Abstract
Acacia species are important trees in arid ecosystems due to their diverse ecological roles, such as providing vegetation cover, community structures, food resources for animals, soil stabilization, and erosion prevention. However, in the Arabian Peninsula, Acacia species are declining due to climate change, overgrazing, and fuelwood harvesting. This study evaluates the effectiveness of various pre-sowing treatments—sulfuric acid soaking and tap and hot water soaking—on breaking seed dormancy to enhance germination in nine Acacia species native to the Al-Baha region of Saudi Arabia. The key germination indicators assessed were the mean germination time (MGT), germination percentage (GP), and germination index (GI). Sulfuric acid treatments for 10–15 min reduced the MGT and increased the GP for A. etbaica, A. hamoulosa, and A. tortilis, while A. origena responded best to 1 min of hot water soaking. Conversely, A. asak, A. ehrenbergiana, and A. johnwoodii showed little to no germination improvement with treatment and A. oerfota and A. gerrardii showed no germination improvement, indicating the need for alternative methods. These findings indicate that the seed germination requirements vary within Acacia spp. from the same geographic region and similar climatic conditions. Further work is required for five of the species tested to develop better seed germination techniques, given the potential utility of Acacia spp., in ecological restoration and sustainable land management in arid regions.
1. Introduction
Acacia trees play a vital and irreplaceable role within the arid ecosystems of the Arabian Peninsula. Their significance stems from their role in providing habitat and food resources for a number of animal species and also as contributors to soil stabilization and erosion control [1,2]. Acacia species are predominantly found in dry tropical regions, particularly in arid and semi-arid areas. These species have evolved various adaptations that enable them to endure harsh climatic conditions, including extreme temperatures and prolonged drought [3]. In recent decades several anthropogenic disturbances, including global climate change, unchecked overgrazing, and Acacia wood exploitation for fuel, have significantly impacted the health and survival of these trees [4]. The profound impacts of these challenges are most evident as escalating temperatures and diminishing precipitation wrought by climate change have exacerbated drought stress and slowed Acacia tree growth. This has decreased their flowering and seed set and increased their mortality rates [2]. The abundance and diversity of Acacia species have been significantly impacted by overgrazing by livestock, which has decreased soil nutrients and changed the structure of plant communities [5].
The potential loss of many ecologically significant Acacia species due to the above-mentioned causes could have an adverse effect on the biodiversity and general health of arid ecosystems [2,6]. Therefore, prioritizing the rejuvenation of degraded ecosystems and the protection of remaining Acacia populations through targeted conservation and restoration initiatives are imperative. These efforts are key to restoring the distribution, abundance, and thus the ecological functional roles of Acacia species within the Arabian Peninsula. Potential restoration strategies include reforestation, habitat restoration, the restriction of grazing, and the implementation of improved grazing management techniques. Importantly, the extensive root systems characteristic of Acacia species can serve as a natural bulwark against soil instability and erosion, particularly in regions where these issues are prevalent [6]. Acacia species have been widely used in restoration efforts in arid and semi-arid regions globally due to their resilience to harsh environments. For example, in the Sahel region of Africa, Acacia senegal has been employed in agroforestry systems to combat desertification, improve soil quality, and provide fuelwood and gum for local communities [7]. These efforts have helped restore degraded lands and support local livelihoods by providing essential resources in challenging environments. Similarly, in Australia, Acacia mearnsii and Acacia saligna were used in reforestation projects to stabilize sand dunes and reduce wind erosion, thereby protecting agricultural lands [8]. The use of Acacia for restoration purposes extends beyond ecosystem rehabilitation to include socio-economic benefits, such as providing fuelwood and building materials, which are crucial for sustainable development in arid regions [4].
The Genus Acacia is classified under the Kingdom Plantae, Family Fabaceae, and Tribe Acacieae [9]. Acacia includes more than 1350 species of “thorn” trees and shrubs, which occur throughout the dry regions of the world [1]. The geographic range of Acacia includes Australia, South Asia, North and South America, and the tropical warm–temperate area of Africa [10]. However, in the Kingdom of Saudi Arabia, only 16 species have been reported [11]. These species occur naturally in the Arabian Peninsula and are most common in western and southwest Saudi Arabia regions [3].
Acacia seeds have a hard outer seed coat that can be impermeable to water, making it difficult for seeds to germinate. Under natural conditions, seeds require exposure to environmental conditions that cause the water gap on the seed coat to open. This scarification process can occur from natural factors, such as heat from the sun or mechanical abrasion over time [12]. While some Acacia seeds can remain dormant in the soil for years, germination can occur once the water gap on the seed coat is open and environmental conditions, such as temperature and moisture, are favorable [2,11]. Therefore, Acacia seeds often require pre-treatment to be made water permeable in the laboratory before they can be used for reforestation, afforestation, or other types of ecological restoration [2,11]. Several studies indicated that the pre-treatment methods help break down the seed coat and stimulate the germination process, thereby increasing the likelihood of a successful seedling establishment. Thus, Acacia seeds can benefit from pre-germination treatments to increase their germination rate and effectiveness in restoration efforts [2].
Some of the common pre-treatment methods for Acacia seeds include scarification with a blade or with acid and by soaking in water at different temperatures, depending on the species [10,11,12]. Among these techniques, soaking in water is a simple but common and effective method for pre-treating Acacia seeds. This approach entails immersing the seeds in water for a certain period to allow the water to penetrate the seed coat, thereby softening it and promoting germination. The length of time required for soaking can vary depending on the species and seed lot; but generally, soaking for 24–48 h is sufficient to achieve good results [13,14]. Another application involves soaking Acacia seeds in a dilute sulfuric acid solution for several minutes to break down the hard seed coat [13]. Our research benefits from the experiences of others who conducted similar experiments under varying conditions. For instance, [13] tested dormancy-breaking methods on Leucaena leucocephala seeds from southern China and Acacia nilotica seeds from western Sudan, while [14] focused on L. leucocephala and Acacia farnesiana seeds from several locations in Jordan, and [2] conducted experiments on the seeds of A. asak, A. etbaica, A. ehrenbergiana, A. gerrardii, and A. origena that were collected from three regions of Saudi Arabia, Asir, Al-Baha, and Al-Madinah.
Breaking seed dormancy and improving seed germination involves several measurable variables used in nursery practice. The mean germination time (MGT) refers to the average elapsed time after the seed treatment required for the experimental batch of seeds to germinate. The germination percentage (GP) indicates the proportion of seeds that successfully germinate within a given period, expressed as a percentage of the total seeds tested. The germination index (GI) assesses both the proportion of seeds that have germinated and the speed at which they do so, providing a combined measure of germination efficiency [15].
