Seed Germination of Garberia heterophylla (W. Bartram) Merr. & F. Harper, a Pollinator Plant with Ornamental Appeal
Abstract
1. Introduction
2. Materials and Methods
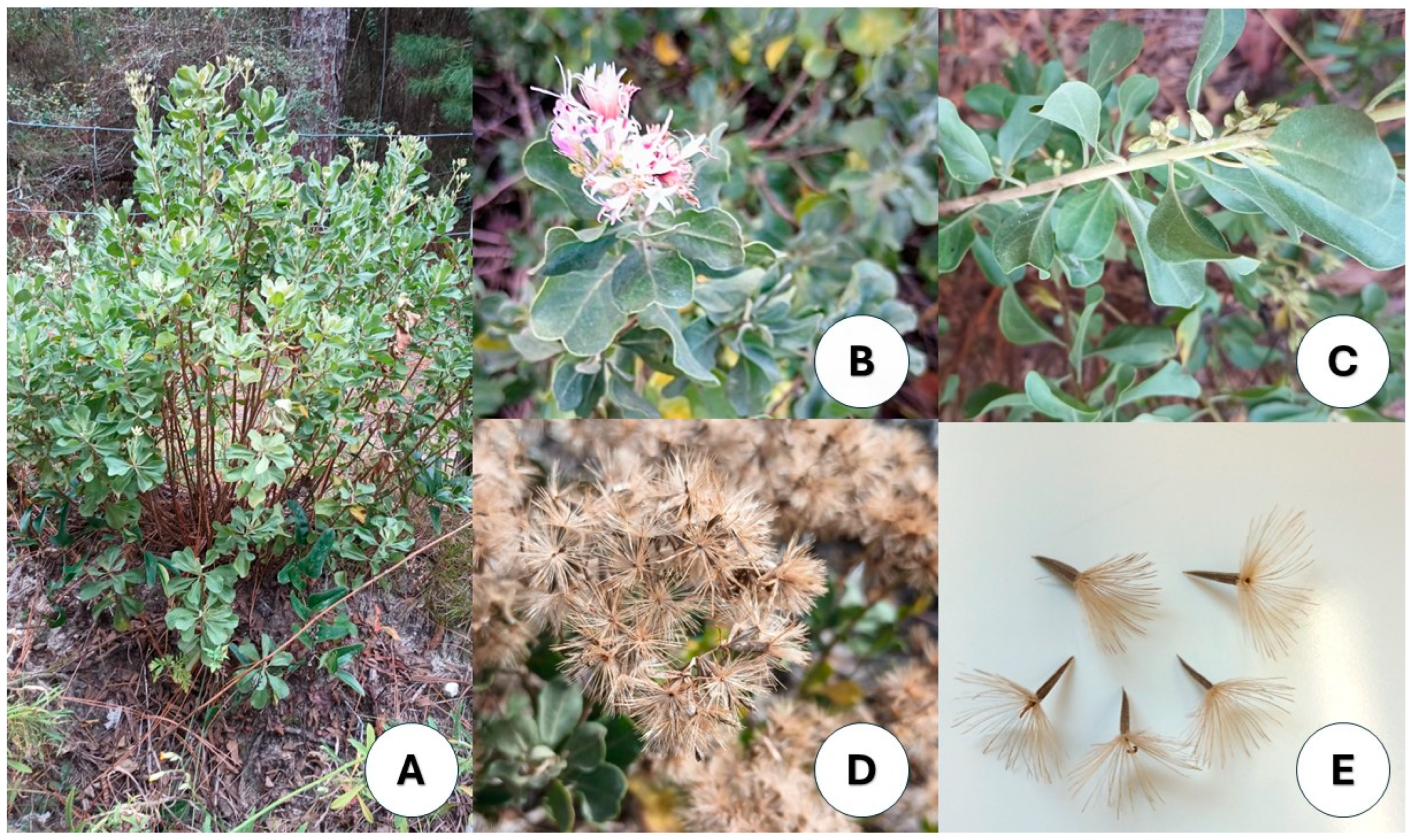
2.1. Seed Collection and Site Descriptions
2.2. Pre-Germination Viability and Imaging
2.3. Seed Germination
2.4. Seed Storability
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Seed Size, Pre-Germination Viability, and Visual Embryo Presence
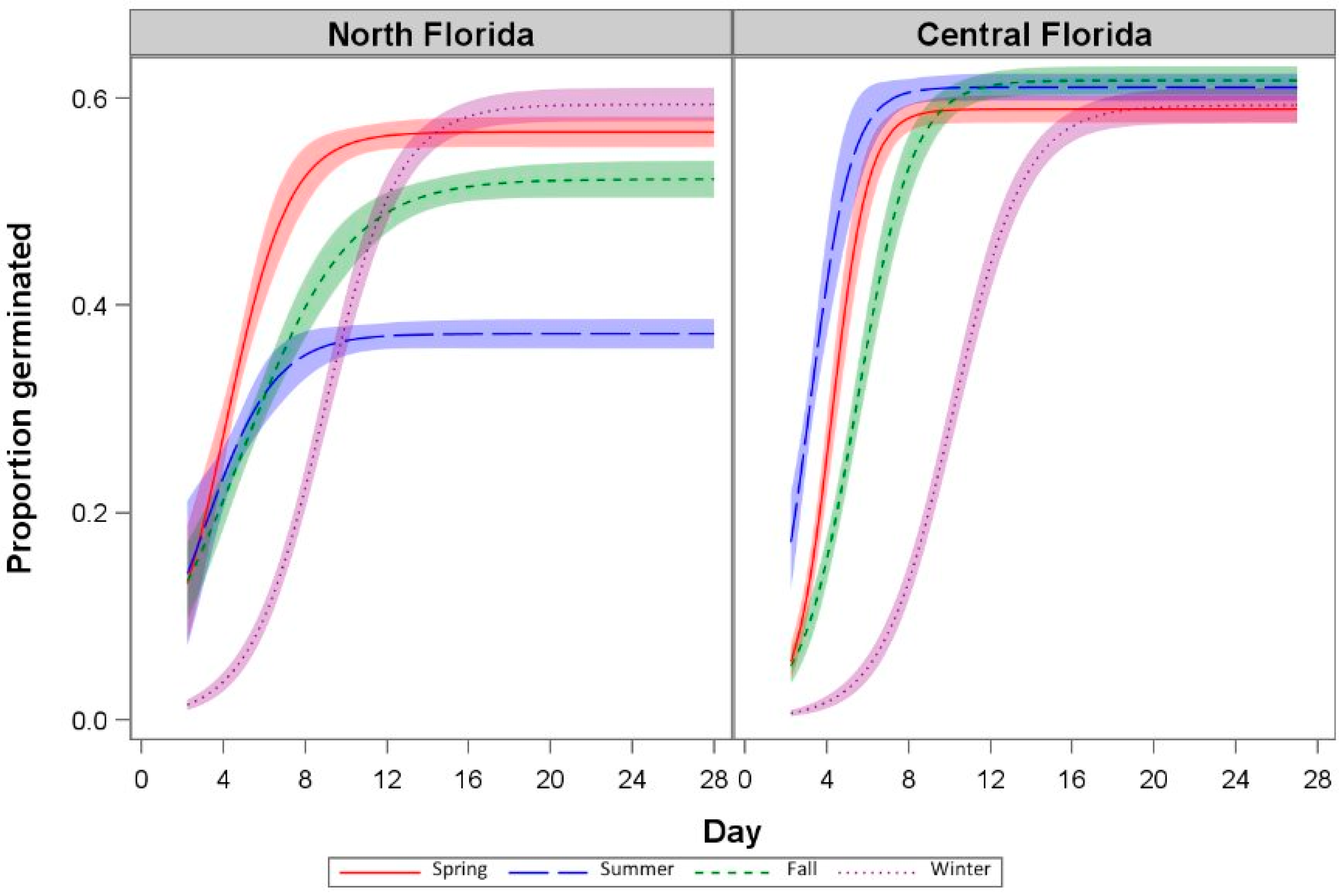
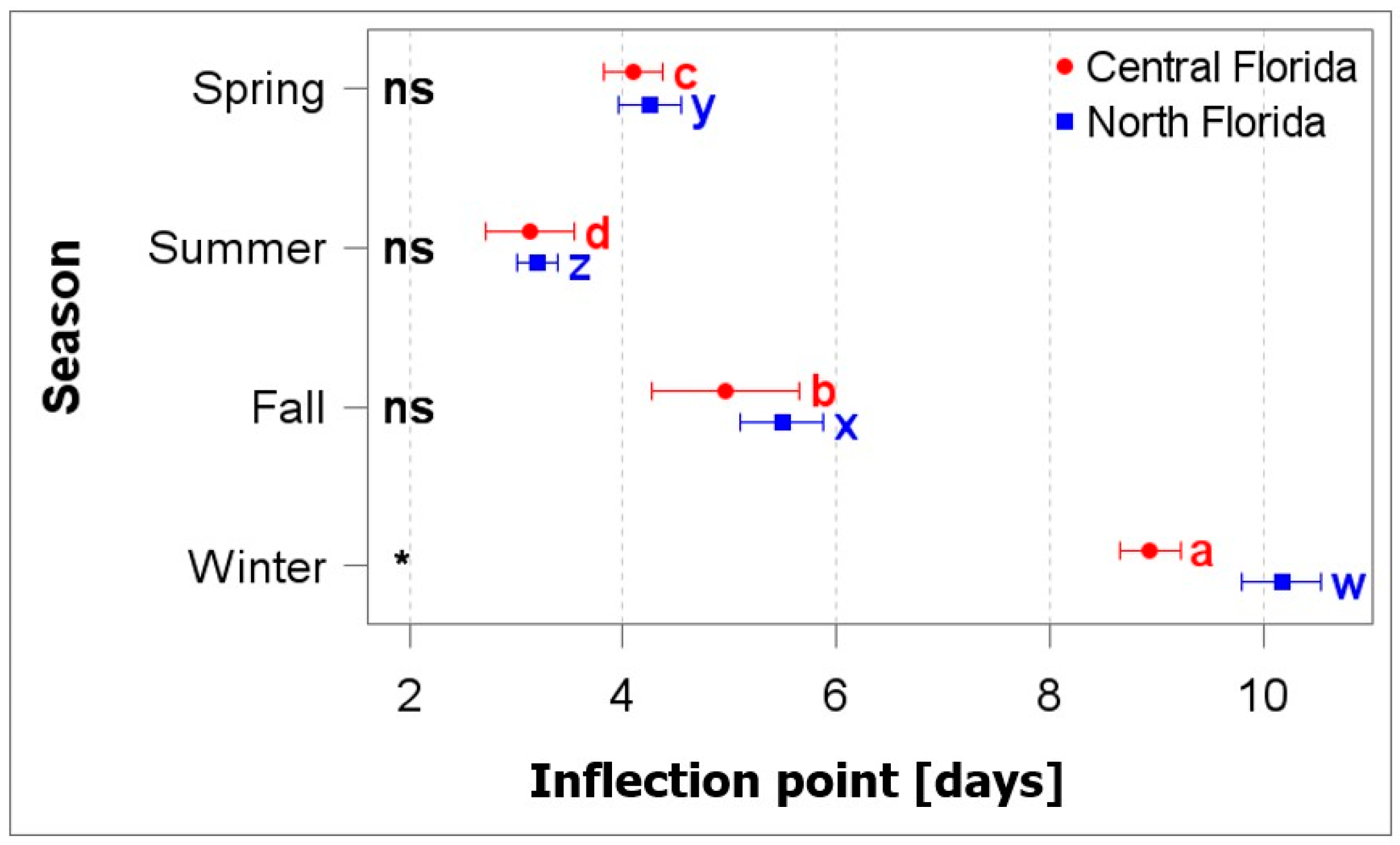
3.2. Germination Response
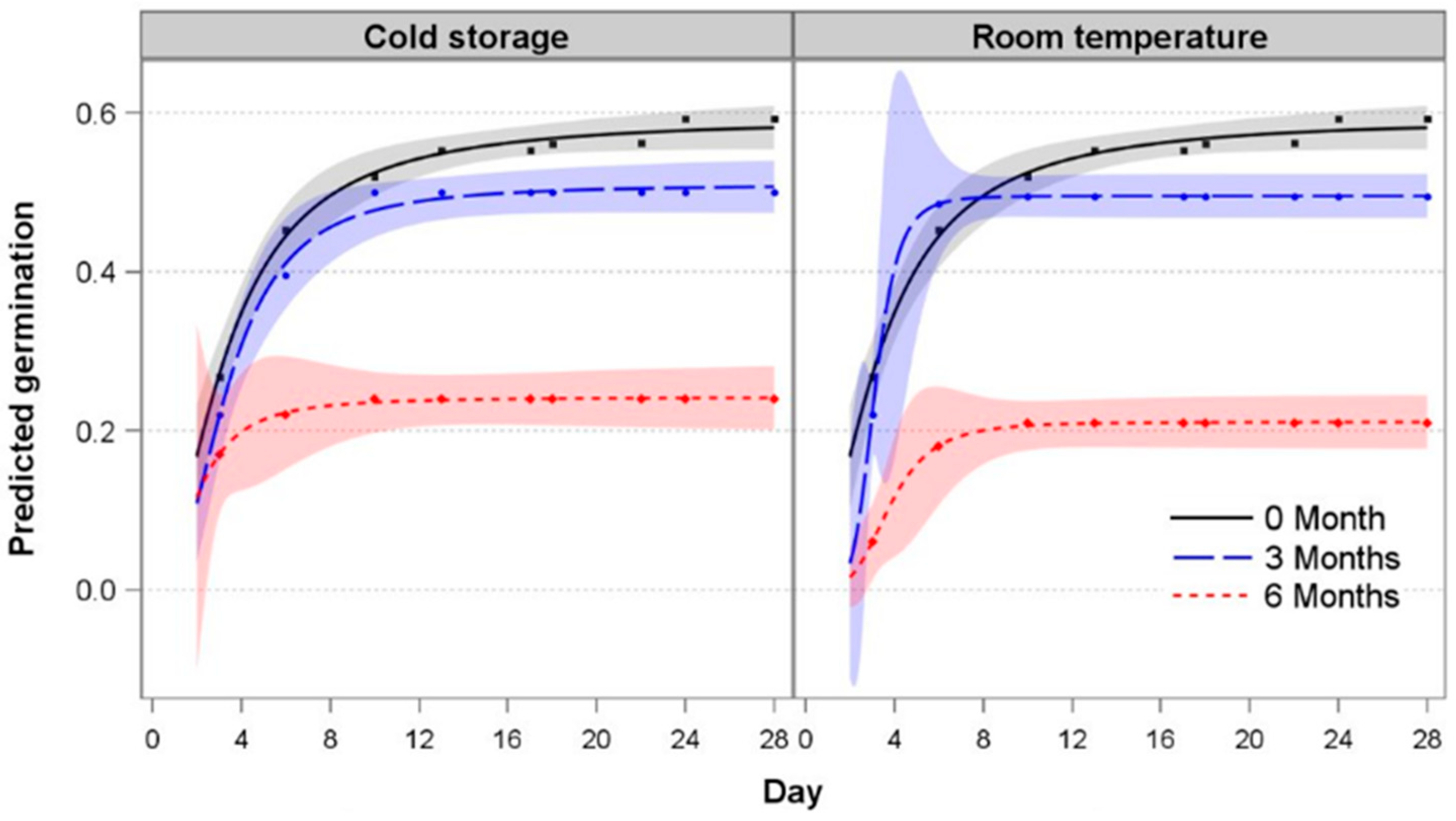
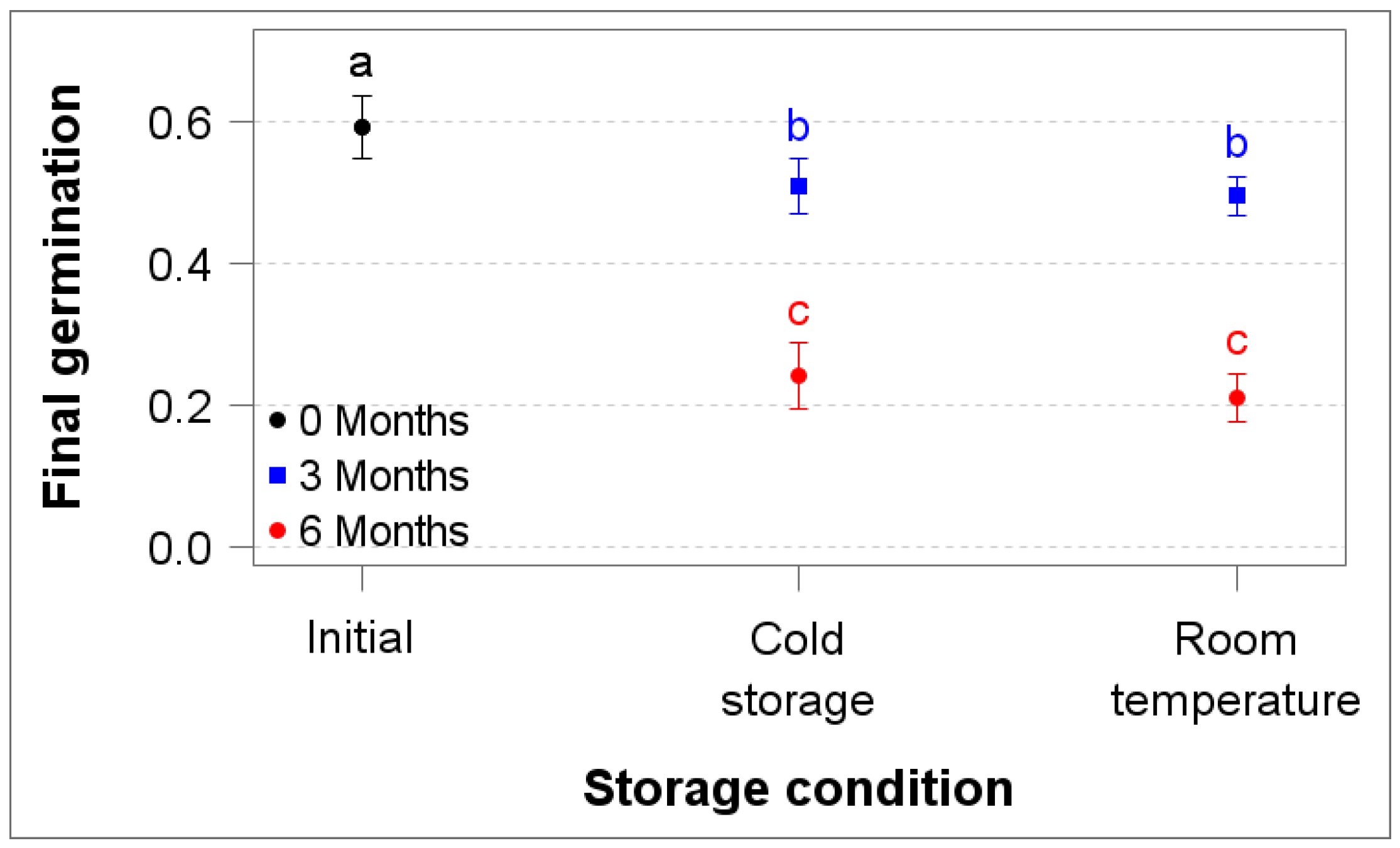
3.3. Seed Storability
4. Discussion
4.1. Initial Seed Fill and Viability
4.2. Seeds Are Non-Dormant
4.3. Population and Temperature Affect Germination Response
4.4. Seeds Lose Viability After Room Temperature or Cold Storage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brzuszek, R.F.; Harkess, R.L.; Mulley, S.J. Landscape architects use of native plants in the southeastern United States. HortTechnology 2007, 17, 78–81. [Google Scholar] [CrossRef]
