Abstract
This study investigates the effect of Gliding Arc Plasma Activated Water (GAPAW) technique on maize germination and growth variables. The effect of GAPAW was evaluated on corn texture, water absorption in Lab conditions, and the pathway Scanning electron microscopy (SEM) analysis of corn seeds was also carry out. Maize seeds were sown 150 m2 and then watered with tap water and with GAPAW. Seed germination, maize growth and chlorophyll content were evaluated in field conditions in a complete randomized block design with four replicates using the Acid Tolerance Population (ATP) variety. Seed texture treated with 15 min of GAPAW making it darker than those of the control (tap water). Pathway SEM analysis showed no change for the 5 min-GAPAW-treated seeds compared to the control but at 15 min-GAPAW significant changes were observed. Germination was 100% at the 15 min-GAPAW compared to the other treatments (0 and 5 min). Stem length, leaf width, collar diameter, chlorophyll content and water uptake have higher values on plants watered with 15 min-GAPAW compared to others treatments. This application could highlight the germination properties of GAPAW in crop production.
1. Introduction
The need for enhancing microbial food safety and quality, without compromising the nutritional, functional, and sensory characteristics of foods, has created an increasing interest in innovative technologies in food industry. Plasma is an emerging, green processing technology offering many potential applications and fulfills the need of the industry [1]. Previously named as “creeping arc” or “glidarc”, plasma was mainly developed for gas treatment. This process is obtained by applying a suitable potential difference from 5 to 10 kV between at least two electrodes with continuously divergent profiles arranged symmetrically around a gas jet [2,3]. A discharge occurs at the minimum spacing; the arc feet formed is pushed towards the ends of the electrodes by a gas flow directed along the axis of the reactor. The ionized channel of the arc lengthens as it progresses, its volume increases and consequently its temperature decreases before it bursts into a hardened plume and is short-circuited by a new arc [4]. The result is a series of sliding discharges that sweep across the inter-electrode space (plume). The transfers of electrical energy into thermal energy supplied to the gas molecules are compensated by a High Voltage Generator. As the arc spreads along the electrodes, its resistance increases as well as its terminal voltage. This technique makes it possible to use high powers (several kW) [5], and thus to produce a much larger quantity of gas and a large number of active species (hydroxyl radical HO• (most reactive), superoxide radical ion O2•− (fairly reactive), ozonide ion O3•− (not very reactive), hydroperoxide radical HO2• (not very reactive), radical HO3• (inert)) [6,7]. The effect, of species present in the plasma depend on the nature of the plasma gas. In the case of humid air, these species are therefore derived from N2, O2 and H2O. Research work has highlighted the presence of ozone, nitrogen oxide and the radicals NO•, HO• and HO2• [8,9]. The glidarc humid air was successfully applied for the inactivation of microorganisms in planktonic form [10,11] or in adherent form to solids [12,13]. In Cameroon, pesticides are normally used for agriculture, including the treatment of livestock, and, to a lesser extent, in the protection of public health via the treatment of stored foodstuffs. Agriculture contributes 21.4% to the Gross National Product [14], with the regulation of pesticides assigned to the National Registration Commission of Phytosanitary Products and Certification of Sprayers, a specialized unit of the Ministry of Agriculture and Rural Development (MINADER). However, exchange between the local populations and neighboring countries facilitates the supply of unauthorized compounds [15]. Pesticide formulations may contain more than one active ingredient and are aimed mainly for protecting crops or storage of foodstuffs to limit annual losses, estimated at 10–40% [16]. In order to implement the effects of nitrogen species generated by the Gliding Arc Plasma Activated Water (GAPAW) technique on corn seed fertilization, plasma technology would offer good prospects for seed germination [17], root and shoot growth [18,19], photosynthesis, nutrients homeostasis, and floral regulation [20,21]. To the best of our knowledge, no studies on germination and fertilizing properties of Gliding Arc Plasma Activated Water on maize have been conducted so far. Therefore, we develop non-thermal plasma in agriculture in order to improve seed germination and maize growth. This paper presents preliminary work on the improvement of maize germination and growth by gliding arc plasma in temporal post discharge action mode. This gliding arc plasma treatment refers to the action of the treated water after the discharge is stopped, and the reactions continue without any further energy source [11,22]. Therefore, the current work includes surface analysis of maize seeds by SEM, physicochemical analysis (pH, conductivity, total dissolved solids, NO3−, H2O2), water uptake, measurement of growth variables (stem length, leaf width, crown diameter) and a physiological parameter as chlorophyll content of activated water sprayed samples compared to control samples.
2. Material and Experimental Methods
2.1. Location of the Study Site
The study was conducted at the Applied Physical and Analytical Chemistry Laboratory of the University of Yaoundé I for the Gliding Arc Plasma activation of water and its characterization at the Dschang branch of the Institute of Agricultural Research for Development research laboratory in terms of growth and physiological parameters of maize plants. Dschang locality is located in the West region of Cameroon, more precisely between 5°25′–5°30′ North Latitude and 10°–10°5′ East Longitude, altitude of 1380 m. During the trial, the average temperature was around 21 °C, and rainfall from 160 mm to 155 mm. The study was conducted in the experimental research farm of the Dschang Agricultural Research Station. Maize seeds (Acid tolerance population variety) were collected from the Institute of Agricultural Research for Development Dschang Station. This variety was chosen because of its adaptability in the agro-ecological zone of humid forest with mono-modal rainfall.
