Abstract
Kentucky bluegrass (KBG) has poor seed establishment in the fall when used as a perennial groundcover in corn production. This study was conducted to investigate the effect of various seed treatments and soil amendments on the establishment of KBG under drought and non-drought conditions, simulated in a growth chamber. The effect of seed treatments, soil amendments, and irrigation frequency on KBG germination and shoot dry weight were measured over 21 days in a controlled environment at 21 °C, 50% RH, and exposure to a constant red light. The treatments were the Hydroloc seed treatment, a lime soil amendment, the Pivot Bio seed treatment, an ammonium nitrate soil amendment, a gibberellic acid seed treatment, osmotic seed priming, and an untreated control. The layout was a randomized complete block design, with two irrigation frequencies (restricted and full irrigation) and four replications (blocks). The irrigation treatments were applied to whole plots and the seed treatments were applied to subplots. The entire experiment was repeated four times. Irrigation affected the germination of all the seed treatments, but the size of the effect depended on the seed treatment applied. The control and Hydroloc treatments did not have significantly different dry shoot weights, while all the other treatments had significantly different dry shoot weights when comparing the irrigation regimes. The Hydroloc treatment significantly outperformed all the other treatments in regard to the restricted and full irrigation regime. These results indicate that the Hydroloc seed treatment improves KBG germination and shoot dry weight in drought and non-drought conditions, promoting KBG establishment in a wide range of soil moisture conditions.
1. Introduction
Over the past two centuries, the Midwest landscape in the USA has changed dramatically from predominantly tallgrass prairie to highly productive corn (Zea mays L.) and soybean (Glycine max L.) fields. This farming system has increased soil erosion and water quality issues in regard to the landscape, due to chemical leachates and winter fallow periods [1]. According to the 2017 National Resources Inventory Report, Iowa had the highest soil erosion rate of any other state at 5.8 tons of soil per acre [2]. Over the years, there have been attempts to mitigate these problems through the use of winter annual cover crops; however, the uptake of this system by farmers has been sparse, due to cover crop activities coinciding with cash crop (corn/soybean) sowing and harvesting [3].
Perennial groundcover (PGC) may provide a potential solution to this problem, wherein perennial grasses like kentucky bluegrass (KBG) (Poa pratensis L.) and perennial ryegrass (PRG) (Lolium perenne L.) are grown alongside cash crops to reduce soil erosion, avoid spring nitrogen loss, and protect the natural ecosystem [4,5]. PGC is planted once and, when managed correctly, the PGC should last many years. Thus, the PGC system has fewer labor requirements compared with an annual cover crop system. As a cool-season grass, KBG has ideal characteristics for use as a PGC. Some of these beneficial characteristics are a low growth habit, rhizome development, summer dormancy, shade tolerance, and good traffic tolerance [6]. When managed correctly, KBG can increase carbon sequestration and help retain soil organic matter [7,8,9]. These characteristics are the reason why KBG is commonly selected as the species of choice for sports turf, lawns, and recreational areas. Cool-season grasses like KBG have active growth periods from March to early May and late August to early November [10]. The periods of inactive growth (or dormancy) coincide with when the cash crop grows most actively.
Despite these favorable characteristics, the management of KBG in a turfgrass system differs significantly from its management in a PGC system due to the use of irrigation for turfgrass establishment. Early observations of the PGC system have shown that a lack of soil moisture in the fall, along with slow KBG germination rates, makes it challenging to establish this species as a PGC [6,11,12]. Seed germination-enhancing treatments, such as seed priming, seed coating, and the use of growth-regulating hormones, have been applied for many years in the turf industry to break seed dormancy and enhance germination rates [13,14,15]. Seed coatings create an ideal micro-environment for germination around the seed and may include macro- and micronutrients, which improve germination in poor seed-bed conditions [16]. Hydrophilic polymer coatings offer limited benefits for larger seeded cool-season grasses, but studies have shown positive results for this coating when applied to smaller seeds, such as KBG [17,18]. Seed treatments are reported to improve grass seed establishment, but have not been tested under drought conditions. There is a need for further identification of treatments that improve seed germination in drought conditions. A growth chamber must be used to determine the efficacy of these seed treatments and soil amendments under both water-restricted and unrestricted growing conditions. A growth chamber enables controlled testing of these seed treatments in conditions where soil moisture is the only variable factor.
The goal of this experiment is to identify a seed treatment or soil amendment capable of expediting seed germination and promoting seedling vigor. To date, much of the research into solutions for improving KBG establishment focuses on sports turf and intensively managed lawns. The application of these solutions to a PGC system could overcome the problem of poor KBG germination due to low soil moisture in the fall. Better established PGC would enhance soil health, and reduce soil erosion and nutrient leaching into waterways, allowing farmers to contribute towards achieving the UN sustainable development goals related to taking climate action, preserving life on land, and improving water quality [19]. Furthermore, this approach would remove the need to resow the PGC in year 2, resulting in reduced annual labor input and increased profit for row-crop farmers [20]. The objective of this study is to evaluate the effectiveness of different seed treatments and soil amendments in regard to improving seed germination and establishment of KBG under drought and non-drought conditions. The results of this research may provide insights that could lead to the development of a more reliable PGC for corn farmers in the Midwest.
2. Materials and Methods
A growth chamber experiment was conducted at the Iowa State University Seed Science Center to identify different seed treatments that would significantly improve the establishment of kentucky bluegrass seed. The KBG variety used was ’Mercury’, purchased from Pawnee Buttes Seed (Greeley, CO, USA). The experiment consisted of the sowing of KBG seeds using six different treatments or soil amendments and an untreated control. The seeds were planted in individual pots filled with Sungro soil (Agawam, MA, USA), and the pots were treated with two different irrigation regimes. The restricted irrigation regime simulated field establishment in a drought scenario where water was limited, while the unrestricted irrigation regime did not have water as a limiting factor. The experiment layout was a randomized complete block design in regard to the restricted and unrestricted irrigation regimes. The irrigation regime was organized in 2 whole plots due to the constraints on applying the irrigation in the growth chamber. There were seven different seed treatments in each block. Each treatment was replicated four times within each of the four blocks. The entire experiment was repeated over four periods, using the same growth chamber. The treatments used in this experiment were: the Pivot Bio nitrogen-fixing microbe seed treatment, a hydrophilic seed coating (Hydroloc), a gibberellic acid (GA3) seed treatment, osmotic priming of the seed in water (H2O), lime applied to the soil with an untreated seed, ammonium nitrate applied to the soil with an untreated seed, and a control involving an untreated seed. These treatments (T1–T7) and the layout of the experiment can be seen below in Figure 1.
