Morphological and Seed Germination Behavior of Three Herba Swertiae Species from Hulunbuir, Inner Mongolia: Temperature and Substrate Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. External Characteristic Observation of Seeds
2.2.2. Measurement of the Weight of 1000 Seeds
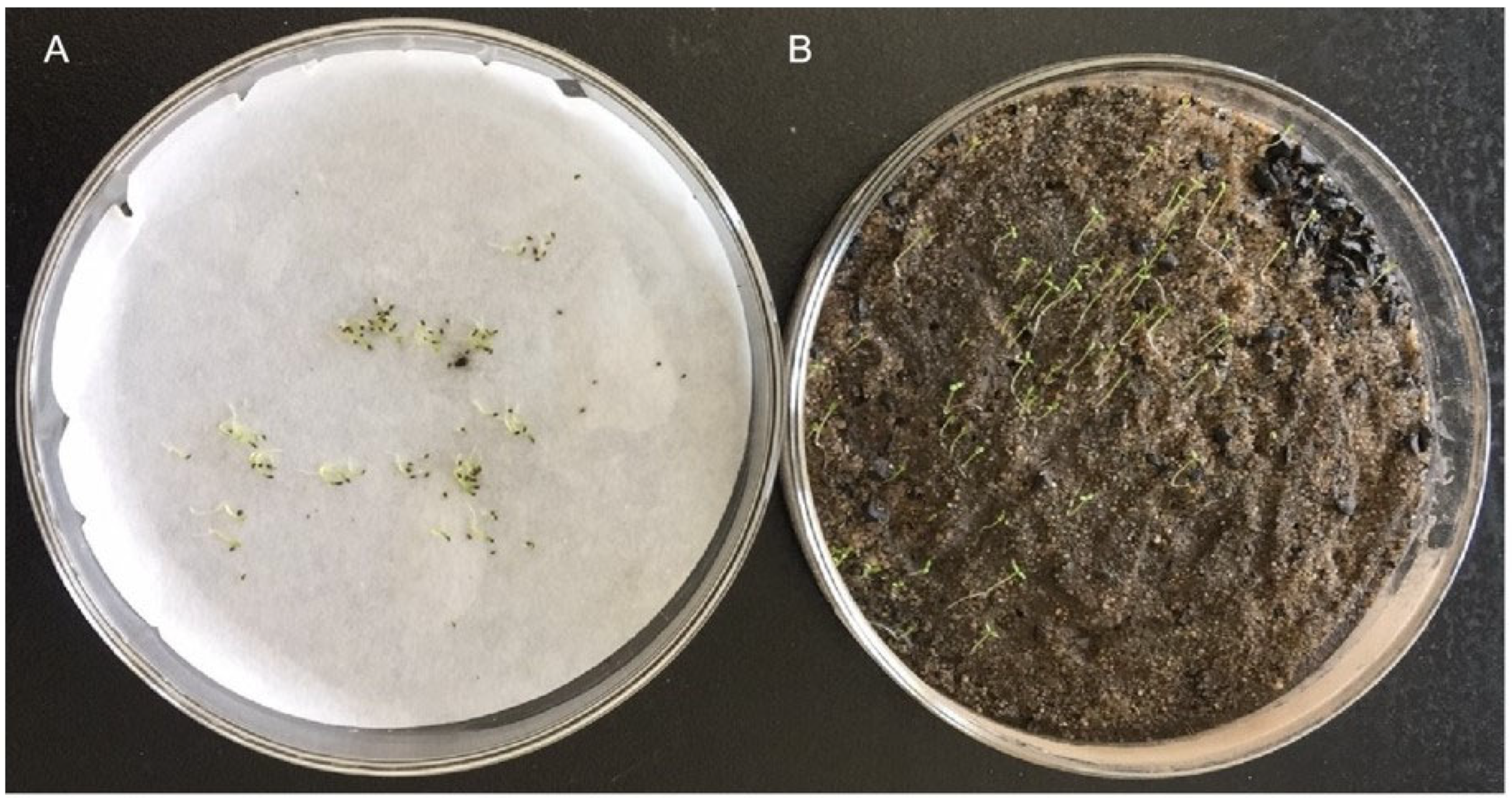
2.2.3. Seed Germination Test
2.3. Indicator Determination
2.4. Statistical Analysis
3. Results
3.1. Morphological Characteristics
3.2. Weight of 1000 Seeds
3.3. Seed Ggermination Characteristics
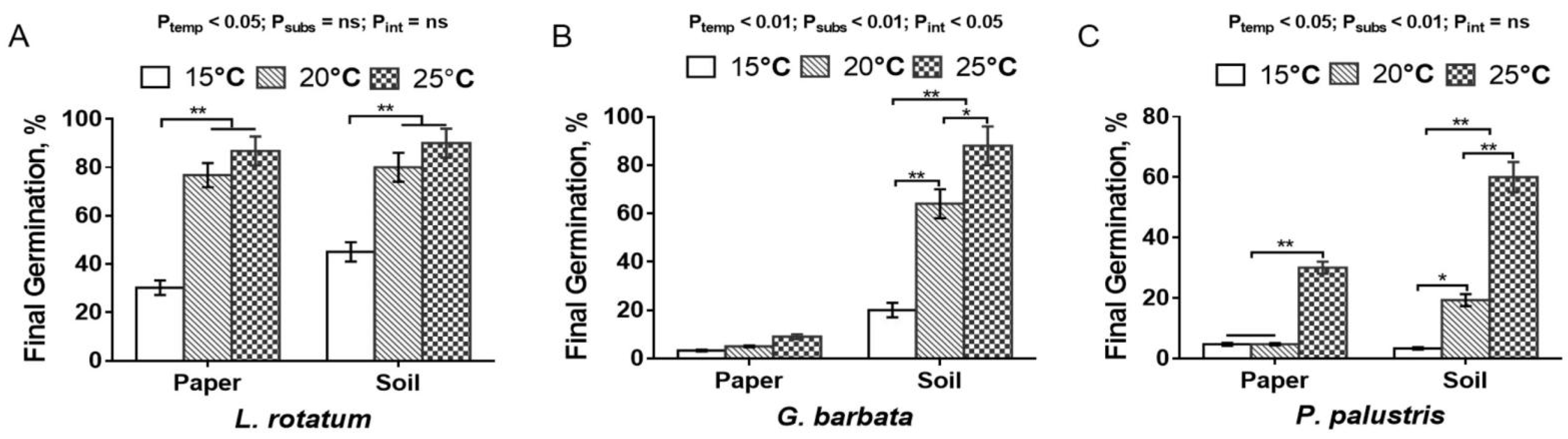
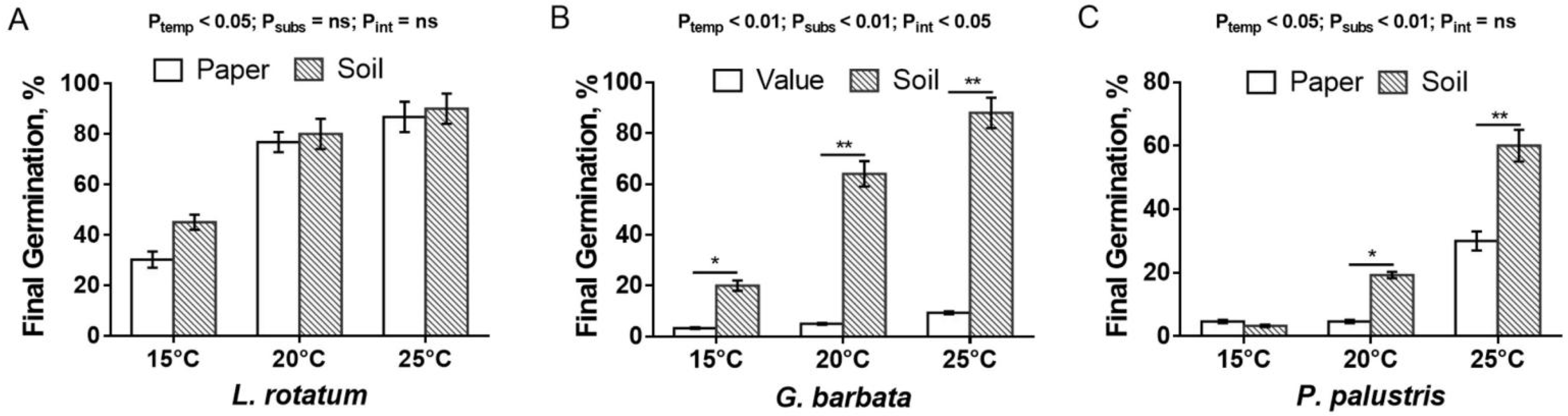
3.3.1. Effect of Temperature on Seed Germination
3.3.2. Effects of Substrates on Seed Germination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pharmacopoeia Committee; Ministry of Health; PRC. Pharmaceutical standards of ministry of health of the People’s Republic of China. Mong. Med. Vol. 1998, 15, 36–91. [Google Scholar]
- Bada, R.H.; Li, J. Encyclopedia of Traditional Mongolian Medicine; Inner Mongolia, People’s Publishing House: Huhhot, China, 2012. [Google Scholar]
- Wurchaih, W.; Huar, H.; Menggenqiqig, M.; Khasbagan, K. Medicinal wild plants used by the Mongol herdsmen in Bairin Area of Inner Mongolia and its comparative study between TMM and TCM. J. Ethnobiol. Ethnomed. 2019, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhu, X.G.; Yin, L.T. Application of Traditional Mongolian Medicine “DIGEDA” in Mongolian Medicine Compound Preparation. Moder. Chin. Med. 2018, 20, 1583–1592. [Google Scholar]
- Chen, R.H.; Wang, Q.; Zhao, L.J.; Yang, S.L.; Li, Z.F.; Feng, Y.L.; Chen, J.Q.; Ong, C.N.; Zhang, H. Lomatogonium Rotatum for Treatment of Acute Liver Injury in Mice: A Metabolomics Study. Metabolites 2019, 9, 227. [Google Scholar] [CrossRef]