In this study, we aimed to identify pre-treatments that break seed dormancy to maximize seed germination in nine species of Acacia that grow on the Arabian Peninsula. Specifically, we proposed to determine which pre-treatments produce the best results in terms of germination speed and germination rates. The seeds used in this study were collected from nine species growing in natural habitats in the Al-Baha region of Saudi Arabia. Our study design examines which pre-treatments are most effective for each Acacia species in accomplishing the following: (1) accelerate the mean germination time (MGT); (2) increase the germination percentage (GP); and (3) increase the germination index (GI).
2. Materials and Methods
2.1. Study Area for Seed Harvest
2.1.1. Collection Sites
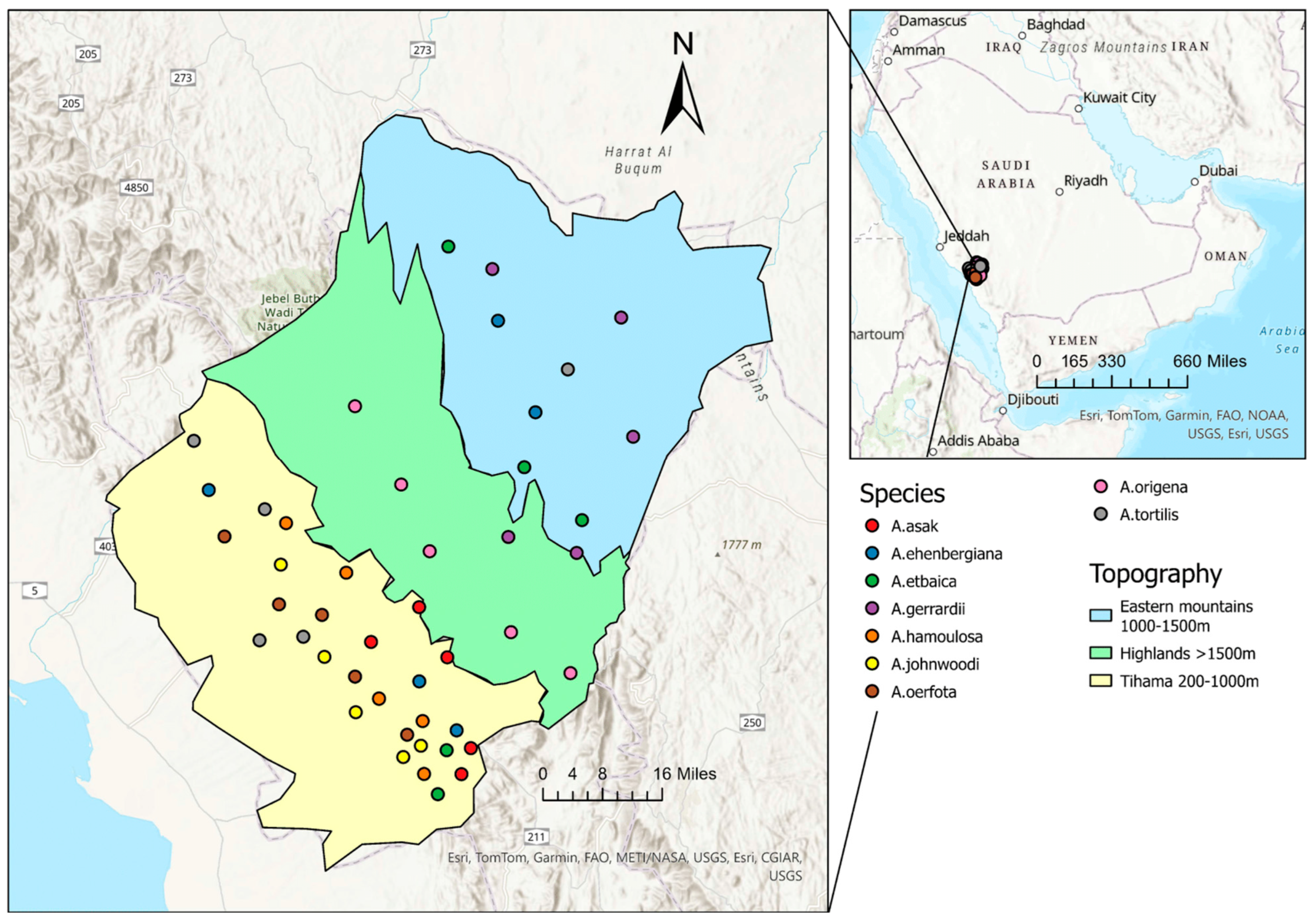
The Al-Baha region (Figure 1) is one of the thirteen regions comprising the Kingdom of Saudi Arabia. It is located in the southwestern part of the Arabian Peninsula between the Makkah and Aseer regions. Al-Baha lies between longitudes 41° and 42° and latitudes 19° and 21° [16].
Figure 1.
The small map shows the location of the Al-Baha region, Saudi Arabia. The large map shows the locations of Acacia trees from which samples were collected. Each colored dot represents a sampled tree. The colors indicate topographic zones: cyan corresponds to the east of the highlands, green to the highlands, and yellow to the Tihama lowlands.
2.1.2. Climate
The climate in the Al-Baha region varies greatly between summer and winter, which is related to topographic and climatic factors, including elevation and the alignment of mountain ranges in relation to prevailing winds that carry moisture. Al-Baha as a region can be divided into three geographic sections, with distinct differences in temperature and rainfall [2].
The Al-Baha region is divided, because of its steep, rocky, topography, into three different parts [17]. First, the highlands, which comprise high mountains that exceed 2000 m above sea level, have temperature averages of 25 °C in the summer and 10 °C in the winter. The temperature may attain lows of 0 °C in some places in the winter. The highlands area is rich in plant biodiversity, due to the relatively high annual rainfall. The lowlands, which are coastal areas located to the west of the highlands, vary in elevation from 200 to 1000 m above sea level and have high summer temperatures, with an average of 40 °C, while in the winter, the average is 25 °C and there is typically very little rainfall. The middle altitude lands are located to the east of the highlands, with heights varying from about 1000 to 1500 m above sea level, temperatures that are moderate in summer, with an average of 30 °C, and temperatures on average of 16 °C in winter (National Center of Meteorology of Saudi Arabia 2006–2015).