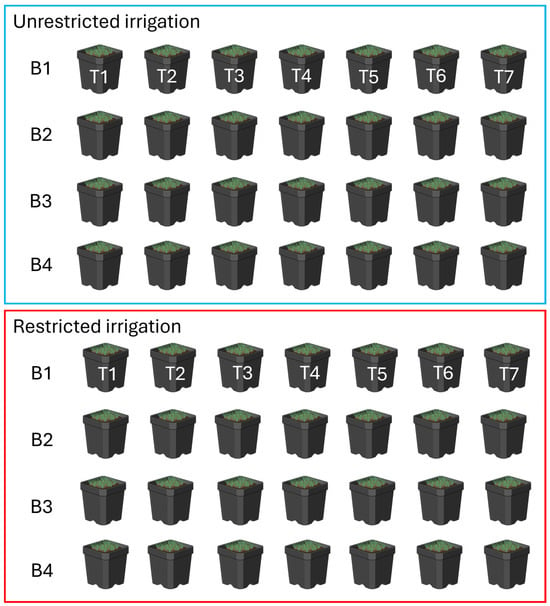
Figure 1.
Layout of the growth chamber, which was a randomized complete design in 2 whole plots on either side of the growth chamber, comparing restricted (red box) and unrestricted (blue box) irrigation regimes. The seven treatments (T), namely Hydroloc, control, ammonium, lime, GA3, Pivot, and H2O are repeated in 4 blocks (B), within each irrigation regime.
Each experiment replication lasted 21 days, and the growth chamber was maintained at a constant temperature of 21 °C and a relative humidity of 50% (±5%). The humidity and temperature were regulated using a Quest dual humidifier 110 (Madison, WI, USA). The humidity was monitored constantly using a WatchDog A110 data logger from Spectrum Technologies (Aurora, IL, USA). The light chosen for the experiment was red light (660 nm), as it promotes the unfolding of leaves [21]. The light in the growth chamber was left on constantly. Before the experiment, the seeds were counted using a vibratory counter from IMD Corp (San Antonio, TX, USA). A preliminary seed count of 100 was conducted to check the accuracy of the vibratory seed counter. It was set at a high sensitivity and a low speed to ensure the seeds were accurately counted. Sungro potting soil was used, which consisted of 45–55% Canadian Sphagnum peat moss, composted bark, perlite, dolomite lime, and silicon dioxide (SiO2) from calcium silicate. The soil was packed into a 10 cm × 10 cm × 9 cm sized pot. Before sowing, 150 g of soil was weighed into each pot, and the soil was compressed. After testing the seeds, 50 seeds were placed into each pot. The seeds were spread equally on the soil and pressed to ensure good seed–soil contact. A thin layer of five grams of soil was scattered on the seeds in all the pots.
2.1. Restricted Irrigation Regime
A preliminary experiment was designed to estimate the volume of water and the irrigation frequency required to maintain a soil moisture content of between 70% and 30% of the water-holding capacity. This was achieved by first calculating the moisture content of the soil medium using the method described in the AOSA Seed Vigor Testing Handbook [22]. The soil medium was weighed, and 150 g of soil was placed into a pot and compacted. The compacted soil was then placed in an oven at 105 °C for 24 h to obtain the dry weight of the soil. This was repeated a second time, and the average moisture percentage of the soil was calculated. The dry weight of the soil was 66.5 g. This meant the soil had a moisture content of 55.7%.
Moisture in medium = (moisture loss/dry weight of medium) × 100
The soil’s water-holding capacity was calculated by flooding the soil with water, placing the pot above a mesh, and waiting until the water stopped draining from the pot. The water-holding capacity was calculated as the moisture loss divided by the dry weight of the medium, expressed as a percentage. This was repeated three times. At this point, all the macropores in the medium have drained, and only the micropores retain water. This can also be called the field capacity, wherein the soil’s maximum water-holding capacity has been reached [23]. The water-holding capacity of different textures of soil can vary considerably. The next step was to weigh the pot to determine the water-holding capacity of 150 g of compacted soil, which was 258 mL. This number was multiplied by 70% and the original moisture content of the soil was subtracted to calculate the amount of irrigation necessary to achieve 70% of the water-holding capacity.
The water evaporation rate from the soil was determined by placing the soil in the growth chamber and irrigating to 70% of the water-holding capacity, which was carried out by adding 100 mL of water to the soil. The container was weighed daily to measure the soil’s moisture evaporation rate. This was repeated in three pots. This value provided an estimation of the number of days necessary for the soil to reach 30% of its water-holding capacity. Under normal conditions, the soil typically took 4 days to reach a water-holding capacity of 30% of field capacity, which equated to a weight of 163 g for the pot and the soil.
After these preliminary results were obtained, it was determined that the pots needed to be weighed every 4 days throughout the experiment to accurately estimate the volume and frequency of water required for the restricted irrigation regime. If the pots reached the desired weight of 163 g after 4 days, 100 mL of DI (de-ionized) water was added. A plastic tray with perforated holes was used to slow the watering rate, so that the seeds were not displaced in the soil. The soil was irrigated before sowing the seeds to prevent them from sinking deeper into the soil, affecting the germination.
Maintaining the correct soil moisture was a challenge during the summer as the relative humidity in the growth chamber increased. This resulted in abandoning one repetition of the experiment due to the high moisture content in the growth chamber. This problem was overcome by installing a second dehumidifier and a separate monitor to control the relative humidity in the growth chamber. These solutions, along with weighing the pots before irrigation, resulted in a more accurate estimation of the soil water content throughout the experiment.
2.2. Unrestricted Irrigation Regime
The unrestricted irrigation regime consisted of each pot having a wick cord connecting the soil to a DI water source below. The DI water was transported via a wick from a plastic tray that held the water to the top of the soil and, thus, was imbibed by the seed. Each pot had an individual tray to hold the DI water, and a wire rack separated the pot and the water tray to ensure the soil did not become saturated, thus creating anaerobic conditions and preventing seed germination. Preliminary tests were carried out to determine the rate of water uptake by the wick that was connected to the soil and were repeated three times. The full apparatus (pot, soil, and wick) was weighed each day and subtracted from the soil dry weight, wick, and pot weight in order to calculate the weight of the water in the soil. The percentage of the water-holding capacity was then calculated for each day (Figure 2). We found by weighing the pots each day that the soil water content increased up to day six, when it stabilized at 86–88% of the water-holding capacity. This allowed for a controlled irrigation regime to be applied. The results of this pre-experiment test are presented below (Figure 2).
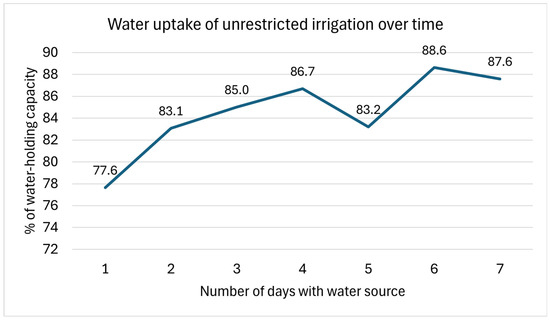
Figure 2.