- Bao, L.d.; Hu, L.X.; Zhang, Y.; Wang, Y.I. Hypolipidemic effects of flavonoids extracted from Lomatogonium rotatum. Exp. Ther. Med. 2016, 11, 1417–1424. [Google Scholar] [CrossRef]
- Guo, C.; Shen, Y.; Shi, F. Effect of Temperature, Light, and Storage Time on the Seed Germination of Pinus bungeana Zucc. ex Endl.: The Role of Seed-Covering Layers and Abscisic Acid Changes. Forests 2020, 11, 300. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Mamani, G.; Chuquillanqui, H.; Chumbiauca, S.; Sahley, C.; Alonso, A.; Linares-Palomino, R. Substrate, moisture, temperature and seed germination of the threatened endemic tree Eriotheca vargasii (Malvaceae). Rev. Biol. Tropical. 2018, 66, 1162. [Google Scholar] [CrossRef]
- GB/T 2930.4-2001; Seed Inspection Regulations of the State Administration of Quality and Technical Supervision. Standards Press of China: Beijing, China, 2001.
- Motulsky, H.J. GraphPad Statistics Guide. Available online: http://www.graphpad.com/guides/prism/7/statistics/index.htm (accessed on 5 March 2016).
- Bareke, T. Biology of seed development and germination physiology. Adv. Plants Agric. Res. 2018, 8, 336–346. [Google Scholar] [CrossRef]
- Xie, Y.J.; He, Z.P. Seed characteristics and seedling breeding techniques of Sophora flavescens. Bullet. Agric. Sci. Tech. 2012, 4, 195–196. [Google Scholar]
- Gao, Y.H.; Liu, X. Study on seed germination of Clematis aethusifolia. Chin. J. Ethnic Med. 2019, 6, 38–40. [Google Scholar]
- Gutterman, Y. Seed Germination in Desert Plants; Springer: Berlin, Germany, 1993. [Google Scholar]