- Tartaglia, E.S.; Aronson, M.F.J. Plant native: Comparing biodiversity benefits, ecosystem services provisioning, and plant performance of native and non-native plants in urban horticulture. Urban Ecosyst. 2024, 27, 2587–2611. [Google Scholar] [CrossRef]
- Gillis, A.; Swim, J.K. Adding native plants to home landscapes: The roles of attitudes, social norms, and situational strength. J. Environ. Psychol. 2020, 72, 101519. [Google Scholar] [CrossRef]
- Rihn, A.L.; Behe, B.K.; Barton, S.; Torres, A. Greater appeal of native plants for environmentally conscious consumers. J. Environ. Hortic. 2023, 41, 7–13. [Google Scholar] [CrossRef]
- Rupp, L.A.; Anderson, R.M.; Klett, J.; Love, S.L.; Goodspeed, J.; Gunnell, J. Native and adapted plant introduction for low-water landscaping. HortTechnology 2018, 28, 431–435. [Google Scholar] [CrossRef]
- Silva, J.J.; Wilson, S.B.; Knox, G.W.; Mallinger, R.E. Evaluation of plant growth and flowering performance of Florida native and non-native ornamentals under varying irrigation. HortTechnology 2024, 34, 629–643. [Google Scholar] [CrossRef]
