Different Traits Affect Salinity and Drought Tolerance during Germination of Citrullus colocynthis, a Potential Cash Crop in Arid Lands
Abstract
1. Introduction
2. Results
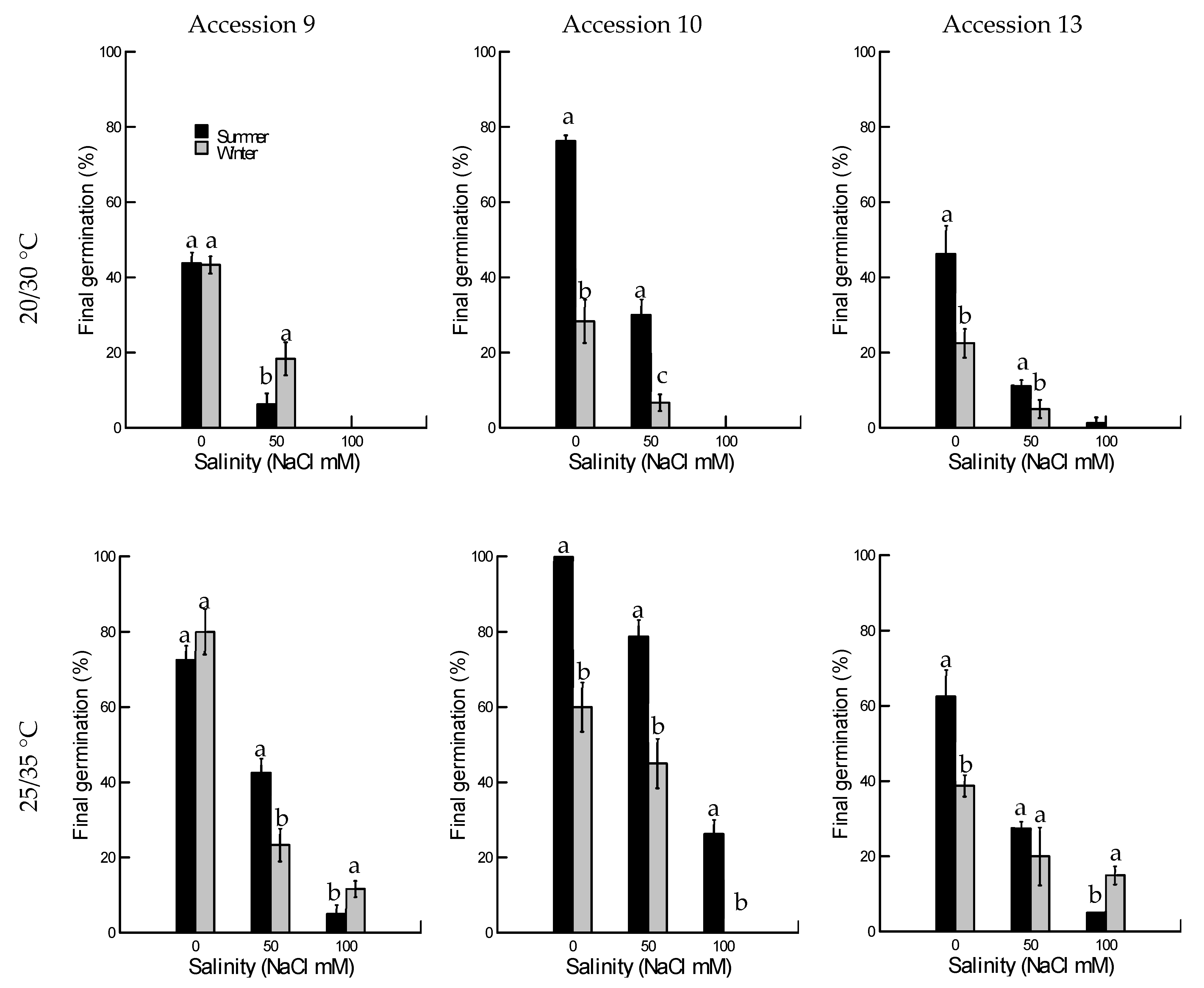
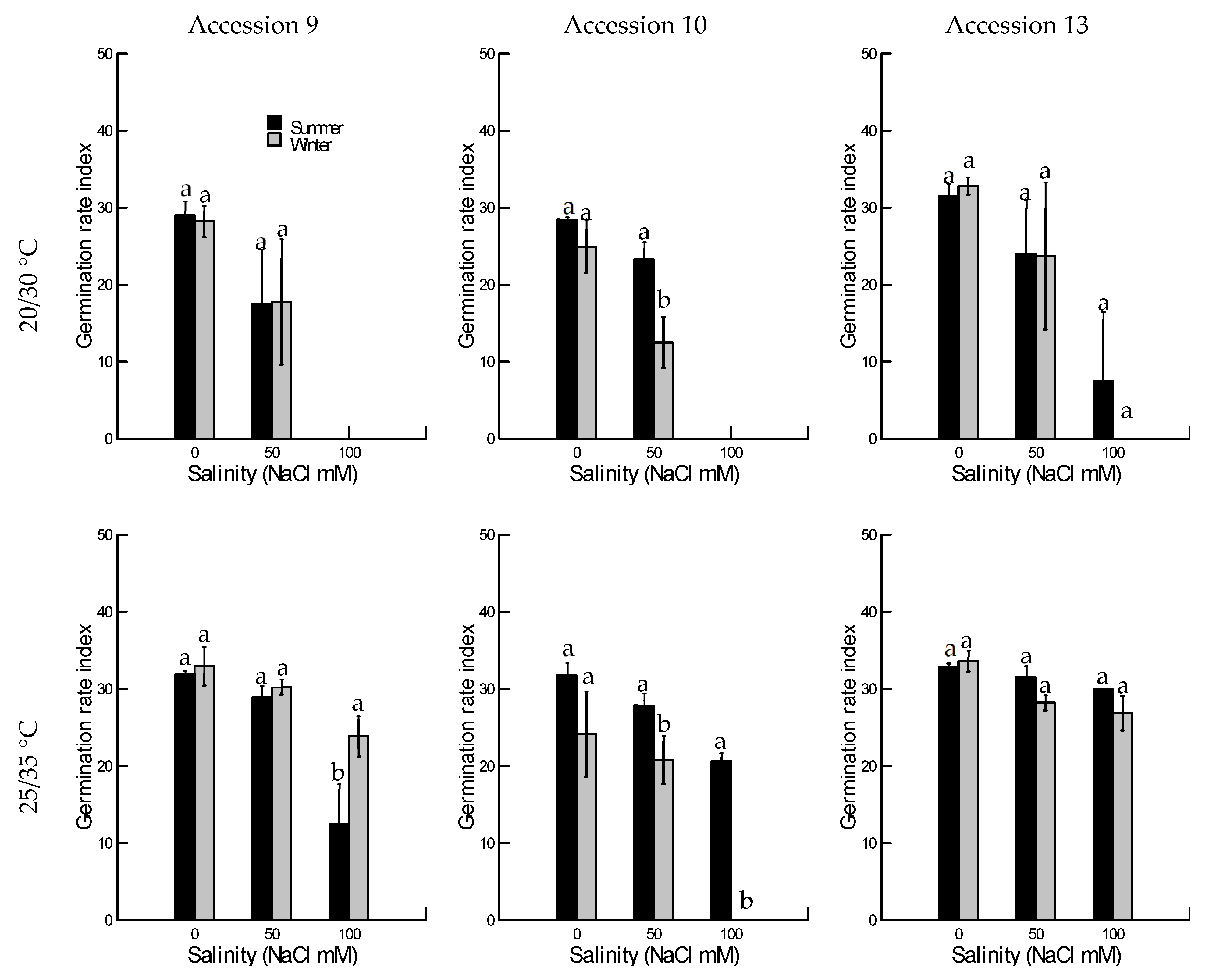
2.1. Salinity Tolerance
2.1.1. Final Germination
2.1.2. Germination Rate Index
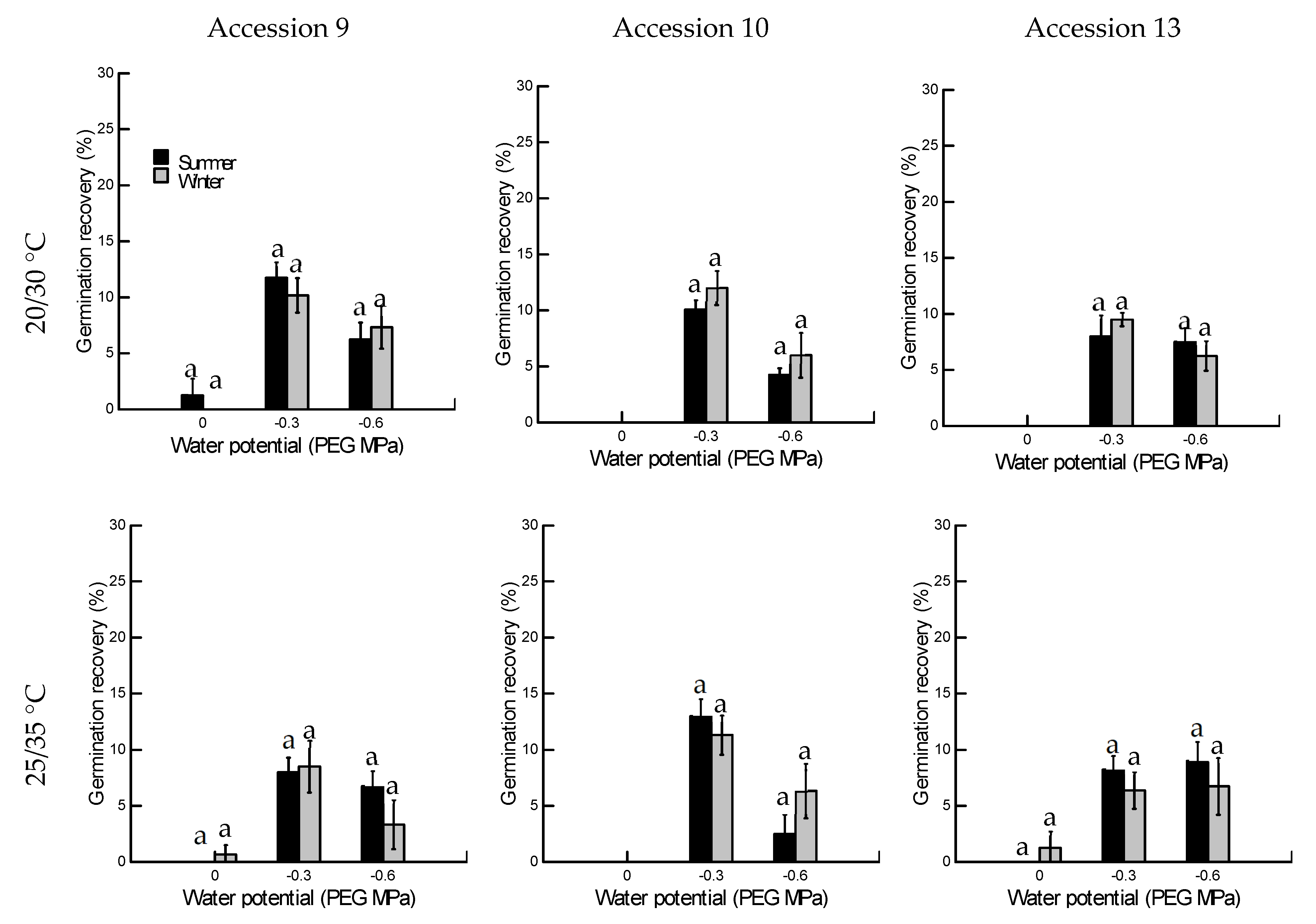
2.1.3. Germination Recovery
2.2. Drought Tolerance
2.2.1. Final Germination
2.2.2. Germination Recovery
3. Discussion
4. Materials and Methods
4.1. Accession Selection and Growth Conditions
4.2. Seed Collection and Germination
4.3. Germination Recovery
4.4. Calculations and Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Keblawy, A.; Al-Shamsi, N.; Mosa, K. Effect of Maternal Habitat, Temperature and Light on Germination and Salt Tolerance of Suaeda Vermiculata, a Habitat-Indifferent Halophyte of Arid Arabian Deserts. Seed Sci. Res. 2018, 28, 140–147. [Google Scholar] [CrossRef]
- Gamalero, E.; Bona, E.; Todeschini, V.; Lingua, G. Saline and Arid Soils: Impact on Bacteria, Plants, and Their Interaction. Biology 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Bradford, K.J. Water Relations in Seed Germination. In Seed Development and Germination; Routledge: Oxfordshire, UK, 2017; pp. 351–396. [Google Scholar]
- Barrios, D.; Flores, J.; Sánchez, J.A.; González-Torres, L.R. Combined Effect of Temperature and Water Stress on Seed Germination of Four Leptocereus spp. (Cactaceae) from Cuban Dry Forests. Plant Species Biol. 2021, 36, 512–522. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Footitt, S. Seed Dormancy Cycling and the Regulation of Dormancy Mechanisms to Time Germination in Variable Field Environments. J. Exp. Bot. 2017, 68, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.S.; Meyer, S.E.; Khan, M.A. Hydrothermal Time as a Tool in Comparative Germination Studies. In Seed Biology; CABI Publishing: Wallingford, UK, 2000; pp. 401–410. [Google Scholar]
- Yi, F.; Wang, Z.; Baskin, C.C.; Baskin, J.M.; Ye, R.; Sun, H.; Zhang, Y.; Ye, X.; Liu, G.; Yang, X. Seed Germination Responses to Seasonal Temperature and Drought Stress are Species-Specific but not Related to Seed Size in a Desert Steppe: Implications for Effect of Climate Change on Community Structure. Ecol. Evol. 2019, 9, 2149–2159. [Google Scholar] [CrossRef]
- Blank, R.R.; Young, J.A.; Martens, E.; Palmquist, D.E. Influence of Temperature and Osmotic Potential on Germination of Allenrolfea occidentalis seeds. J. Arid Environ. 1994, 26, 339–347. [Google Scholar] [CrossRef]