- Aubert, L.; Decamps, C.; Jacquemin, G.; Quinet, M. Comparison of Plant Morphology, Yield and Nutritional Quality of Fagopyrum esculentum and Fagopyrum tataricum Grown under Field Conditions in Belgium. Plants 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Siqin, B.T.; Hasi, B.G.; Wu, R.N.; Wei, X.J. Study on physiological characteristics of seed germination of Ephedra sinica. J. Chin. Med. Mater. 2009, 32, 656–659. [Google Scholar] [CrossRef]
- Mi, Y.W.; Gong, C.W.; Wang, G.X.; Qi, Y.H. Effects of Angelica sinensis fruit wing on water absorption and germination of seeds. Bull. Bot. Res. 2021, 41, 174–179. [Google Scholar]
- Thompson, K. Seed and seed banks. New Phytol. 1987, 106, 23–24. [Google Scholar] [CrossRef]
- Honarmand, S.J.; Nosratti, I.; Nazari, K.; Heidari, H. Factors affecting the seed germination and seedling emergence of muskweed (Myagrum perfoliatum). Weed Biol. Manag. 2016, 16, 186–193. [Google Scholar] [CrossRef]
- Powell, A.A. Morphological and physiological characteristics of seeds and their capacity to germinate and survive. Ann. Bot. 2010, 105, 975–976. [Google Scholar] [CrossRef]
- Li, X.X.; Yang, H.S.; Ba, G.N.; Bao, J.H.; Sun, D.Z. Seed Germination Condition of Mongolia Medicine Plant Lomatogonium rotatum. J. Agronomy 2017, 7, 48–51. [Google Scholar]
- Abe, M.; Kurashima, A.; Maegawa, M. Temperature requirements for seed germination and seedling growth of Zostera marina from central Japan. Fish Sci. 2008, 74, 589–593. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Badola, H.K. Chemical Stimulation of Seed Germination in ex situ Produced Seeds in Swertia chirayita, A Critically Endangered Medicinal Herb. Res. J. Seed Sci. 2010, 3, 139–149. [Google Scholar] [CrossRef][Green Version]
