Abstract
The knowledge of the physiological aspects of Caatinga’s vegetal species is extremely important for preserving this biome, which suffers with human impacts, mainly to select propagation methods. Erythrina velutina is a Brazilian tree, generally found in Caatinga, with medical and forestry potential. The objective of this paper was to determine the best soaking period in gibberellin solution to achieve the highest germination and to evaluate the internal anatomy by digital microtomography of E. velutina seeds. The design was completely randomized and consisted of eight treatments: 1, 3, 6, 12, 24 and 48 h of soaking, scarified seeds and the control with no-scarified seeds. Digital microtomography was efficient in describing the anatomy of the seeds and distinguishing their tissues. There was no significant difference between the treatments at different soaking times, as the gibberellic acid did not influence the germination; only the control presented a lower germination percentage, differing from the other treatments. The study presents evidence that E. velutina seeds do not require the exogenous use of gibberellic acid, but only the scarification process. In addition, the use of digital microtomography can be useful in understanding the anatomy of seeds, especially forest species, which can contribute to the future studies of other plant species.
1. Introduction
The native vegetation of the Caatinga biome is characterized by great floristic and physiognomic spatial variability, with a predominance of shrub, woody, and xerophytic species, rich in biodiversity and with approximately 3150 catalogued species, of which 23% are endemic [1,2]. The Caatinga is a patchwork of thorny bushes and seasonally dry forests which stretch across 844.453 km² of the semiarid region from the north of Brazil [3]. The natural beauty of this biome, according to [4], is losing its geographical and ecological characteristics due to its intense usage in socioeconomics activities, and it is suffering from these anthropic activities. Although 50% of the original Caatinga vegetal covering is already modified by human action [5], only 1.75% of the original Caatinga vegetal covering is protected by Integral Protection Conservation Units, and 7% by Sustainable Use Conservation Units [6]. Considering this, studies about the species that are a part of this vegetal complex are incredibly necessary, especially those focused on increasing knowledge about species, including mulungu (Erythrina velutina Willd).
The E. velutina is a pioneer specie, heliophile and intolerable to cold [7], with a wide geographic distribution in the northeast region of Brazil [8]. This tree is very appreciated in ornamental usage [9,10], in the recovering of a degraded area, is used in popular medicine [11,12], and has an allelopathic and insecticide effect [12,13,14], and these are the reasons why it deserves special attention considering its physiology. For this purpose, studies focusing on its seeds must be realized, for example, on the gibberellic acid effects on its germination and vigor.
For most Fabaceae species, an impermeability to water in the integument can also be caused by the presence of one or more layers of elongated Malpighi cells (macrosclereids) impregnated with water-repellent chemicals [15]. The integument of E. velutina seeds is anatomically similar to most other legume seeds, consisting of a palisade layer, composed of compacted macrosclereid cells, a layer of osteosclereids, and several layers of sclerified parenchyma [16]. The seeds of E. velutina are exotestal, according to [17], as they present a rigid palisade layer (exotest) as the external epidermis that constitutes the forehead, whose cells are provided with thick lignified walls.
Gibberellin acts at different stages of plant development, including the height and diameter, at first node height, the foliar area and phytomass production [18], root extension, the size and format of the leaves, and the development of flowers and fruits. It participates in the activation of the embryo vegetative growth, in the weakening of the endosperm that involves the embryo and restricts its development, and in the mobilization of the endosperm energy stocks [19]; therefore, an exogenous application can contribute to improving the germination parameters of E. velutina, since this species presents difficulties, as previously explained.
Combined with other efficient techniques used in seed technology, X-ray computed microtomography (micro-CT) is a non-destructive, high-resolution image analysis method that provides evaluations in 3D space, based on sample projections at different angles, and allowing the internal reconstruction of the same; thus, the technique makes it possible to visualize and measure the internal morphological aspects in seeds, without causing any damage or losses. The use of image analysis in seeds has evolved as a technological resource for individual, accurate and non-destructive assessment, thus enabling the observation of their characteristics and subsequent performance assessments [20].