- Rihn, A.L.; Knuth, M.J.; Peterson, B.J.; Torres, A.P.; Campbell, J.H.; Boyer, C.R.; Palma, M.A.; Khachatryan, H. Investigating drivers of native plant production in the United States green industry. Sustainability 2022, 14, 6774. [Google Scholar] [CrossRef]
- Nabors, A.; Hung, K.L.J.; Corkidi, L.; Bethke, J.A. California native perennials attract greater native pollinator abundance and diversity than nonnative, commercially available ornamental in southern California. Environ. Entomol. 2022, 51, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Seitz, N.; vanEngelsdorp, D.; Leonhardt, S.D. Are native and non-native pollinator friendly plants equally valuable for native wild bee communities? Ecol. Evol. 2020, 10, 12838–12850. [Google Scholar] [CrossRef]
- Kalaman, H.; Wilson, S.B.; Mallinger, R.E.; Knox, G.W.; van Santen, E. Evaluating native and non-native ornamentals as pollinator plants in Florida: I. Floral abundance and insect visitation. HortScience 2022, 57, 126–136. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Mata, L.; Mackie, J.A.; Hahs, A.K.; Stork, N.E.; Williams, N.S.G.; Livesley, S.J.; Beggs, J. Increasing biodiversity in urban green spaces through simple vegetation interventions. J. Appl. Ecol. 2017, 54, 1874–1883. [Google Scholar] [CrossRef]
