Innovative Approaches for Engineering the Seed Microbiome to Enhance Crop Performance
Abstract
1. Introduction
2. Seed Microbiome Composition and Transmission
3. Beneficial Effects of Seed-Associated Microbes
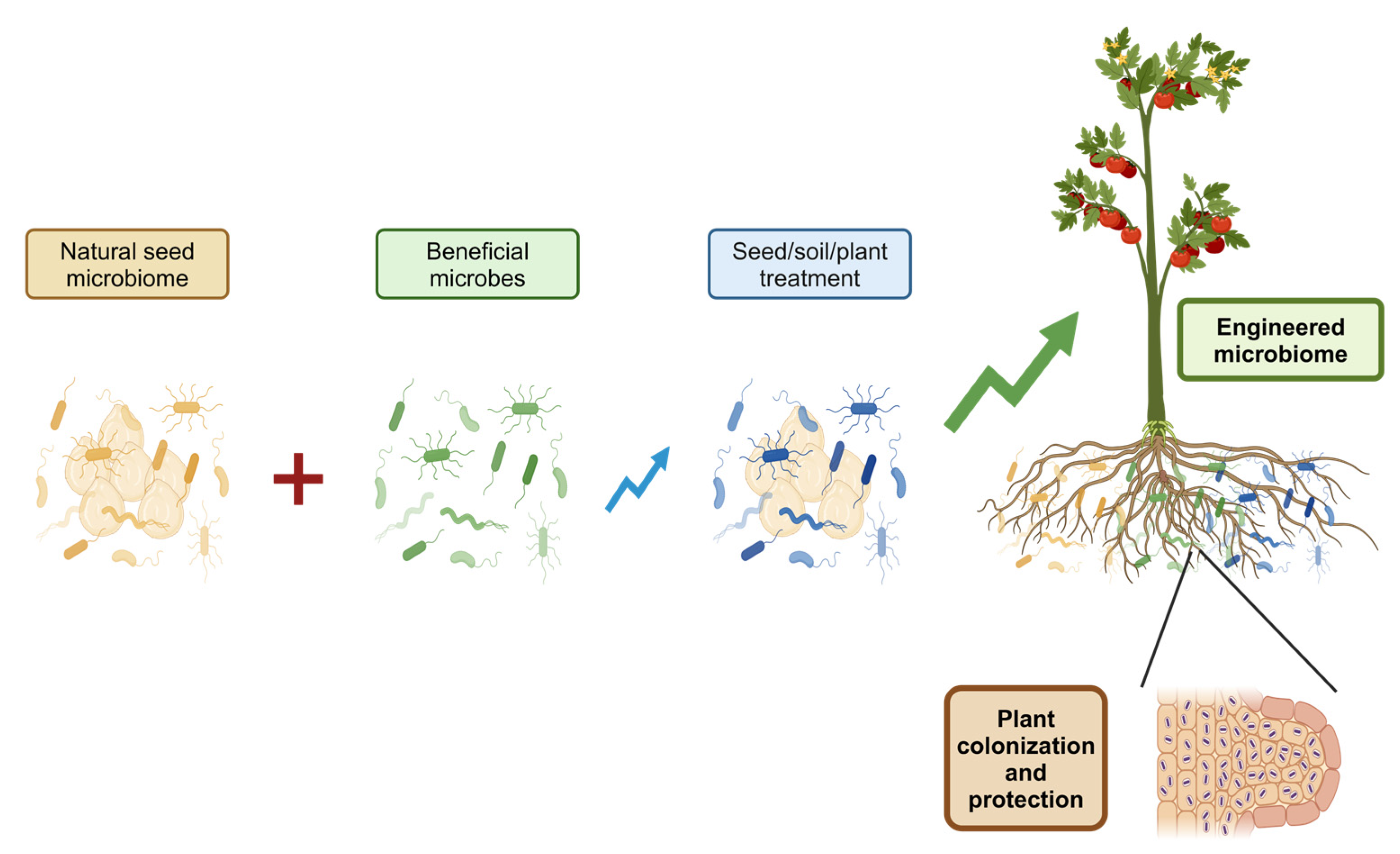
4. Innovative Approaches to Seed Microbiome Engineering
5. Seed Biopriming with Beneficial Microorganisms
6. Specific Microbial Strains and Their Effects
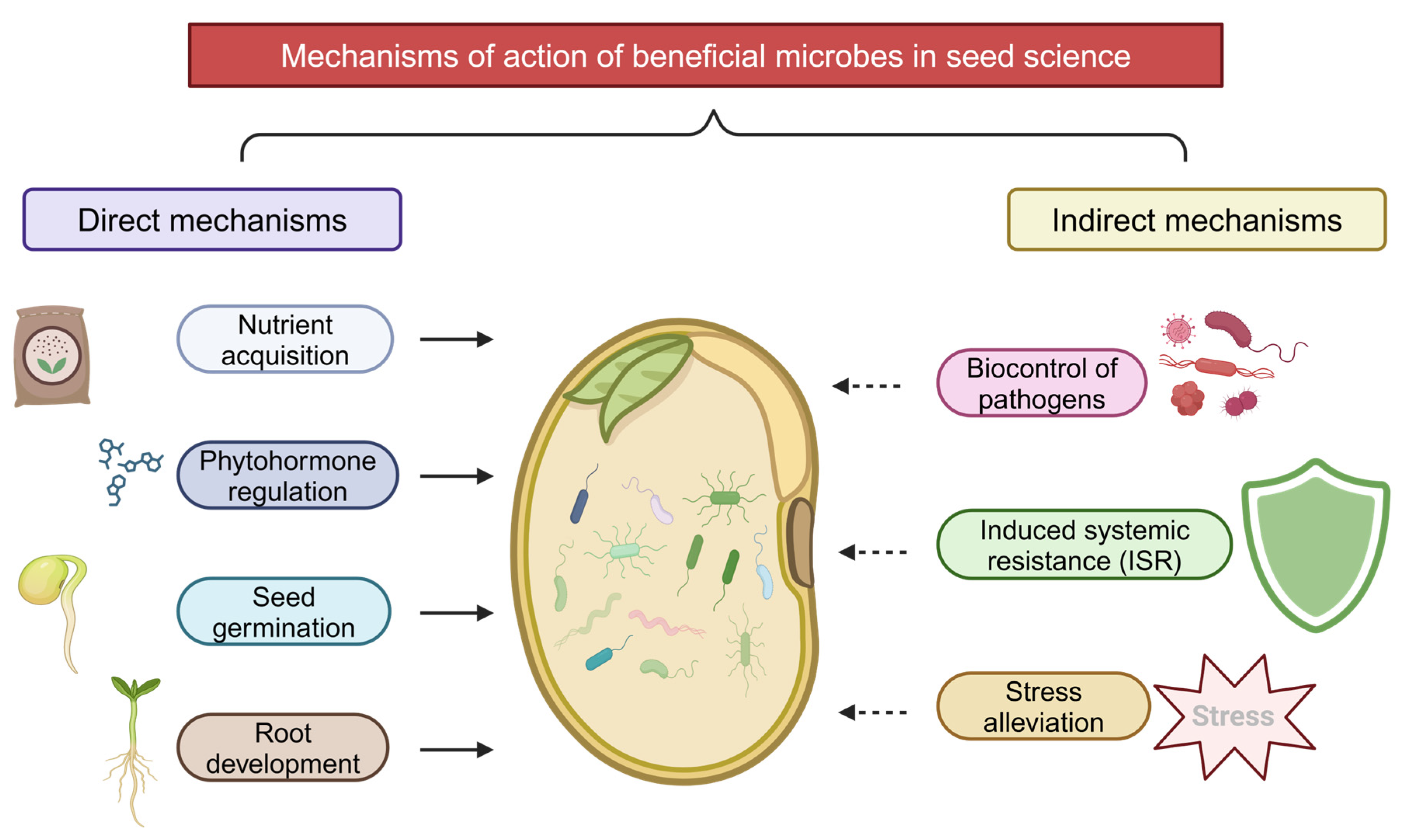
7. Mechanisms of Action
7.1. Direct Growth Promotion
7.2. Stress Tolerance Enhancement
7.3. Disease Suppression
8. Conclusions and Future Directions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Adeleke, B.S.; Shi, Y.; Li, C. The Seed Microbiomes of Staple Food Crops. Microb. Biotechnol. 2023, 16, 2236–2249. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Tack, A.J.M.; Lobato, C.; Wassermann, B.; Berg, G. From Seed to Seed: The Role of Microbial Inheritance in the Assembly of the Plant Microbiome. Trends Microbiol. 2023, 31, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Hone, H.; Mann, R.; Yang, G.; Kaur, J.; Tannenbaum, I.; Li, T.; Spangenberg, G.; Sawbridge, T. Profiling, Isolation and Characterisation of Beneficial Microbes from the Seed Microbiomes of Drought Tolerant Wheat. Sci. Rep. 2021, 11, 11916. [Google Scholar] [CrossRef]
- Yang, P.; Condrich, A.; Lu, L.; Scranton, S.; Hebner, C.; Sheykhhasan, M.; Ali, M.A. Genetic Engineering in Bacteria, Fungi, and Oomycetes, Taking Advantage of CRISPR. DNA 2024, 4, 427–454. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, F.; Wang, F.; Le, L.; Pu, L. Synthetic Biology and Artificial Intelligence in Crop Improvement. Plant Commun. 2025, 6, 101220. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, P.; Liu, W.; Zhao, Z.; Bernier, M.C.; Zhang, C.; Adhikari, A.; Opiyo, S.O.; Zhao, L.; Banks, F.; et al. Plant Growth Promotion and Plant Disease Suppression Induced by Bacillus amyloliquefaciens Strain GD4a. Plants 2024, 13, 672. [Google Scholar] [CrossRef]
- Jonkers, W.; Gundel, P.E.; Verma, S.K.; White, J.F. Editorial: Seed Microbiome Research. Front. Microbiol. 2022, 13, 943329. [Google Scholar] [CrossRef]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed Treatments with Microorganisms Can Have a Biostimulant Effect by Influencing Germination and Seedling Growth of Crops. Plants 2022, 11, 259. [Google Scholar] [CrossRef]
- Fiodor, A.; Ajijah, N.; Dziewit, L.; Pranaw, K. Biopriming of Seed with Plant Growth-Promoting Bacteria for Improved Germination and Seedling Growth. Front. Microbiol. 2023, 14, 1142966. [Google Scholar] [CrossRef]