2.2. Seeds for Study
The mature seeds of nine different Acacia species were collected from the Al-Baha region: Acacia asak, Acacia ehenbergiana, Acacia etbaica, Acacia gerrardii, Acacia hamoulosa, Acacia johnwoodi, Acacia oerfota, Acacia origena, and Acacia tortilis (Figure S1). Seed pods were collected during 2015 over the course of an entire year because species differed in their flowering and seed pod maturation phenology. Seeds were extracted manually from pods, and seeds obviously infected by pathogens were discarded (Table 1).

Table 1.
Flowering and pod maturation periods for the Acacia species in the study area are based on our field observations. Scientific names and authorities follow [18,19].
2.3. Seed Treatments
Following the extraction of seeds from pods, they were stored in sealed, airtight containers in a cool, dark, and dry environment to preserve their viability until the experiments were conducted. The treatments were performed within approximately 3 months of seed collection, ensuring minimal impact on seed quality due to storage time. A water test was performed to test seed viability before the experiments. Seeds were placed in a bowl of water and left to sit for 1 min. Seeds that sank were considered viable and selected for the study, while floating seeds were discarded to minimize the potential influence of non-viable seeds on the germination results.
After performing the seed viability, we carried out the treatment applications using a randomized complete block design (RCBD), where blocks correspond to Acacia species and were carried out consecutively. For each treatment, healthy seeds were randomly selected from different individuals of the same species and then randomized to sub-treatments. For each sub-treatment, 25 seeds were placed in a 100 mL flask, and four flasks (replicates) were prepared for each sub-treatment, resulting in a total of 100 seeds per sub-treatment for each Acacia species. The seeds were subjected to one of four main treatments, each comprising multiple sub-treatments, summarized in Table 2. All treatments were conducted at room temperature. The four main treatments included: (1) tap (room-temperature) water: seeds were soaked in two tap water treatments for 24 and 48 h, respectively, inside a 100 mL flask; (2) hot water: seeds were soaked in three hot distilled water treatments at 100 °C for 1, 2, and 3 min, respectively, inside a 100 mL flask; (3) acid soaking: seeds were soaked in three concentrated sulfuric acid treatments (80%) for 5, 10, and 15 min, respectively, within a 100 mL flask, after which the seeds were retrieved and thoroughly rinsed in tap water and distilled water; and (4) control: seeds were not treated.

Table 2.
The nine treatments that were used in the germination experiment included a control treatment and sub-treatments using tap water, hot water, and sulfuric acid.
2.4. Germination Experiments
Seed germination experiments for all nine species were performed on moist filter papers in sterilized Petri dish using seeds that had been subjected to all 8 sub-treatments (a total of 72 sub-treatments for nine species) plus untreated controls (9 controls). Filter papers were kept wet, and distilled water was added as needed for the duration of the experiments. Petri dishes were maintained in the plant growth chamber at constant temperature (25 ± 1 °C) and 12 h of light and dark. Seeds were monitored daily with all new germination recorded for 30 days.
Germination time was recorded starting from the time of placement of seeds on filter paper in Petri dishes under these standardized germination conditions. For seeds that underwent water soaking (24 or 72 h), this initial soaking time was not included in the germination time calculations. Instead, the germination time reflects the period following soaking in Petri dishes. This approach provided a consistent basis for comparing the effects of all treatments.
2.5. Data Recording and Germination Assessment
The following describes how germination parameters were calculated:
- (1)
- Mean germination time (MGT) measures in days the average time it takes for a cohort of 25 seeds to germinate and produce a radical and is based on the equation of [15]: MGT (days) = ∑(Ti Ni)/S, where Ti is the number of days from the beginning of the experiment to the ith day (i = 1, 2, …, 30), Ni is the number of seeds germinated on the ith day, and S = ∑Ni is the total number of seeds germinated over all 30 days of the experiment.
- (2)
- Germination percentage (GP) measures the proportion of seeds of the 25-seed sample that germinated over a 30-day period. It is calculated as the number of seeds that have germinated, divided by the total number of seeds (25) that were treated. The formula for GP is as follows [20]: GP = (germinated seeds/total treated seeds) × 100%.
- (3)
- Germination index (GI) measures both the proportion of seeds that have germinated and the speed of germination, which was calculated for each sub-treatment based on the formula: GI = ∑Ni/Ti, where Ni and Ti are defined as above [21]. The GI value will be large when large numbers of seeds germinate in short amounts of time.
For each of the nine Acacia species and the nine sub-treatments per species, four values of MGT, GP, and GI were obtained, one for each 25-seed replicate of the experiment.
2.6. Statistical Analysis
To determine whether germination (sub-) treatments had any effect on MGT or GI in comparison to the control, we performed a one-way analysis of variance (ANOVA) separately for each species, with MGT or GI as the response and treatment as the factor. In each ANOVA, we checked the normal distribution and homoscedasticity assumptions using plots of residuals. If the ANOVA indicated statistically significant differences among the treatment groups, we carried out Dunnett’s post hoc test procedure (p ≤ 0.05) to compare the mean MGT or GI for each treatment group to that of the control group.