Using the micro-CT in studies which involve the internal morphology of seeds, to date, has been limited [21], due to the cost of equipment and the reduced number of professionals trained to carry out the measurements and analyses, although this technique is already efficient in the characterization of mechanical damage, insect injuries and the anatomical characterization of seeds.
Given the relevance of such studies and the scarcity of information on the propagation techniques for native forest species in the Caatinga, this work aimed to characterize the internal anatomy and evaluate the effect of different soaking times in a gibberellin solution on the germination and vigor of E. velutina seeds.
2. Material and Methods
This research took place at the Seed Analysis Laboratory of the Agrarian Science Center at the State University of Londrina, in Londrina/PR. A batch of E. velutina seeds, collected in 2018, from matrices without a known genotype in the city of Guanambi/BA (Latitude 14°13′01′ S, Longitude 42°46′40′ W) was used.
The three-dimensional images of the seeds were carried out in the Laboratory of X-ray Applications (LARX) in a SkyScan-Bruker microtomograph, model 1173, using a voltage of 45 kV, 140 µA of current and a resolution of 10 µm, without using a filter. Each projection was taken using an exposure time of one second, with an angular step of 0.3° in 180°. The projections were reconstructed using the NRecon software and the images evaluated using the CTVox software. Five seeds were used to verify the integument, cotyledons and structures such as the hilum, rafe and micropyle.
Gibberellic acid (GA3) (90%) powder was dissolved at a concentration of 200 mg L−1. After separating the necessary amount of GA3 (200 g), it was initially diluted completely in 70% alcohol and then distilled water was added to complete 1 L. Subsequently, all the seeds were disinfected and manually scarified with #80 wood sandpaper, in order to break the integument on the opposite side of the hilum. Only the control seeds (intact seeds and without imbibition) were not scarified.
The trial design was completely randomized with eight treatments and four replications, with 25 seeds each. After this procedure, the following treatments were composed: control; mechanical scarification; mechanical scarification + soaking in gibberellin solution for 1 h, 3 h, 6 h, 12 h, 24 h and 48 h.
The imbibition took place in the dark, in 500 mL beakers, lined with aluminum foil sheets. After the soaking time was completed, the seeds were placed in laboratory plastic trays at a depth of 2 cm with only sand as the substrate. The sand was sieved and sterilized in an oven at 100 °C for two days, for ten hours a day. The seeds were considered germinated when the plumular hook was exposed through the sand. At 15 days after sowing, with daily data on the number of emerged seeds, the following variables were calculated:
- % of germination (G) or germinability, calculated through [22] formula:in which: N = the number of germinated seeds by the end of the test; A = the total number of seeds placed to germinate.G = (N/A) × 100
- Germination speed index (GSI), calculated by the formula of [23]:in which: ESI = the germination speed index; G1, G2, Gn = the number of seeds germinated in the first, second and last counting; N1, N2, Nn = the number of sowing days at first, second and last counting.GSI = G1/N1 + G2/N2 + ⋯ + Gn/Nn
- Mean germination time (t) in days, calculated by the formula [24]:in which: ni = the number of seeds germinated by day; ti = the time of incubation.t = (∑niti)/∑ni
- Average germination speed (AGS) in days, calculated by the formula [24]:in which: t = the medium germination time.AGS = 1/t
- Relative germination frequency, in percentage, according to the formula [22]:in which: Fr = the germination relative frequency; ni = the number of seeds germinated by day; ∑ni = the total amount of germinated seeds.
The experiment was conducted under natural temperature conditions with a temperature of 25 °C and a relative humidity of 80%, in a greenhouse, with water supplementation replenished daily. The seedlings mass was obtained by drying in a forced air circulation oven at 70 °C until a constant mass was obtained.
The data were subjected to one-way analysis of variance at a 5% significance. The assumptions of normality of errors and homoscedasticity of variances were tested by the Shapiro–Wilk and Bartlett tests, respectively. If significant, a linear or non-linear regression analysis was performed (p < 0.05). The germination curve was analyzed using a three-parameter logistic regression using the drc package [25]. All analyses were performed using the R software [26].