- Yang, P.; Condrich, A.; Scranton, S.; Hebner, C.; Lu, L.; Ali, M.A. Utilizing Plant Growth-Promoting Rhizobacteria (PGPR) to Advance Sustainable Agriculture. Bacteria 2024, 3, 434–451. [Google Scholar] [CrossRef]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; von Maltzahn, G.; et al. A New Approach to Modify Plant Microbiomes and Traits by Introducing Beneficial Bacteria at Flowering into Progeny Seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Arnault, G.; Marais, C.; Préveaux, A.; Briand, M.; Poisson, A.-S.; Sarniguet, A.; Barret, M.; Simonin, M. Seedling Microbiota Engineering Using Bacterial Synthetic Community Inoculation on Seeds. FEMS Microbiol. Ecol. 2024, 100, fiae027. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Meza, C.; Valenzuela, F.; Echeverría-Vega, A.; Gomez, A.; Sarkar, S.; Cabeza, R.A.; Arencibia, A.D.; Quiroz, K.; Carrasco, B.; Banerjee, A. Plant-Growth-Promoting Bacteria from Rhizosphere of Chilean Common Bean Ecotype (Phaseolus vulgaris L.) Supporting Seed Germination and Growth against Salinity Stress. Front. Plant Sci. 2022, 13, 1052263. [Google Scholar] [CrossRef]
- Efthimiadou, A.; Katsenios, N.; Chanioti, S.; Giannoglou, M.; Djordjevic, N.; Katsaros, G. Effect of Foliar and Soil Application of Plant Growth Promoting Bacteria on Growth, Physiology, Yield and Seed Quality of Maize under Mediterranean Conditions. Sci. Rep. 2020, 10, 21060. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Xiong, C.; Egidi, E.; Singh, B.K. Formulation Challenges Associated with Microbial Biofertilizers in Sustainable Agriculture and Paths Forward. J. Sustain. Agric. Environ. 2024, 3, e70006. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.M.; Parra Cota, F.I.; Cira Chávez, L.A.; García Ortega, L.F.; Estrada Alvarado, M.I.; Santoyo, G.; de los Santos-Villalobos, S. Microbial Inoculants in Sustainable Agriculture: Advancements, Challenges, and Future Directions. Plants 2025, 14, 191. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, J.; Lee, K.K.; Lee, Y.-H. Longitudinal Transmission of Bacterial and Fungal Communities from Seed to Seed in Rice. Commun. Biol. 2022, 5, 1–14. [Google Scholar] [CrossRef]
- Simonin, M.; Briand, M.; Chesneau, G.; Rochefort, A.; Marais, C.; Sarniguet, A.; Barret, M. Seed Microbiota Revealed by a Large-Scale Meta-Analysis Including 50 Plant Species. New Phytol. 2022, 234, 1448–1463. [Google Scholar] [CrossRef]