Results of pre-experiment test in regard to the uptake of water by the soil via a wick connected to a water source. The Y axis is the percentage of the water-holding capacity, while the X axis is the number of days when the wick was connecting the soil to a water source. The test showed that the full irrigation regime plateaued at a water-holding capacity of ~88% after 6 days.
2.3. Seed Germination Testing
The seed label stated that the seed lot had a germination rate of 76% and a purity of 98.31%, with 1.69% inert matter. The seeds were re-tested before commencing the experiment. The germination and seed purity tests were carried out according to the AOSA standards [22]. To determine the seed purity, a sample was taken randomly from the bag of seeds, and the seed was separated from the inert matter using a HMC 67 seed blower (Hoffman Manufacturing, Corvallis, OR, USA), calibrated according to the AOSA standards. The percentage of pure seed and inert matter in the sample was 99.45% pure seed and 0.55% inert matter. The seeds were planted on moist blotter paper, inside a transparent plexiglass box (Hoffman Manufacturing, Corvallis, OR, USA). The blotter paper was moistened with KNO3, which is used to break KBG seed dormancy and promote germination. We counted one hundred pure seeds and placed them equidistantly apart on top of the blotter paper inside the transparent box, and this was repeated 4 times. All the boxes were placed in a growth chamber, with an alternating temperature of 15 °C for 16 h to 25 °C for eight hours. The growth chamber from Hoffman Manufacturing (Corvallis, OR, USA) was subject to a constant cool white LED (light-emitting diode), with an illuminance of 807–1346 lux.
The KBG sample was counted after ten, seventeen, and twenty-four days. Only ‘normal’ (germinated) seedlings, according to the AOSA rules, were counted and removed in the first count. In the final count, the seedlings were deemed ‘normal’, ‘abnormal’, ‘dormant’, ‘hard’, or ‘dead’, according to the AOSA rules [22]. A normal seedling is described as having “no defects or only slight defects that will not impair the continued development of the seedling or plant when grown in soil under favorable conditions”. Seedlings are classified as abnormal if they have “defects that will prevent them from developing into mature plants when grown in favorable soil conditions”. A dormant seed is classified as a “viable seed, other than a hard seed that fails to germinate when provided with the specified germination conditions”. Tests, such as the tetrazolium (TZ) test, can be used to tell whether an ungerminated seed is viable or not. If the seed is dormant, it must be reported in the test report, along with the percentage germination. A dead seed is defined as having not produced any part of a seedling by the end of the test period and is not a hard or dormant seed. After the test had ceased, the germination percentage for the KBG seed lot of the variety ‘Mercury’ was 81.7%.
2.4. Seed Coatings/Treatments
The hydrophilic coating was applied by Summit Seeds Coatings (Caldwell, ID, USA). This treatment is called Hydroloc QS plus (Summit Seed Coatings). This coating was applied at a ratio of 1:1, seed/coating. The coating consists of finely ground limestone, which helps to neutralize the pH around the seed [24]. It also has Fe, Zn, and Mg micronutrients and a super hydrating polymer called Stock‘O’Sorb, which can hold up to 10 times its weight in water around the seed. The coated seed was sowed uniformly in the soil for both irrigation regimes.
Lime treatment was applied as a comparison treatment to the Hydroloc coating. The soil was dusted with lime, which contained 22% calcium (Ca) and 12% magnesium (Mg). The lime was crushed and dusted at a rate of 3.83 g/pot on each pot. This is the equivalent of a field application rate of 3833.3 g/ha. This rate was decided by calculating the amount of lime applied to the Hydroloc seed surface. The untreated seed was placed above the crushed lime and pressed into the soil.
The Pivot Bio (Ames, IA, USA) nitrogen-fixing microbe seed treatment, called Proven 40, was applied to the seed before the experiment. This treatment was applied with an extender/microbe powder ratio of 32:3. The extender adheres the microbes to the surface of the KBG seed. To make the Proven 40 solution, 0.86 g of microbe powder was measured using lab weighing scales. A volume of 9.65 mL of the extender liquid and microbe powder were combined in a test tube and shaken vigorously for 1 min to ensure the powder was dissolved in the solution. To treat the seed, 7 μL of the Proven 40 solution was pipetted into test tubes and then vortexed for 3 min to thoroughly coat the seed. The test tube was left uncapped for 5 h to allow the seed to dry. Prior to planting the seeds in the pots, a steel spatula was used to separate any seeds that had stuck together during the treatment process. The treated seeds were placed into the soil using tweezers and pressed to ensure good seed-soil contact.
Ammonium nitrate (NH4NO3) is made up of ammonia (NH3) and nitric acid (HNO3). It consists of 34% nitrogen, while the remaining chemical composition is made up of hydrogen and oxygen. Ammonium nitrate is a commonly used nitrogen fertilizer source in agriculture, because it is readily available to plants in the soil. In this experiment, ammonium nitrate was used as a comparison treatment in regard to the Pivot Bio treatment. Pivot Bio claims that their product can fix N at a 45 kg/ha rate. Hence, the equivalent rate of ammonium N was applied to the soil’s surface in the pot. The ammonium nitrate granules were weighed to a value of 0.132 g/pot and ground in a pestle and mortar. It was then dusted onto the soil before the seeds were sown and pressed into the soil.
Another treatment involved priming the seed in a GA3 solution before commencing the experiment. The GA3 solution had a concentration of 400 mg L−1 [25]. The GA3 solution consisted of 125 mL of DI water, with 0.05 g of GA3. This solution was stirred for 3 min with a stir plate. Each seed sample of 50 seeds was placed in a test tube with 1 mL of GA3 solution and it was ensured that the seed was fully submerged. The caps were put on the test tubes to ensure that none of the solution evaporated. The seed was soaked for 24 h at room temperature (21 °C). The seeds in the GA3 solution were removed from the test tubes after 24 h and washed with DI water in a fine sieve to clean off any remaining GA3 from the seed surface. They were then placed, using tweezers, into the soil and pressed to ensure good seed-soil contact.
A comparison to the GA3 treatment was the treatment involving osmotically priming seeds in DI water (H2O treatment). To osmotically prime the KBG seed, the untreated seed was placed in a small tube with 1 mL of DI water, measured with a 1000 µL pipette. The caps were put on the test tubes to ensure no water evaporated. The seed was soaked for 24 h in a chamber at 21 °C [13]. The seed was removed from the test tube and washed with a fine sieve before being planted in the soil. The control treatment consisted of planting untreated KBG seeds into the first inch of the soil and pressing well.
2.5. Measurements
The germination speed was measured by counting the number of germinated seedlings on days 7 and 8 (first signs of germination) and every second day after that. The experiment was terminated on day 21, following the AOSA standards for KBG seed germination tests.