- Smallwood, N.; Wood, E. The ecological role of native-plant landscaping in residential yards to birds during the nonbreeding period. Ecosphere 2023, 14, e4360. [Google Scholar] [CrossRef]
- Torres, A.P.; Rihn, A.L.; Barton, S.S.; Behe, B.K. Perceptions and socioeconomic status influence purchases of native plants. HortTechnology 2024, 34, 153–160. [Google Scholar] [CrossRef]
- Rihn, A.L.; Torres, A.; Behe, B.K.; Barton, S. Unwrapping the native plant black box: Consumer perceptions and segments for target market strategies. HortTechnology 2024, 34, 361–371. [Google Scholar] [CrossRef]
- White, A.; Fant, J.B.; Havens, K.; Kramer, A.T. Restoring species diversity: Assessing capacity in the U.S. native plant industry. Restor. Ecol. 2018, 26, 605–611. [Google Scholar] [CrossRef]
- Wilson, S.B. Expanding Our Plant Palette: The Role of Natives and Non-Invasive Cultivars. In Proceedings of the Florida State Horticultural Society, Virtual, Bartow, FL, USA, 19 October 2020. [Google Scholar]
- Smith, T.P.; Wilson, S.B.; Marble, C.S.; Xu, J. Propagation for commercial production of sweet acacia (Vachellia farnesiana): A native plant with ornamental potential. Nativ. Plants J. 2022, 23, 337. [Google Scholar] [CrossRef]