Additionally, we performed a chi-squared (χ2) test of homogeneity separately for each species to determine whether the treatments had differing effects on GP. For each species, the 900 seeds of that species were cross-tabulated by treatment received (100 seeds per treatment) and germination status (yes/no), and the resulting nine-by-two contingency table was used in the chi-squared test. In each case, we checked the large sample size assumption required for the chi-squared test by verifying that all expected counts were larger than five. If the chi-squared test found statistically significant differences in GP among the nine treatment groups for a given species, we carried out post hoc tests to compare each treatment’s GP to the control group’s. For this, we used a Holm p-value adjustment for multiple comparisons on eight Fisher’s Exact Tests, each on a two-by-two contingency table summarizing germination statuses for 100 treated seeds and the 100 control seeds. Fisher’s Exact Tests were used (instead of chi-squared tests) because the large sample size assumption (needed for a chi-squared test) was not met for all the two-by-two tables. All statistical analyses were performed using the software JMP Pro 15 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Effect of Pre-Treatments on Breaking Dormancy to Accelerate MGT for Acacia Species
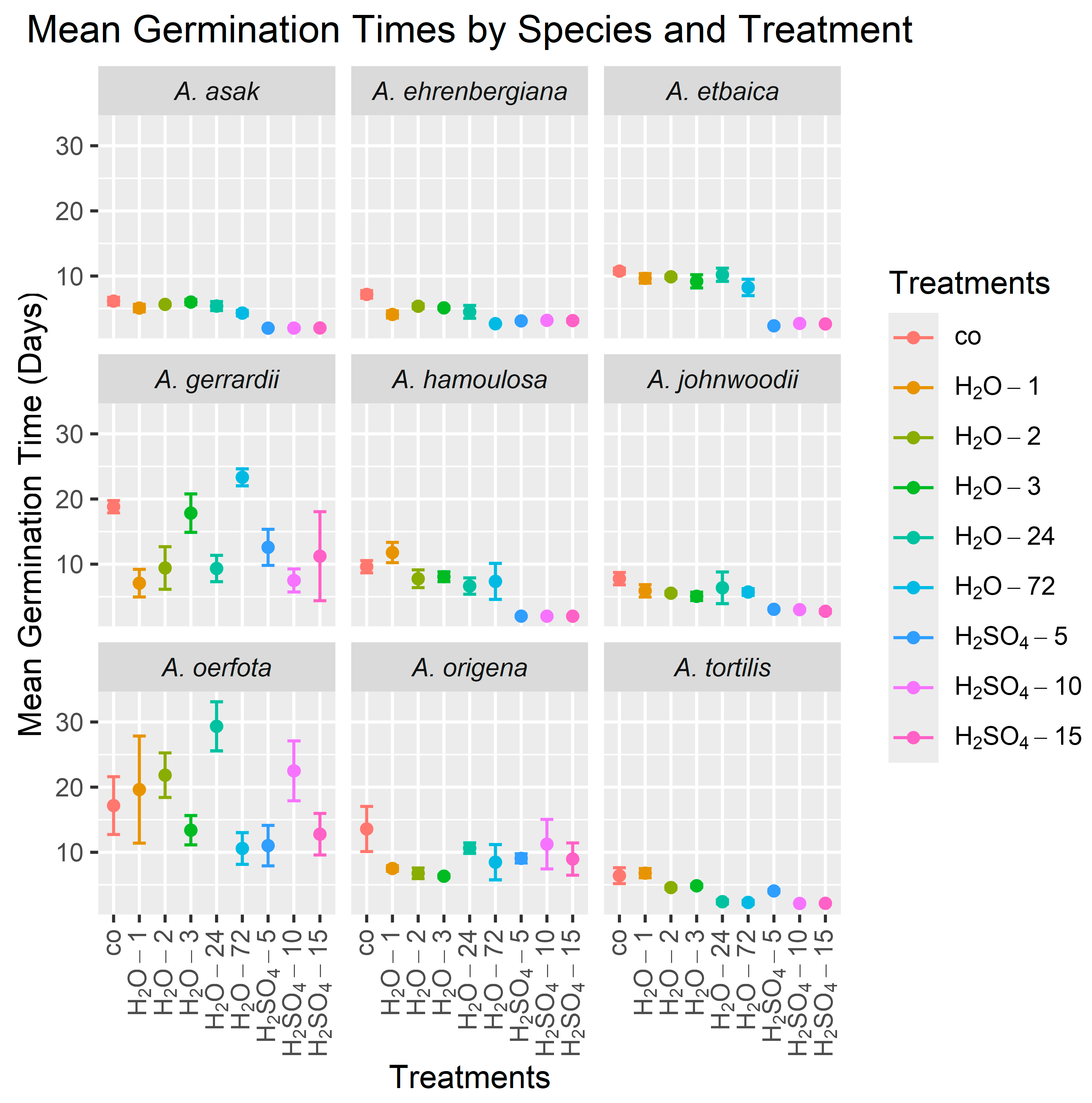
Sulfuric acid treatments exhibited the highest efficacy in accelerating the germination of six out of the nine species studied, although the amount of treatment time required varied among species (Table 3, Figure 2). Specifically, soaking the seeds in sulfuric acid for 5 min (H2SO4-5) was found to be the most effective for decreasing the MGT in A. etbaica to a mean (±standard deviation) over the four replicates of 2.36 ± 0.17 days, compared to a mean of 10.75 ± 0.93 days for the control, and in A. asak to 2.00 ± 0.00 days compared to 6.15 ± 1.17 days for the control. Soaking in sulfuric acid for 10 min (H2SO4-10) was the most effective for decreasing the MGT in A. tortilis to 2.14 ± 0.08 days compared to 6.41 ± 2.44 days for the control. Significant improvements in the MGT for A. hamoulosa were observed following both the 10 min (H2SO4-10) and 15 min (H2SO4-15) treatments, with the MGT reduced to 2.01 ± 0.02 days compared to 9.60 ± 1.88 days for the control. Soaking the seeds in sulfuric acid for 15 min (H2SO4-15) was also the most effective treatment in inducing rapid germination in A. johnwoodii, reducing the MGT to 2.77 ± 0.56 days compared to 7.78 ± 1.89 days for the control. Interestingly, the most effective treatment for A. ehrenbergiana was soaking in tap water for 72 h (H2O-72), resulting in an MGT of 2.68 ± 0.21 days compared to 7.18 ± 1.17 days for the control. There were no significant treatment effects on the MGT for A. gerrardii, A. oerfota, and A. origena.

Table 3.
The mean germination times (MGTs, in days), results of ANOVA (F statistic value) for each species (to decide if the MGTs differ across treatment groups), and results of post hoc Dunnett’s tests comparing each treatment’s MGT to the control’s. Each reported MGT value is actually the mean of the four MGT values (the four replicates). Post hoc tests were not conducted for A.gerrardii, A.oerfota, and A.origena (given the nonsignificant ANOVA). Significance codes: a: p < 0.0001, b: p < 0.001, c: p < 0.01, d: p < 0.05, and none: p ≥ 0.05.
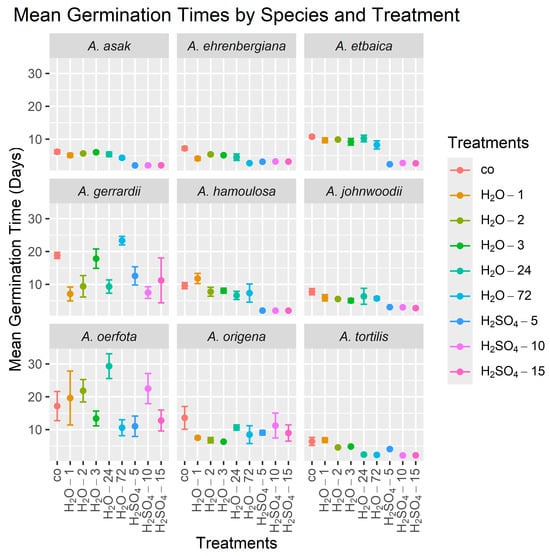
Figure 2.