3. Results
3.1. Anatomical Aspects
Figure 1A,B show the images obtained, which are efficient for anatomical observations in seeds, enabling a clear identification of their structures. Micro-CT made it possible to observe the seeds in 3D, reconstructing the image in several sessions, allowing the evaluation of the internal structures and identification of tissues, through a non-destructive internal analysis.
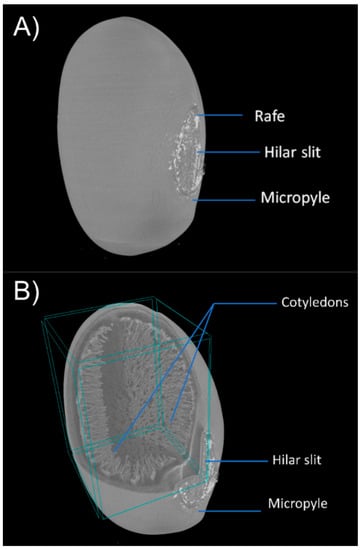
Figure 1.
Anatomical characterization of E. velutina seeds by microtomography demonstrating the structure of the raphe, hilar slit, micropyle of the seed externally (A) and internally (B).
The seed is stenospermic, elongated, reniform, with a rounded apex and rounded-truncated base (Figure 1A), oblong hilum, slightly sunken, and a brownish-brown longitudinal hilar cleft. The micropyle is punctiform, the raphe is small as an enlarged and raised stripe, with brown tones, located above the hilum and opposite the micropyle (Figure 1A and Figure 2B). The raphe is an extension of the funiculus, seen in the integument as a bulge. The integument of E. velutina seeds is cartaceous-coriaceous, with a smooth surface, reddish-orange color, and vernish with a smooth surface. The integuments of the ovum originate the integument, resulting in the forehead and the tegma or tegmen, through the primin and secundine, respectively, with a clear differentiation between the forehead and the tegma, present in a bitegumented seed (Figure 2A,B).
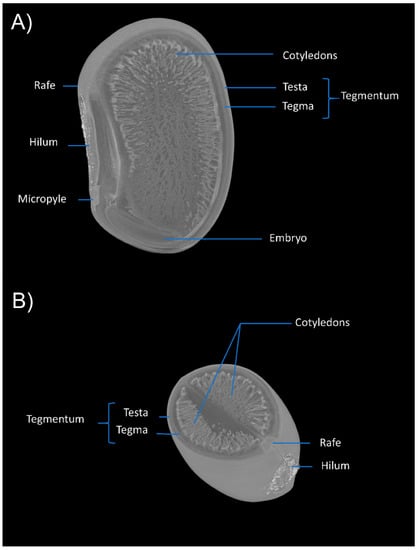
Figure 2.
Three-dimensional anatomical characterization of E. velutina seeds demonstrating the structures of the rafe, hilum, micropyle, cotyledons, tegmentum (testa, tegma) and embryo in vertical (A) and horizontal (B) positions.
The Embryo is axial curved, dominant and papillary, reniform, and cream-colored, (Figure 2A) consisting of fleshy, large, thick, whitish, asymmetric, plane-convex, mucronate cotyledons at the apex, which completely cover the hypocotyl–radicle axis, with a venation that reaches throughout the cotyledonary area, extending from a point at its base. The seed is exalbuminous, with all the reserve material stored in the cotyledons, so that they occupy the entire length of the seed (Figure 2A). The sectioning of the image in several observation sessions makes it possible to analyze the anatomical differentiations of the seed in the raphe–embryo axis, exposing the tissue configuration and arrangement.
3.2. Germination Aspects
Figure 3 shows the cumulative results of the germination over time using logistic models. In general, there was a need to carry out the scarification, since the control treatment had a lower germination percentage than the other treatments. Increasing the soaking time at the concentration of 200 mg L−1 of GA3 reduced the time for germination, especially the treatments with 12, 24 and 48 h; however, they were the ones with the lowest germination percentage. On the other hand, the treatments scarified with 0 or 1 h of imbibition, although presenting a delay in germination, were the ones with the highest final percentage (Figure 4B).