- Thetford, M.; O’Donoughue, A.; Wilson, S.B.; Pérez, H.E. Softwood cutting propagation of three Polygonella wildflower species native to Florida. Propag. Ornam. Plants 2012, 12, 58–62. [Google Scholar] [CrossRef]
- Mikell, L.; Wilson, S.B.; Marbel, C.S.; Vendrame, W.; van Santen, E. Sexual and asexual reproduction of Wild lime (Zanthoxylum fagara L. Sarg), a native Florida plant with ornamental and ecological value. J. Environ. Hortic. 2024, 42, 131–139. [Google Scholar] [CrossRef]
- Young, T.; Wilson, S.B.; Thetford, M.; Coole, J. Cutting propagation of four Florida native taxa of wild coffee (Psychotria sp.) for ornamental use. Nativ. Plants J. 2022, 23, 288–297. [Google Scholar] [CrossRef]
- Xu, J.; Wilson, S.B.; Vendrame, W.A.; Beleski, D.G. Micropropagation of sweet acacia (Vachellia farnesiana), an underutilized tree with ornamental value. Plant Tissue Cult. 2023, 59, 74–82. [Google Scholar] [CrossRef]
- Wunderlin, R.P.; Hansen, B.F.; Franck, A.R.; Essig, F.B. Atlas of Florida Plants. Available online: https://florida.plantatlas.usf.edu (accessed on 15 August 2024).