- Uçarlı, C. Effects of Salinity on Seed Germination and Early Seedling Stage. In Abiotic Stress in Plants; IntechOpen: London, UK, 2020; p. 211. [Google Scholar]
- Ungar, I.A. Seed Germination and Seed-Bank Ecology in Halophytes. In Seed Development and Germination; Routledge: Oxfordshire, UK, 2017; pp. 599–628. [Google Scholar]
- Hu, Y.; Schmidhalter, U. Drought and Salinity: A Comparison of Their Effects on Mineral Nutrition of Plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Rawai, A. Impacts of the Invasive Exotic Prosopis juliflora (Sw.) DC on the Native Flora and Soils of the UAE. Plant Ecol. 2007, 190, 23–35. [Google Scholar] [CrossRef]
- Aljasmi, M.; El-Keblawy, A.; Mosa, K.A. Abiotic Factors Controlling Germination of the Multipurpose Invasive Prosopis pallida: Towards Afforestation of Salt-Affected Lands in the Subtropical Arid Arabian Desert. Trop. Ecol. 2021, 62, 116–125. [Google Scholar] [CrossRef]
- El-Keblawy, A.E.; Al Neyadi, S.S.; Rao, M.V.; Al-Marzouqi, A.H. Interactive Effects of Salinity, Light and Temperature on Seed Germination of Sand Dunes Glycophyte Cyprus conglomeratus Growing in the United Arab Emirates Deserts. Seed Sci. Technol. 2011, 39, 364–376. [Google Scholar] [CrossRef]
- Gulzar, S.; Khan, M.A. Germination Responses of Sporobolus ioclados: A Potential Forage Grass. J. Arid Environ. 2003, 53, 387–394. [Google Scholar]
- Alvarado, V.; Bradford, K.J. A Hydrothermal Time Model Explains the Cardinal Temperatures for Seed Germination. Plant Cell Environ. 2002, 25, 1061–1069. [Google Scholar] [CrossRef]
- Zhang, H.; Irving, L.J.; McGill, C.; Matthew, C.; Zhou, D.; Kemp, P. The Effects of Salinity and Osmotic Stress on Barley Germination Rate: Sodium as an Osmotic Regulator. Ann. Bot. 2010, 106, 1027–1035. [Google Scholar] [CrossRef]
- Alzarah, M.I.; Alaqil, A.A.; Abbas, A.O.; Nassar, F.S.; Mehaisen, G.M.; Gouda, G.F.; Abd El-Atty, H.K.; Moustafa, E.S. Inclusion of Citrullus colocynthis Seed Extract into Diets Induced a Hypolipidemic Effect and Improved Layer Performance. Agriculture 2021, 11, 808. [Google Scholar] [CrossRef]
- Farooq, M.; Azadfar, E.; Trif, M.; Jabaleh, R.A.; Rusu, A.; Bahrami, Z.; Sharifi, M.; Bangar, S.P.; Ilyas, N.; Ștefănescu, B.E. Soybean Oil Enriched with Antioxidants Extracted from Watermelon (Citrullus colocynthis) Skin Sap and Coated in Hydrogel Beads via Ionotropic Gelation. Coatings 2021, 11, 1370. [Google Scholar] [CrossRef]