The results measured at the end of the experiment were the seed germination rate and shoot dry weight. The germination percentage was measured according to the number of germinated seeds/number of seeds planted (total) × 100. The germination of a seed was determined according to the AOSA guidelines [22]. A normal grass seedling must have a shoot that extends more than halfway up into the coleoptile, that is not split, shredded, or spindly. The primary roots of KBG must be 0.16 cm or more in length to be classified as a normal seedling. The shoot dry weight of each pot was measured to compare the treatment’s effect on seed vigor. The shoots were harvested by removing the seedling from the pot with tweezers and cutting the seedling at the crown to remove the shoot. The shoots for each pot were placed in an aluminum tray and labeled according to their pot number. The aluminum trays were weighed before starting the experiment and placed in an oven at 80 °C for 18 h and then weighed again. The weight of the tray + shoots after drying was subtracted from the weight of the tray to get the dry weight of the shoots only.
2.6. Statistical Analysis
All the data were analyzed using SAS version 9.4 software [26]. All the variables were fixed except the blocks, period, and their interactions with the fixed effects. The blocks were nested within the periods. The GLM procedure was used to calculate the analysis of variance (ANOVA), comparing the irrigation regime and treatment effect on the number of germinated seeds, percentage germination, and dry weight of the germinated plants. A test statement was included to assess the main effect of irrigation, using the block-by-irrigation interaction as the error term. The assumptions of normality, homogeneity of variances, and independence of residuals were verified prior to the statistical analysis to ensure the validity of the applied tests. The least square means (LSMEANS) function was used to analyze the effect of the treatment-by-irrigation interaction, which was further examined using the slice function. The mixed procedure in SAS was used to further investigate each treatment’s dry weight and percentage germination under each irrigation regime. The LSMEANS function was performed with pairwise differences (PDIFF) for the treatment-by-irrigation interaction. The mixed procedure was used to analyze the standard error for each treatment under the two irrigation regimes. All tests of significance were made at α = 0.05 unless otherwise noted. The model used was as follows:
Yjklm = µ + Pj + B(P)jk + Il + B(P) × Ijkl + Tm + B(P) × Tjkm + I × Tlm + Ƹjklm
- µ = Germination mean;
- Pj = Effect of period on germination;
- B(P)jk = Effect of block in period on germination;
- Il = Effect of irrigation on germination;
- Tm = Effect of treatment on germination;
- Ƹjklm = Error term.
The speed of germination was analyzed using the mixed procedure in SAS. All the factors except the blocks and periods were fixed, and the blocks were nested within a period. The model investigated the effect of the treatment, irrigation regime, and the days since planting on the number of seeds germinated. The least square means function was applied to compare the treatment and irrigation effects over time. Slicing options were used to investigate the interaction effects at specific levels of treatment-by-day and irrigation-by-day. This allowed for a detailed comparison of the interactions between these factors across different time points. Repeated measures were tested using an unstructured model. Errors were determined to be independent and random.
3. Results
3.1. Speed of Germination
The data used in the analysis, presented in Figure 3, Figure 4 and Figure 5, were an average from four repetitions of the growth chamber experiment (n = 4), carried out across 6 months.
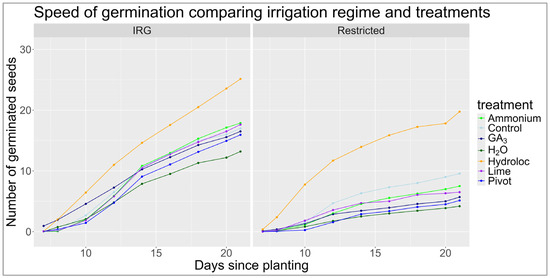
Figure 3.
Speed of germination of KBG seeds with different seed treatments/soil amendments and under two different irrigation regimes between 7–21 days. The X axis is the number of days since planting, starting on day 7. Seed germination was recorded on day seven, eight, and every second day after that up to day 21, when the experiment was terminated. The Y axis is the number of germinated seeds. The seed treatments are compared using different colored lines.
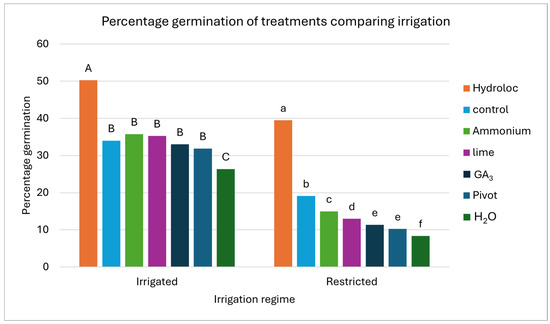
Figure 4.
The percentage seed germination for each treatment under two irrigation regimes. The percentage germination of the 50 seeds planted after 21 days (Y-axis) and the irrigation regime (X-axis) are represented in the graph. The treatments applied are represented by different colored bars. The treatments are Hydroloc, control, ammonium, lime, gibberellic acid (GA3), Pivot Bio (Pivot), and water immersion (H2O). Different letters above each bar represent differences in terms of least square means (LSMEANS; p < 0.05), indicating whether the means of the treatments are significantly different within the irrigation regime. The capital letters are used to represent the full irrigation regime, and the lower case letters are used to represent the restricted irrigation regime.
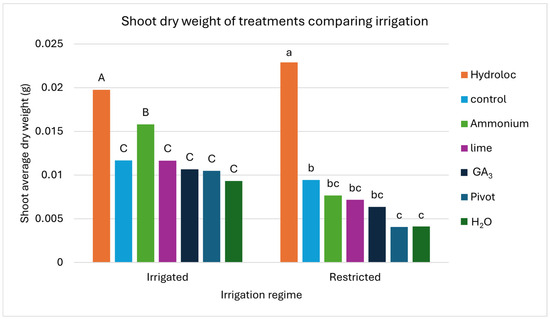
Figure 5.
The shoot dry weight of each treatment comparing irrigation regime. The shoot dry weight in grams of the emerged seedlings after 21 days (Y-axis) and the irrigation regime (X-axis) are presented. The treatments applied are represented by different colored bars. The letters above each bar compare the LSMEANS for each treatment within an irrigation regime. Capital letters are used for the full irrigation regime, and non-capitalized ones are used for the restricted irrigation regime.
Seed germination under full irrigation was better (p < 0.0001) than that under restricted irrigation over 21 days. There were no significant seed germination differences between the two irrigation factors for the initial 7 and 8 days (p > 0.05). Likewise, no significant seed germination differences were observed among the seed treatments for the same period.
After the first week, there was a significant difference in the seed germination between the two irrigation regimes at days 10 to 21 (p < 0.05). The three-way interaction of the irrigation, seed treatment, and days after planting was not significant, but the two-way interaction between the irrigation regime and days after planting was significant. The effect of the repeated measures was tested to ensure that the residuals from the repeated measures did not affect the results. The results were not affected when tested using SAS.