- Deyrup, M.; Edirisinghe, J.; Norden, B. The diversity and floral hosts of bees at the Archbold Biological Station, Florida (Hymenoptera: Apoidea). Insecta Mundi 2002, 16, 87–120. [Google Scholar]
- Florida Association of Native Nurseries (FANN). Native Plant and Service Directory. Available online: https://www.fann.org/info/native-plant-service-directory/ (accessed on 29 August 2024).
- Association of Official Seed Analysts (AOSA). Rules for Testing Seeds, Volume 1; Association of Official Seed Analysts, Inc.: Washington, DC, USA, 2022; pp. 8-1–8-3. [Google Scholar]
- Campbell-Martínez, G.; Steppe, C.; Wilson, S.B.; Thetford, M.; Miller, D. Effect of temperature, light, and seed provenance on germination of Paronychia erecta (squareflower): A native plant with ornamental potential. Nativ. Plants J. 2022, 23, 56–64. [Google Scholar] [CrossRef]
- Vijayalakshmi, B.; Raveendran, T.S. Postfertilization barriers in crosses between Gossypium triphyllum and Gossypium hirsutum. Mod. Phytomorphol. 2000, 50, 287–289. [Google Scholar]
- Hong, S.K.; Aoki, T.; Kitano, H.; Satoh, H.; Nagato, Y. Temperature-sensitive mutation, embryoless 1, affects both embryos and endosperm development in rice. Plant Sci. 1995, 108, 165–172. [Google Scholar] [CrossRef]
- Sangiorgio, S.; Carabelli, L.; Gabotti, D.; Manzotti, P.; Persico, M.; Gavazzi, G. A mutational approach for the detection of genetic factors affecting seed size in maize. Plant Reprod. 2016, 29, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, T. Fruit and seed development in Sesili rigidum. Fitologija 1977, 7, 59–74. [Google Scholar]
- Erdelska, O. The recycling processes in unfertilized ovules. Acta. Biol. Cracov. 1999, 41, 14. [Google Scholar]
- Krinski, D.; Foerster, L.A. Quantitative and qualitative damage caused by Oebalus poecilus (Hemiptera, Pentatomidae) to upland rice cultivated in new agricultural frontier of the Amazon rainforest (Brazil). Cienc. Agrotechnologia 2017, 41, 300–311. [Google Scholar] [CrossRef]
- von Aderkas, P.; Rouault, G.; Wagner, R.; Rohr, R.; Roques, A. Seed parasitism redirects ovule development in douglas fir. Proc. R. Soc. B. 2005, 272, 1491–1496. [Google Scholar] [CrossRef]
- Baskin, C.; Baskin, J. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; p. 292. [Google Scholar]
- Kodym, A.; Turner, S.; Delpratt, J. In situ seed development and in vitro regeneration of three difficult-to-propagate Lepidosperma species (Cyperaceae). Aust. J. Bot. 2010, 58, 107–114. [Google Scholar] [CrossRef]
- Perea, R.; Venturas, M.; Gil, L. Empty seeds are not always bad: Simultaneous effect of seed emptiness and masting on animal seed predation. PLoS ONE 2013, 8, e65573. [Google Scholar] [CrossRef]
- Müller, M.; Siles, L.; Cela, J.; Munné-Bosch, S. Perennially young: Seed production and quality in controlled and natural populations of Cistus albidus reveal compensatory mechanisms that prevent senescence in terms of seed yield and viability. J. Exp. Bot. 2014, 65, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.B.; Perez, H.; Thetford, M. Propagation, Production, and Landscape Evaluation of Native Wildflowers in West, Central and South Florida. Florida Wildflower Foundation Condensed Progress Report, 2010. Available online: https://www.flawildflowers.org/wp-content/resources/pdfs/Research/Wildflower%20year-end%20progress%20report-Wilson%20et%20al.pdf (accessed on 15 January 2025).