- Nkoana, D.K.; Mashilo, J.; Shimelis, H.; Ngwepe, R.M. Nutritional, Phytochemical Compositions and Natural Therapeutic Values of Citron Watermelon (Citrullus lanatus Var. Citroides): A Review. S. Afr. J. Bot. 2021, 145, 65–77. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Munawar, M.; Saeed, M.; Shen, J.-Q.; Khan, M.S.; Noreen, S.; Alagawany, M.; Naveed, M.; Madni, A.; Li, C.-X. Citrullus colocynthis (L.) Schrad (Bitter Apple Fruit): Promising Traditional Uses, Pharmacological Effects, Aspects, and Potential Applications. Front. Pharmacol. 2021, 12, 791049. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.; El-Agamy, D.S.; Elsaed, W.M.; Sirwi, A.; Asfour, H.Z.; Koshak, A.E.; Elhady, S.S. Cucurbitacin E Glucoside Alleviates Concanavalin A-Induced Hepatitis through Enhancing SIRT1/Nrf2/HO-1 and Inhibiting NF-ĸB/NLRP3 Signaling Pathways. J. Ethnopharmacol. 2022, 292, 115223. [Google Scholar] [CrossRef]
- Giwa, S.; Abdullah, L.C.; Adam, N.M. Investigating “Egusi” (Citrullus colocynthis L.) Seed Oil as Potential Biodiesel Feedstock. Energies 2010, 3, 607–618. [Google Scholar] [CrossRef]
- Ajenu, C.O.; Ukhun, M.E.; Imoisi, C.; Imhontu, E.E.; Irede, L.E.; Orji, U.R. Characterization and Stability Studies of Egusi Melon Seed Oil (Citrullus colocynthis L.). J. Chem. Soc. Niger. 2021, 46. [Google Scholar] [CrossRef]
- Sawaya, W.N.; Daghir, N.J.; Khalil, J.K. Citrullus colocynthis Seeds as a Potential Source of Protein for Food and Feed. J. Agric. Food Chem. 1986, 34, 285–288. [Google Scholar] [CrossRef]
- Singh, N.P.; Matta, N.K. Levels of Seed Proteins in Citrullus and Praecitrullus Accessions. Plant Syst. Evol. 2010, 290, 47–56. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Muscolo, A.; Rehman, A. Crop Diversification and Saline Water Irrigation as Potential Strategies to Save Freshwater Resources and Reclamation of Marginal Soils—A Review. Environ. Sci. Pollut. Res. 2020, 27, 28695–28729. [Google Scholar] [CrossRef]
- Qasim, M.; Gulzar, S.; Khan, M.A. Halophytes as Medicinal Plants. In Urbanisation, Land Use, Land Degradation and Environment; Institute of Sustainable Halophyte Utilization, University of Karachi: Karachi, Pakistan, 2011; pp. 330–343. [Google Scholar]
- Chauhan, S.S. Desertification Control and Management of Land Degradation in the Thar Desert of India. Environmentalist 2003, 23, 219–227. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Abdelfattah, M.A.; Khedr, A.-H.A. Relationships between Landforms, Soil Characteristics and Dominant Xerophytes in the Hyper-Arid Northern United Arab Emirates. J. Arid Environ. 2015, 117, 28–36. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Kafhaga, T.; Navarro, T. Live and Dead Shrubs and Grasses Have Different Facilitative and Interfering Effects on Associated Plants in Arid Arabian Deserts. J. Arid Environ. 2016, 125, 127–135. [Google Scholar] [CrossRef]