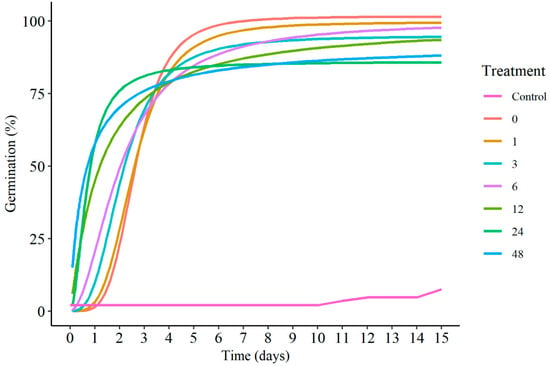
Figure 3.
Cumulative germination of E. velutina seeds during the 15 day monitoring period for the different immersion treatments in GA3 solution. Control: without scarification and gibberellin; 0, 1, 3, 6, 12, 24 and 48 indicate the scarified treatment under a different soaking time (in hours).
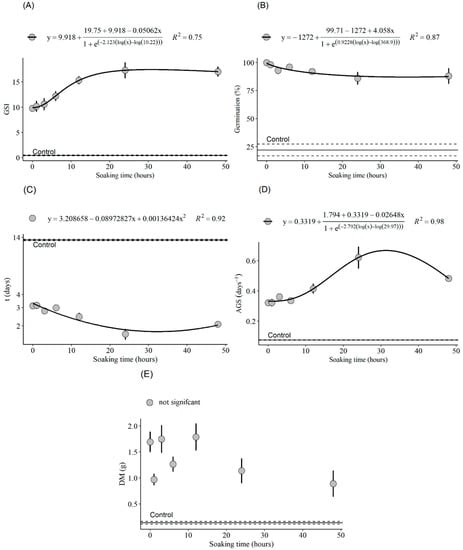
Figure 4.
GSI (A), total germination (B), mean germination time (C), germination speed (D) and dry mass (E) as a function of soaking time. Bars indicate standard deviation.
The use of GA3 during the imbibition showed a statistical difference for the germination speed index (Figure 4A), with logistic behavior and with a maximum response close to 20 h, then a slight decrease from that point on. Regarding the average germination time (Figure 4C), it was found that it presented a quadratic behavior with a minimum response in the estimated time of 32.89 h. The average germination speed, inversely proportional to the average germination time, was higher in the seeds germinated under immersion for the longest time (Figure 4D) and showed a maximum response in the estimated time of 31.32 h of imbibition. In relation to the seedling dry mass, no significant trend was observed (Figure 4E). As for the germination over time, in the variables GSI, t, AGS and dry mass, there was a pronounced difference between the scarified treatments compared to the non-scarified control.
The results of the germination frequency of E. velutina seeds are shown in Figure 5. The seed germination was distributed differently, in the studied immersion times, over time. In 1 h, 3 h, 12 h, 24 h, 48 h and the mechanical scarification immersions, the graphics had a unimodal character, and in the intact seeds and a 6 h immersion they acquired a polymodal character. According to [27], polymodal curves mean treatments in which the germination is heterogeneous and extended over a long period of time and the unimodal curves present a germination peak, showing a uniform germination pointing to an efficient treatment, capable of anticipating the germination process in the seeds of a certain species.
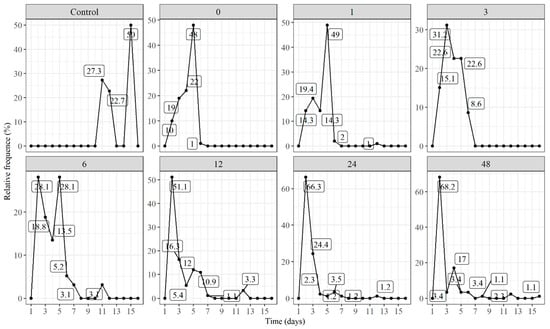
Figure 5.
Relative frequency of germination of E. velutina seeds under immersion in gibberellic acid solution. Control: without scarification and gibberellin; 0, 1, 3, 6, 12, 24 and 48 indicate the scarified treatment under a different soaking time (in hours).