Mean germination times (MGTs) of seeds from nine Acacia species over 30 days using nine treatments. The height of each point is the mean of the four MGT values (the four replicates), and lines emanating from a point indicate ±1 standard error. In all graphs, the control treatment is on the left side. Treatment abbreviations are as defined in Table 2.
3.2. Efficacy of Various Pre-Treatments for Breaking Dormancy and Increasing GP in Different Acacia Seed Species
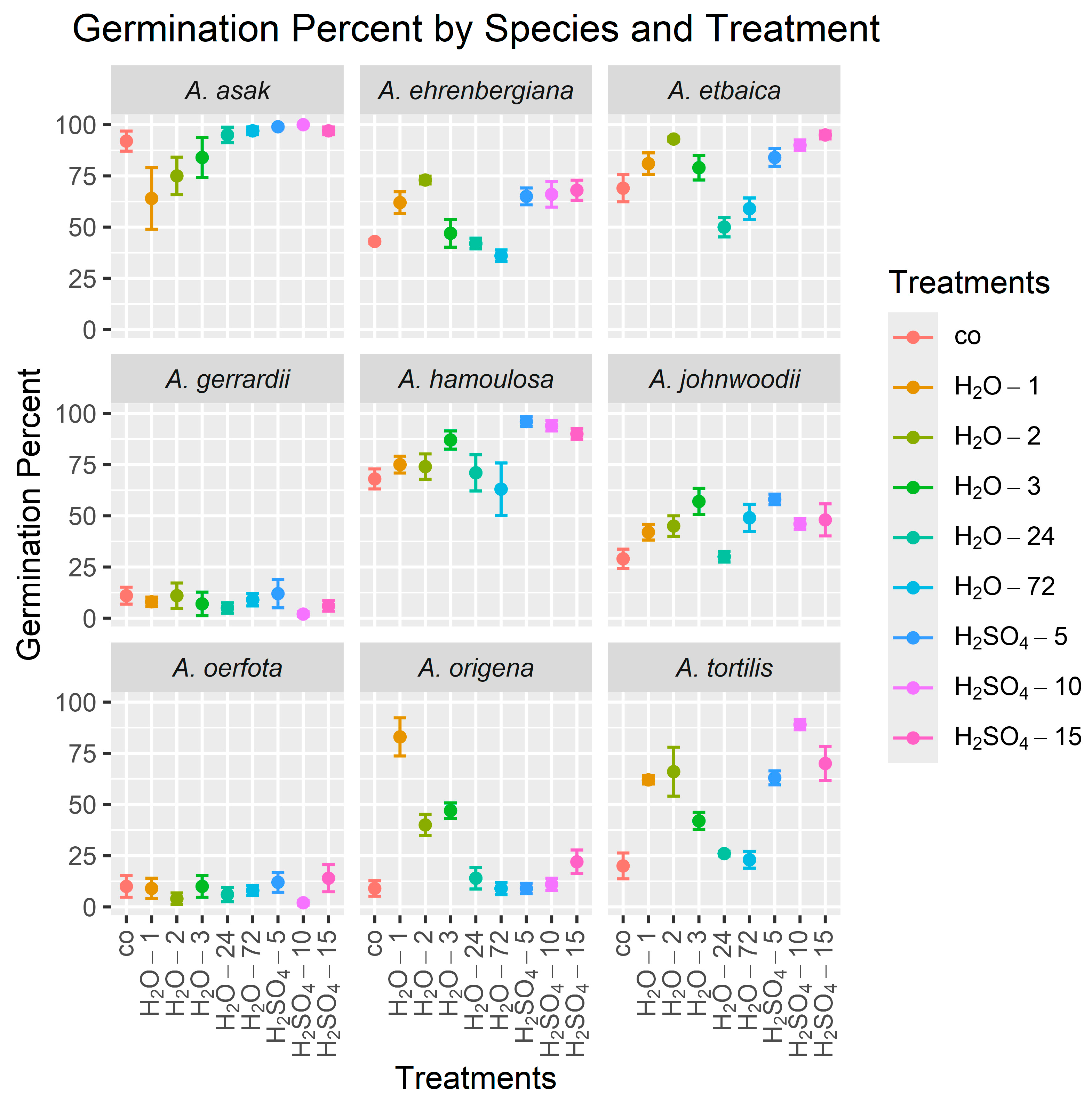
The results showed that sulfuric acid treatments were also highly effective in improving the GP in most species studied compared to untreated control seeds (Table 4, Figure 3). Specifically, soaking seeds in sulfuric acid for 5 min (H2SO4-5) was the most effective treatment for increasing the GP in A. johnwoodii to a mean (±standard deviation) over the four replicates of 58% ± 5.2, compared to a mean of 29% ± 9.5 for the control, and in A. hamoulosa to 96% ± 4.6, compared to 68% ± 9.8 for the control. Additionally, a 10 min sulfuric acid treatment (H2SO4-10) significantly increased the GP in A. asak to 100% ± 0.0, compared to 92% ± 9.8 for the control, and in A. tortilis to 89% ± 5.0, compared to 20% ± 12.6 for the control. The 15 min sulfuric acid treatment (H2SO4-15) was particularly effective in increasing the GP in A. etbaica to 95% ± 3.8, compared to 69% ± 13.2 for the control. Furthermore, soaking seeds in tap water for 2 min (H2O-2) significantly increased the GP in A. ehrenbergiana to 73% ± 3.8, compared to 43% ± 2.0 for the control. In A. origena, the optimal treatment for achieving the highest GP was soaking seeds in hot water for 1 min (H2O-1), which remarkably increased the GP to 83% ± 18.6, compared to 9% ± 7.6 for the control. There were no significant treatment effects on the GP for A. gerrardii and A. oerfota.

Table 4.
Germination percentages (GPs), results of omnibus chi-squared (χ2) test (χ2 statistic value) for each species (to decide if GPs differ across treatment groups), and results of post hoc Fisher’s Exact Tests comparing each treatment’s GP to the control’s GP. Each reported GP value is based on pooling the four replicates’ GP values. The nine χ2 tests are each based on 8 degrees of freedom. For each species, the post hoc p-values used to determine the statistical significance were adjusted for multiple comparisons using the Holm method. Post hoc tests were not conducted for A. gerrardii and A. oerfota given the nonsignificant χ2 test results. Significance codes: a: p < 0.0001, b: p < 0.001, c: p < 0.01, d: p < 0.05, and none: p ≥ 0.05.