- Chesneau, G.; Laroche, B.; Préveaux, A.; Marais, C.; Briand, M.; Marolleau, B.; Simonin, M.; Barret, M. Single Seed Microbiota: Assembly and Transmission from Parent Plant to Seedling. mBio 2022, 13, e01648-22. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Gutiérrez, J.P.; Lopez-Lavalle, L.A.B. Seed-Transmitted Bacteria and Fungi Dominate Juvenile Plant Microbiomes. Front. Microbiol. 2021, 12, 737616. [Google Scholar] [CrossRef]
- Yang, P. Exploring Plant-Microbe Interactions Through the Lens of Beneficial Bacteria; The Ohio State University: Columbus, OH, USA, 2023. [Google Scholar]
- Yang, P.; Bokros, N.; Debolt, S.; Zhao, Z.; Xia, Y. Genome Sequence Source of Bacillus amyloliquefaciens Strain GD4a, a Bacterial Endophyte Associated with Switchgrass Plants. Phytobiomes J. 2022, 6, 354–357. [Google Scholar] [CrossRef]
- Singam, R.; Azhari, A.; Patmanathan, S.N. Microbes Cultured from Garden Soil Positively Impact Seed Germination and Plant Growth. J. Emerg. Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- L’Hoir, M.; Duponnois, R. Combining the Seed Endophytic Bacteria and the Back to the Future Approaches for Plant Holonbiont Breeding. Front. Agron. 2021, 3, 724450. [Google Scholar] [CrossRef]
- Srivastava, S.; Tyagi, R.; Sharma, S. Seed Biopriming as a Promising Approach for Stress Tolerance and Enhancement of Crop Productivity: A Review. J. Sci. Food Agric. 2024, 104, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Kumar, A.; Babalola, O.O. The Role of Microbial Seed Endophytes in Agriculture: Mechanisms and Applications. Cereal Res. Commun. 2024, 53, 43–55. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Yang, P.; Liu, W.; Yuan, P.; Zhao, Z.; Zhang, C.; Opiyo, S.O.; Adhikari, A.; Zhao, L.; Harsh, G.; Xia, Y. Plant Growth Promotion and Stress Tolerance Enhancement through Inoculation with Bacillus proteolyticus OSUB18. Biology 2023, 12, 1495. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, Z.; Fan, J.; Liang, Y.; Bernier, M.C.; Gao, Y.; Zhao, L.; Opiyo, S.O.; Xia, Y. Bacillus proteolyticus OSUB18 Triggers Induced Systemic Resistance against Bacterial and Fungal Pathogens in Arabidopsis. Front. Plant Sci. 2023, 14, 1078100. [Google Scholar] [CrossRef]
- War, A.F.; Bashir, I.; Reshi, Z.A.; Kardol, P.; Rashid, I. Insights into the Seed Microbiome and Its Ecological Significance in Plant Life. Microbiol. Res. 2023, 269, 127318. [Google Scholar] [CrossRef]