- Frischie, S.; Miller, A.L.; Pedrini, S.; Kildisheva, O.A. Ensuring seed quality in ecological restoration: Native seed cleaning and testing. Restor. Ecol. 2020, 28, 239–248. [Google Scholar] [CrossRef]
- Robinson, R.; Beck, L.; Kettenring, K.M. The effects of native seed mix composition and sowing density on plant community reassembly in wetlands. Ecosphere 2024, 15, 4829. [Google Scholar] [CrossRef]
- Lavallee, K.; Soti, P.G.; Rodrigo, H.; Kariyat, R.; Racelis, A. Pre-Sowing Treatments Improve Germinability of South Texas Native Plant Seeds. Plants 2021, 10, 2545. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seed dormancy in Asteraceae: A global vegetation zone and taxonomic/phylogenetic assessment. Seed Sci. Res. 2023, 33, 135–169. [Google Scholar] [CrossRef]
- de Waal, C.; Anderson, B.; Ellis, A.G. Dispersal, dormancy and life-history tradeoffs at the individual, population and species levels in southern African Asteraceae. New Phytol. 2016, 210, 356–365. [Google Scholar] [CrossRef]
- Pagano, A.; Doria, E.; Mondoni, A.; White, F.J.; Balestrazzi, A.; Macovei, A. Oxidant and antioxidant profiling in Viscaria alpina seed populations following the temporal dynamics of an alpine climate. Seeds 2023, 2, 357–369. [Google Scholar] [CrossRef]
- Dell, N.D.; Albrecht, M.A.; Long, Q.G. Effects of light and temperature on germination of Eggert’s Sunflower (Helianthus eggertii). Am. Midland Nat. 2021, 185, 49–56. [Google Scholar] [CrossRef]
- Graves, N.; Campbell-Martínez, G.; Thetford, M.; Miller, D.; Wilson, S.B. Conservation and re-establishment of Florida panhandle goldenasters (Chrysopsis): I. Reproduction characteristics and germination requirements. Nativ. Plants J. 2021, 22, 315–322. [Google Scholar] [CrossRef]
- Trigiano, R.N.; Boggess, S.L.; Wyman, C.R.; Hadziabdic, D.; Wilson, S.B. Propagation methods for the conservation and preservation of the endangered whorled sunflower (Helianthus verticillatus). Plants 2021, 10, 1565. [Google Scholar] [CrossRef]
- Laghmouchi, Y.; Belmehdi, O.; Bouyahya, A.; Skali-Senhaji, N.; Abrini, J. Effect of temperature, salt stress, and pH on seed germination of medicinal plant Origanum compactum. Biocatal. Agric. Biotechnol. 2017, 10, 156–160. [Google Scholar] [CrossRef]
- Catão, H.; Gomes, L.; Guimarães, R.M.; Fonseca, P.H.F.; Caixeta, F.; Marodin, J.C. Physiological and isozyme alterations in lettuce seeds under different conditions and storage periods. J. Seed Sci. 2016, 38, 305–313. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Q.; Zhang, Y.; Yang, L.; Zeng, Z.; Zhou, Y.; Chen, B. Advance in thermoinhibition of lettuce (Lactuca sativa L.) seed germination. J. Seed Sci. 2024, 13, 2051. [Google Scholar] [CrossRef] [PubMed]
- Corbineau, F. The effects of storage time on seed deterioration and ageing: How to improve seed longevity. Seeds 2024, 3, 56–75. [Google Scholar] [CrossRef]
- Guo, C.; Shen, Y.; Shi, F. Effect of temperature, light, and storage time on the seed germination of Pinus bungeana Zucc. Ex Endl.: The role of seed-covering layers and abscisic acid changes. Forests 2020, 11, 300. [Google Scholar] [CrossRef]
- Klose, C.; Nagy, F.; Schäfer, E. Thermal reversion of plant phytochromes. Mol. Plant 2020, 13, 386–397. [Google Scholar] [CrossRef]
- Saux, M.; Bleys, B.; André, T.; Bailly, C.; El-Maarouf-Bouteau, H. A correlative study of sunflower seed vigor as related to genetic background. Plants 2020, 9, 386. [Google Scholar] [CrossRef]
- Bisht, V.K.; Kuniyal, C.P.; Negi, J.S.; Bhandari, A.K.; Bhatt, V.P. Variations in the seed germination in Sapindus mukorossi in relation to tree age dependent seed vigour. Natl. Acad. Sci. Lett. 2016, 39, 379–382. [Google Scholar] [CrossRef]
- LeFait, B.M.; Qaderi, M.M. Maternal environmental effects of temperature and exogenous gibberellic acid on seed and seedling traits of four populations of evening primrose (Oenothera biennis). Seeds 2022, 1, 110–125. [Google Scholar] [CrossRef]
