabria, O.H. Priming ‘Santa Isabel’ Pea (Pisum sativum L.) Seeds with NaCl and H2O2 as a Strategy to Promote Germination. Seeds 2025, 4, 34. https://doi.org/10.3390/seeds4030034
Álvarez-Herrera JG, Lozano JS, Alvarado-Sanabria OH. Priming ‘Santa Isabel’ Pea (Pisum sativum L.) Seeds with NaCl and H2O2 as a Strategy to Promote Germination. Seeds. 2025; 4(3):34. https://doi.org/10.3390/seeds4030034
Chicago/Turabian StyleÁlvarez-Herrera, Javier Giovanni, Julián Stiven Lozano, and Oscar Humberto Alvarado-Sanabria. 2025. "Priming ‘Santa Isabel’ Pea (Pisum sativum L.) Seeds with NaCl and H2O2 as a Strategy to Promote Germination" Seeds 4, no. 3: 34. https://doi.org/10.3390/seeds4030034
APA StyleÁlvarez-Herrera, J. G., Lozano, J. S., & Alvarado-Sanabria, O. H. (2025). Priming ‘Santa Isabel’ Pea (Pisum sativum L.) Seeds with NaCl and H2O2 as a Strategy to Promote Germination. Seeds, 4(3), 34. https://doi.org/10.3390/seeds4030034