The germination peaks showed different behavior between the treatments, with peaks as early as the 1st days after planting (D.A.P.) for treatments with an immersion of 12, 24 and 48 h. The treatments with an immersion for 3 and 6 h showed a peak between the 1st and 4th days after planting, and the treatments only under mechanical scarification and with an immersion for 1 h showed a peak on the 4th D.A.P. Only the treatment with intact seeds showed a peak between the 14th and 15th D.A.P. For all treatments, the characteristic of a leptokurtic distribution was maintained, that is, when seed germination occurs more concentrated in time.
4. Discussion
The X-ray digital microtomography method allowed the identification of the structures that compose the seed, with characteristics similar to those reported by [28], demonstrating that such methodology can be used to study the anatomy of seeds. The structure of the integument of E. velutina seeds is maple-leather, with a smooth surface, red-orange color, and vernish with a smooth surface. This integument performs fundamental functions to guarantee the vitality of the seeds [20], as an aid in the gas exchange between the seed and the environment, for the protection of the developing embryo against climatic, biotic, mechanical or chemical agents, keeping the parts of the seed together, regulating germination and dormancy, and transferring growth substances to the embryo. In the case of E. velutina, the integument prevents the exchange of moisture between the seed and the medium [29], and in this way, imbibition and scarification are important processes for the successful germination of this species.
Results showing that there is no need to use gibberellin in seed germination were also verified by [30] in pomegranate, by [31] in Passiflora ligularis JUSS and Passiflora edulis F. flavicarpa, by [32] in Pfaffia glomerata, by [33] in Psidium guineense, in clove lemon, by [34] and in Tabernaemontana catharinensis, by [35].
The lower percentage of germination in the control treatment without scarification was due to the fact that E. velutina seeds have tegumentary dormancy, which causes the tegument to be impermeable to water, preventing the exchange of seed moisture with the medium [29]. This is especially attributed to the palisade cell layer that prevents water absorption and imposes a mechanical restriction on the embryo’s growth, thus preventing germination [20].
The impermeability of the seed coat comprises cells rich in hydrophobic substances (e.g., cutin, lignin, quinones, suberin and wax) forming a protective barrier between the embryo and the environment [36].
The germination of seeds submitted to the non-scarified control treatment took place from the eleventh day, reaching the percentage of 22%, as found by [37] in which the control treatment involved minor percentage rates of E. velutina germination; thus, some scarification is necessary to enable germination, in contrast to [38], who recommended planting seeds without any pre-germination treatment.
The reduced germination percentage values in E. velutina seeds without a pre-germination treatment intervention were also pointed out by [27] with 14%, by [39] with 5%, by [40] with 12% and [41] with 10%. T2, in which GA3 was not applied, but only scarification, had the highest germination percentage, with 100% germinated seeds. A possible hypothesis for this result is the fact that the germination process of the seeds in the present study required aeration, that is, the presence of oxygen to germinate, with the oxygen content in the atmosphere being totally sufficient, so that germination may decrease if its tension is lower than normal in the atmosphere [42,43]. Hydration was continuous, as all the necessary water was added to the germination tray daily, a fact that also allowed the rapid activation of the germination process.
The hormonal balance of E. velutina seeds is probably favorable for germination, which may explain the fact that the supply of GA3 did not influence the percentage of germination. This fact was also evidenced by [30] in a study with pomegranate seeds immersed in a solution of different concentrations of GA3.
In the case of the GSI, the abundant availability of water provided the seed with a greater imbibition speed, a fact that benefited the early emergence of the radicle [43]. The decrease in the GSI value in the longer immersion times in the GA3 concentration may be related to the fact that this gibberellin, when diluted in water, changed the osmotic potential of the solution in the run time, consequently modifying the water potential and, thus, making it difficult for the entrance of water into the seed. Another aspect that should be highlighted is that, probably, when subjected to solutions with a longer immersion time, the seeds may have suffered phytotoxicity due to a hormonal imbalance.
In addition, [24] commented that the average time corresponds to the average of the time required for a set of seeds to germinate, giving the process a kinetic character, with the average being a measure of the central tendency of a given set of values, in this case, the number of germinated seeds. These results follow the same understanding of the GSI, in which the treatments with longer immersion times had the best mean time values, that is, the lowest mean germination time values.