Figure 3 shows each treatment’s germination rate over 21 days. Eight days after planting, the treatment with the most germinated seeds was the Hydroloc treatment, with an average of two seedlings for the full irrigation and 2.38 seedlings for the restricted irrigation regimes. The seeds treated with GA3 that had been subject to the full irrigation regime had an average germination rate of 1.93 seedlings, while the restricted irrigation regime had an average of 0.37 emerged seedlings. Fourteen days after planting, the treatments with a higher number of germinated seeds for the irrigated regime were the Hydroloc (14.6 seedlings), ammonia (10.8 seedlings), and control (10.6 seedlings) treatments. Seed germination under restricted irrigation showed similar trends to the full irrigation regime. The Hydroloc treatment (13.9 seedlings) outperformed the control (6.3 seedlings) and lime (4.7 seedlings) treatments. These results show that the hydrophilic polymer treatment, Hydroloc, had the fastest seed germination and the highest germination percentage on each day after planting, measured throughout the experiment. Hydroloc also had the lowest seed germination differences recorded between the full and restricted irrigation regimes, showing an average difference of 0.7 seedlings after 14 days. GA3 had the largest seed germination differences between the irrigation regimes. After 14 days, the average seed germination differences were 6.9 seedlings greater for the full irrigation regime for the GA3 treatment, followed by ammonia and Pivot Bio, both with a difference of 6.2 seedlings.
3.2. Percentage Seed Germination
The analysis of the final percentage of seed germination (Table 1) after 21 days showed that the period, irrigation regime, and seed treatment were all significant (p < 0.0001). The block effect was not significant (p = 0.7215), and neither was the block-by-irrigation interaction (p = 0.6883). The treatment-by-irrigation interaction was not significant (p = 0.1469).

Table 1.
Analysis of variance (ANOVA) for percentage seed germination after 21 days of growing (DF = degrees of freedom). For Irrigation x Treatment, “x” represents the interaction of these two factors.
Figure 4 is a graphic representation of the percentage germination under each irrigation regime. Table S1 in the Supplementary Materials gives the corresponding numerical values of the data in Figure 4. The different letters above the columns represent the means that are different (LSMEANS). The Hydroloc treatment significantly outperformed all the other treatments under full and restricted irrigation. The H2O treatment performed significantly poorer than the control for full irrigation, while all the other treatments performed similar to the control. Under restricted irrigation, all the treatments except the Hydroloc treatment had a significantly lower percentage germination than the control.
The percentage seed germination for each treatment had a standard error of 3.35%. The treatment with the highest germination percentage after 21 days for the irrigated regime was the Hydroloc treatment (50.25%), followed by the ammonium (35.7%), lime (35.25%), control (34%), GA3 (33%), and Pivot Bio (31.9%) treatments, while the lowest value was observed for the H2O treatment (26.4%). The Hydroloc treatment also had the highest germination percentage for the restricted irrigation regime (39.5%), outperforming the control (19.13%) and ammonium (15%), lime (13%), GA3 (11.4%), Pivot (10.2%), and H2O (8.4%) treatments. The Hydroloc treatment had the smallest difference in the percentage germination of 10.8% when comparing the irrigation regimes, but this was still a significant difference (p = 0.0019). The control had the second smallest difference between the irrigation regimes of 14.9%, while all the other treatments had a difference of 18% or greater (p < 0.0001).
3.3. Shoot Dry Weight
The results for the shoot dry weight (Table 2) after 21 days showed that periods, irrigation regime, and treatment were all significant (p < 0.0001). The treatment-by-irrigation interaction was significant (p = 0.0023). The block effect was not significant (p = 0.3168).

Table 2.
Analyses of variance (ANOVA) for shoot dry weight after 21 days of growing (DF = degrees of freedom). For Irrigation x Treatment, “x” represents the interaction of these two factors.
Figure 5 is a graphic representation of the shoot dry weight of treatments under each irrigation regime after 21 days of growing. Table S2 in the Supplementary Materials gives the corresponding numerical values of the data in Figure 5. The Hydroloc treatment significantly outperformed all the other treatments, with the highest dry weight for both the irrigated and restricted regimes. The ammonium treatment had the second highest shoot dry weight in regard to the full irrigation regime, followed by the control, lime, GA3, Pivot, and H2O treatments. The control treatment had the second highest shoot dry weight in regard to the restricted irrigation regime, followed by the ammonium, lime, GA3, Pivot, and H2O treatments. The letters above the bars (Figure 5) representing the LSMEANS show how the irrigated regime, the Hydroloc, and the ammonium treatments performed significantly better than the control, with the other treatments having similar dry weights to the control. Regarding the restricted irrigation regime, only the Hydroloc treatment performed significantly better than the control, with the Pivot and H2O treatments performing significantly worse than the control and the remaining treatments having similar shoot dry weights to the control.
When comparing the restricted and full irrigation regimes, the shoot dry weight of the Hydroloc treatment with restricted irrigation was similar to the Hydroloc treatment subject to full irrigation (p = 0.1047). The control treatment also had similar shoot dry weights under both irrigation regimes (p = 0.2476). The irrigation regime significantly differed in regard to the ammonium, lime, GA3, Pivot, and H2O (p < 0.05) treatments, with the full irrigation regime having higher dry weights in each of these treatments.
4. Discussion
All the treatments subject to the restricted irrigation regime had a significantly lower germination percentage than the full irrigation regime. These results indicate that water was a limiting factor for KBG germination in this experiment. However, many other factors influence the speed of germination of KBG. Folck (2023) tested the seedling vigor of 21 KBG cultivars in a growth chamber and reported large variances in germination among the cultivars [27]. Their study also showed that there was no relationship between the thousand seed weight (TSW) and germination time, which contradicts the claims by other authors that a heavier seed reduces the germination time of cool-season perennials, including KBG [28]. Thus, many difficult-to-control factors may be the reason for slow germination of KBG, and this reinforces the need for solutions, such as seed treatments, to improve the germination of KBG.
4.1. Hydroloc and Lime
This study found that a hydrophilic and lime seed coating (Hydroloc) increased the speed of germination, percentage seed germination, and shoot dry weight of KBG seeds after 21 days of growing in restricted and unrestricted soil moisture conditions. This shows that the Hydroloc coating creates an ideal micro-environment around the seed that improves seedling vigor and germination, especially in situations where the seed-bed conditions would result in poor germination. Hydrophilic polymer applications previously had limited benefits in regard to larger cool-season grass seeds, such as tall fescue, compared to their application on smaller seeds, such as those of KBG [18]. In agreement with the results from this study, the authors concluded that the benefits of hydrophilic polymers are more pronounced when applied to small seeds planted in sandy soils, where drought is more prevalent. Leinauer (2010) similarly found that applying a hydrophilic polymer to KBG seeds could improve their establishment when the planting density or water is a limiting factor. The coating also protected the seed from winter kill and desiccation stress, both important characteristics in a late fall-planted PGC scenario [17]. The current study observed a difference between the Hydroloc treatments under different irrigation regimes, with the unrestricted irrigation regime having a significantly higher germination percentage. Interestingly, this difference was not seen in the shoot dry weight measurements, with the Hydroloc treatment having a similar shoot dry weight under each irrigation regime. Increasing the shoot dry weight or biomass production of perennial cover crops is important to the provision of natural ecosystem services [29]. The price of applying the Hydroloc treatment to grass seed is USD 0.26 per kg of seed treated. This is a cost-effective solution for increasing PGC establishment.