- Compant, S.; Cassan, F.; Kostić, T.; Johnson, L.; Brader, G.; Trognitz, F.; Sessitsch, A. Harnessing the Plant Microbiome for Sustainable Crop Production. Nat. Rev. Microbiol. 2025, 23, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Chen, K.; Cao, W.; Meng, L.; Yang, B.; Xu, M.; Xing, Y.; Li, P.; Freilich, S.; Chen, C.; et al. Engineering Natural Microbiomes toward Enhanced Bioremediation by Microbiome Modeling. Nat. Commun. 2024, 15, 4694. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Ahmed, H.I.; Parween, S.; Sheikh, A.H.; Saad, M.M.; Krattinger, S.G.; Hirt, H. Host Genotype, Soil Composition, and Geo-Climatic Factors Shape the Fonio Seed Microbiome. Microbiome 2024, 12, 11. [Google Scholar] [CrossRef]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed Biopriming with Plant Growth Promoting Rhizobacteria: A Review. FEMS Microbiol. Ecol. 2016, 92, fiw112. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Vaishnav, A.; Liu, H.; Xiong, C.; Singh, H.B.; Singh, B.K. Seed Biopriming for Sustainable Agriculture and Ecosystem Restoration. Microb. Biotechnol. 2023, 16, 2212–2222. [Google Scholar] [CrossRef]
- Swain, H.; Adak, T.; Mukherjee, A.K.; Sarangi, S.; Samal, P.; Khandual, A.; Jena, R.; Bhattacharyya, P.; Naik, S.K.; Mehetre, S.T.; et al. Seed Biopriming with Trichoderma Strains Isolated from Tree Bark Improves Plant Growth, Antioxidative Defense System in Rice and Enhance Straw Degradation Capacity. Front. Microbiol. 2021, 12, 633881. [Google Scholar] [CrossRef]
- Hanif, M.S.; Tayyab, M.; Baillo, E.H.; Islam, M.M.; Islam, W.; Li, X. Plant Microbiome Technology for Sustainable Agriculture. Front. Microbiol. 2024, 15, 1500260. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Gupta, M. Effect of Bioagents on Cucumber Seed Mycoflora, Seed Germination, and Seedling Vigour. Sci. Rep. 2023, 13, 6052. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed Treatment with Trichoderma harzianum Alleviates Biotic, Abiotic, and Physiological Stresses in Germinating Seeds and Seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef]
- Mahmoodian, S.; Kowsari, M.; Motallebi, M.; Zamani, M.; Jahromi, Z.M. Effect of Improved Trichoderma harzianum on Growth and Resistance Promotion in Bean Plant. Braz. Arch. Biol. Technol. 2022, 65, e22210671. [Google Scholar] [CrossRef]