- Pradhan, B.K.; Badola, H.K. Effects of microhabitat, light and temperature on seed germination of a critically endangered Himalayan medicinal herb, Swertia chirayita: Conservation implications. Plant Biosyst. 2012, 146, 345–351. [Google Scholar] [CrossRef]
- Lee, J.; Chauhan, B.S.; Johnson, D.E. Germination of fresh horse purslane (Trianthema portulacastrum) seeds in response to different environmental factors. Weed Sci. 2011, 59, 495–499. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Xu, H.; Dong, L. Factors affecting seed germination and seedling emergence of Asia minor bluegrass (Polypogon fugax). Weed Sci. 2015, 63, 440–447. [Google Scholar] [CrossRef]
- Ghersa, C.M.; Arnold, R.L.B.; Martinez-Ghersa, M.A. The role of fluctuating temperatures in germination and establishment of Sorghum halepense. Regulation of germination at increasing depths. Funct. Ecol. 1992, 6, 460–468. [Google Scholar] [CrossRef]
- Lei, L.; Zhu, X.; Wang, S.; Zhu, M.; Carver, B.F.; Yan, L.L. TaMFT-A1 is associated with seed germination sensitive to temperature in winter wheat. PLoS ONE 2013, 8, e73330. [Google Scholar] [CrossRef]
- Liao, C.K.; Mao, R.F.; Zhang, W.L.; Zheng, S.W.; Zhang, S.L.; Jiang, J.H.; Xiao, D.Q. Effects of meteorological factors on Tripterygium wilfordii seedling and greenhouse control. Chin. Agric. Sci. Bull. 2011, 27, 214–218. [Google Scholar]
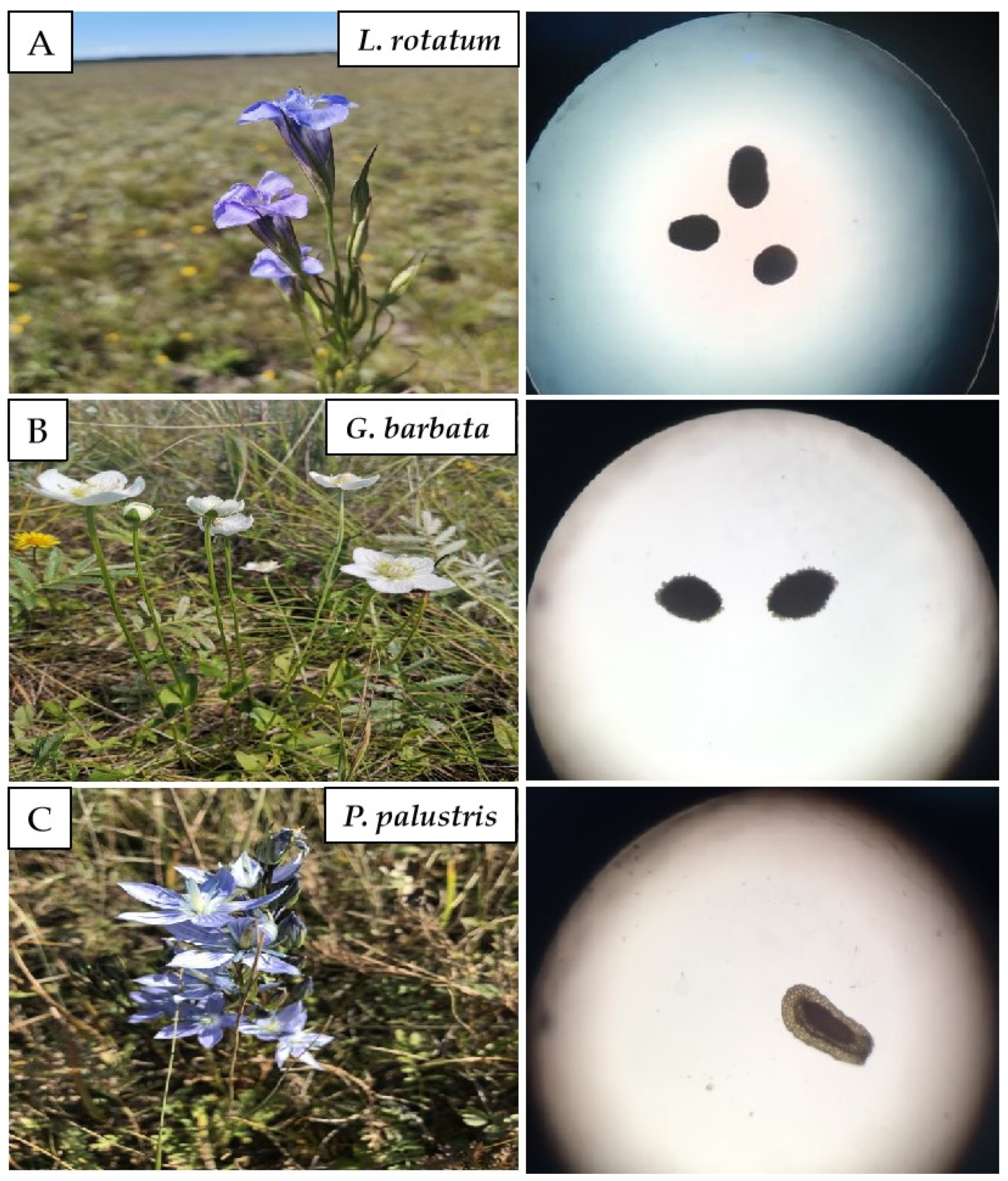
| Plant Name | Geographic Location | Time | Parts |
|---|---|---|---|
| Lomatogonium rotatum (L.) Fries ex Nym | 49°11′27″ N 119°44′1″ E | 10 August 2021 | Mature seeds |