Probably, the period of the time that the plantlets remained in the germination trays allowed all of them to reach their maximum size, because, as the medium did not provide any nutrients, the plantlets grew using only their reserves, assuming that these were practically the same.
5. Conclusions
Digital microtomography is efficient to characterize the anatomy of seeds, allowing a clear and accurate evaluation of the internal structures, such as the cotyledons, integument forming layers, embryo, micropyle and hilar cleft, thus being a promising technique for seed technology. The germination of E. velutina seeds was little influenced by the different periods of imbibition of these seeds in gibberellic acid, with the greatest differences occurring as a function of the scarification process.
Author Contributions
Conceptualization, H.R.G. and H.C.O.; methodology, H.R.G., M.H.S.P., H.V.S., W.A.R.J. and J.C.B.P.; validation, H.R.G., H.C.O., G.D.S., R.Y.P.M., E.I.J. and A.C.A.; formal analysis, G.D.S., R.Y.P.M., E.I.J. and A.C.A.; investigation, H.R.G., J.R., A.P.S.C. and K.A.M.M.; writing—original draft preparation, H.R.G., J.C.B.P., G.D.S. and M.H.S.P.; writing—review and editing, H.C.O. and H.R.G.; visualization, J.R.; A.P.S.C., K.A.M.M. and W.A.R.J.; supervision, H.C.O., E.I.J. and A.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Queiroz, L.P.; Cardoso, D.; Fernandes, M.F.; Moro, M.F. Diversity and Evolution of Flowering Plants of the Caatinga Domain. In Caatinga; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Junior, C.R.P.; Salvador, C.A.; Tavares, T.R.; Abreu, M.C.; Fagundes, H.S.; Almeida, W.S.; Neto, E.C.S.; Anjos, L.H.C.; Pereira, M.G. Lithic soils in the semi-arid region of Brazil: Edaphic characterization and susceptibility to erosion. J. Arid. Land 2022, 14, 56–69. [Google Scholar] [CrossRef]
- Salazar, A.A.; Arellano, E.C.; Muñoz-Sáez, A.; Miranda, M.D.; da Silva, F.O.; Zielonka, N.B.; Crowther, L.P.; Silva-Ferreira, V.; Oliveira-Reboucas, P.; Dicks, L.V. Restoration and conservation of priority areas of Caatinga’s semi-arid forest remnants can support connectivity within an agricultural landscape. Land 2021, 10, 550. [Google Scholar] [CrossRef]
- Ribeiro, E.M.S.; Arroyo-Rodríguez, V.; Santos, B.; Tabarelli, M.; Leal, I.R. Chronic anthropogenic disturbance drives the biological impoverishment of the Brazilian Caatinga vegetation. J. Appl. Ecol. 2015, 52, 611–620. [Google Scholar] [CrossRef]
- Schulz, K.; Voigt, K.; Beusch, C.; Almeida-Cortez, J.S.; Kowarik, I.; Walz, A.; Cierjacks, A. Grazing deteriorates the soil carbon stocks of Caatinga forest ecosystems in Brazil. For. Ecol. Manag. 2016, 367, 62–70. [Google Scholar] [CrossRef]
- Antongiovanni, M.; Venticinque, E.M.; Fonseca, C.R. Fragmentation patterns of the Caatinga drylands. Landsc. Ecol. 2018, 33, 1353–1367. [Google Scholar] [CrossRef]
- Carvalho, P.E.M. Mulungu (Erythrina velutina); Circular Técnica 160; Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA, Embrapa Florestas: Colombo, PR, Brazil, 2008; pp. 1–8. [Google Scholar]