2.2. Glidarc Plasma Device and Treatment Procedure
The experimental apparatus used is a cylinder containing 430 mL of tap water, a glass reactor equipped with a water cooling jacket, and a magnetic stirrer (Figure 1). The sliding discharge device held by the lid has two divergent aluminum electrodes, symmetrically arranged around a wet air nozzle according to the founding father Czernichowski et al. [2]. The moist air is obtained by bubbling the air flow supplied by a compressor through a Durand bottle filled with water. The gas flow rate is controlled by a flow meter set at Q = 800 L/h. Energy is supplied by a 40 kHz, 9–10 kV HV Generator. For this purpose 430 mL of tap water was exposed to the plasma for 5 and 15 min and then 100 mL of activated water respectively was taken and brought into contact with one hundred corn seeds in a beaker. After a contact time (1 h), seeds were dried and reduced to a moisture content of 14%.
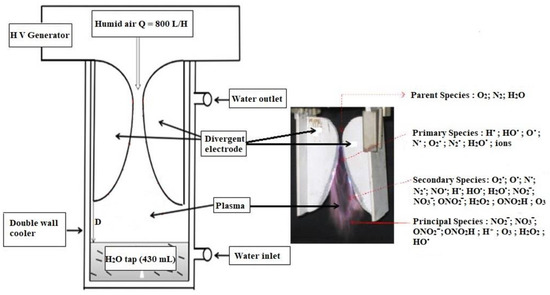
Figure 1.
Experimental-processing system to have plasma activated water.
2.3. Physicochemical Analysis
The determination of pH, Conductivity and TDS was carried out using a HANNA HI 9811-5 pH/°C/EC/TDS multimeter with digital display.
The colorimetric method has been used for the determination of nitrates (515 nm) and hydrogen peroxide [23].
To assess possible morphological changes after contact between seeds and plasma-activated water, scanning electron microscopy (SEM) imaging was performed on three sets of seeds: seeds in contact with tap water (control); seeds in contact with plasma-activated water for 5 min; and seeds in contact with plasma-activated water for 15 min. We produced SEM images of the pericarp using a Philips FEI XL γ0 FEG equipped with a field emission gun. The nonmetal-coated samples were exposed to electrons with an accelerating voltage of 3 and 5 kV.
2.4. Evaluation the Effect of Plasma on Water Absorption and Seed Germination
Concerning water absorption, 100 mL of plasma-treated (5 and 15 min) and untreated (0 min) tap water were placed in glass jars containing 100 maize seeds. The mixtures (seeds + water) were left at a laboratory temperature of 25 °C for forty-eight hours (Figure 2). The seeds were dried and weighed within the first and last twelve hours at six hours intervals. For this purpose, an electronic analytical balance SARTORIUS 1264MP with an accuracy of 0.1 mg was used. Amount of water absorbed was determined from the actual increase in seed weight. Water absorption (WA) was expressed by Equation (1) [18,24]:

Figure 2.
Maize seeds soaked in plasma-activated water for forty-eight hours.
With FW the fresh weight of samples stored in distilled water and DW the dry weight of samples.
Seed germination test was carry out in a fully randomized design with four replications according to International Seed Testing Association procedures [25]. Seeds were sown on an area of 150 m2 at a rate of three seeds per pocket at a spacing of 40 cm in the line and 80 cm between the lines. During the germination period, 100 mL of activated (5, 15 min) and untreated (control) water were sprinkled every six hours on each pocket to maintain soil moisture. Three hundred seeds were sown at three replicates of one hundred seeds per treatment. After ten days, germination rate (GR) was calculated as follow (Equation (2)). The seed was considered to be germinated when the radicle protrusion was 5 cm.
where SG is the number of germinated seeds and ST is the total number of seeds.
2.5. Pathway Scanning Electron Microscopy (SEM) Analysis
Scanning electron microscopy (SEM) imaging was conducted on three sets of seeds: seeds in contact with tap water (control), seeds in contact with plasma activated water treated for 5 min and 15 min. We produced SEM images of pericarp using a Philips FEI XL γ0 FEG brand apparatus equipped with a field emission gun. The nonmetal-coated samples were exposed to electrons with an acceleration voltage of 3 and 5 kV.
2.6. Effects of Plasma on Maize Growth Variables and Chlorophyll Content
Growth variables measured were stem length, leaves width and collar diameter on seedlings receiving 800 mL of plasma activated water weekly. These variables were measured weekly after germination using a graduated ruler. Stem length was measured from the collar to the beginning of the first unfolded leaf while the width of leaves was measured in the middle of the leaf. Collar diameter was measured one month after emergence using a caliper. Chlorophyll content was taken using a chlorophyll meter SPAD-502, on three selected leaves for each plant on thirty days [26].
2.7. Statistical Analysis
All these data based on the average of the four replicates using Microsoft Excel software. Analysis of variances was performed using SPSS software version 2021 at p < 0.05. In the figures, the spread of values is shown as error bars representing standard errors of the means.