- Kthiri, Z.; Jabeur, M.B.; Machraoui, M.; Gargouri, S.; Hiba, K.; Hamada, W. Coating Seeds with Trichoderma Strains Promotes Plant Growth and Enhance the Systemic Resistance against Fusarium Crown Rot in Durum Wheat. Egypt. J. Biol. Pest Control 2020, 30, 139. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus Subtilis: A Plant-Growth Promoting Rhizobacterium That Also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Song, P.; Zhao, B.; Sun, X.; Li, L.; Wang, Z.; Ma, C.; Zhang, J. Effects of Bacillus Subtilis HS5B5 on Maize Seed Germination and Seedling Growth under NaCl Stress Conditions. Agronomy 2023, 13, 1874. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, R.; Liu, J. Effects of Bacillus subtilis QM3 on Germination and Antioxidant Enzymes Activities of Wheat Seeds under Salt Stress. Open Access Libr. J. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Patel, M.; Islam, S.; Husain, F.M.; Yadav, V.K.; Park, H.-K.; Yadav, K.K.; Bagatharia, S.; Joshi, M.; Jeon, B.-H.; Patel, A. Bacillus subtilis ER-08, a Multifunctional Plant Growth-Promoting Rhizobacterium, Promotes the Growth of Fenugreek (Trigonella Foenum-graecum L.) Plants under Salt and Drought Stress. Front. Microbiol. 2023, 14, 1208743. [Google Scholar] [CrossRef]
- Mehmood, N.; Saeed, M.; Zafarullah, S.; Hyder, S.; Rizvi, Z.F.; Gondal, A.S.; Jamil, N.; Iqbal, R.; Ali, B.; Ercisli, S.; et al. Multifaceted Impacts of Plant-Beneficial Pseudomonas Spp. in Managing Various Plant Diseases and Crop Yield Improvement. ACS Omega 2023, 8, 22296–22315. [Google Scholar] [CrossRef] [PubMed]
- Nilmat, A.; Thepbandit, W.; Chuaboon, W.; Athinuwat, D. Pseudomonas fluorescens SP007S Formulations in Controlling Soft Rot Disease and Promoting Growth in Kale. Agronomy 2023, 13, 1856. [Google Scholar] [CrossRef]
- Rivera-Conde, M.I.; Aranda-Ocampo, S.; Carrillo-Castañeda, G.; Gijón-Hernández, A.R.; Bueno-Aguilar, G.M.; Rivera-Conde, M.I.; Aranda-Ocampo, S.; Carrillo-Castañeda, G.; Gijón-Hernández, A.R.; Bueno-Aguilar, G.M. Effect of Fluorescent Pseudomonas on Tomato Seed Germination and Seedling Vigor. Rev. Chapingo Ser. Hortic. 2018, 24, 121–131. [Google Scholar] [CrossRef]
- Lally, R.D.; Galbally, P.; Moreira, A.S.; Spink, J.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Application of Endophytic Pseudomonas fluorescens and a Bacterial Consortium to Brassica napus Can Increase Plant Height and Biomass under Greenhouse and Field Conditions. Front. Plant Sci. 2017, 8, 2193. [Google Scholar] [CrossRef]
- Langendries, S.; Goormachtig, S. Paenibacillus polymyxa, a Jack of All Trades. Environ. Microbiol. 2021, 23, 5659–5669. [Google Scholar] [CrossRef]
- Soni, R.; Rawal, K.; Keharia, H. Genomics Assisted Functional Characterization of Paenibacillus polymyxa HK4 as a Biocontrol and Plant Growth Promoting Bacterium. Microbiol. Res. 2021, 248, 126734. [Google Scholar] [CrossRef]