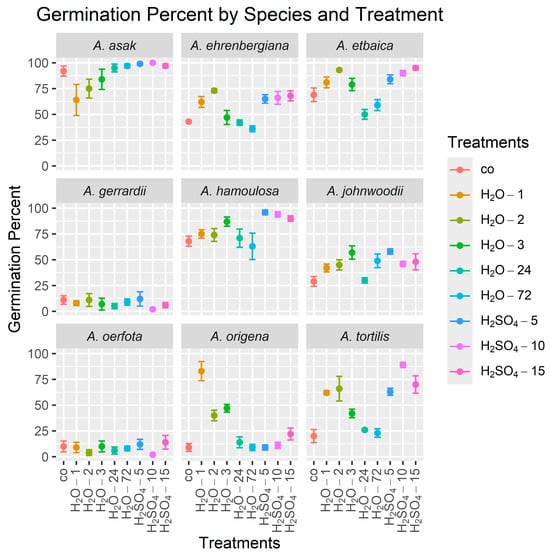
Figure 3.
Mean germination percents (GPs) for seeds from nine Acacia species over 30 days using nine treatments. The height of each point is the mean of the four GP values (the four replicates), and lines emanating from a point indicate ±1 standard error. In all graphs, the control treatment is on the left side. Treatment abbreviations are as defined in Table 2.
3.3. Effect of Pre-Treatments on Breaking Dormancy to Increase GI for Various Acacia Seeds
Sulfuric acid treatments led to a higher success in germination indicators in general than no treatments in most species studied (Table 5, Figure 4). Seeds were effectively treated by soaking in sulfuric acid for 5 min (H2SO4-5) to produce the highest GI for A. hamoulosa at a mean (±standard deviation) over the four replicates of 11.91 ± 0.67, compared to a mean of 4.17 ± 0.90 for the control, and A. johnwoodii at 4.92 ± 0.16, compared to 1.82 ± 1.08 for the control. Additionally, soaking in sulfuric acid for 10 min (H2SO4-10) significantly improved the GI of A. tortilis to 10.60 ± 0.60 compared to 1.06 ± 0.78 for the control and A. asak to 12.46 ± 0.08 compared to 4.85 ± 0.83 for the control. The results demonstrated that 15 min of soaking in sulfuric acid (H2SO4-15) successfully improved the GI in A. ehrenbergiana to 6.05 ± 0.40, compared to 2.34 ± 0.56 for the control, and A. etbaica to 9.68 ± 0.95, compared to 2.60 ± 0.20 for the control. However, soaking the seeds in hot water for a minute (H2O-1) rather than in sulfuric acid increased the germination index of A. origena to 3.14 ± 0.60, compared to 0.22 ± 0.17 for the control. There were no significant treatment effects on the GI for A. gerrardii and A. oerfota.

Table 5.
Germination index (GI), results of ANOVA (F statistic value) for each species (to determine if the GI differs across treatment groups), and results of post hoc Dunnett’s tests comparing each treatment’s GI to the control’s GI. Each reported GI value is actually the mean of the four replicates’ GI values. Post hoc tests were not conducted for A. gerrardii and A. oerfota (due to nonsignificant ANOVA test results). Significance codes: a: p < 0.0001, b: p < 0.001, c: p < 0.01, d: p < 0.05, and none: p ≥ 0.05.
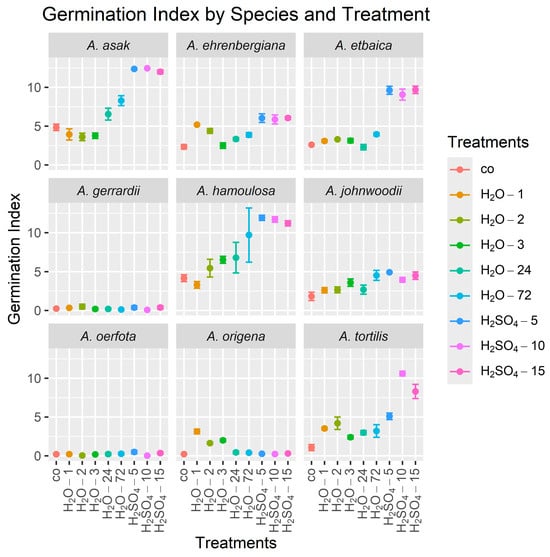
Figure 4.
Mean germination index (GI) values for seeds from nine Acacia species over 30 days using nine treatments. The height of each point is the mean of the four GI values (the four replicates), and lines emanating from a point indicate ±1 standard error. In all graphs, the control treatment is on the left side. Treatment abbreviations are as defined in Table 2.
4. Discussion
Acacia trees are important species for arid land restoration, providing habitats and sustenance for various animal species and contributing to soil stabilization and erosion control [1,2]. In this study, we investigated the effectiveness of different pre-germination treatments to overcome seed dormancy and improve the germination of nine Acacia species native to the Arabian Peninsula. The aim was to identify treatments that could significantly reduce the mean germination time (MGT), enhance the germination percentage (GP), and increase the germination index (GI) for practical uses in ecological restoration.
Our study found that the sulfuric acid treatments (H2SO4), for 5, 10, and 15 min, were particularly effective at reducing the MGT, improving the GP, and thus increasing the GI for some Acacia species in the study area, including A. asak, A. etbaica, A. hamoulosa, and A. johnwoodii. These findings align with the results of [2], who reported that seeds from A. asak, A. etbaica, A. gerradii, and A. origena germinated more effectively and rapidly after being treated with concentrated sulfuric acid (98%) for 5, 10, and 15 min. However, the effectiveness of these treatments varied across species, and our results differ in some respects from those reported in the previous study [2], which involved similar treatments on overlapping species. For example, [2] observed a significant improvement in the GP for A. gerrardii and A. origena following the sulfuric acid treatment, while our study found minimal to no effect of the same treatment on these species. Similarly, [13] confirmed that concentrated sulfuric acid (98%) treatments ranging from 10 to 60 min effectively broke the seed dormancy and promoted germination in L. leucocephala and A. nilotica. [22]. This discrepancy can likely be attributed to differences in the concentration of the sulfuric acid used in the two studies. The former study [2] utilized concentrated sulfuric acid (98%) for their pre-treatment, while our study employed an 80% concentration. Higher acid concentrations are known to produce a more aggressive scarification of the seed coat, potentially leading to greater permeability and improved germination. In contrast, an 80% solution may provide milder scarification, which might not be sufficient to overcome dormancy in certain species like A. gerrardii and A. origena. This highlights the sensitivity of germination responses to specific treatment parameters and underscores the need for the species-specific optimization of pre-treatments. Our findings emphasize that while sulfuric acid treatments are effective for many Acacia species, the choice of the acid concentration and exposure duration must be carefully tailored to achieve optimal results for each species. These differences also illustrate the importance of reporting detailed experimental conditions in germination studies, as slight variations can lead to significantly different outcomes.