Another reason for the significantly better performance of the Hydroloc treatment may be attributed to the lime and micronutrient constituents of the coating. Lime neutralizes the pH around the seed, allowing for better uptake of nutrients [24]. Previous studies show that lime soil amendment leads to greater seedling survival, as a result of increasing the soil pH in the top soil [30]. An earlier study by Hathcock (1984) on the effects of seed adhesives, fertilizers, and lime on KBG seed establishment found that the application of nutrients and lime to the seeds was preferable to soil amendments [16]. This advantage is more significant over large acres of land, wherein applying soil amendments consisting of lime and fertilizers is time consuming and expensive compared to seed-applied treatments. In the current study, the seed germination percentage and shoot dry weight of KBG seedlings were greater when lime was applied in the form of the Hydroloc coating rather than as a soil amendment. Lime was applied to the soil at a similar rate (3.8317 g/pot) to that of the Hydroloc coating on the seed surface. The difference in germination and growth of the Hydroloc-coated seed could be attributed to the hydrophilic polymer or the application of lime and micronutrients directly on the seed surface. This study confirms the results from previous studies suggesting that the hydrophilic polymer can significantly improve germination when soil moisture is limited.
4.2. Nitrogen
The application of nitrogen to grass seeds is essential for synthesizing amino acids and proteins, both vital for the germination of the seed embryo. Nitrogen has also been proven to influence or break seed dormancy by interacting with hormones, such as gibberellins, that promote germination [31]. Previous studies have shown that nitrogen soil amendments increase root and shoot growth rates in perennial grasses, while also improving the germination speed [32,33]. In this study, the ammonium nitrate soil amendment resulted in a significantly higher shoot dry weight than the control under the full irrigation regime, but this result was still significantly lower than that for the Hydroloc treatment. This result, along with many other studies, has proven nitrogen to be an essential nutrient for the germination of seeds. However, high nitrogen concentrations around the seed could lead to toxic effects on seed germination, and this sensitivity is species dependent [34].
Nitrifying microbes are found naturally on the root nodules of leguminous crops, like soybean and clover, fixing atmospheric N2 and converting it into a plant-available form. This mutualistic relationship reduces the need for synthetic nitrogen use on legume crops and is the main benefit of their incorporation into a crop rotation system [35]. In recent years, much research has been conducted to identify and isolate nitrifying microbes. This research also involves successfully inoculating non-leguminous seeds with nitrogen-fixing microbes, resulting in the successful colonization of the rhizosphere in plants like rice, wheat, and maize [36]. The efficiency of nitrogen-fixing microbes on non-legume crops has varying results depending on the soil pH and oxygen levels. Early studies on the use of the Pivot Bio nitrogen-fixing microbe on corn seeds showed some evidence of greater N mineralization, but no significant increase in corn yield [37].
This study showed that inoculating KBG seeds with nitrifying microbes (Pivot Bio Proven 40) did not improve germination. It is important to bear in mind that the duration of this experiment may have limited the success of this treatment. It may take longer than 21 days for the microbe to colonize the rhizosphere and successfully fix plant-available nitrogen. Another issue is the low survival rate of nitrogen-fixing microbes that are inoculated onto non-leguminous seeds [38]. The Pivot Bio seed treatment had a significantly lower shoot dry weight under full irrigation than the ammonium soil amendment, showing that the nitrogen-fixing microbes had limited effects on the germinated KBG seedlings. The Pivot Bio nitrogen-fixing microbe was primarily designed for corn seed applications, and there is a need for further research into the use of nitrogen-fixing microbes on cool-season grass seeds.
4.3. Seed Priming
The least effective treatment in this experiment is osmotic priming or the H2O treatment of the KBG seeds. Seed priming involves the controlled hydration (10–20% of full imbibition) of the seed before planting. Seed osmotic priming enhances germination rates, increases the speed of seedling emergence, breaks seed dormancy, and improves overall plant performance [15,39]. H2O osmotic priming decreased the mean germination time in KBG seeds in comparison to non-primed seeds [13]. However, similar to the results in this study, the authors found that the final germination percentage of the osmotically primed seeds was not higher than that of the control. These authors hypothesized that these seed germination differences might be more substantial in adverse seed-bed conditions, such as when there is low water availability and low temperature [13].
Priming can also involve growth-regulating hormones, like gibberellic acid (GA3), which affects germination. GA3 is a growth-regulating hormone naturally found in plants. Exogenous GA3 is often used as a seed treatment to improve seed germination, break seed dormancy, and enhance shoot and root elongation [29]. GA3 is naturally released from the embryo and stimulates the production of hydrolytic enzyme α-amylase in the aleurone layer, which breaks down starch in the endosperm into soluble sugars to fuel the growth of the germinating seed. GA3 in the embryo is activated by the imbibition of water into the seed [14]. Along with improving germination, GA3 has been applied to many plant species, resulting in increased fruit production [40]. Studies have shown that using exogenous GA3 on seeds can improve plant water use efficiency, which may allow for the growth of plants in saline or low-moisture soils [41].
Studies have demonstrated that GA3 is associated with the plant hormone Abscisic acid (ABA). Both hormones antagonistically regulate seed germination [42]. ABA is synthesized in the chloroplast and cytoplasm to maintain seed dormancy and restrict precocious germination during seed maturation, allowing the seed to adapt to abiotic stressors [43]. The ABA content in seeds increases as a result of dehydration and a high temperature. For example, a study conducted on aleurone cells from Barley showed that ABA blocked GA synthesis, maintaining the seed in a dormant state [44]. Along with seed germination, ABA controls stomata closure and root growth when plants are under drought stress. When KBG seeds were exposed to drought stress, ABA may have inhibited the germination of the KBG seeds subject to the restricted irrigation regime in our experiment [45]. Another link between drought stress and poor germination is that water is essential for activating GA3. Water facilitates GA3’s mobility in the aleurone layer, and the restricted irrigation regime may have inhibited GA3 from activating.
A previous study looking into the use of GA3 on grass seed found that KBG germination increased by 6% when the seed was soaked in a GA3 solution with a concentration of 400 mg L−1 [25]. In our study, the GA3 seed treatment with the same concentration did not improve the germination percentage or shoot dry weight, performing similarly or worse than the untreated control. GA3 had the largest difference in the number of seeds germinated between the irrigation regimes, indicating that it is an ineffective solution for improving germination under low soil-moisture conditions. Although the values in this study showed no increase in the germination rate, the increase in germination in a similar study was very small for KBG when compared to other cool-season species, such as perennial ryegrass [25]. A potential reason for low germination is that fluctuations from dry to wet moisture conditions around the seed, paired with the room temperature conditions, can lead to increased seed respiration, harmful microbes colonizing the seed, and the eventual deterioration of the seed [46]. Seed deterioration due to moisture fluctuation results in decreased seed enzyme activity. This may be a reason for the GA3 and H2O treatment not increasing seed germination compared to the control treatment in this experiment [47]. Regardless, this experiment identified GA3 as an unsuitable seed treatment for stimulating the germination of KBG under drought conditions.