- Jeong, H.; Choi, S.-K.; Ryu, C.-M.; Park, S.-H. Chronicle of a Soil Bacterium: Paenibacillus Polymyxa E681 as a Tiny Guardian of Plant and Human Health. Front. Microbiol. 2019, 10, 467. [Google Scholar] [CrossRef]
- Acuña, J.J.; Rilling, J.I.; Inostroza, N.G.; Zhang, Q.; Wick, L.Y.; Sessitsch, A.; Jorquera, M.A. Variovorax Sp. Strain P1R9 Applied Individually or as Part of Bacterial Consortia Enhances Wheat Germination under Salt Stress Conditions. Sci. Rep. 2024, 14, 2070. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.M.; Humm, E.; Rubbi, L.; Del Vecchio, G.; Ha, S.M.; Pellegrini, M.; Gunsalus, R.P. Complete Genome of Variovorax sp. EBFNA2, Isolated from a Surface-Sterilized Fava Bean Nodule. Microbiol. Resour. Announc. 2024, 13, e00762-24. [Google Scholar] [CrossRef]
- José, J.F.B.d.S.; Hernandes, M.A.S.; Lisboa, B.B.; Volpiano, C.G.; Schlindwein, G.; da Trindade, J.K.; Lattuada, D.S.; Beneduzi, A.; Vargas, L.K. Seed Size and Azospirillum brasilense Ab-V5 and Ab-V6 Inoculation Influences Germination and Early Seedling Vigor of Acacia mearnsii. Ciênc. Florest. 2024, 34, e85546. [Google Scholar] [CrossRef]
- Méndez-Gómez, M.; Castro-Mercado, E.; López-Bucio, J.; García-Pineda, E. Azospirillum brasilense Sp245 Triggers Cytokinin Signaling in Root Tips and Improves Biomass Accumulation in Arabidopsis through Canonical Cytokinin Receptors. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2021, 27, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Imran, M.; Kang, S.-M.; Khan, M.A.; Asaf, S.; Kim, W.-C.; Lee, I.-J. Seed Bio-Priming of Wheat with a Novel Bacterial Strain to Modulate Drought Stress in Daegu, South Korea. Front. Plant Sci. 2023, 14, 1118941. [Google Scholar] [CrossRef]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial Seed Coating: An Attractive Tool for Sustainable Agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Vij, S.; Sharma, N.; Sharma, M.; Mohanta, T.K.; Kaushik, P. Application of Trichoderma viride and Pseudomonas fluorescens to Cabbage (Brassica oleracea L.) Improves Both Its Seedling Quality and Field Performance. Sustainability 2022, 14, 7583. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and Its Role in Biological Control of Plant Fungal and Nematode Disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular Aspects of Plant Growth Promotion and Protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Bokros, N.; DeBolt, S.; Yang, P.; Xia, Y. Genome Sequence Resource of Bacillus sp. RRD69, a Beneficial Bacterial Endophyte Isolated from Switchgrass Plants. Mol. Plant. Microbe Interact. 2021, 34, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Dumigan, C.R.; Deyholos, M.K. Soil and Seed Both Influence Bacterial Diversity in the Microbiome of the Cannabis sativa Seedling Endosphere. Front. Plant Sci. 2024, 15, 1326294. [Google Scholar] [CrossRef]