3. Results
3.1. Physicochemical Properties of Plasma Activated Water
Data from the physicochemical analyses of plasma activated water are presented in Table 1. It is shown that after a few minutes of exposure to humid air plasma (5 min), the pH of the tap water decreases from 6.4 to 3.4 and then continues to decrease to reach a value of 3.3 after 15 min of treatment. The conductivity and total dissolved solids (TDS) in tap water begin to increase after 5 min of exposure to the electrical discharge. Nitrate ions evolve gradually, increasing with exposure time and becoming more significant after 15 min of treatment. It is found that the concentration of hydrogen peroxide (H2O2) in solution increases with the time of exposure to the electric discharge.

Table 1.
Physicochemical analysis of plasma-activated tap water.
3.2. Effect of Plasma on Water Absorption, Maize Seed Color and Seed Germination
3.2.1. Water Absorption
There was an increase in water absorption of GAPAW fortreated corn seeds compared to untreated one (Figure 3). Water uptake indicated that seeds absorbed rapidly at the onset of imbibition (the first twelve hours), then became slowly in the final hours to reach the values of 41.96%, 38.89%, and 35.13% respectively for seeds soaked at 15, 5 and 0 min. The GAPAW-treated samples for 5 min and 15 min absorbed more water than the control samples, regardless of the imbibition time. In addition, no significant difference was recorded in the two groups of seeds treated at 5 min and 15 min, indicating that the duration of the treatment had no significant influence on water absorption.
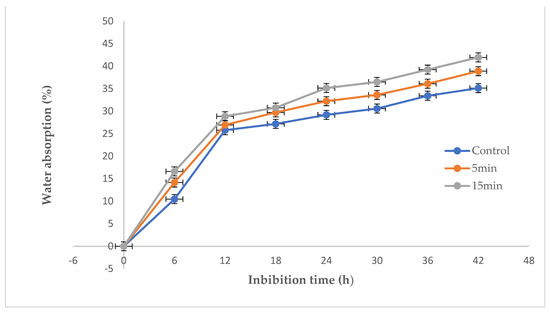
Figure 3.
Water absorption as a function of the imbibition time at 25 °C.
3.2.2. Maize Seed Color
Maize seeds color did not change after 5 min of treatment, but from 15 min of treatment, change in color appears on the surface of maize seeds treated with GAPAW which became darker compared to others treatments. At that treatment time, the pulp of the kernels was darker in color (Figure 4). These photos were taken to explain the change in the in seeds color. In fact, neutral oxygen species, especially atomic oxygen, induce a minor structural change or functional inhibition of the cell membranes of seeds, which leads to the oxidation of intracellular organelles via the penetration of ROS into the cell.
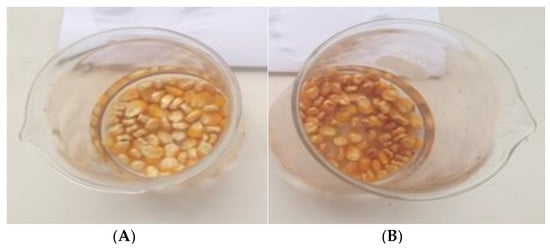
Figure 4.
Color change of GAPAW treated seeds at 0 min (A) and 15 min (B).
3.2.3. Seed Germination
Growth variables of untreated (0 min) and treated (5 and 15 min) seeds after ten days are shown in Figure 5. The most significant response was obtained with seeds treated for 15 min with 100% germination rate, compared to untreated seeds (83.3%). No significant difference was recorded in germination at 5 min treatment time compared to the control (p > 0.05).
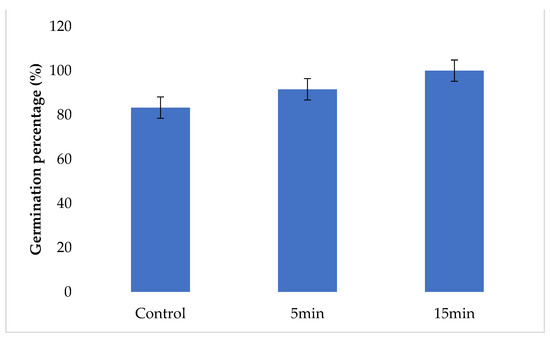
Figure 5.
Seeds germination (%) after being treated during 5 and 15 min with GAPAW.
3.3. Pathway Scanning Electron Microscopy
SEM micrographs showed that the 15 min treatment significantly affected the crystal structure, molecular structure and rheological properties of maize starch (Figure 6). It seems that some debris-like contamination was noted on the seeds treated by activated water. Due to the presence of pinhole-like structures in the starch granules, the GAPAW treatment not only modified the surface of the starch granules but also penetrated into the granules, resulting in water holes and large channels. On the other hand, the 5 min GAPAW did not cause any degradation of the maize seeds surface (Figure 7).
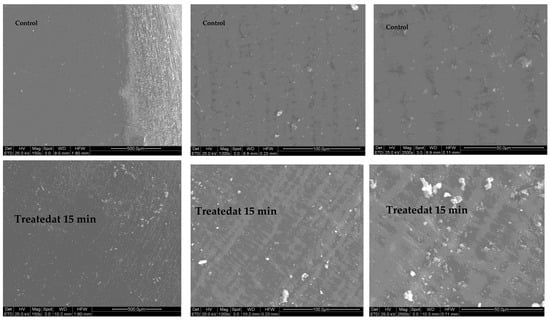
Figure 6.