5. Conclusions
Despite the favorable characteristics of KBG for use in a PGC system, previous studies and observations have shown stand establishment to be a problem for its adoption. In this study, we have shown soil moisture to be a limiting factor in the germination speed and establishment of KBG. Seed treatments such as the Hydroloc treatment, consisting of a hydrophilic polymer and lime, can hold moisture and nutrients around the seed that are needed for germination when soil moisture is limited. Future research should investigate the impact of other variables, such as temperature, on the germination of KBG seeds.
Improving KBG establishment in the fall would allow farmers to protect the soil from erosion and nutrient leaching, while eliminating the financial burden of reseeding the following year. Improving the efficiency of the PGC system may also persuade growers to adopt this system due to the possibility of reducing the yearly labor input required by annual cover crops during the busy seasons of harvesting and planting row crops. This study shows that the Hydroloc treatment can improve the establishment of KBG in a growth chamber, regardless of whether soil moisture is limited. Future research should include field-based experiments for screening the effect of seed treatments and soil amendments on KBG germination over a longer period of time.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/seeds4010016/s1, Table S1: Percentage germination comparing each treatment between full and restricted irrigation regimes; Table S2: Shoot dry weight in grams (g) of each treatment comparing full irrigation and restricted irrigation regime.
Author Contributions
Conceptualization, A.S.G., S.-z.F. and K.J.M.; methodology, A.S.G. and K.J.M.; formal analysis, J.M.; investigation, J.M.; data curation, K.J.M.; writing—original draft preparation, J.M.; writing—review and editing, A.S.G., S.-z.F. and K.J.M.; supervision, A.S.G. All authors have read and agreed to the published version of the manuscript.
Funding
RegenPGC is supported by the Agriculture and Food Research Initiative Competitive Grant no. 2021-68012-35923 from the USDA National Institute of Food and Agriculture. Any opinions, findings, conclusions, or recommendations expressed in this article are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Data Availability Statement
The dataset for this experiment can be found under the Perennial groundcover consortium in Zenodo. These data are publicly available at https://doi.org/10.5281/zenodo.14783080.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Comer, P.J.; Hak, J.C.; Kindscher, K.; Muldavin, E.; Singhurst, J. Continent-Scale Landscape Conservation Design for Temperate Grasslands of the Great Plains and Chihuahuan Desert. Nat. Areas J. 2018, 38, 196–211. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Summary Report: 2017 National Resources Inventory; Center for Survey Statistics and Methodology, Iowa State University: Ames, IA, USA; Natural Resources Conservation Service: Washington, DC, USA, 2020.
- Moore, K.J.; Karlen, D.L. Double Cropping Opportunities for Biomass Crops in the North Central USA. Biofuels 2013, 4, 605–615. [Google Scholar] [CrossRef]
- Hall, J.K.; Hartwig, N.L.; Hoffman, L.D. Cyanazine Losses in Runoff from No-Tillage Corn in “Living” and Dead Mulches vs. Unmulched, Conventional Tillage. J. Environ. Qual. 1984, 13, 105–110. [Google Scholar] [CrossRef]
- Moore, K.J.; Anex, R.P.; Elobeid, A.E.; Fei, S.; Flora, C.B.; Goggi, A.S.; Jacobs, K.L.; Jha, P.; Kaleita, A.L.; Karlen, D.L.; et al. Regenerating Agricultural Landscapes with Perennial Groundcover for Intensive Crop Production. Agronomy 2019, 9, 458. [Google Scholar] [CrossRef]
- Flynn, E.S.; Moore, K.J.; Singer, J.W.; Lamkey, K.R. Evaluation of Grass and Legume Species as Perennial Ground Covers in Corn Production. Crop Sci. 2013, 53, 611–620. [Google Scholar] [CrossRef]
- Schlautman, B.; Bartel, C.; Diaz-Garcia, L.; Fei, S.; Flynn, S.; Haramoto, E.; Moore, K.; Raman, D.R. Perennial Groundcovers: An Emerging Technology for Soil Conservation and the Sustainable Intensification of Agriculture. Emerg. Top. Life Sci. 2021, 5, 337–347. [Google Scholar]
- Bartel, C.A.; Archontoulis, S.V.; Lenssen, A.W.; Moore, K.J.; Huber, I.L.; Laird, D.A.; Dixon, P.M. Modeling Perennial Groundcover Effects on Annual Maize Grain Crop Growth with the Agricultural Production Systems sIMulator. Agron. J. 2020, 112, 1895–1910. [Google Scholar] [CrossRef]
- Qian, Y.; Follett, R.F.; Kimble, J.M. Soil Organic Carbon Input from Urban Turfgrasses. Soil Sci. Soc. Am. J. 2010, 74, 366–371. [Google Scholar] [CrossRef]
- Beard, J.B. Turfgrass: Science and Culture; Prentice-Hall: Englewood Cliffs, NJ, USA, 1972; ISBN 0-13-933002-X. [Google Scholar]
- Chen, A.; Fei, S.-Z.; Lenssen, A.W.; Moore, K.J. Evaluating Cool-season Grass Species as Potential Perennial Groundcover for Maize Production. Agron. J. 2022, 114, 2415–2429. [Google Scholar] [CrossRef]
- Bartel, C.A.; Banik, C.; Lenssen, A.W.; Moore, K.J.; Laird, D.A.; Archontoulis, S.V.; Lamkey, K.R. Establishment of Perennial Groundcovers for Maize-Based Bioenergy Production Systems. Agron. J. 2017, 109, 822–835. [Google Scholar] [CrossRef]
- Campbell, J.H.; Henderson, J.J.; Inguagiato, J.C.; Wallace, V.H.; Minniti, A. Optimizing Pre-Germination Techniques for Kentucky Bluegrass and Perennial Ryegrass. J. Environ. Hortic. 2019, 37, 19–23. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic Acid in Plant: Still a Mystery Unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef]
- Nelson, M.N.; Nesi, N.; Barrero, J.M.; Fletcher, A.L.; Greaves, I.K.; Hughes, T.; Laperche, A.; Snowdon, R.; Rebetzke, G.J.; Kirkegaard, J.A. Chapter Two—Strategies to Improve Field Establishment of Canola: A Review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 175, pp. 133–177. ISBN 0065-2113. [Google Scholar]
- Hathcock, A.L.; Dernoeden, P.H.; Turner, T.R.; McIntosh, M.S. Tall Fescue and Kentucky Bluegrass Response to Fertilizer and Lime Seed Coatings. Agron. J. 1984, 76, 879–883. [Google Scholar] [CrossRef]
- Leinauer, B.; Serena, M.; Singh, D. Seed Coating and Seeding Rate Effects on Turfgrass Germination and Establishment. HortTechnology Hortte 2010, 20, 179–185. [Google Scholar] [CrossRef]
- Richardson, M.D.; Hignight, K.W. Seedling Emergence of Tall Fescue and Kentucky Bluegrass, as Affected by Two Seed Coating Techniques. HortTechnology Hortte 2010, 20, 415–417. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs. The Sustainable Development Goals Report 2024; United Nations: New York, NY, USA, 2024; ISBN 978-92-1-358975-5. [Google Scholar]
- Bartel, C.A.; Jacobs, K.L.; Moore, K.J.; Raman, D.R. Anticipatory Technoeconomic Evaluation of Kentucky Bluegrass-Based Perennial Groundcover Implementations in Large-Scale Midwestern US Corn Production Systems. Sustainability 2024, 16, 7112. [Google Scholar] [CrossRef]
- Virgin, H.I. Light-Induced Unfolding of the Grass Leaf. Physiol. Plant. 1962, 15, 380–389. [Google Scholar] [CrossRef]
- Assn of Official Seed. AOSA Rules For Testing Seeds: Principles and Procedures, Effective October 1, 2019; Assn of Official Seed: Wichita, KS, USA, 2019. [Google Scholar]
- Curell, C. Why Is Soil Water Holding Capacity Important? Available online: https://www.canr.msu.edu/news/why_is_soil_water_holding_capacity_important (accessed on 28 October 2024).