| Gentianopsis barbata (Froel.) Ma. | 49°11′27″ N 119°44′1″ E | 10 August 2021 | Mature seeds |
| Parnassis palustris L. | 49°11′27″ N 119°44′1″ E | 10 August 2021 | Mature seeds |
| Plant Name | Number of Samples | Average Weight of 1000 Seeds | Average Length (mm) | Average Width (mm) |
|---|---|---|---|---|
| Lomatogonium rotatum (L.) Fries ex Nym | 6 | 0.02 a | 0.36 b | 0.22 a |
| Gentianopsis barbata (Froel.) Ma. | 6 | 0.04 a | 1.00 a | 0.50 b |
| Parnassis palustris L. | 6 | 0.03 a | 0.95 a | 0.10 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ta, N.; A, L.; Bai, S.; Mu, X.; Bai, L.; A, R.; Feng, L.; Fu, M. Morphological and Seed Germination Behavior of Three Herba Swertiae Species from Hulunbuir, Inner Mongolia: Temperature and Substrate Effects. Seeds 2022, 1, 221-229. https://doi.org/10.3390/seeds1040019
Ta N, A L, Bai S, Mu X, Bai L, A R, Feng L, Fu M. Morphological and Seed Germination Behavior of Three Herba Swertiae Species from Hulunbuir, Inner Mongolia: Temperature and Substrate Effects. Seeds. 2022; 1(4):221-229. https://doi.org/10.3390/seeds1040019
Chicago/Turabian StyleTa, Na, Lisha A, Siriguleng Bai, Xiyele Mu, Li Bai, Rure A, Lan Feng, and Minghai Fu. 2022. "Morphological and Seed Germination Behavior of Three Herba Swertiae Species from Hulunbuir, Inner Mongolia: Temperature and Substrate Effects" Seeds 1, no. 4: 221-229. https://doi.org/10.3390/seeds1040019
APA StyleTa, N., A, L., Bai, S., Mu, X., Bai, L., A, R., Feng, L., & Fu, M. (2022). Morphological and Seed Germination Behavior of Three Herba Swertiae Species from Hulunbuir, Inner Mongolia: Temperature and Substrate Effects. Seeds, 1(4), 221-229. https://doi.org/10.3390/seeds1040019