- da Silva, J.A.T.; Hussain, A.I. Citrullus colocynthis (L.) Schrad. (Colocynth): Biotechnological Perspectives. Emir. J. Food Agric. 2017, 29, 83–90. [Google Scholar] [CrossRef]
- Elouafi, I.; Shahid, M.A.; Begmuratov, A.; Hirich, A. The Contribution of Alternative Crops to Food Security in Marginal Environments. In Emerging Research in Alternative Crops; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–23. [Google Scholar]
- Saberi, M.; Shahriari, A.; Tarnian, F.; Noori, S. Comparison the Effect of Different Treatments for Breaking Seed Dormancy of Citrullus colocynthis. J. Agric. Sci. 2011, 3, 62. [Google Scholar] [CrossRef]
- Menon, K.; Jayakumar, A.P.; Shahid, M.; Sood, N.; Rao, N.K. Seed Dormancy and Effect of Salinity on Germination of Citrullus colocynthis. Int. J. Environ. Sci. Dev. 2014, 5, 566. [Google Scholar] [CrossRef]
- Parveen, B.; Neeta, S. Seed Dormancy and Effect of Salinity on Germination of Citrullus colocynthis L. Int. J. Res. Appl. Sci. Eng. Technol. 2016, 4, 525–528. [Google Scholar]
- Koller, D.; Poljakoff-Mayber, A.; Berg, A.; Diskin, T. Germination-regulating Mechanisms in Citrullus colocynthis. Am. J. Bot. 1963, 50 Pt 1, 597–603. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Shabana, H.A.; Navarro, T.; Soliman, S. Effect of Maturation Time on Dormancy and Germination of Citrullus colocynthis (Cucurbitaceae) Seeds from the Arabian Hyper-Arid Deserts. BMC Plant Biol. 2017, 17, 263. [Google Scholar] [CrossRef] [PubMed]
- El-Keblawy, A.; Soliman, S.; Al-Khoury, R.; Ghauri, A.; Al Rammah, H.; Hussain, S.E.; Rashid, S.; Manzoor, Z. Effect of Maturation Conditions on Light and Temperature Requirements during Seed Germination of Citrullus colocynthis from the Arabian Desert. Plant Biol. 2019, 21, 292–299. [Google Scholar] [CrossRef]
- Al-Nablsi, S.; El-Keblawy, A.; Mosa, K.A.; Soliman, S. Variation among Individuals of Citrullus colocynthis from a Desert Population in Morphological, Genetic, and Germination Attributes. Trop. Ecol. 2022, 63, 171–182. [Google Scholar] [CrossRef]
- Niknahad Gharmakher, H.; Saberi, M.; Heshmati, G.; Barani, H.; Shahriyari, A. Effects of Different Drought and Salinity Levels on Seed Germination of Citrullus colocynthis. Ecopersia 2017, 5, 903–1917. [Google Scholar]