One possible explanation for these differences is the variation in seed collection seasons. The timing of the seed maturation and environmental conditions prior to collection can significantly impact the seed coat thickness, dormancy levels, and overall viability. So, the study [2] may have collected seeds following consecutive wet seasons, in which case the seeds might have exhibited lower dormancy, making them more responsive to treatments. Conversely, our seed collection could have followed prolonged dry conditions, potentially leading to the development of harder, more dormant seeds. Another critical factor is the elevation of the seed collection sites. Elevation gradients influence microclimatic conditions, such as temperature and humidity, which can impact seed development and dormancy traits [23]. Seeds collected from higher elevations are often exposed to cooler and more humid conditions, potentially reducing their dormancy levels compared to those from lower elevations, where harsher conditions may promote the development of thicker seed coats [24]. These environmental differences could explain why certain species required more intensive pre-germination treatments in our study but not in the study by [2].
However, our findings also indicate that the effectiveness of sulfuric acid treatments varies across different Acacia species in terms of improving germination outcomes. For instance, in A. asak, the H2SO4-5 treatment reduced the MGT by 4.15 days while increasing the GP by 8%, which is considered insufficient for restoration purposes. Similarly, in A. johnwoodii, although the MGT was reduced by 5.01 days and the GP increased by 19%, the reduction in the germination time is not useful for practical restoration. Conversely, for A. etbaica and A. hamoulosa, the treatments were much more effective, with A. etbaica showing an 8.39-day reduction in the MGT and a 26% increase in the GP, while A. hamoulosa experienced a 7.58-day reduction in the MGT and a 28% increase in the GP. These results highlight that the utility of pre-germination treatments depends on achieving significant improvements in either the germination speed or percentage. For restoration efforts where rapid germination and establishment is critical, even small reductions in the MGT are valuable if accompanied by significant increases in the GP, especially when those increases approach or exceed 90%.
Furthermore, our investigation highlights the species-specific nature of pre-treatment strategies. In line with [2], soaking Acacia seeds in tap water for 72 h (H2O-72) proved effective for promoting germination in A. ehrenbergiana. This extended hydration period likely facilitated the imbibition process, breaking dormancy and enhancing germination in these particular species. Reference [25] demonstrated that immersing Acacia nilotica and Tamarindus indica seeds in hot water and leaving them for 24 to 48 h results in moderately successful germination. Similarly, [23] concluded that pre-sowing treatments, such as hot water immersion (80 °C for 10 min and 100 °C for 12 min), enhance germination under nursery conditions. For instance, the best treatment for A. catechu involved immersing seeds in hot water (80 °C) for 10 min, followed by soaking them in cold water for 24 h [23]. These cost-effective methods, which are widely accessible, offer a practical solution for efficiently producing seedlings of these species. On the other hand, a brief exposure to hot water for 1 min (H2O-1) was found to be optimal for enhancing germination in A. origena, supporting the conclusions drawn by [2] for Acacia species regarding the effectiveness of water-based treatments.
The thermal shock caused by hot water treatments likely stimulated the opening of the water gap and facilitated germination in these species. Similarly, sulfuric acid treatments are believed to work by chemically scarifying the hard seed coat, a common feature in many Acacia species. This scarification process weakens the seed coat, making it more permeable to water, which is essential for the initiation of germination. Concentrated sulfuric acid can soften the seed coat without changing its thickness, effectively allowing for a faster water uptake, thereby accelerating the germination process [26]. Given that A. origena thrives at highland elevations within the study area, it is probable that the seeds of this species rely on the warm summer season to break dormancy and promote successful germination naturally. Our collective experience working in this system suggests that the variation in germination responses among the Acacia species studied can be attributed to differences in the environmental conditions required to open the water gap on the seed coat. Some species typically require direct scarification to overcome dormancy.
While our study provides valuable insights, further investigations are warranted to deepen our understanding of the underlying mechanisms involved in breaking seed dormancy and promoting germination in Acacia species. However, there are avenues for further exploration, as suggested by [4]. Future research that explores the physiological and molecular processes underlying the responses to different pre-treatments would allow for more targeted and refined approaches. Additionally, studies focusing on the long-term effects of these pre-treatments on the seedling growth, survival, and overall fitness would provide a more comprehensive assessment of their practical applications.
5. Conclusions
This study demonstrated the variability of Acacia species’ responses to pre-germination treatments, highlighting the necessity of tailoring methods to individual species. Given the ecological and socio-economic significance of Acacia species, our findings provide useful information for ecological restoration and conservation efforts in the Arabian Peninsula and other arid regions worldwide. By identifying effective pre-treatment methods, practitioners can significantly improve seedling production for habitat restoration and reforestation, aiming to restore the distribution, abundance, and ecological functions of Acacia species in these fragile landscapes, thereby alleviating soil instability and erosion [2,6].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/seeds4020022/s1, Figure S1: Pictures of the nine Acacia tree species in the Al-Baha region used in this study, each picture including by a small inset depicting the characteristic shape of their seeds.
Author Contributions
Conceptualization, A.A.A. and I.M.A.; methodology, A.A.A.; software, A.A.A.; validation, A.A.A., I.M.A. and N.G.; formal analysis, A.A.A.; investigation, A.A.A.; resources, A.A.A.; data curation, A.A.A. and N.G.; writing—original draft preparation, A.A.A.; writing—review and editing, A.A.A., I.M.A. and N.G.; visualization, A.A.A. and N.G.; supervision, I.M.A.; project administration, A.A.A.; funding acquisition, A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was by King Saud University, Deanship of Scientific Research, College of Science Research Center during Mr. Alzandi’s master’s studies.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research, College of Science Research Center, in King Saud University for funding and supporting this research through the initiative of DSR Graduate Students Research Support (GSR). We would like to thank Diana Tomback for her unwavering support and insightful feedback; she has been instrumental in refining the context and scope of this research.
Conflicts of Interest
There are no conflicts of interest for the authors in this research, and the research was funded by the Student Fund at the Research Center at the College of Science at King Saud University during Mr. Alzandi’s master’s studies. There was no interference from the supporting source on the research results.
References
- Maslin, B.R.; McDonald, M. Acacia Search: Evaluation of Acacia as a Woody Crop Option for Southern Australia; Rural Industries Research and Development Corporation: Canberra, Australia, 2004.