SEM micrographs taken for 0 and 15 min after treated with GAPAW.
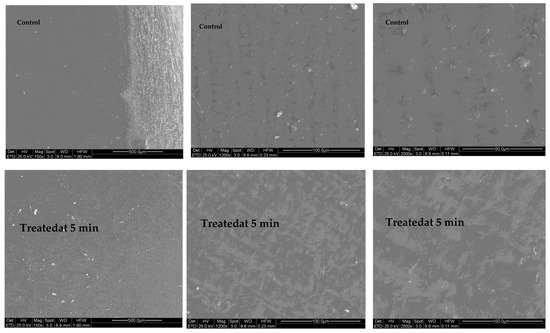
Figure 7.
SEM micrographs taken for 0 and 5 min after treated with GAPAW.
3.4. Effect of Plasma on Maize Growth Variables and Chlorophyll Content
3.4.1. Stem Length
Regarding stem length (Figure 8), the results indicated an average length of 11.58 cm for seeds not watered with GAPAW four weeks after sowing. However, the stem length increased with treatment time to reach the values of 12.84, 12.15 cm when maize plants were watered with GAPAW 15 and 5 min respectively. GAPAW treatment did not negatively affect stem length, as a slight increase was obtained, although it was not significantly different (p > 0.05) regardless of treatment time (compared to control).
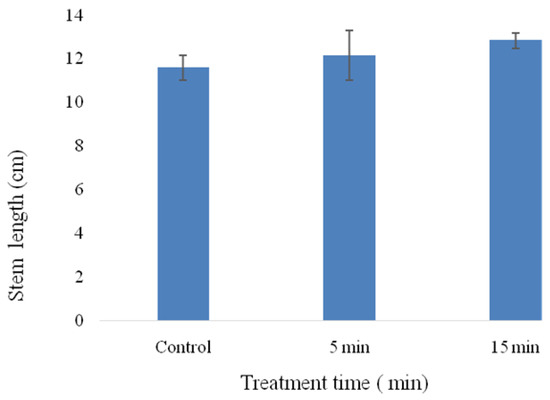
Figure 8.
Stem length of one month old maize seedlings treated different time with GAPAW.
3.4.2. Leaf Width and Collar Diameter
Leaf width increases thirty days after sowing (Figure 9). Values obtained were 1.58, 1.61 and 1.74 cm respectively for 0, 5 and 15 min after four weeks of watering (Figure 10). Collar diameter also varies with the GAPAW processing time. In fact, collar diameter was 0.37, 0.37 and 0.38 cm respectively for 0, 5 and 15 min.

Figure 9.
Maize with seeds not treated (A) and treated for 15 min (B) with activated water plasma, thirty days after sowing.
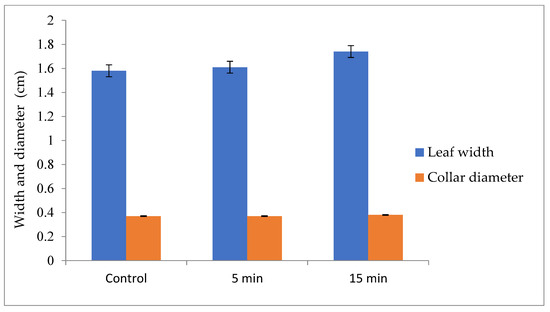
Figure 10.
Leaf width (cm) and collar diameter (cm) at thirty days after sowing.
3.4.3. Chlorophyll Content
Plants watered with plasma activated water showed an increase in their chlorophyll content with treatment time, chlorophyll content were 23.91, 25.32 and 29.66 mg/g on plants treated with GAPAW at 0, 5 and 15 min respectively (Figure 11).
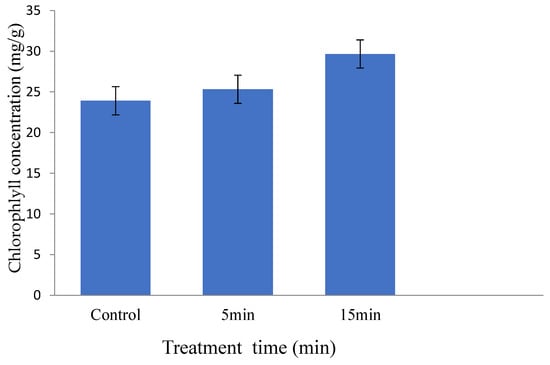
Figure 11.
Chlorophyll concentration on maize plants watered for 0, 5 and 15 min during four weeks.