- Hamurcu, M.; Khan, M.; Pandey, A.; Ozdemir, C.; Avsaroglu, Z.Z.; Elbasan, F.; Gezgin, S. Nitric oxide regulates watermelon (Citrullus lanatus) responses to drought stress. 3 Biotech 2020, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Al-Nablsi, S.; El-Keblawy, A.; Ali, M.A.; Mosa, K.A.; Hamoda, A.M.; Shanableh, A.; Almehdi, A.M.; Soliman, S.S. Phenolic Contents and Antioxidant Activity of Citrullus colocynthis Fruits, Growing in the Hot Arid Desert of the UAE, Influenced by the Fruit Parts, Accessions, and Seasons of Fruit Collection. Antioxidants 2022, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Z.; Shabelsky, E.; Schafferman, D. Colocynth: Potential Arid Land Oilseed from an Ancient Cucurbit. In Perspectives; New Crops New Uses ASHS Press: Alexandria, VA, USA, 1999; pp. 257–261. [Google Scholar]
- Edwards, B.R.; Burghardt, L.T.; Zapata-Garcia, M.; Donohue, K. Maternal Temperature Effects on Dormancy Influence Germination Responses to Water Availability in Arabidopsis Thaliana. Environ. Exp. Bot. 2016, 126, 55–67. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B. Halophyte Seed Germination. In Ecophysiology of High Salinity Tolerant Plants; Springer: Berlin/Heidelberg, Germany, 2006; pp. 11–30. [Google Scholar]
- Lacey, E.P.; Smith, S.; Case, A.L. Parental Effects on Seed Mass: Seed Coat but Not Embryo/Endosperm Effects. Am. J. Bot. 1997, 84, 1617–1620. [Google Scholar] [CrossRef]
- Galloway, L.F. The Effect of Maternal Phenology on Offspring Characters in the Herbaceous Plant Campanula americana. J. Ecol. 2002, 90, 851–858. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Cavers, P.B.; Bernards, M.A. Pre-and Post-dispersal Factors Regulate Germination Patterns and Structural Characteristics of Scotch Thistle (Onopordum acanthium) Cypselas. New Phytol. 2003, 159, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The Signalling Role of ROS in the Regulation of Seed Germination and Dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-B.; Kim, Y.-H.; Lee, H.-S.; Kim, K.-Y.; Deng, X.-P.; Kwak, S.-S. Analysis of Antioxidant Enzyme Activity during Germination of Alfalfa under Salt and Drought Stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Krauss, K.W.; Chambers, J.L.; Allen, J.A. Salinity Effects and Differential Germination of Several Half-Sib Families of Baldcypress from Different Seed Sources. New For. 1998, 15, 53–68. [Google Scholar] [CrossRef]
- Elnaggar, A.; El-Keblawy, A.; Mosa, K.A.; Soliman, S. Drought Tolerance during Germination Depends on Light and Temperature of Incubation in Salsola imbricata, a Desert Shrub of Arabian Deserts. Flora 2018, 249, 156–163. [Google Scholar] [CrossRef]