- Aref, I.M.; El Atta, H.A.; Shahrani, T.A.; Mohamed, A.I. Effects of Seed Pretreatment and Seed Source on Germination of Five Acacia spp. Afr. J. Biotechnol. 2011, 10, 15901–15910. [Google Scholar] [CrossRef]
- Aref, I.M.; El-Juhany, L.I.; Hegazy, S.S. Comparison of the Growth and Biomass Production of Six Acacia Species in Riyadh, Saudi Arabia after 4 Years of Irrigated Cultivation. J. Arid Environ. 2003, 54, 783–792. [Google Scholar] [CrossRef]
- Raddad, E.Y.; Luukkanen, O. Dryland Rehabilitation with Acacia senegal in the Central Clay Plain of the Sudan: Implications for Ecological Sustainability and Management Interventions. Sudan J. Agric. Res. 2013, 22, 31–48. [Google Scholar]
- Shaltout, K.H.; El-Halawany, E.F.; El-Kady, H.F. Consequences of Protection from Grazing on Diversity and Abundance of the Coastal Lowland Vegetation in Eastern Saudi Arabia. Biodivers. Conserv. 1996, 5, 27–36. [Google Scholar] [CrossRef]
- Mayence, C.E.; Stevens, J.C.; Courtney, P.; Dixon, K.W. Edaphic Constraints on Seed Germination and Emergence of Three Acacia Species for Dryland Restoration in Saudi Arabia. Plant Ecol. 2017, 218, 55–66. [Google Scholar] [CrossRef]
- Fagg, C.; Allison, G. Acacia senegal and the Gum Arabic Trade: Monograph and Annotated Bibliography; Oxford Forestry Institute: Oxford, UK, 2004. [Google Scholar]
- Midgley, S.; Turnbull, J. Domestication and Use of Australian Acacias: Case Studies of Five Important Species. Aust. Syst. Bot. 2003, 16, 89–102. [Google Scholar] [CrossRef]
- Maslin, B.R.; Miller, J.T.; Seigler, D.S. Overview of the Generic Status of Acacia (Leguminosae: Mimosoideae). Aust. Syst. Bot. 2003, 16, 1–18. [Google Scholar] [CrossRef]
- Lorenzo, P.; González, L.; Reigosa, M.J. The Genus Acacia as Invader: The Characteristic Case of Acacia Dealbata Link in EuropeLe Genre Acacia Comme Envahisseur: Caractéristiques Du Cas Acacia Dealbata Link En Europe. Ann. For. Sci. 2010, 67, 101. [Google Scholar] [CrossRef]
- Al-Watban, A. Pollen Morphology of Seven Wild Species of Acacia in Saudi Arabia. Afr. J. Plant Sci. 2013, 7, 602–607. [Google Scholar] [CrossRef]
- Kelly, K.M.; Van Staden, J.; Bell, W.E. Seed Coat Structure and Dormancy. Plant Growth Regul. 1992, 11, 201–209. [Google Scholar] [CrossRef]
- Yousif, M.A.I.; Wang, Y.R.; Dali, C. Seed Dormancy Overcoming and Seed Coat Structure Change in Leucaena leucocephala and Acacia nilotica. For. Sci. Technol. 2020, 16, 18–25. [Google Scholar] [CrossRef]
- Tadros, M.J.; Samarah, N.H.; Alqudah, A.M. Effect of Different Pre-Sowing Seed Treatments on the Germination of Leucaena leucocephala (Lam.) and Acacia farnesiana (L.). New For. 2011, 42, 397–407. [Google Scholar] [CrossRef]
- Scott, S.J.; Jones, R.A.; Williams, W.A. Review of Data Analysis Methods for Seed Germination1. Crop Sci. 1984, 24, 1192–1199. [Google Scholar] [CrossRef]
- Alahmed, A.M.; Naeem, M.; Kheir, S.M.; Sallam, M.F. Ecological Distribution Modeling of Two Malaria Mosquito Vectors Using Geographical Information System in Al-Baha Province, Kingdom of Saudi Arabia. Pak. J. Zool. 2015, 47, 1797–1806. [Google Scholar]
- Al-Aklabi, A.; Al-Khulaidi, A.W.; Hussain, A.; Al-Sagheer, N. Main Vegetation Types and Plant Species Diversity along an Altitudinal Gradient of Al Baha Region, Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Migahid, A.M. Flora of Saudi Arabia, 3rd ed.; University Libraries, King Saud University: Riyadh, Saudi Arabia, 1989; Volume 2, pp. 1–282. [Google Scholar]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. Available online: https://powo.science.kew.org/ (accessed on 22 April 2025).
- Tanaka-Oda, A.; Kenzo, T.; Fukuda, K. Optimal Germination Condition by Sulfuric Acid Pretreatment to Improve Seed Germination of Sabina Vulgaris Ant. J. For. Res. 2009, 14, 251–256. [Google Scholar] [CrossRef]
- Esechie, H.A. Interaction of Salinity and Temperature on the Germination of Sorghum. J. Agron. Crop Sci. 1994, 172, 194–199. [Google Scholar] [CrossRef]
- Oj, O.; Om, D.; Td, I.; Aa, B. Evaluation of Different Pre-Germination Treatments and Growth Media on Seed Emergence and Early Seedling Growth of Gum Arabic (Acacia senegal). J. Agric Earth Environ. Sci. 2024, 1, 1–6. [Google Scholar]
- Das, N. The Effect of Seed Sources Variation and Presowing Treatments on the Seed Germination of Acacia Catechu and Elaeocarpus floribundus Species in Bangladesh. Int. J. For. Res. 2024, 2024, 984194. [Google Scholar] [CrossRef]
- Sugiyama, A.; Friday, J.B.; Giardina, C.P.; Jacobs, D.F. Intraspecific Variation Along an Elevational Gradient Alters Seed Scarification Responses in the Polymorphic Tree Species Acacia Koa. Front. Plant Sci. 2021, 12, 716678. [Google Scholar] [CrossRef]
- Barthelemy, Y.; Abdoulaye, T.; Jonas, K.; Rebecca, Z. Effects of Different Seeds Pretreatments on the Germination of Five Local Trees: Four From The Fabaceae Family and One From the Bombacacea. Eur. Sci. J. ESJ 2021, 17, 89–101. [Google Scholar] [CrossRef]
- Fu, C.; Xing, S.; Zhao, D.; Zang, D.; Wei, Y.; Zhao, L.; Yu, X.; Fu, C.; Xing, S.; Zhao, D.; et al. A Study on the Aseptic Germination Method for Rosa rugose Seeds. Agric. Sci. 2017, 8, 426–434. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).