4. Discussion
The physicochemical analyses of Plasma activated water shown that after a few minutes of exposure to humid air plasma, the pH of the tap water decreases. This result is in line with those obtained by some researchers who explained the gradual decrease in pH during plasma treatment due to the increase in treatment time, which further promotes the production of active species that diffuse into the aqueous solution [27]. Indeed, the presence of the NO• radicalsin the discharge in contact with air leads to the formation of NOx which can hydrolyze into nitrous, nitric and peroxonitric acids in solution [23]. The conductivity and total dissolved salts in tap water only begin to increase significantly after 5 min of exposure to the electrical discharge. The relative variations of these two parameters can be explained on the one hand by the change in pH. Indeed, the decrease in pH is accompanied by an increase in conductivity and total dissolved solids, as these two parameters are closely related. Furthermore, the study of the effect of ionizing radiation in the liquid phase [28], electrolysis of the discharge at liquid-gas interfaces [29] and electronic collisions with water vapor in moist gases [30] suggest the following reactions [31]:
These reactions lead to the formation of •OH and H3O+. Also the high mobility ofoxonium ion H3O+ in water contributes to the increase in conductivity due to these cations compared to other cations of similar size [32]. Table 1 shows that the release of ions and NO3- begins within the first few minutes of exposure of tap water to the plasma discharge. Nitrate ions evolve gradually, increasing with exposure time and becoming more significant after 15 min of treatment. This phenomenon could be explained by the oxidation of nitrite ions to nitrate ions using the following equation [33,34]:
Nitrate plays an important role in the photosynthesis process. Without nitrates, the amount of chlorophylls in leaves is reduced, which means leaves turn pale green or yellow.
Based on the values obtained at different concentrations of hydrogen peroxide in solution, it is found that the concentration of H2O2 decreases with increasing exposure time. These results were similar to some previous work showing that the radicals formed upstream in the landfill (HO• and HO2•) responsible for the formation of H2O2 would not recombine sufficiently or that these radicals would preferentially participate in other reactions [35]. Also, the high solubility of hydrogen peroxide in water would contribute kinetically to make it react spontaneously in aqueous solution as soon as it is formed in the gas phase in the landfill [31]. The GAPAW treated samples for 5 min and 15 min absorbed more water than the control samples, regardless of the imbibition time. The initial absorption of water is necessarily related to the hydrophilic of the seed surface. Previously, we have shown an increase in the hydrophilicity of the surface of kaolinitic clay after a humid air plasma treatment using the same device, due to the increase of hydroxyl groups at the surface of the material [36]. Other previous studies using the gliding arc plasma with the sample placed in the discharge area have also shown an increase in the hydrophilicity of polymeric and metallic materials [37]. These results were obtained on wheat seeds [38] and on soybeans [39]. A reduction in the contact angle, but on non-living materials was also observed using Gliding plasma [40,41].
Results on the color change of maize seeds could be explained by the plasma treatment temporal post-discharge mode changed color of the plant organs surfaces. For example, the effect of atmospheric double barrier discharge (DBD) of kiwifruit exhibited a lesser degree of darkening during storage compared to untreated samples, suggesting that the change in texture is likely due to physiological events that include enzymatic degradation of hemicelluloses [42,43]. Furthermore, it is known that RONS and UV-photons generated by NTP react with the components of the seed surface or penetrate the external layer of the seed coat, inducing significant changes in the elemental composition of the seed [44].
Germination is the beginning of the development for a new plant individual, from a seed placed in favorable conditions. There are various events associated with germination ranging from the initial absorption of water by dry seeds to the emergence of the radicle through the seed coat. Seed germination is accompanied by intensive production of oxidizing species, such as superoxide anion, hydrogen peroxide, NO, and its derivatives. Protein contents can be an indicator for faster germination [45]. Concerning the germination of maize seeds with GAPAW the result corroborates previous studies when using glidarc plasma treatment in spatial post discharge (gas transfer) mode [46]. Consistent with these authors, the plasma treatment had an influence on seed germination of 96% for maize seeds treated at 5 min, which was much higher than the 78% obtained for untreated seeds.
SEM photographs showed that the 15 min treatment significantly affected the morphology of maize starch (Figure 6). This change has been observed previously [47], using the low-pressure plasma to modify the microstructure and textural properties of brown rice. Their study revealed an etching of brown rice surface after the plasma treatment for 18 min. The shorter treatment time (5 min) and the low concentration of chemical species in solution, especially NO3-, H2O2, may explain this observation. This result is consistent with that obtained on maize seeds using DBD cold atmospheric plasma [48] and on another cereal seeds [49,50]. In fact, the activated species of the plasma did not cause any surface degradation, erosion or scratches for short treatment time.
The increase of stem length 30 days after sowing by GAPAW corroborates previous results [51] which show an improvement stem length of 1.33 and 1.31 times compared to the control, respectively for the treatment times of 1 and 2 min of the corn plants after four weeks of growth. In addition, similar results were obtained with the standardized lengths of radish seedlings; for 0 min 1.25; for 5 min 1.52 and 10 min 1.62 [52]. The differences between the studies may be caused by the different maize cultivars used, the different type of working gas, the nature of the instruments used, and the type of plasma discharge [19]. This suggests that stimulating effect of GAPAW increases the nitrogen concentration in the leaves, thus promoting seedling growth. Therefore, the more a plant is rich in nitrogenous elements, the larger are the leaves.
Plants sprayed with plasma-activated water showed an increase in chlorophyll content with treatment time, probably due to the increased concentration of RONS in the water. Indeed, it was clearly establish that an increase in chlorophyll content of to 1.11 mg/g compared to control for corn watered non-thermal air plasma at 1 min [51]. Moreover, the chlorophyll level increased with the activity of the water activated plasma and the increasing concentrations of RONS; compared to the control, the plants watered for 2 min showed an increase of 17% [53,54]. This suggests that the species (H2O2, NO2− and NO3−) generated by the GAPAW treatment increases the total metabolism of the plant modulates the process of photosynthesis, affects the synthesis of starch and chlorophyll [55].