- Andersson, S. Seed size as a determinant of germination rate in Crepis tectorum (Asteraceae): Evidence from a seed burial experiment. Can. J. Bot. 1996, 74, 568–572. [Google Scholar] [CrossRef]
- Gairola, S.; Mahmoud, T.; Shabana, H.A.; Alketbi, A.; Phartyal, S.S. Effect of seed size on germination in three species from arid Arabian deserts. Botany 2021, 99, 69–74. [Google Scholar] [CrossRef]
- Rich, S.M.; Berger, J.; Lawes, R.; Fletcher, A. Chickpea and lentil show little genetic variation in emergence ability and rate from deep sowing, but small-sized seed produces less vigorous seedlings. Crop Pasture Sci. 2022, 73, 1042–1055. [Google Scholar] [CrossRef]
- Campbell-Martínez, G.; Thetford, M.; Wilson, S.B.; Steppe, C.; Pérez, H.; Miller, D. Germination of coastalplain honeycombhead (Balduina angustifolia) in response to photoperiod, temperature, and gibberellic acid. Seed Sci. Technol. 2021, 49, 287–303. [Google Scholar] [CrossRef]
- Jiménez-Vázquez, A.M.; Flores-Palacios, A.; Flores-Morales, A.; Perea-Arango, I.; Gutiérrez, M.D.; Arellano-García, J.D.; Valencia-Díaz, S. Seed longevity, viability and germination of four weed-ruderal Asteraceae species of ethnobotanic value. Bot. Sci. 2021, 99, 279–290. [Google Scholar] [CrossRef]
- Liava, V.; Ntatsi, G.; Karkanis, A. Seed germination of three milk thistle (Silybum marianum (L.) Gaertn.) populations of greek origin: Temperature, duration, and storage conditions effects. Plants 2023, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Adegbola, Y.; Pérez, H.E. Extensive desiccation and aging stress tolerance characterize Gaillardia pulchella (Asteraceae) seeds. HortScience 2016, 51, 159–163. [Google Scholar] [CrossRef]
- Cuena-Lombraña, A.; Sanna, M.; Porceddu, M.; Bacchetta, G. Does storage under gene bank conditions affect seed germination and seedling growth? The case of Senecio morisii (Asteraceae), a vascular plant exclusive to sardinian water meadows. Plants 2020, 9, 581. [Google Scholar] [CrossRef]
- Desheva, G. The longevity of crop seeds stored under long-term conditions in the National Gene Bank of Bulgaria. Agriculture 2016, 62, 90–100. [Google Scholar] [CrossRef]
- Salazar, A.; Maschinski, J.; Possley, J.; Heineman, K. Seed germination of 53 species from the globally critically imperiled pine rockland ecosystem of South Florida, USA: Effects of storage, phylogeny, and life-history traits. Seed Sci. Res. 2018, 28, 82–92. [Google Scholar] [CrossRef]
- Morgan, J.W.; Salmon, K.L. Message in a bottle: Inadvertent loss of seeds of native grassland species as a result of rudimentary long-term storage. Ecol. Manag. Restor. 2019, 20, 159–161. [Google Scholar] [CrossRef]
- Duong, T.H.; Shen, J.L.; Luangviriyasaeng, V.; Ha, H.T.; Pinyopusarerk, K. Storage behavior of Jatropha curcas seeds. J. Trop. For. Sci. 2013, 25, 193–199. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carapezza, G.; Wilson, S.B.; McMillan, M.; van Santen, E. Seed Germination of Garberia heterophylla (W. Bartram) Merr. & F. Harper, a Pollinator Plant with Ornamental Appeal. Seeds 2025, 4, 23. https://doi.org/10.3390/seeds4020023
Carapezza G, Wilson SB, McMillan M, van Santen E. Seed Germination of Garberia heterophylla (W. Bartram) Merr. & F. Harper, a Pollinator Plant with Ornamental Appeal. Seeds. 2025; 4(2):23. https://doi.org/10.3390/seeds4020023
Chicago/Turabian StyleCarapezza, Grace, Sandra B. Wilson, Mica McMillan, and Edzard van Santen. 2025. "Seed Germination of Garberia heterophylla (W. Bartram) Merr. & F. Harper, a Pollinator Plant with Ornamental Appeal" Seeds 4, no. 2: 23. https://doi.org/10.3390/seeds4020023
APA StyleCarapezza, G., Wilson, S. B., McMillan, M., & van Santen, E. (2025). Seed Germination of Garberia heterophylla (W. Bartram) Merr. & F. Harper, a Pollinator Plant with Ornamental Appeal. Seeds, 4(2), 23. https://doi.org/10.3390/seeds4020023