- Vazquez, E.; Benito, M.; Espejo, R.; Teutscherova, N. Effects of No-Tillage and Liming Amendment Combination on Soil Carbon and Nitrogen Mineralization. Eur. J. Soil Biol. 2019, 93, 103090. [Google Scholar] [CrossRef]
- Abdullah, B.S.; Abdulrahman, Y.A. Effect of Different Concentrations of Gibberellic Acid on Seeds Germination and Growth in Different Turf Grass Genera. Kufa J. Agric. Sci. 2017, 9, 226–247. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.4 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Folck, A.J.; Bigelow, C.A.; Jiang, Y.; Patton, A.J. Genotypic Variation in Germination Rate, Seedling Vigor, and Seed Phenotype of Kentucky Bluegrass Cultivars. Crop Sci. 2023, 63, 3065–3078. [Google Scholar] [CrossRef]
- Larsen, S.U.; Andreasen, C.; Kristoffersen, P. The Variation in Seed Weight within and among Cultivars of Slender Creeping Red Fescue (Festuca rubra ssp. litoralis), Perennial Ryegrass (Lolium perenne) and Kentucky Bluegrass (Poa pratensis) and Its Importance for the Composition of Seed Mixtures. Seed Sci. Technol. 2004, 32, 135–147. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Zhao, D.-D.; Ning, Q.-R.; Wei, J.-P.; Li, Y.; Wang, M.-M.; Liu, X.-L.; Jiang, C.-J.; Liang, Z.-W. A Multi-Year Beneficial Effect of Seed Priming with Gibberellic Acid-3 (GA3) on Plant Growth and Production in a Perennial Grass, Leymus Chinensis. Sci. Rep. 2018, 8, 13214. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R. The Effect of Lime Application to an Acid Soil on Perennial Grass Establishment; NSW Department of Primary Industries: Canberra, Australia, 2010. [Google Scholar]
- Bethke, P.C.; Libourel, I.G.L.; Jones, R.L. Nitric Oxide Reduces Seed Dormancy in Arabidopsis. J. Exp. Bot. 2006, 57, 517–526. [Google Scholar] [CrossRef]
- Monaco, T.A.; Mackown, C.T.; Johnson, D.A.; Jones, T.A.; Norton, M.; Norton, J.B.; Redinbaugh, M.G. Nitrogen Effects on Seed Germination and Seedling Growth. J. Range Manag. 2003, 56, 646–653. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, M.; Huang, X.; Hu, W.; Qiao, N.; Song, H.; Zhang, B.; Zhang, R.; Yang, Z.; Liu, Y.; et al. Direct Effects of Nitrogen Addition on Seed Germination of Eight Semi-arid Grassland Species. Ecol. Evol. 2020, 10, 8793–8800. [Google Scholar] [CrossRef]
- Davis, A.S. Nitrogen Fertilizer and Crop Residue Effects on Seed Mortality and Germination of Eight Annual Weed Species. Weed Sci. 2007, 55, 123–128. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef]
- Bhattacharjee, R.B.; Singh, A.; Mukhopadhyay, S.N. Use of Nitrogen-Fixing Bacteria as Biofertiliser for Non-Legumes: Prospects and Challenges. Appl. Microbiol. Biotechnol. 2008, 80, 199–209. [Google Scholar] [CrossRef]
- Davis, W.G.; Bonini Pires, C.A.; Ruiz Diaz, D.A.; Roozeboom, K.L.; Rice, C.W. Pivot Bio Proven Inoculant as a Source of Nitrogen in Corn. Kans. Agric. Exp. Stn. Res. Rep. 2020, 6, 7. [Google Scholar] [CrossRef]
- O’Callaghan, M. Microbial Inoculation of Seed for Improved Crop Performance: Issues and Opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. In New Challenges in Seed Biology-Basic and Translational Research Driving Seed Technology; InTechOpen: London, UK, 2016; Volume 46. [Google Scholar]
- El-Tohamy, W.A.; Dasgan, H.Y.; Gruda, N.S. Impact of Gibberellic Acid on Water Status, Growth, and Development of Cape Gooseberry in Newly Reclaimed Sandy Lands within Arid Regions. Horticulturae 2023, 9, 1283. [Google Scholar] [CrossRef]
- Maggio, A.; Barbieri, G.; Raimondi, G.; De Pascale, S. Contrasting Effects of GA3 Treatments on Tomato Plants Exposed to Increasing Salinity. J. Plant Growth Regul. 2010, 29, 63–72. [Google Scholar] [CrossRef]
- Gubler, F.; Millar, A.A.; Jacobsen, J.V. Dormancy Release, ABA and Pre-Harvest Sprouting. Curr. Opin. Plant Biol. 2005, 8, 183–187. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Antagonistic Regulation of ABA and GA in Metabolism and Signaling Pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef]
- Gubler, F.; Chandler, P.M.; White, R.G.; Llewellyn, D.J.; Jacobsen, J.V. Gibberellin Signaling in Barley Aleurone Cells. Control of SLN1 and GAMYB Expression. Plant Physiol. 2002, 129, 191–200. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M.; Halmer, P. The Encyclopedia of Seeds: Science, Technology and Uses; Cabi: Oxfordshire, UK, 2006. [Google Scholar]
- Magan, N.; Sanchis, V.; Aldred, D. The Role of Spoilage Fungi in Seed Deterioration. Mycol. Ser. 2004, 21, 311–324. [Google Scholar]
- Cj, V.; Nethra, N. Seed Deterioration: An Overview. Seed Res. 2022, 50, 91–99. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).