5. Conclusions
The objective of this study was to improve seed germination and maize growth variables using Gliding Arc Plasma Activated Water. The study shows that when maize seeds are watered with GAPAW during5 to 15 min seed germination and growth variables are improved. These results correlate well with SEM analyses, and physicochemical analyses (pH, NO3-, H2O2, conductivity, TDS) carry out on un-watered and watered seeds. It will be more interesting to change the nature of the plasmagenic gas and optimize the plasma treatment time in order to obtain better results.
Author Contributions
Conceptualization, S.L., G.K.Y. and J.P.K.M.; methodology, J.P.K.M., J.D.F., B.S.-T. and G.K.Y.; software, J.D.F.; validation, S.L. and J.D.F.; formal analysis, J.P.K.M. and H.N.A.M.; investigation, M.J.T. and M.C.N.N.; resources, M.C.N.N. and M.J.T.; data curation, B.S.-T. and J.P.K.M.; writing—original draft preparation, J.P.K.M. and B.S.-T.; writing—review and editing, J.D.F., S.L., G.K.Y. and H.N.A.M.; reading, H.N.A.M. and J.P.K.M.; supervision, S.L. and J.D.F.; project administration, J.P.K.M.; funding acquisition, J.P.K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Authors are grateful to Kamseu Elie for SEM photography and the Engineering Department “Enzo Ferrari” of the Università degli Studi di Modena Reggio Emilia, Italy for scientific collaboration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shabir, A.M.; Manzoor, A.S.; Mohammad, M.M. Understanding the role of plasma technology in food industry. Food Bioprocess Technol. 2016, 9, 734–750. [Google Scholar] [CrossRef]
- Czernichowski, A. Gliding arc: Applications to engineering and environment control. Pure Appl. Chem. 1994, 66, 1301–1310. [Google Scholar] [CrossRef]
- Lesueur, H.; Czernichowski, A.; Chapelle, J. Electrically assisted partial oxidation of methane. Int. J. Hydrog. Energy 1994, 19, 139–144. [Google Scholar] [CrossRef]
- Brisset, J.L. Décharges Electriques Glissantes à la Pression Atmosphérique et Leurs Applications à L’environnement; Union des Professeurs de Physique et de Chimie: Paris, France, 2009; p. 103. [Google Scholar]
- Fridman, A.A.; Petrousov, A.; Chapelle, J.; Cormier, J.M.; Czernichowski, A.; Lesueur, H.; Stevefelt, J. Modèle physique de l’arc glissant. J. Phys. III 1994, 4, 1449–1465. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Prados, M.; Paillard, H.; Roche, P. Hydroxyl Radical Oxidation Processes for the Removal of Triazine from Natural Water. Ozone Sci. Eng. 1995, 17, 183–194. [Google Scholar] [CrossRef]
- Clements, J.S.; Sato, M.; Davis, R.H. Preliminary investigation of pre-breakdown phenomena and chemical reactions using a pulsed high voltage discharge in water. IEEE Trans. Ind. Appl. 1985, 23, 1372–1379. [Google Scholar]
- Burlica, R.; Kirkpatrick, M.J.; Locke, B.R. Formation of reactive species in gliding arc discharges with liquid water. J. Electrost. 2006, 64, 35–43. [Google Scholar] [CrossRef]
- Moreau, M.; Feuilloley, M.G.J.; Veron, W.; Meylheuc, T.; Chevalier, S.; Brisset, J.L.; Orange, N. Gliding arc discharge in the potato pathogen Erwinia carotovora subsp. atroseptica: Mechanism of lethal action and effect on membrane-associated molecules. Appl. Environ. Microbiol. 2007, 73, 5904–5910. [Google Scholar]
- Kamgang-Youbi, J.; Herry, M.; Bellon-Fontaine, M.; Brisset, J.; Doubla, A.; Naiïtali, M. Evidence of temporal post discharge decontamination of bacteria by gliding electric discharges: Application to Hafniaalvei. Appl. Environ. Microbiol. 2007, 73, 4791–4796. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, J.O.; Briandet, R.; Herry, J.M.; Brisset, J.L.; Naïtali, M. Destruction of planktonic, adherent and biofilm cells of Staphylococcus epidermidis using a gliding discharge in humid air. J. Appl. Microbiol. 2007, 103, 621–628. [Google Scholar] [CrossRef]
- Dasan, B.G.; Onal-Ulusoy, B.; Pawlat, J.; Diatczyk, J.; Sen, Y.; Mutlu, M. A New and Simple Approach for Decontamination of Food Contact Surfaces with Gliding Arc Discharge Atmospheric Non-Thermal Plasma. Food Bioprocess Technol. 2016, 10, 650–661. [Google Scholar] [CrossRef]
- INS. Quatrième Enquête Camerounaise Auprès des Ménages-Présentation des Bases de Données. Institut National de la Sta-tistique, Yaoundé (Cameroun). [Cameroon Fourth Household Survey. National Institute of Statistic]. 2014. Available online: http://www.statistics-cameroon.org/ins/publications.htm (accessed on 10 September 2022).