- Al-Shamsi, N.; El-Keblawy, A.; Mosa, K.A.; Navarro, T. Drought Tolerance and Germination Response to Light and Temperature for Seeds of Saline and Non-Saline Habitats of the Habitat-Indifferent Desert Halophyte Suaeda vermiculata. Acta Physiol. Plant. 2018, 40, 200. [Google Scholar] [CrossRef]
- Bhatt, A.; Gairola, S.; Carón, M.M.; Santo, A.; Murru, V.; El-Keblawy, A.; Mahmoud, T. Effects of Light, Temperature, Salinity, and Maternal Habitat on Seed Germination of Aeluropus lagopoides (Poaceae): An Economically Important Halophyte of Arid Arabian Deserts. Botany 2020, 98, 117–125. [Google Scholar] [CrossRef]
- Wilson, T.B.; Witkowski, E.T.F. Water Requirements for Germination and Early Seedling Establishment in Four African Savanna Woody Plant Species. J. Arid Environ. 1998, 38, 541–550. [Google Scholar] [CrossRef]
- Thanos, C.A.; Mitrakos, K. Watermelon Seed Germination. 1. Effects of Light, Temperature and Osmotica. Seed Sci. Res. 1992, 2, 155–162. [Google Scholar] [CrossRef]
- Hameed, A.; El-Keblawy, A.; Aljasmi, M.; Gairola, S.; Phartyal, S.S.; Mosa, K.A.; Soliman, S. Seed Provenance, Thermoperiod, and Photoperiod Affect Low Water Potential Tolerance during Seed Germination of the Multipurpose Exotic Tree Prosopis juliflora. J. Arid Environ. 2021, 195, 104627. [Google Scholar] [CrossRef]
- Tobe, K.; Li, X.; Omasa, K. Effects of Five Different Salts on Seed Germination and Seedling Growth of Haloxylon ammodendron (Chenopodiaceae). Seed Sci. Res. 2004, 14, 345–353. [Google Scholar] [CrossRef]
- Cony, M.A.; Trione, S.O. Inter-and Intraspecific Variability in Prosopis flexuosa and P. chilensis: Seed Germination under Salt and Moisture Stress. J. Arid Environ. 1998, 40, 307–317. [Google Scholar] [CrossRef]
- Amin, M.T.; Mahmoud, S.H.; Alazba, A.A. Observations, Projections and Impacts of Climate Change on Water Resources in Arabian Peninsula: Current and Future Scenarios. Environ. Earth Sci. 2016, 75, 864. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Castañeda, V.; González, E.M. Strategies to Apply Water-Deficit Stress: Similarities and Disparities at the Whole Plant Metabolism Level in Medicago Truncatula. Int. J. Mol. Sci. 2021, 22, 2813. [Google Scholar] [CrossRef]
- Gorai, M.; El Aloui, W.; Yang, X.; Neffati, M. Toward Understanding the Ecological Role of Mucilage in Seed Germination of a Desert Shrub Henophyton Deserti: Interactive Effects of Temperature, Salinity and Osmotic Stress. Plant Soil 2014, 374, 727–738. [Google Scholar] [CrossRef]
- Rasheed, A.; Hameed, A.; Gul, B.; Khan, M.A. Perianth and Abiotic Factors Regulate Seed Germination of Haloxylon stocksii—A Cash Crop Candidate for Degraded Saline Lands. Land Degrad. Dev. 2019, 30, 1468–1478. [Google Scholar] [CrossRef]