- Brasileiro, A.C.; Lima-Araujo, F.; Alcântara, J.A.; Pontes, A.S.M.; Neto, J.A.; de Oliveira Tavares, R.; Silvino, A.S.; Vizentin-Bugoni, J. Birds of the Parque Ecológico Lagoa da Fazenda, Sobral, Ceará state, northeastern Brazil. Check List 2017, 13, 2037. [Google Scholar] [CrossRef]
- Rodrigues, D.R.; da Silva, A.F.; Cavalcanti, M.I.P.; Escobar, I.E.C.; Fraiz, A.C.R.; Ribeiro, P.R.D.A.; Neto, R.A.F.; de Freitas, A.D.S.; Fernandes-Júnior, P.I. Phenotypic, genetic and symbiotic characterization of Erythrina velutina rhizobia from Caatinga dry forest. Braz. J. Microbiol. 2018, 49, 503–512. [Google Scholar] [CrossRef]
- Silva, P.A.; Silva, L.L.; Brito, L. Using bird-flower interactions to select native tree resources for urban afforestation: The case of Erythrina velutina. Urban For. Urban Green. 2020, 51, 126677. [Google Scholar] [CrossRef]
- da Silva, M.M.; Santana, A.S.; Pimentel, R.M.; Silva, F.C.; Randau, K.P.; Soares, L.A. Anatomy of leaf and stem of Erythrina velutina. Rev. Bras. Farm. 2013, 23, 200–206. [Google Scholar] [CrossRef]
- Oliveira, A.K.; Coelho, M.F.B.; Maia, S.S.S.; Diógenes, F.E.P.; Medeiros Filho, S. Allelopathy of extracts of different organs of coral tree on the germination of lettuce. Hortic. Bras. 2012, 30, 480–483. [Google Scholar] [CrossRef]
- Santos, L.W.; Coelho, M.F.B.; Azevedo, R.A.B.; Lima, A.K.B.; Souza, J.W.N. Erythrina velutina Willd: Fabaceae: Árvore de múltiplos usos no nordeste brasileiro. Rev. Verde Agroecol. E Desenvolv. Sustentável 2013, 8, 72–80. [Google Scholar]
- Agra, M.F.; Queiroz, R.T. Erythrina velutina Mulungu. In Espécies Nativas da Flora Brasileira de Valor Econômico Atual ou Potencial: Plantas Para o Futuro: Região Nordeste; Coradin, L., Camillo, J., Pareyn, F.G.C., Eds.; Ministério do Meio Ambiente, Secretaria de Biodiversidade: Brasília, DF, Brazil, 2018; pp. 789–900. [Google Scholar]
- Geisler, G.E.; Pinto, T.T.; Santos, M.; Paulilo, M.T.S. Seed structures in water uptake, dormancy release and germination of two tropical forest Fabaceae species with physically dormant seeds. Braz. J. Bot. 2016, 40, 67–77. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [PubMed]
- Gloria, B.A.; Guerreiro, S.M.C. Anatomia Vegetal; Editora UFV: Viçosa, MG, Brazil, 2012. [Google Scholar]
- Almeida, G.M.; Rodrigues, J.G.L. Development of plants by interference auxins, cytokinins, gibberellins and ethylene. Braz. J. Appl. Technol. Agric. Sci. 2016, 9, 111–117. [Google Scholar]
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and seed germination. Annu. Plant Rev. 2016, 49, 253–284. [Google Scholar]
- Filho, J.M. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Gomes Junior, F.G.; Cicero, S.M. X-ray analysis to assess mechanical damage in sweet corn seeds. J. Seed Sci. 2012, 34, 78–85. [Google Scholar] [CrossRef]
- Labouriau, L.G.; Valadares, M.E.B. On the germination of seeds Calotropis procera (Ait.) Ait.f. An. Da Acad. Bras. De Ciências 1976, 48, 263–284. [Google Scholar]
- Maguire, J.D. Speeds of germination-aid selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Ferreira, A.G.; Borghetti, F. Germinação—do Básico ao Aplicado; Artmed: Porto Alegre, RS, Brazil, 2013. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 21 October 2021).