- Sonchieu, J. Selling pesticides in Ngaoundere, Cameroon. Crop Prot. 2006, 48, 180–181. [Google Scholar]
- Shaaya, E.; Kostjukovski, M.; Eilberg, J.; Sukprakarn, C. Plant oils as fumigants and contact pesticides for the control of stored-product insects. J. Stored Prod. Res. 1997, 33, 7–15. [Google Scholar] [CrossRef]
- Randeniya, L.K.; de-Groot, G.J.J.B. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A review. Plasma Chem. Plasma Process. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.D. The effects of non-thermal plasma treatment on wheat germination. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Ling, L.; Jiangang, L.; Minchong, S.; Chunlei, Z.; Yuanhua, D. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 13033. [Google Scholar] [CrossRef] [PubMed]
- Sera, B.; Sery, M.; Gavril, B. Seed germination and early growth responses to seed pre-treatment by non-thermal plasma in hemp cultivars (Cannabis sativa L.). Plasma Chem. Plasma Process. 2017, 37, 207–221. [Google Scholar] [CrossRef]
- Šerá, B.; Vanková, R.; Roháček, K.; Šerý, M. Gliding Arc Plasma Treatment of Maize (Zea mays L.) Grains Promotes Seed Germination and Early Growth, Affecting Hormone Pools, but Not Significantly Photosynthetic Parameters. Agronomy 2021, 11, 2066. [Google Scholar] [CrossRef]
- Kolbert, Z.; Feigl, G.; Freschi, L.; Poór, P. Gasotransmitters inaction: Nitric oxide-ethylene crosstalk during plant growth and abiotic stress responses. Antioxidants 2019, 8, 167. [Google Scholar] [CrossRef]
- Khan, M.N.; Alamri, S.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedi, B.; Ali, H.M.; Siddiqui, M.H. Effect of nitric oxide on seed germination and seedling development of tomato under chromium toxicity. J. Plant Growth Regul. 2021, 40, 2358–2370. [Google Scholar] [CrossRef]
- Brisset, J.-L.; Moussa, D.; Doubla, A.; Hnatiuc, E.; Hnatiuc, B.; Youbi, G.K.; Herry, J.-M.; Naïtali, M.; Bellon-Fontaine, M.-N. Chemical Reactivity of Discharges and Temporal Post-Discharges in Plasma Treatment of Aqueous Media: Examples of Gliding Discharge Treated Solutions. Ind. Eng. Chem. Res. 2008, 47, 5761–5781. [Google Scholar] [CrossRef]
- Hoang, L.V. Comparaison des Rendements Synergétiques de Dégradation de Trois Composés Organiques par Plusieurs Pro-cédés D’oxydations Avancées en Milieu Aqueux. PhD Thesis, Poitiers University, Poitiers, France, 2009; p. 57. [Google Scholar]
- Molina, R.; López-Santos, C.; Gómez-Ramírez, A.; Vílchez, A.; Espinós, J.P.; González-Elipe, A.R. Influence of irrigation conditions in the germination of plasma treated Nasturtium seeds. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). Seed Sci. Tech. 2001, 29, 1–127. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing-1168.html (accessed on 10 September 2022).
- Sarinont, T.; Amano, T.; Koga, K. Effects of atmospheric air plasma irradiation to seeds of radish sprouts on chlorophyll and carotenoids concentrations in their leaves. MRS Online Proc. Libr. 2014, 1723, 34–38. [Google Scholar] [CrossRef]
- Benstaali, B.; Moussa, D.; Addou, A.; Brisset, J.-L. Plasma treatment of aqueous solutes: Some chemical properties of a glid-ing arc in humid air. Eur. Phys. J. Appl. Phys. 1998, 4, 171–179. [Google Scholar] [CrossRef]
- Buxton, G.V. Radiation chemistry of the liquid state (1) water and homogeneous aqueous solutions. In Radiation Chemistry, Principles and Applications; Farhataziz, A., Rodgers, M.A.J., Eds.; VCH: Weinheim, Germany, 1987; pp. 321–376. [Google Scholar]
- Hickling, A. Electrochemical processes in glow discharge at the gas–solution interface. In Modern Aspects of Electrochemistry; O’Bockris, J.M., Conway, B.E., Eds.; Plenum Press: New York, NY, USA, 1971; pp. 329–373. [Google Scholar]
- Dolan, T. Electron and ion collisions with water-vapor. J. Phys. 1993, 26, 4–8. [Google Scholar]
- Brisset, J.L.; Hnatiuc, E. Peroxynitrite are-examination of the chemical properties of non-thermal discharges burning in air over aqueous solutions. Plasma Chem. Plasma Process. 2012, 32, 655–674. [Google Scholar] [CrossRef]
- Kornyshev, A.A.; Kuznetsov, A.M.; Spohr, E.; Ulstrup, J. Kinetics of Proton Transport in Water. J. Phys. Chem. B 2003, 107, 3351–3366. [Google Scholar] [CrossRef]
- Brisset, J.L.; Benstaali, B.; Moussa, D.; Fanmoe, J.; Njoyim-Tamungang, E. Acidity control of plasma chemical oxidation: Application to dye removal, urban wastes abatement and microbial inactivation. Plasma Source Sci. Technol. 2011, 20, 034021. [Google Scholar] [CrossRef]