- Mishra, A.; Tanna, B. Halophytes: Potential Resources for Salt Stress Tolerance Genes and Promoters. Front. Plant Sci. 2017, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, A.; Li, W. How Membranes Organize during Seed Germination: Three Patterns of Dynamic Lipid Remodelling Define Chilling Resistance and Affect Plastid Biogenesis. Plant Cell Environ. 2015, 38, 1391–1403. [Google Scholar] [CrossRef]
- Nerson, H. Seed Production and Germinability of Cucurbit Crops. Seed Sci. Biotechnol. 2007, 1, 1–10. [Google Scholar]
- El-Keblawy, A.; Al-Shamsi, N. Salinity, Temperature and Light Affect Seed Germination of Haloxylon salicornicum, a Common Perennial Shrub of the Arabian Deserts. Seed Sci. Technol. 2008, 36, 679–688. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, C.; Meng, S.; Dane, F. Gene Expression Changes in Response to Drought Stress in Citrullus colocynthis. Plant Cell Rep. 2009, 28, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rashotte, A.M.; Moss, A.G.; Dane, F. Two NAC Transcription Factors from Citrullus colocynthis, CcNAC1, CcNAC2 Implicated in Multiple Stress Responses. Acta Physiol. Plant. 2014, 36, 621–634. [Google Scholar] [CrossRef]



| df | Final Germination | Germination Rate Index | Germination Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ms | F-Ratio | p | Ms | F-Ratio | p | Ms | F-Ratio | p | ||
| Season (S) | 1 | 0.882 | 117.836 | <0.001 | 241.23 | 6.987 | <0.05 | 0.029 | 15.535 | <0.001 |
| Accession (A) | 2 | 0.581 | 77.578 | <0.001 | 608.0 | 17.609 | <0.001 | 0.013 | 6.871 | <0.01 |
| Temperature (T) | 1 | 2.271 | 303.461 | <0.001 | 2915.1 | 84.428 | <0.001 | 0.014 | 7.720 | <0.01 |
| NaCl | 2 | 4.219 | 563.858 | <0.001 | 4731.0 | 137.022 | <0.001 | 0.579 | 311.4 | <0.001 |
| S*A | 2 | 0.528 | 70.537 | <0.001 | 299.74 | 8.681 | <0.001 | 0.006 | 3.460 | <0.05 |
| S*T | 1 | 0.075 | 10.041 | <0.01 | 3.712 | 0.108 | ns | 0.004 | 2.199 | ns |
| S*NaCl | 2 | 0.256 | 34.212 | <0.001 | 13.274 | 0.384 | ns | 0.010 | 5.548 | <0.01 |
| A*T | 2 | 0.165 | 22.020 | <0.001 | 87.978 | 2.548 | ns | 0.002 | 1.314 | ns |
| A*NaCl | 4 | 0.154 | 20.533 | <0.001 | 42.200 | 1.222 | ns | 0.006 | 2.986 | <0.05 |
| T*NaCl | 2 | 0.231 | 30.811 | <0.001 | 702.4 | 20.344 | <0.001 | 0.019 | 9.990 | <0.001 |
| S*A*T | 2 | 0.066 | 8.878 | <0.001 | 95.77 | 2.774 | ns | 0.003 | 1.553 | ns |
| S*A*NaCl | 4 | 0.116 | 15.549 | <0.001 | 42.08 | 1.219 | ns | 0.004 | 2.222 | ns |
| S*T*NaCl | 2 | 0.017 | 2.253 | ns | 3.23 | 0.093 | ns | 0.000 | 0.163 | ns |