- Matheus, M.T.; Guimarães, R.M.; Bacelar, M.; Oliveira, S.A.D.S. Dormancy overcome of two Erythrina species seeds. Rev. Caatinga 2010, 23, 48–53. [Google Scholar]
- Silva, K.B.; Alves, E.U.; Bruno, R.L.A.; Matos, V.P.; Gonçalves, E.P. Morfologia de frutos, sementes, plântulas e plantas de Erythrina velutina willd, Leguminoseae-Papilionideae. J. Seed Sci. 2008, 30, 104–114. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Gomes, S.E.V.; Dantas, B.F. Physiological quality of Erythrina velutina Willd. seeds (Fabaceae) under different storage conditions. Sci. For. 2018, 46, 562–570. [Google Scholar] [CrossRef]
- Takata, W.; Silva, E.G.; Corsato, J.M.; Ferreira, G. Germination of pomegranate (Punica granatum L.) seeds under gibberellin concentrations. Rev. Bras. Frutic. 2014, 36, 254–260. [Google Scholar] [CrossRef]
- Cárdenas, J.; Carranza, C.; Miranda, D.; Magnitskiy, S. Effect of GA3, KNO3, and removing of basal point of seeds on germination of sweet granadilla (Passiflora ligularis JUSS) and yellow passion fruit (Passiflora edulis F. flavicarpa). Rev. Bras. Frutic. 2013, 35, 853–859. [Google Scholar] [CrossRef]
- Nobrega, M.A.S.; Pontes, M.S.; Santiago, E.F. Aplicação exógena de GA3 e tiametoxam sobre a dinâmica da germinação de sementes de Psidium guineense Swartz (Myrtaceae). Acta Biomed. Bras. 2018, 9, 58–66. [Google Scholar] [CrossRef]
- De Sousa, H.U.; Ramos, J.D.; Pasqual, M.; Ferreira, E.A. Efeito do ácido giberélico sobre a germinação de sementes de porta-enxertos cítricos. Rev. Bras. Frutic. 2002, 24, 496–499. [Google Scholar] [CrossRef]
- Afonso, M.V.; Paranhos, J.T.; Tabaldi, L.A.; Soriani, H.H. Germinação in vitro de sementes e parâmetros morfofisiológicos de microestacas de Tabernaemontana catharinensis A. DC. Iheringia. Série Botânica 2018, 73, 39–45. [Google Scholar] [CrossRef]
- Junior, C.A.; de Oliveira Vitoriano, J.; Da Silva, D.L.S.; de Lima Farias, M.; de Lima Dantas, N.B. Water uptake mechanism and germination of Erythrina velutina seeds treated with atmospheric plasma. Sci. Rep. 2016, 6, srep33722. [Google Scholar] [CrossRef]
- Siqueira, J.V.G.; Barros, J.P.A.; Araújo, Y.P.; Silva, T.G.F.; Souza, L.S.B. Tratamentos pré-germinativos em sementes de espécies da Caatinga. J. Environ. Anal. Prog. 2017, 2, 499–508. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Editora Plantarum: Nova Odessa, SP, Brazil, 2016. [Google Scholar]
- Silva, K.B.; Alves, E.U.; Bruno, R.L.A.; Pereira, E.; Gonçalves, M.D.S.S.B.; Viana, J.S. Quebra de dormência em sementes de Erythina velutina Willd. Rev. Bras. De Biociências 2007, 5, 180–182. [Google Scholar]
- Rissi, R.N.; Júnior, R.F.G. Escarificação de sementes e quebra de dormência de mulungu (Erythrina velutina WILLD.—Leguminosae). Rev. Biol. FAFIBE 2011, 1, 1–11. [Google Scholar]
- Souza, V.N.; Araújo, A.V.; Pinto, M.A.D.C.; Brito, A.S. Tratamentos físicos e químicos para acelerar e uniformizar a emergência de plântulas de Erythrina velutina Willd. Enciclopédia Biosf. 2016, 13, 1732–1741. [Google Scholar] [CrossRef]
- Phartyal, S.S.; Rosbakh, S.; Ritz, C.; Poschlod, P. Ready for change: Seed traits contribute to the high adaptability of mudflat species to their unpredictable habitat. J. Veg. Sci. 2019, 31, 331–342. [Google Scholar] [CrossRef]
- Rosbakh, S.; Phartyal, S.S.; Poschlod, P. Seed germination traits shape community assembly along a hydroperiod gradient. Ann. Bot. 2019, 125, 67–78. [Google Scholar] [CrossRef]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a Mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).