- Kamgang, G. Propriétés Réactives en Post-Décharge Temporelle des Décharges Electriques Glissantes dans l’air Humide: Ap-plication à la Dégradation de Colorant Azoïque et à la Décontamination Microbienne. [Reactive Properties in Temporal after Discharge of Sliding Electric Discharges in Humid Area: Application to azo dye Degradation and Microbial Decontamination]. Ph.D. Thesis, Université de Rouen et de Yaoundé I, Rouen, France, 2008. [Google Scholar]
- Tamo, B.S.; Kamgang-Youbi, G.; Acayanka, E.; Simo, L.M.; Tiya-Djowe, A.; Kuete-Saa, D.; Laminsi, S.; Tchadje, L. Plasma chemical functionalization of a Cameroonian kaolinite clay for a greater hydrophilicity. Plasma Chem. Plasma Process. 2016, 36, 1449–1469. [Google Scholar] [CrossRef]
- Kamgang, J.O.; Naitali, M.; Herry, J.M.; Bellon-Fontaine, M.N.; Brisset, J.L.; Briandet, R. Increase in the hydrophilicity and Lewis acid base properties of solid surfaces achieved by electric gliding discharge in humid air: Effects on bacterial ad-herence. Plasma Sci. Technol. 2009, 11, 187. [Google Scholar] [CrossRef]
- Velichko, I.; Gordeev, I.; Shelemin, A.; Nikitin, D.; Brinar, J.; Pleskunov, P.; Choukourov, A.; Pazderů, K.; Pulkrábek, J. Plasma Jet and Dielectric Barrier Discharge Treatment of Wheat Seeds. Plasma Chem. Plasma Process 2019, 39, 913–928. [Google Scholar] [CrossRef]
- Faubert, F.; Wartel, M.; Pellerin, N.; Cochet, V.; Regnier, E.; Hnatiuc, B. Treatment by gliding arc of epoxy resin: Prelimi-nary analysis of surface modifications. In Advanced Topics in Optoelectronics, Microelectronics, and Nanotechnologies VIII; SPIE: Bellingham, WA, USA, 2016; Volume 10010, pp. 884–894. [Google Scholar] [CrossRef]
- Varoquaux, P.; Wiley, R.C. Biological and biochemical changes in minimally processed refrigerated fruits and vegeta-bles. In Minimally Processed Refrigerated Fruits & Vegetables; Springer: Boston, MA, USA, 1994; pp. 226–268. [Google Scholar]
- Rocculi, P.; Romani, S.; Dalla Rosa, M. Effect of MAP with argon and nitrous oxide on quality maintenance of minimally processed kiwifruit. Postharvest Biol. Technol. 2005, 35, 319–328. [Google Scholar] [CrossRef]
- Ramazzina, I.; Berardinelli, A.; Rizzi, F.; Tappi, S.; Ragni, L.; Sacchetti, G.; Rocculi, P. Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit. Postharvest Biol. Technol. 2015, 107, 55–65. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Henselová, M.; Slováková, L.; Martinka, M.; Zahoranová, A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 2012, 67, 490–497. [Google Scholar] [CrossRef]
- Kamseu Mogo, J.-P.; Kamgang Youbi, G.; Djepang, S.A.; Tamo, B.S.; Laminsi, S. Treatment of Maize Seeds (Zea mays L.) by Nonthermal Plasma Generated by Gliding Electric Discharge for Application in Agriculture. IEEE Trans. Plasma Sci. 2021, 49, 2318–2328. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, Y.K.; Chang, H.C. Evaluation of physicochemical properties of plasma treated brown rice. Food Chem. 2012, 135, 74–79. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface mi-croorganisms inactivation. Plasma Chem. Plasma Process 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Šerá, B.; Špatenka, P.; Šerý, M.; Vrchotová, N.; Hrušková, I. Influence of plasma treatment on wheat and oat germination and earlygrowth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Starič, P.; GrobelnikMlakar, S.; Junkar, I. Response of Two Different Wheat Varieties to Glow and Afterglow Oxygen Plasma. Plants 2021, 10, 1728. [Google Scholar] [CrossRef]
- Ndiffo-Yemeli, G.B.; Švubová, R.; Kostolani, D.; Kyzek, S.; Machala, Z. The effect of water activated by non-thermal air plasma on the growth of farm plants: Case of maize and barley. Plasma Process. Polym. 2020, 18, 2000205. [Google Scholar] [CrossRef]
- Thapanut, S.; Ryu, K.; Yosuke, W.; Kazunori, K.; Masaharu, S. Plant growth enhancement of seeds immersed in plasma activated water. Mater. Res. Soc. 2017, 2, 995–1000. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, L.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Marques, N.C.; Semedo, J.N.; Matos, M.C.; Quartin, V.L. Photosynthetic performance and pigment com-position of leaves from two tropical species is determined by light quality. Plant Biol. 2002, 4, 112–120. [Google Scholar] [CrossRef]
- Danilejko, Y.K.; Belov, S.V.; Egorov, A.B.; Lukanin, V.I.; Sidorov, V.A.; Apasheva, L.M.; Gudkov, S.V. Increase of Productivity and Neutralization of Pathological Processes in Plants of Grain and Fruit Crops with the Help of Aqueous Solutions Activated by Plasma of High-Frequency Glow Discharge. Plants 2021, 10, 2161. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).