- Fanai, A.; Bohia, B.; Lalremruati, F.; Lalhriatpuii, N.; Lalrokimi; Lalmuanpuii, R.; Singh, P.K. Zothanpuia Plant Growth Promoting Bacteria (PGPB)-Induced Plant Adaptations to Stresses: An Updated Review. PeerJ 2024, 12, e17882. [Google Scholar] [CrossRef] [PubMed]
- Song, G.C.; Choi, H.K.; Kim, Y.S.; Choi, J.S.; Ryu, C.-M. Seed Defense Biopriming with Bacterial Cyclodipeptides Triggers Immunity in Cucumber and Pepper. Sci. Rep. 2017, 7, 14209. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, Z.; Virag, A.; Becker, T.; Zhao, L.; Liu, W.; Xia, Y. Botrytis Cinerea In-Vivo Inoculation Assays for Early-, Middle- and Late-Stage Strawberries. Bio Protoc. 2023, 13, e4859. [Google Scholar] [CrossRef]
- Khatoon, Z.; Orozco-Mosqueda, M.d.C.; Santoyo, G. Microbial Contributions to Heavy Metal Phytoremediation in Agricultural Soils: A Review. Microorganisms 2024, 12, 1945. [Google Scholar] [CrossRef]
- Montreemuk, J.; Stewart, T.N.; Prapagdee, B. Bacterial-Assisted Phytoremediation of Heavy Metals: Concepts, Current Knowledge, and Future Directions. Environ. Technol. Innov. 2024, 33, 103488. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil Microbial Inoculants for Sustainable Agriculture: Limitations and Opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
| Beneficial Microbes | Beneficial Effects | Mode of Action | Example Strains | References |
|---|---|---|---|---|
| Trichoderma harzianum | Improves seed germination and enhances plant growth; provides stress tolerance against biotic and abiotic factors; alleviates physiological stresses in germinating seeds and seedlings; promotes root colonization and enhances disease resistance. | Stress Tolerance Enhancement; Seed Germination and Seedling Vigor Enhancement | T. harzianum strain T22; T. harzianum strain S. INAT | [39,40,41,42] |
| Bacillus subtilis | Enhances seed germination rates and promotes plant growth; improves stress tolerance by increasing chlorophyll content and root length under saline conditions; acts as a biocontrol agent against pathogens; stimulates plant growth through production of phytohormones and stress-related metabolites. | Seed Germination and Seedling Vigor Enhancement; Disease Suppression; | B. subtilis HS5B5; B. subtilis ER-08; B. subtilis QM3 | [43,44,45,46] |