| A*T*NaCl | 4 | 0.042 | 5.624 | <0.001 | 75.65 | 2.191 | ns | 0.001 | 0.616 | ns |
| S*A*T*NaCl | 4 | 0.027 | 3.594 | <0.01 | 65.95 | 1.910 | ns | 0.002 | 1.216 | ns |
| Error | 102 | 0.007 | 34.528 | 0.002 | ||||||
| df | Final Germination | Germination Recovery | |||||
|---|---|---|---|---|---|---|---|
| Ms | F-Ratio | p | Ms | F-Ratio | p | ||
| Season (S) | 1 | 0.589 | 127.040 | <0.001 | 0.000 | 0.011 | ns |
| Accession (A) | 2 | 0.280 | 60.320 | <0.001 | 0.000 | 0.116 | ns |
| Temperature (T) | 1 | 0.706 | 152.163 | <0.001 | 0.001 | 1.465 | ns |
| PEG | 2 | 5.864 | 1263.792 | <0.001 | 0.099 | 210.780 | <0.001 |
| S*A | 2 | 0.286 | 61.597 | <0.001 | 0.001 | 1.761 | ns |
| S*T | 1 | 0.010 | 2.130 | ns | 0.000 | 0.565 | ns |
| S*PEG | 2 | 0.302 | 65.024 | <0.001 | 0.000 | 0.052 | ns |
| A*T | 2 | 0.078 | 16.880 | <0.001 | 0.001 | 2.142 | ns |
| A*PEG | 4 | 0.172 | 37.121 | <0.001 | 0.004 | 7.809 | <0.001 |
| T*PEG | 2 | 0.492 | 105.938 | <0.001 | 0.000 | 0.722 | ns |
| S*A*T | 2 | 0.028 | 6.024 | <0.01 | 0.000 | 0.114 | ns |
| S*A*PEG | 4 | 0.183 | 39.519 | <0.001 | 0.001 | 1.571 | ns |
| S*T*PEG | 2 | 0.003 | 0.686 | ns | 0.001 | 1.178 | ns |
| A*T*PEG | 4 | 0.034 | 7.305 | <0.001 | 0.001 | 1.216 | ns |
| S*A*T*PEG | 4 | 0.020 | 4.368 | <0.01 | 0.001 | 1.998 | ns |
| Error | 102 | 0.005 | 0.000 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abushamleh, N.H.; El-Keblawy, A.; Mosa, K.A.; Soliman, S.S.M.; Tsombou, F.M. Different Traits Affect Salinity and Drought Tolerance during Germination of Citrullus colocynthis, a Potential Cash Crop in Arid Lands. Seeds 2022, 1, 244-259. https://doi.org/10.3390/seeds1040021
Abushamleh NH, El-Keblawy A, Mosa KA, Soliman SSM, Tsombou FM. Different Traits Affect Salinity and Drought Tolerance during Germination of Citrullus colocynthis, a Potential Cash Crop in Arid Lands. Seeds. 2022; 1(4):244-259. https://doi.org/10.3390/seeds1040021
Chicago/Turabian StyleAbushamleh, Noor Hilal, Ali El-Keblawy, Kareem A. Mosa, Sameh S. M. Soliman, and François Mitterand Tsombou. 2022. "Different Traits Affect Salinity and Drought Tolerance during Germination of Citrullus colocynthis, a Potential Cash Crop in Arid Lands" Seeds 1, no. 4: 244-259. https://doi.org/10.3390/seeds1040021
APA StyleAbushamleh, N. H., El-Keblawy, A., Mosa, K. A., Soliman, S. S. M., & Tsombou, F. M. (2022). Different Traits Affect Salinity and Drought Tolerance during Germination of Citrullus colocynthis, a Potential Cash Crop in Arid Lands. Seeds, 1(4), 244-259. https://doi.org/10.3390/seeds1040021