| Pseudomonas fluorescens | Enhances seed germination and promotes plant growth by producing phytohormones; improves nutrient acquisition; suppresses various plant diseases through production of antimicrobial compounds. | Seed Germination and Seedling Vigor Enhancement; Direct Growth Promotion | P. fluorescens SP007S; P. fluorescens F113 | [47,48,49,50] |
| Paenibacillus polymyxa | Promotes plant growth and enhances stress tolerance; suppresses diseases by producing various antibiotics; improves germination and protects plants against pathogenic fungi, oomycetes, and bacteria. | Direct Growth Promotion; Disease Suppression | P. polymyxa HK4; Paenibacillus polymyxa E681 | [51,52,53] |
| Variovorax sp. | Enhances wheat germination under salt stress conditions; improves biomass; reduces lipid peroxidation. | Direct Growth Promotion; Stress Tolerance Enhancement; Seed Germination and Seedling Vigor Enhancement | Variovorax sp. P1R9; Variovorax sp. EBFNA2 | [54,55] |
| Azospirillum brasilense | Enhances seed germination; increases root length; promotes plant growth through auxin production, stimulating root development and improving nutrient uptake; enhances stress tolerance in plants. | Direct Growth Promotion; Seed Germination and Seedling Vigor Enhancement | Azospirillum brasilense Ab-V5; Azospirillum brasilense Ab-V6; Azospirillum brasilense Sp245 | [56,57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Lu, L.; Condrich, A.; Muni, G.A.; Scranton, S.; Xu, S.; Xia, Y.; Huang, S. Innovative Approaches for Engineering the Seed Microbiome to Enhance Crop Performance. Seeds 2025, 4, 24. https://doi.org/10.3390/seeds4020024
Yang P, Lu L, Condrich A, Muni GA, Scranton S, Xu S, Xia Y, Huang S. Innovative Approaches for Engineering the Seed Microbiome to Enhance Crop Performance. Seeds. 2025; 4(2):24. https://doi.org/10.3390/seeds4020024
Chicago/Turabian StyleYang, Piao, Ling Lu, Abraham Condrich, Gavin A. Muni, Sean Scranton, Shixiang Xu, Ye Xia, and Shuai Huang. 2025. "Innovative Approaches for Engineering the Seed Microbiome to Enhance Crop Performance" Seeds 4, no. 2: 24. https://doi.org/10.3390/seeds4020024
APA StyleYang, P., Lu, L., Condrich, A., Muni, G. A., Scranton, S., Xu, S., Xia, Y., & Huang, S. (2025). Innovative Approaches for Engineering the Seed Microbiome to Enhance Crop Performance. Seeds, 4(2), 24. https://doi.org/10.3390/seeds4020024









